Abstract
Background:
Observational studies have reported a modest association between obesity and risk of ovarian cancer; however, whether it is also associated with survival and whether this association varies for the different histologic subtypes are not clear. We undertook an international collaborative analysis to assess the association between body mass index (BMI), assessed shortly before diagnosis, progression-free survival (PFS), ovarian cancer-specific survival and overall survival (OS) among women with invasive ovarian cancer.
Methods:
We used original data from 21 studies, which included 12 390 women with ovarian carcinoma. We combined study-specific adjusted hazard ratios (HRs) using random-effects models to estimate pooled HRs (pHR). We further explored associations by histologic subtype.
Results:
Overall, 6715 (54%) deaths occurred during follow-up. A significant OS disadvantage was observed for women who were obese (BMI: 30–34.9, pHR: 1.10 (95% confidence intervals (CIs): 0.99–1.23); BMI: ⩾35, pHR: 1.12 (95% CI: 1.01–1.25)). Results were similar for PFS and ovarian cancer-specific survival. In analyses stratified by histologic subtype, associations were strongest for women with low-grade serous (pHR: 1.12 per 5 kg m−2) and endometrioid subtypes (pHR: 1.08 per 5 kg m−2), and more modest for the high-grade serous (pHR: 1.04 per 5 kg m−2) subtype, but only the association with high-grade serous cancers was significant.
Conclusions:
Higher BMI is associated with adverse survival among the majority of women with ovarian cancer.
Keywords: ovarian cancer, obesity, overall survival, progression-free survival, ovarian cancer-specific survival
Ovarian cancer survival is poor, with only ∼30–50% of women alive 5 years after diagnosis and over 140 000 deaths globally per year (Jemal et al, 2011). The key prognostic factors – age, stage and grade of tumour – are not modifiable at diagnosis. Understanding how potentially modifiable factors such as obesity influence survival following a diagnosis of ovarian cancer could potentially be harnessed as a means of reducing a woman's risk of cancer progression or recurrence.
Women who are overweight or obese have increased risks of developing many types of cancer, including ovarian, when compared with women of normal weight (Reeves et al, 2007). Two large pooled analyses recently confirmed that this increased risk for ovarian cancer is modest (odds ratios ∼10% increase in risk per 5 kg m−2 increase in body mass index (BMI) (Collaborative Group on Epidemiological Studies of Ovarian Cancer, 2012; Olsen et al, 2013) and may be restricted to non-high-grade serous subtypes (Olsen et al, 2013). Evidence that obesity is a poor prognostic factor for several malignancies including the breast, prostate and colon is increasing (Calle et al, 2003; Parekh et al, 2012) and several lines of evidence suggest that obesity may also be associated with poor survival among women with ovarian cancer (Ptak et al, 2013; Diaz et al, 2013; Makowski et al, 2014). A recent meta-analysis of 14 studies concluded that women with ovarian cancer, who were obese, had 17% worse survival compared with those of normal weight (Protani et al, 2012). However, the studies in this meta-analysis varied greatly in the timing of obesity measurement: from usual adult weight to weight at the time of diagnosis, or at the commencement of chemotherapy. Most of the studies included had a relatively small sample size (median=301) and, as a consequence, variation by histologic subtype could not be investigated. Furthermore, few studies had examined progression-free survival (PFS) or ovarian cancer-specific survival.
Using data from 21 case–control studies from the Ovarian Cancer Association Consortium (OCAC), we sought to evaluate the association between BMI and survival (PFS, ovarian cancer-specific and overall survival (OS)), overall and by histologic subtype, in over 12 000 women with invasive epithelial ovarian cancer.
Materials and Methods
The OCAC consortium was founded in 2005 to combine data from individual case–control studies, to provide increased accuracy of estimates of genetic associations with ovarian cancer (Ramus et al, 2008). Twenty-one studies summarized in Table 1 provided BMI data and clinical follow-up information, allowing calculation of 5-year survival estimates for invasive ovarian, fallopian tube or peritoneal cancer cases (full study name and acronym are listed in Supplementary Table 1). All of the studies were approved by their institutional review board and all study participants provided informed consent.
Table 1. Characteristics of 21 OCAC studies included in analysis.
| Site | Country | Source of cases | Diagnosis years | Age range | Number of cases | Reference period for BMI measurement (before diagnosis) | Median (range) BMI (kg m−2) | Median (range) follow-up time among living (years) | Number (%) dead | 5-Year survival (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| AUS (Merritt et al, 2008) | Australia | Population | 2002–2006 | 20–80 | 1404 | 1 Year | 26.1 (46.7) | 7.3 (8.0) | 875 (62.3) | 48.5 |
| BAV (Song et al, 2009) | Germany | Hospital/Clinic | 2002–2006 | 22–84 | 431 | 5 Years | 25.9 (34.2) | 5.6 (25.1) | 236 (54.8) | 47.4 |
| BEL (Song et al, 2009) | Belgium | Hospital/Clinic | 2007–2012 | 18–85 | 477 | 1 Year | 24.7 (34.5) | 3.5 (28.8) | 133 (27.9) | 70.0 |
| CON (Risch et al, 2006) | USA | Population | 1998–2003 | 36–81 | 388 | 5 Years | 24.6 (43.6) | 8.3 (10.1) | 224 (57.7) | 57.6 |
| DOV (Rossing et al, 2007; Bodelon et al, 2012) | USA | Population | 2002–2005 | 35–74 | 1146 | 5 Years | 25.1 (44.8) | 4.4 (8.8) | 486 (42.4) | 55.0 |
| GER (Royar et al, 2001) | Germany | Population | 1993–1996 | 21–75 | 240 | At diagnosis | 24.4 (40.6) | 14.5 (3.9) | 167 (69.6) | 47.1 |
| HAW (Goodman et al, 2008; Lurie et al, 2008) | USA | Population | 1993–2008 | 24–85 | 429 | 5 Years | 25.1 (36.9) | 7.3 (16.5) | 217 (50.6) | 62.2 |
| HOP (Lo-Ciganic et al, 2012) | USA | Population | 2003–2009 | 25–85 | 652 | 1 Year | 27.4 (51.1) | 5.1 (9.1) | 335 (51.4) | 51.3 |
| HSK (du Bois et al, 2003; Harter et al, 2011) | Germany | Hospital/clinic | 2000–2007 | 29–80 | 111 | At diagnosis | 24.2 (21.3) | 5.0 (10.7) | 65 (58.6) | 48.2 |
| JPN (Hamajima et al, 2001) | Japan | Hospital/clinic | 2001–2005 | 23–75 | 65 | 1 Year | 22.4 (12.5) | 3.6 (9.2) | 29 (44.6) | 44.1 |
| MAC+MAY (Kelemen et al, 2008; Goode et al, 2010, 2011) | USA | Hospital/clinic | 1999–2012 | 21–85 | 944 | 1 Year | 26.6 (41.3) | 3.3 (22.0) | 503 (53.3) | 44.0 |
| MAL (Glud et al, 2004; Soegaard et al, 2007) | Denmark | Population | 1994–1999 | 32–80 | 573 | 5 Years | 23.6 (42.2) | 13.8 (4.5) | 438 (76.4) | 43.5 |
| NCO (Schildkraut et al, 2008, 2010) | USA | Population | 1999–2008 | 22–74 | 916 | 1 Year | 26.6 (47.4) | 7.2 (8.6) | 551 (60.2) | 50.2 |
| NEC (Terry et al, 2005; Merritt et al, 2013) | USA | Population | 1992–2003 | 21–77 | 847 | 1 Year | 24.6 (50.3) | 13.3 (10.6) | 490 (57.9) | 58.7 |
| NJO (Bandera et al, 2011; Chandran et al, 2011; Gifkins et al, 2012) | USA | Population | 2002–2008 | 25–81 | 240 | 1 Year | 25.8 (51.4) | 6.2 (7.4) | 113 (47.1) | 61.0 |
| POL (Garcia-Closas et al, 2007) | Poland | Population | 2000–2003 | 24–74 | 268 | 5 Years | 23.9 (21.9) | 5.3 (7.1) | 142 (53.0) | 49.0 |
| PVD (Hogdall et al, 2010; Risum et al, 2011) | Denmark | Hospital/clinic | 2004–2012 | 30–84 | 191 | At diagnosis | 24.2 (32.1) | 4.8 (4.9) | 102 (53.4) | 46.6 |
| STA (McGuire et al, 2004) | USA | Population | 1997–2001 | 21–64 | 499 | At diagnosis | 24.4 (44.7) | 11.0 (13.3) | 284 (56.9) | 54.6 |
| TBO (Pal et al, 2005, 2007; Lacour et al, 2011) | USA | Population | 2000–2012 | 26–85 | 245 | At diagnosis | 24.8 (32.9) | 5.9 (7.9) | 131 (53.5) | 49.9 |
| UCI (Ziogas et al, 2000) | USA | Population | 1993–2005 | 21–84 | 394 | 1 Year | 24.1 (41.2) | 8.7 (17.6) | 179 (45.4) | 73.5 |
| USC (Pike et al, 2004; Wu et al, 2009) | USA | Population | 1992–2009 | 20–84 | 1930 | 1 Year | 24.6 (42.4) | 8.0 (17.7) | 1015 (52.6) | 57.4 |
| TOTAL | 18–85 | 12 390 | 25.1 (54.6) | 6.9 (28.8) | 6715 (54.2) | 53.6 |
Analysis variables
In all but two studies (HSK and PVD), both height and weight were self-reported. For 10 studies, women recalled their weight ∼1 year before diagnosis (AUS (Merritt et al, 2008), BEL (Song et al, 2009), HOP (Lo-Ciganic et al, 2012), JPN (Hamajima et al, 2001), MAC (Goode et al, 2011) and MAY (Kelemen et al, 2008; Goode et al, 2010), NCO (Schildkraut et al, 2008, 2010), NEC (Terry et al, 2005; Merritt et al, 2013), NJO (Bandera et al, 2011; Chandran et al, 2011; Gifkins et al, 2012), UCI (Ziogas et al, 2000) and USC (Pike et al, 2004; Wu et al, 2009)); for six studies, they were asked to recall their weight ∼5 years before diagnosis (BAV (Song et al, 2009), CON (Risch et al, 2006), DOV (Rossing et al, 2007; Bodelon et al, 2012), HAW (Goodman et al, 2008; Lurie et al, 2008), MAL (Glud et al, 2004; Soegaard et al, 2007) and POL (Garcia-Closas et al, 2007)); for three studies, it was recalled weight around the time of diagnosis (GER (Royar et al, 2001), STA (McGuire et al, 2004) and TBO (Lacour et al, 2011; Pal et al, 2005, 2007)); and for two studies, it was reported in the medical records around the time of diagnosis (PVD (Hogdall et al, 2010; Risum et al, 2011) and HSK (du Bois et al, 2003; Harter et al, 2011). This information was used to calculate BMI as weight in kilograms divided by the square of height in metres (kg m−2), which was classified using the World Health Organization (WHO) definitions of obesity (<18.5 ‘underweight' 18.5–24.9 ‘normal weight' 25–29.9 ‘overweight' 30–34.9 ‘class I obesity' 35–39.9 ‘class II obesity' and ⩾40 ‘class III obesity') (World Health Organisation, 1995). Among the 21 OCAC studies, 756 (5.3%) women were with missing BMI information. Of the 12 390 women included in the analyses, BMI ranged from 13.7 to 68.3 kg m−2. Three hundred and seven women were underweight (BMI <18.5) and 71 (0.6%) had BMI values >50 kg m−2.
Covariate information
Each OCAC study submitted their data to Duke University using a standard format. Here the variables were reviewed, discrepancies were checked with individual studies and data merged where necessary. The data included information about variables potentially associated with BMI and/or survival: age (at diagnosis), tumour stage summarized from International Federation of Gynecology and Obstetrics or the Surveillance, Epidemiology, and End Results (SEER) stage (localized, regional, distant and unknown) and tumour grade (well, moderately, poorly undifferentiated and unknown). Residual disease remaining after primary cytoreductive surgery (no macroscopic disease, macroscopic disease ⩽2 cm, macroscopic disease, macroscopic disease, size unknown and tumour not resected) was reported in 9 of the studies and cigarette smoking status (never, current and former smoker) was reported in 17 studies.
Clinical data, definitions and analysis
Vital status and survival time were determined by each study and OS time was calculated from date of diagnosis to date of death, or last follow-up. Cause of death information was available for 1511 of the 6715 women who had died (23%) and, of these, most (94%) had died from ovarian cancer; thus, all-cause mortality was used as the primary outcome. Where time from diagnosis to study recruitment was provided (19 of the 21 studies, not available for HSK and PVD), the left truncation was used to account for time elapsed between date of diagnosis and date of study recruitment, in order to reduce potential survivorship biases arising from the exclusion of eligible women who had died before recruitment. Additional analyses were conducted using death from ovarian cancer as the end point and PFS (defined by each study as time from date of diagnosis to the first confirmed sign of disease progression (clinical, biochemical (i.e., CA125) or radiological progression), death or last follow-up date) where such data were available.
Statistical analysis
We used a two-stage method of analysis. In the first stage, each study was analysed separately. For each study, we used Cox proportional hazards regression models to compute adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between BMI (standard WHO categories and per 5 kg m−2 increase) and survival. The HR for ovarian cancer per 5 kg m−2 increase in BMI was estimated by fitting a log linear trend across intervals of BMI (2.5 units) using the overall median value for each interval, except for the top interval where we used site-specific median values rounded to the nearest integer, to account for the greater variability of BMI in the upper category across study sites. We repeated this analysis using the continuous value of BMI for each woman. We expected that the relationship between BMI and survival might not be linear at very low BMI levels; hence, we excluded women in the ‘underweight' range (BMI <18.5 kg m−2) from these analyses. All study-specific models were adjusted for age (continuous), tumour stage (classified as localized, regional and distant) and tumour grade (well differentiated, moderately differentiated and poorly/undifferentiated). We also adjusted study-specific models for race/ethnicity (non-Hispanic White, Hispanic White, Black, Asian and others) where more than 5% of the study population was not classified as the predominant race. Data on residual disease and cigarette smoking status were not available for all studies; however, we did include these variables in models restricted to studies where they were available. For each study-specific model, we examined possible violation of the proportional hazards assumption by evaluating whether covariate associations with BMI varied over time and allowing them to vary if needed. In the second stage, the pooled BMI effect (pooled HR (pHR)) was calculated using random-effects meta-analysis according to the method of DerSimonian and Laird (1986).
We computed pHRs for BMI for all invasive ovarian cases. Where between-study heterogeneity was evident, we examined the data for potential sources of this heterogeneity considering sample size, study design, study site/region, diagnosis years and median follow-up times (calculated using the reverse Kaplan–Meier method (Clark et al, 2003; Schemper and Smith, 1996)), median BMI, 5-year survival per cent, race/ethnicity and timing of BMI measurement. We also conducted subgroup analyses to examine whether associations between BMI and survival were modified by histologic subtype (low- (well differentiated) and high-grade tumours (moderate/poorly/undifferentiated), serous, mucinous, endometrioid and clear cell cancers), where these data were available. The statistical significance of any observed stratum differences was assessed by including a cross-product term in survival models. All P-values are two-sided. Analyses were performed using SAS 9.2 (SAS Institute, Cary, NC, USA) and Stata 11 (College Station, TX, USA).
Results
Table 1 shows the characteristics of the 21 studies that contributed data from a total of 12 390 women with invasive epithelial ovarian cancer to this analysis. Twelve studies were conducted in the United States, seven in Europe and one each in Australia and Japan. The number of women with invasive epithelial ovarian cancer participating in the studies ranged from 65 (JAP) to 1930 (USC). Women were 18–85 years of age at diagnosis between 1992 and 2012 (most of the OCAC studies capped recruitment at 80 or 85 years and also excluded women younger than 18 years; thus, our cases are slightly younger than those reported in the SEER programme); 15 studies were population based and 6 were hospital/clinic based (two studies from the same population, MAC and MAY, were combined). During the follow-up period, half (54%) of the women had died. The median follow-up time among living women was 6.9 years (maximum 14.5 years). The median BMI was 25.1 kg m−2 (interquartile range: 22.3–29.3 kg m−2).
The clinical characteristics of all the women included in this analysis are shown in Table 2. Women had a mean age of 58 years at diagnosis and the majority had serous (62%), poorly differentiated (56%) and/or distant stage (64%) cancers. Among those with residual disease data available (n=3856), 47% had no macroscopic disease after cytoreductive surgery.
Table 2. Clinical characteristics of 12 390 participants included in analysis.
| Characteristica | Nb | % |
|---|---|---|
|
Age | ||
| Age, years (median) | 58.0 | |
| <40 | 741 | 6.0 |
| 40– <50 | 2286 | 18.5 |
| 50– <60 | 3847 | 31.1 |
| 60– <70 | 3515 | 28.4 |
| 70– <85 | 2001 | 16.2 |
|
Histology | ||
| Serous | 7530 | 62.4 |
| Serous low-grade | 500 | 4.1 |
| Serous high-grade | 6443 | 53.4 |
| Mucinous | 733 | 6.1 |
| Endometrioid | 1683 | 14.0 |
| Clear cell | 896 | 7.4 |
| Other histology | 1222 | 10.1 |
|
Stage | ||
| Localized | 2066 | 17.0 |
| Regional | 2285 | 18.8 |
| Distant | 7809 | 64.2 |
|
Grade | ||
| Well-differentiated | 1336 | 12.1 |
| Moderately differentiated | 2597 | 23.5 |
| Poorly differentiated | 6158 | 55.7 |
| Undifferentiated | 975 | 8.8 |
|
Residual disease after surgery | ||
| No macroscopic disease | 1804 | 46.8 |
| Macroscopic disease ⩽2 cm | 974 | 25.3 |
| Macroscopic disease >2 cm | 250 | 6.5 |
| Macroscopic disease, size unknown | 713 | 18.5 |
| Tumour not resected | 115 | 3.0 |
Participants with unknown histology (n=326), unknown serous high- or low-grade status (n=587), stage (n=230), grade (n=1324) and residual disease (n=8534) were not included in percentages.
Numbers may not add to 12 390 due to missing data.
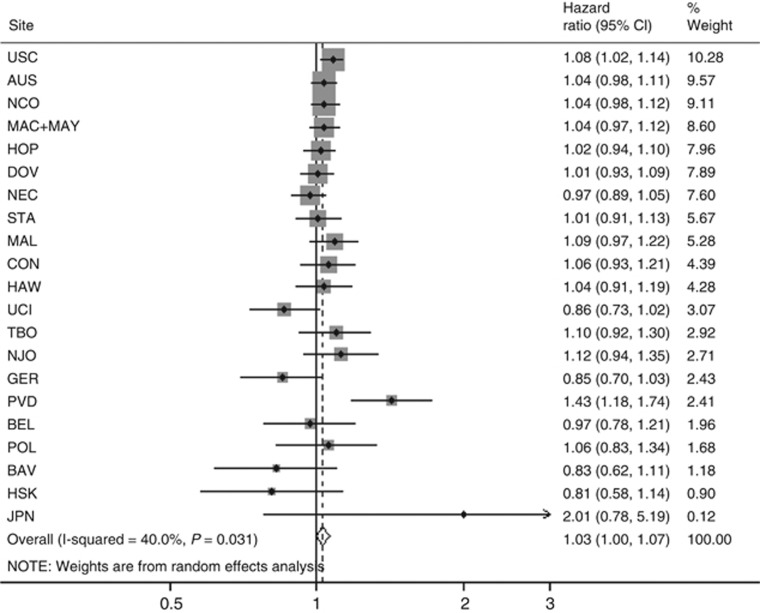
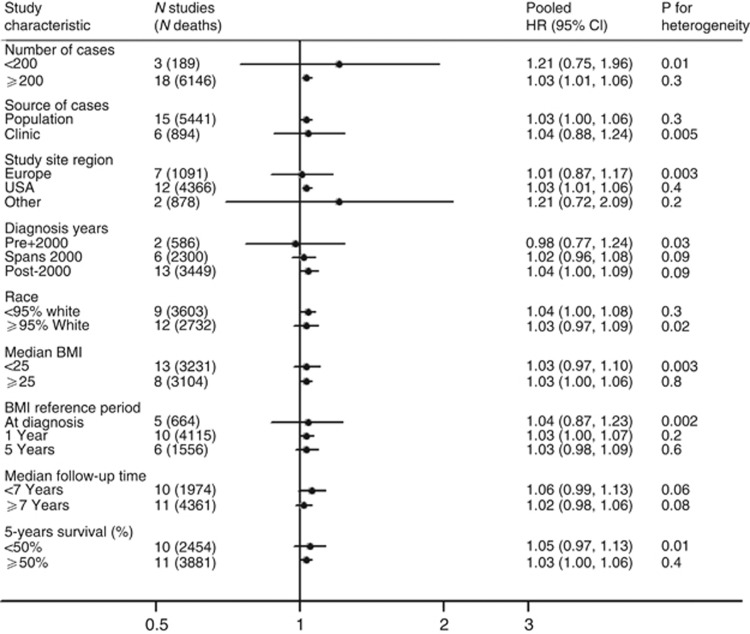
In multivariate analyses (all histologies combined), we found that women who were overweight (BMI: 25–29.9), obese (BMI: 30–34.9) and morbidly obese (BMI: ⩾35) experienced worse survival compared with women within the normal weight range (pHRs: 1.05 (95% CIs: 0.96–1.15), 1.10 (95% CIs: 0.99–1.22) and 1.15 (95% CIs: 0.98–1.37), respectively). However, in the overweight and morbidly obese groups there was significant heterogeneity between the studies (all P<0.05). Using BMI as a continuous variable, risk of death increased by 3% for each 5-unit increase in BMI over 18.5 kg m−2 (HR: 1.03, (95% CIs: 1.00–1.07)); however, again significant heterogeneity was present between the studies (I2 40%, P=0.03) (Figure 1). Exploration of the heterogeneity showed that the largest difference in pHR was seen for study size with no apparent heterogeneity among the 18 studies, with ⩾200 participants but significant heterogeneity among those with fewer women (Figure 2). The pHR per 5-unit increase in BMI kg m−2 was 1.03 (95% CIs: 1.01–1.06) for studies with ⩾200 women vs 1.21 for studies with <200 women (95% CIs: 0.75–1.96). We also saw significant heterogeneity in other strata, in all except one instance (diagnosis years); the group with significant heterogeneity included at least two, if not all three of the small studies. When we repeated these analyses excluding the three small studies (HSK, JPN and PVD), we found that women who were obese still experienced worse survival (pHR: 1.10 (95% CIs: 0.99–1.23)) than women within the normal weight range (Table 3). This association was similar for those who were morbidly obese (pHR 1.12 (95% CIs: 1.01–1.25)) and the pHR per 5-unit increase in BMI kg m−2 was 1.03 (95% CIs: 1.00–1.06); I2 9%, P=0.35) (Table 3). The evidence of heterogeneity disappeared in the obese and morbidly obese groups; however, there remained significant heterogeneity between the studies in the overweight group (I2 46%, P=0.02). When we further excluded the study site where the confidence interval did not include the pooled estimate (MAL), there was no remaining heterogeneity in the overweight group (I2: 10.4%, P=0.3).
Figure 1.
The association between BMI (per 5 kg m−2) and OS following a diagnosis of invasive ovarian cancer, all subtypes, overall and by study site. Estimates are adjusted for age at diagnosis (continuous), stage (local/regional/distant/unknown), grade (well-/moderately-/poorly plus undifferentiated/unknown) and ethnicity (if <95% of participants at a site shared a common ethnicity) estimates are further adjusted for the interaction of age, stage, grade and/or race with time as appropriate at each site.
Figure 2.
The association between BMI (per 5 kg m−2) and OS following a diagnosis of invasive ovarian cancer, all subtypes in 21 studies, stratified by study characteristics. Estimates are adjusted for age at diagnosis (continuous), stage (local/regional/distant/unknown), grade (well-/moderately-/poorly plus undifferentiated/unknown) and ethnicity (if <95% of participants at a site shared a common ethnicity) estimates are further adjusted for the interaction of age, stage, grade and/or race with time as appropriate at each site. Study site region ‘Other'=AUS and JPN.
Table 3. The association between BMI and OS following a diagnosis of invasive ovarian cancer, all subtypes, two-stage pooled analysis, studies where N⩾200 (18 studies).
| BMI (kg m−2) | Study sites (n)a | Cases (n) | I2 (%) | pHRb | 95% CI |
|---|---|---|---|---|---|
| <18.5 | 18 | 284 | 29.7 | 1.18 | 0.94–1.48 |
| 18.5–24.9 (Ref) | 18 | 5385 | – | REF | |
| 25–29.9 | 18 | 3374 | 46.0 | 1.03c | 0.95–1.13 |
| 30–34.9 | 18 | 1547 | 34.9 | 1.10 | 0.99–1.23 |
| ⩾35 | 17 | 1097 | 9.5 | 1.12 | 1.01–1.25 |
| Per 5 kg m−2 d | 18 | 11 403 | 9.1 | 1.03 | 1.00–1.06 |
Abbreviations: BMI=body mass index; CI=confidence interval; HR=hazard ratio; pHR=pooled HR; OS=overall survival.
Excludes study sites HSK, JPN, PVD.
Pooled HR combined study site-specific estimates adjusting for age at diagnosis (continuous), stage (local/regional/distant/unknown), grade (well-/moderately-/poorly plus undifferentiated/unknown) and ethnicity (if <95% of participants at a site shared a common ethnicity) estimates are further adjusted for the interaction of age, stage, grade and/or race with time as appropriate at each site.
Significant heterogeneity noted (P-value for heterogeneity 0.017).
Excludes participants with BMI <18.5 kg m−2.
Data on residual disease were not available for all studies; however, including this variable in models restricted to studies where the data were available did not result in appreciable changes to the pooled estimates (Supplementary Table 2). Similarly, data on cigarette smoking status were not available for all studies and including this variable in models did not result in appreciable changes (Supplementary Table 3).
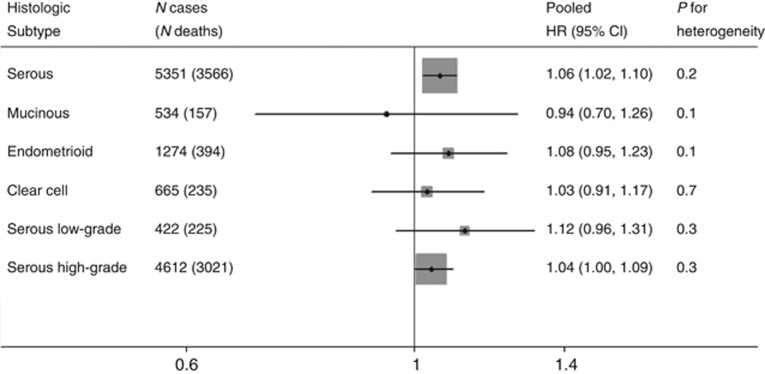
The results stratified by histologic subtype are shown in Figure 3. This analysis included only the 12 studies with adequate numbers of cases and events to generate estimates for each histologic subtype. The strongest associations were seen for the low-grade serous and endometrioid subtypes, but neither result was significant (pHR: 1.12 (95% CIs: 0.96–1.31) and 1.08 (95% CIs: 0.95–1.23), respectively, per 5-unit increase in BMI). A more modest but significant association was observed for the high-grade serous subtype (pHR per 5-unit increase in BMI: 1.04 (95% CIs: 1.00–1.09)). No association was noted between BMI and survival among women with mucinous or clear cell tumours. Tests for heterogeneity (between the four main subtypes and for low- vs high-grade serous subtypes) did not reach statistical significance (both P=1.0).
Figure 3.
The association between BMI (per 5 kg m−2) and OS following a diagnosis of invasive ovarian cancer, by histologic subtype, two-stage pooled analysis. Pooled HR combined study site-specific estimates adjusting for age at diagnosis (continuous), stage (local/regional/distant/unknown), grade (well-/moderately-/poorly plus undifferentiated/unknown) (except for low- and high-grade serous estimates) and ethnicity (if <95% of participants at a site shared a common ethnicity) estimates are further adjusted for the interaction of age, stage, grade and/or race with time as appropriate at each site. Excludes participants with BMI <18.5 kg m−2. Includes study sites with adequate numbers of cases and events to generate an estimate for each histologic group. Pooled HR for serous, mucinous, endometrioid and clear-cell includes study sites: AUS, BAV, CON, DOV, HAW, HOP, MAL, NCO, NEC, STA, TBO and USC. Pooled HR for serous low-grade and serous high-grade includes study sites: AUS, BAV, BEL, DOV, HOP, MAL, NCO, NEC, NJO, STA, UCI and USC.
We also assessed PFS (in 11 studies, n=4133 cases) and ovarian cancer-specific survival (in 9 studies, n=3091) and similar results were noted to those for OS. For the 11 studies where we had both PFS and OS data, the pHR for obese women (BMI: ⩾30) compared with women within the normal weight range was 1.10 (95% CIs: 0.99–1.23) for PFS and 1.12 (955 CIs: 1.01–1.26) for OS. In the nine studies with the cause of death data, the pHR for obese women (BMI: ⩾30) compared with women within the normal weight range was 1.17 (95% CIs: 1.00–1.37) for ovarian-cancer specific survival and 1.16 (95% CIs: 1.03–1.31) for OS.
Discussion
This study is, to our knowledge, the largest evaluation to date, of BMI and survival following a diagnosis of ovarian cancer. We found that obesity was associated with a 10–12% OS disadvantage among women with ovarian cancer and results were similar for PFS and ovarian cancer-specific mortality. In subtype analyses, associations were strongest for women with low-grade serous and endometrioid cancers, and more modest for high-grade serous cancers (12%, 8% and 4% increases in mortality per 5-unit increase in BMI, respectively), but only the association with high-grade serous cancers, by far the largest subgroup, reached statistical significance. No increase in risk was noted for the less common clear-cell or mucinous subtypes, which are estimated to account for ∼8% of all epithelial ovarian cancers.
Several mechanisms have been proposed to underlie the effects of obesity on ovarian cancer outcomes. Makowski et al (2014) recently showed that the obese state promotes tumour progression in animal models of serous ovarian cancer and concluded that metabolic consequences of obesity may be involved in the pathogenesis of ovarian cancer. Aberrant adipokine production, specifically upregulation of leptin and downregulation of adiponectin in the obese state, may explain an association between obesity and ovarian cancer outcomes. Leptin has both mitogenic and anti-apoptotic properties in cancer cell lines and is involved in promoting angiogenesis (Khandekar et al, 2011; Chen et al, 2013; Ptak et al, 2013). Conversely, adiponectin has anti-proliferative effects through the induction of apoptosis (Kelesidis et al, 2006; Barb et al, 2007). In a recent cohort study of 161 women with advanced-stage ovarian cancer, Diaz et al (2013) found that women with increased leptin to adiponectin (L:A) ratios experienced significantly shorter disease-free survival time than those with low L:A ratio. Obesity may also affect ovarian cancer survival through its effect on inflammatory cytokines, markers of insulin resistance and obesity-related hormones such as oestrogen, through the conversion of androgens to oestrogen in adipose tissue. In-vitro studies have shown that oestrogens have pro-proliferative actions on ovarian cancer cells (Galtier-Dereure et al, 1992; Langdon et al, 1994; Karlan et al, 1995). The oestrogen receptor is expressed in up to 80% of epithelial ovarian cancers with the highest expression in serous and endometrioid tumours (Modugno et al, 2012; Sieh et al, 2013), the two subtypes in this study with the strongest associations. Finally, oestrogen may also have a role in the motility and invasion of ovarian cancer cells (Hua et al, 2008; Zhu et al, 2012).
From a treatment perspective, obese women may have worse survival because of the practice of dose capping when prescribing chemotherapy (Modesitt and van Nagell, 2005; Pavelka et al, 2006; Poniewierski et al, 2008; Au-Yeung et al, 2014). Dose capping involves the use of ideal rather than the actual body weight when calculating the dose to be given or dose capping at a body surface area of 2.0 m2 (equivalent to a BMI of ∼30 kg m−2) and occurs largely, it is thought, due to concerns regarding the potential for chemotherapy-related toxicities if the full dose is given (Field et al, 2008). Evidence from an Australian study of 330 women with late-stage ovarian cancer has shown that underdosing of carboplatin was common among the obese women (Au-Yeung et al, 2014). They also reported that reduced dose intensity of carboplatin was associated with worse PFS. Recently, published guidelines from the American Society of Clinical Oncology recommend that full weight-based doses of chemotherapy be used to treat obese patients with cancer, in particular where the goal of treatment is cure (Griggs et al, 2012).
The strengths of our study include the large sample size, which allowed us to examine associations both overall and separately for the different histologic subtypes of ovarian cancer. We included age, ethnicity, clinical factors and study site in our models, and sensitivity analysis suggested that any residual confounding by cigarette smoking would have been minimal. We also assessed PFS and ovarian cancer-specific survival, where such data were available, and the results were essentially unchanged, suggesting that obesity is not just increasing non-ovarian cancer deaths, but progression and cause-specific survival. However, most studies relied on retrospective self-reports of weight and height; hence, there is some potential for recall error; however, it is unlikely to have differed by outcome and thus our results may underestimate the true magnitude of the association. It is possible that our measure of usual weight (before diagnosis) may be influenced by weight loss due to cachexia or weight gain due to the presence of ascites, both of which may be presenting symptoms for ovarian cancer, in particular in women with advanced disease. However, the adverse association between obesity and ovarian cancer survival appeared consistent regardless of when BMI was measured, suggesting this is not a major problem. One potential limitation of our analysis is that the data were not originally collected to look at survival and, as a result, clinical data were not always available and/or complete. However, the major advantage of using the data in this way is cost effectiveness; the time, effort and cost associated with collecting similar data from an equal number of women with ovarian cancer for a new study specifically looking at survival would be prohibitive.
In conclusion, this analysis of data from OCAC has shown that obesity before or at ovarian cancer diagnosis is associated with worse survival, when compared with women within the normal-weight range. As ovarian cancer remains a highly fatal disease and the prevalence of obesity continues to increase, studies focusing on causal mechanisms involved in adverse survival are needed.
Acknowledgments
We are grateful to the family and friends of Kathryn Sladek Smith for their generous support of Ovarian Cancer Association Consortium through their donations to the Ovarian Cancer Research Fund. The Australian Ovarian Cancer Study Management Group (D Bowtell, G Chenevix-Trench, A deFazio, D Gertig, A Green and PM Webb) and ACS Investigators (A Green, P Parsons, N Hayward, PM Webb and D Whiteman) thank all the clinical and scientific collaborators (see http://www.aocstudy.org/) and the women who participated in these studies for their contribution. We thank Gilian Peuteman, Thomas Van Brussel and Dominiek Smeets for technical assistance (for BEL). The cooperation of the 32 Connecticut hospitals, including Stamford Hospital, in allowing patient access, is gratefully acknowledged. This study was approved by the State of Connecticut Department of Public Health Human Investigation Committee. Certain data used in this study were obtained from the Connecticut Tumor Registry in the Connecticut Department of Public Health. We assume full responsibility for analyses and interpretation of these data (for CON). The German Ovarian Cancer Study (GER) thank Ursula Eilber for competent technical assistance (for GER). Funding: The Ovarian Cancer Association Consortium is supported by a grant from the Ovarian Cancer Research Fund. This work was supported by the following: National Institutes of Health (R01-CA61107 (for MAL); R01-CA95023 and R01-CA126841 (for HOP); P01-CA17054 (for USC); NIH-K07 CA095666, R01-CA83918, NIH-K22-CA138563 and P30-CA072720 (for NJO); (R01-CA074850 and R01-CA080742 (for CON); R01-CA112523 and R01-CA087538 (for DOV); R01-CA058598, N01-CN-55424 and N01-PC-67001 (for HAW); R01-CA122443, P50-CA136393 and P30-CA15083 (for MAC and MAY); R01-CA076016 (for NCO); R01-CA054419 and P50-CA105009 (for NEC); U01-CA71966, R01-CA016056 and K07-CA143047 (for STA); R01-CA106414 (for TBO); R01CA058860, R01CA092044 and PSA042205 (for UCI); Department of Defense (DAMD17-02-1-0666 (for NCO); W81XWH-10-1-02802 (for NEC); DAMD17-02-1-0669 (for HOP); DAMD17-01-1-0729 (for AUS); DAMD17-98-1-8659 (for TBO); National Cancer Institute K07-CA80668 (for HOP); NIH/National Center for Research Resources/General Clinical Research Center grant MO1-RR000056 and P50-CA159981 (for HOP); National Health and Medical Research Council of Australia, Cancer Councils of New South Wales, Victoria, Queensland, South Australia and Tasmania, Cancer Foundation of Western Australia; National Health and Medical Research Council of Australia (199600 and 400281) (for AUS); Danish Cancer Society, Copenhagen, Denmark (94 222 52) and the Mermaid I project (for MAL); FM supported by funding from K07-CA080668 (for HOP); California Cancer Research Program (00-01389V-20170, R03-CA113148, R03-CA115195, N01-CN25403 and 2II0200) (for USC); ELAN Funds of the University of Erlangen-Nuremberg (for BAV); Nationaal Kankerplan (for BEL); German Federal Ministry of Education and Research, Programme of Clinical Biomedical Research (01 GB 9401) and data management by the German Cancer Research Center (for GER); Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports, Culture and Technology of Japan, by a Grant-in-Aid for the Third Term Comprehensive 10-Year Strategy for Cancer Control from Ministry Health, Labour and Welfare of Japan, and by a grant from Takeda Science Foundation (for JPN); Mayo Foundation, Minnesota Ovarian Cancer Alliance, Fred C. and Katherine B. Andersen Foundation (for MAC and MAY); Cancer Institute of New Jersey (for NJO); Intramural Research Program of the National Cancer Institute (for POL); Cancer Prevention Institute of California (U01-CA69417) (for STA); American Cancer Society (CRTG-00-196-01-CCE), Celma Mastry Ovarian Cancer Foundation (for TBO); and Lon V Smith Foundation grant LVS-39420 (for UCI). PW is supported by a Fellowship from NHMRC and CN is supported by NHMRC Program grant 552429 (for AUS). AdF is supported by the University of Sydney Cancer Research Fund and the Cancer Institute NSW through the Sydney-West Translational Cancer Research Centre (for AUS).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License
Supplementary Material
References
- Au-Yeung G, Webb PM, Defazio A, Fereday S, Bressel M, Mileshkin L. Impact of obesity on chemotherapy dosing for women with advanced stage serous ovarian cancer in the Australian Ovarian Cancer Study (AOCS) Gynecol Oncol. 2014;133 (1:16–22. doi: 10.1016/j.ygyno.2014.01.030. [DOI] [PubMed] [Google Scholar]
- Bandera EV, King M, Chandran U, Paddock LE, Rodriguez-Rodriguez L, Olson SH. Phytoestrogen consumption from foods and supplements and epithelial ovarian cancer risk: a population-based case control study. BMC Womens Health. 2011;11:40. doi: 10.1186/1472-6874-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 2007;86 (3:s858–s866. doi: 10.1093/ajcn/86.3.858S. [DOI] [PubMed] [Google Scholar]
- Bodelon C, Cushing-Haugen KL, Wicklund KG, Doherty JA, Rossing MA. Sun exposure and risk of epithelial ovarian cancer. Cancer Causes Control. 2012;23 (12:1985–1994. doi: 10.1007/s10552-012-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348 (17:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- Chandran U, Bandera EV, Williams-King MG, Paddock LE, Rodriguez-Rodriguez L, Lu SE, Faulkner S, Pulick K, Olson SH. Healthy eating index and ovarian cancer risk. Cancer Causes Control. 2011;22 (4:563–571. doi: 10.1007/s10552-011-9728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chang YC, Lan MS, Breslin M. Leptin stimulates ovarian cancer cell growth and inhibits apoptosis by increasing cyclin D1 and Mcl-1 expression via the activation of the MEK/ERK1/2 and PI3K/Akt signaling pathways. Int J Oncol. 2013;42 (3:1113–1119. doi: 10.3892/ijo.2013.1789. [DOI] [PubMed] [Google Scholar]
- Clark TG, Bradburn MJ, Love SB, Altman DG. Survival analysis part I: basic concepts and first analyses. Br J Cancer. 2003;89 (2:232–238. doi: 10.1038/sj.bjc.6601118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborative Group on Epidemiological Studies of Ovarian Cancer Ovarian cancer and body size: individual participant meta-analysis including 25,157 women with ovarian cancer from 47 epidemiological studies. PLoS Med. 2012;9 (4:e1001200. doi: 10.1371/journal.pmed.1001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7 (3:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Diaz ES, Karlan BY, Li AJ. Obesity-associated adipokines correlate with survival in epithelial ovarian cancer. Gynecol Oncol. 2013;129 (2:353–357. doi: 10.1016/j.ygyno.2013.02.006. [DOI] [PubMed] [Google Scholar]
- du Bois A, Luck HJ, Meier W, Adams HP, Mobus V, Costa S, Bauknecht T, Richter B, Warm M, Schroder W, Olbricht S, Nitz U, Jackisch C, Emons G, Wagner U, Kuhn W, Pfisterer J, Arbeitsgemeinschaft Gynakologische Onkologie Ovarian Cancer Study G A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95 (17:1320–1329. doi: 10.1093/jnci/djg036. [DOI] [PubMed] [Google Scholar]
- Field KM, Kosmider S, Jefford M, Michael M, Jennens R, Green M, Gibbs P. Chemotherapy dosing strategies in the obese, elderly, and thin patient: results of a nationwide survey. J Oncol Pract. 2008;4 (3:108–113. doi: 10.1200/JOP.0832001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtier-Dereure F, Capony F, Maudelonde T, Rochefort H. Estradiol stimulates cell growth and secretion of procathepsin D and a 120-kilodalton protein in the human ovarian cancer cell line BG-1. J Clin Endocrinol Metab. 1992;75 (6:1497–1502. doi: 10.1210/jcem.75.6.1464654. [DOI] [PubMed] [Google Scholar]
- Garcia-Closas M, Brinton LA, Lissowska J, Richesson D, Sherman ME, Szeszenia-Dabrowska N, Peplonska B, Welch R, Yeager M, Zatonski W, Chanock SJ. Ovarian cancer risk and common variation in the sex hormone-binding globulin gene: a population-based case-control study. BMC Cancer. 2007;7:60. doi: 10.1186/1471-2407-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifkins D, Olson SH, Paddock L, King M, Demissie K, Lu SE, Kong AN, Rodriguez-Rodriguez L, Bandera EV. Total and individual antioxidant intake and risk of epithelial ovarian cancer. BMC Cancer. 2012;12:211. doi: 10.1186/1471-2407-12-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glud E, Kjaer SK, Thomsen BL, Hogdall C, Christensen L, Hogdall E, Bock JE, Blaakaer J. Hormone therapy and the impact of estrogen intake on the risk of ovarian cancer. Arch Intern Med. 2004;164 (20:2253–2259. doi: 10.1001/archinte.164.20.2253. [DOI] [PubMed] [Google Scholar]
- Goode EL, Chenevix-Trench G, Hartmann LC, Fridley BL, Kalli KR, Vierkant RA, Larson MC, White KL, Keeney GL, Oberg TN, Cunningham JM, Beesley J, Johnatty SE, Chen X, Goodman KE, Armasu SM, Rider DN, Sicotte H, Schmidt MM, Elliott EA, Hogdall E, Kjaer SK, Fasching PA, Ekici AB, Lambrechts D, Despierre E, Hogdall C, Lundvall L, Karlan BY, Gross J, Brown R, Chien J, Duggan DJ, Tsai YY, Phelan CM, Kelemen LE, Peethambaram PP, Schildkraut JM, Shridhar V, Sutphen R, Couch FJ, Sellers TA, Ovarian Cancer Association C Assessment of hepatocyte growth factor in ovarian cancer mortality. Cancer Epidemiol Biomarkers Prev. 2011;20 (8:1638–1648. doi: 10.1158/1055-9965.EPI-11-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode EL, Maurer MJ, Sellers TA, Phelan CM, Kalli KR, Fridley BL, Vierkant RA, Armasu SM, White KL, Keeney GL, Cliby WA, Rider DN, Kelemen LE, Jones MB, Peethambaram PP, Lancaster JM, Olson JE, Schildkraut JM, Cunningham JM, Hartmann LC. Inherited determinants of ovarian cancer survival. Clin Cancer Res. 2010;16 (3:995–1007. doi: 10.1158/1078-0432.CCR-09-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman MT, Lurie G, Thompson PJ, McDuffie KE, Carney ME. Association of two common single-nucleotide polymorphisms in the CYP19A1 locus and ovarian cancer risk. Endocr Relat Cancer. 2008;15 (4:1055–1060. doi: 10.1677/ERC-08-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs JJ, Mangu PB, Anderson H, Balaban EP, Dignam JJ, Hryniuk WM, Morrison VA, Pini TM, Runowicz CD, Rosner GL, Shayne M, Sparreboom A, Sucheston LE, Lyman GH, American Society of Clinical O Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2012;30 (13:1553–1561. doi: 10.1200/JCO.2011.39.9436. [DOI] [PubMed] [Google Scholar]
- Hamajima N, Matsuo K, Saito T, Hirose K, Inoue M, Takezaki T, Kuroishi T, Tajima K. Gene-environment Interactions and Polymorphism Studies of Cancer Risk in the Hospital-based Epidemiologic Research Program at Aichi Cancer Center II (HERPACC-II) Asian Pac J Cancer Prev. 2001;2 (2:99–107. [PubMed] [Google Scholar]
- Harter P, Muallem ZM, Buhrmann C, Lorenz D, Kaub C, Hils R, Kommoss S, Heitz F, Traut A, du Bois A. Impact of a structured quality management program on surgical outcome in primary advanced ovarian cancer. Gynecol Oncol. 2011;121 (3:615–619. doi: 10.1016/j.ygyno.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Hogdall E, Fung ET, Christensen IJ, Yip C, Nedergaard L, Engelholm SA, Risum S, Petri AL, Lundvall L, Lomas L, Hogdall C. Proteomic biomarkers for overall and progression-free survival in ovarian cancer patients. Proteomics Clin Appl. 2010;4 (12:940–952. doi: 10.1002/prca.200900171. [DOI] [PubMed] [Google Scholar]
- Hua K, Feng W, Cao Q, Zhou X, Lu X, Feng Y. Estrogen and progestin regulate metastasis through the PI3K/AKT pathway in human ovarian cancer. Int J Oncol. 2008;33 (5:959–967. [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61 (2:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Karlan BY, Jones J, Greenwald M, Lagasse LD. Steroid hormone effects on the proliferation of human ovarian surface epithelium in vitro. Am J Obstet Gynecol. 1995;173 (1:97–104. doi: 10.1016/0002-9378(95)90176-0. [DOI] [PubMed] [Google Scholar]
- Kelemen LE, Sellers TA, Schildkraut JM, Cunningham JM, Vierkant RA, Pankratz VS, Fredericksen ZS, Gadre MK, Rider DN, Liebow M, Goode EL. Genetic variation in the one-carbon transfer pathway and ovarian cancer risk. Cancer Res. 2008;68 (7:2498–2506. doi: 10.1158/0008-5472.CAN-07-5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. Br J Cancer. 2006;94 (9:1221–1225. doi: 10.1038/sj.bjc.6603051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11 (12:886–895. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- Lacour RA, Westin SN, Meyer LA, Wingo SN, Schorge JO, Brooks R, Mutch D, Molina A, Sutphen R, Barnes M, Elder J, Teoh D, Powell CB, Choubey V, Blank S, Macdonald HR, Brady MF, Urbauer DL, Bodurka D, Gershenson DM, Lu KH. Improved survival in non-Ashkenazi Jewish ovarian cancer patients with BRCA1 and BRCA2 gene mutations. Gynecol Oncol. 2011;121 (2:358–363. doi: 10.1016/j.ygyno.2010.12.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon SP, Hirst GL, Miller EP, Hawkins RA, Tesdale AL, Smyth JF, Miller WR. The regulation of growth and protein expression by estrogen in vitro: a study of 8 human ovarian carcinoma cell lines. J Steroid Biochem Mol Biol. 1994;50 (3-4:131–135. doi: 10.1016/0960-0760(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Lo-Ciganic WH, Zgibor JC, Bunker CH, Moysich KB, Edwards RP, Ness RB. Aspirin, nonaspirin nonsteroidal anti-inflammatory drugs, or acetaminophen and risk of ovarian cancer. Epidemiology. 2012;23 (2:311–319. doi: 10.1097/EDE.0b013e3182456ad3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie G, Wilkens LR, Thompson PJ, McDuffie KE, Carney ME, Terada KY, Goodman MT. Combined oral contraceptive use and epithelial ovarian cancer risk: time-related effects. Epidemiology. 2008;19 (2:237–243. doi: 10.1097/EDE.0b013e31816334c5. [DOI] [PubMed] [Google Scholar]
- Makowski L, Zhou C, Zhong Y, Kuan PF, Fan C, Sampey BP, Difurio M, Bae-Jump VL. Obesity increases tumor aggressiveness in a genetically engineered mouse model of serous ovarian cancer. Gynecol Oncol. 2014;133 (1:90–97. doi: 10.1016/j.ygyno.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire V, Felberg A, Mills M, Ostrow KL, DiCioccio R, John EM, West DW, Whittemore AS. Relation of contraceptive and reproductive history to ovarian cancer risk in carriers and noncarriers of BRCA1 gene mutations. Am J Epidemiol. 2004;160 (7:613–618. doi: 10.1093/aje/kwh284. [DOI] [PubMed] [Google Scholar]
- Merritt MA, De Pari M, Vitonis AF, Titus LJ, Cramer DW, Terry KL. Reproductive characteristics in relation to ovarian cancer risk by histologic pathways. Hum Reprod. 2013;28 (5:1406–1417. doi: 10.1093/humrep/des466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt MA, Green AC, Nagle CM, Webb PM. Talcum powder, chronic pelvic inflammation and NSAIDs in relation to risk of epithelial ovarian cancer. Int J Cancer. 2008;122 (1:170–176. doi: 10.1002/ijc.23017. [DOI] [PubMed] [Google Scholar]
- Modesitt SC, van Nagell JR., Jr The impact of obesity on the incidence and treatment of gynecologic cancers: a review. Obstet Gynecol Surv. 2005;60 (10:683–692. doi: 10.1097/01.ogx.0000180866.62409.01. [DOI] [PubMed] [Google Scholar]
- Modugno F, Laskey R, Smith AL, Andersen CL, Haluska P, Oesterreich S. Hormone response in ovarian cancer: time to reconsider as a clinical target. Endocrine Relat Cancer. 2012;19 (6:R255–R279. doi: 10.1530/ERC-12-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CM, Nagle CM, Whiteman DC, Ness R, Pearce CL, Pike MC, Rossing MA, Terry KL, Wu AH, Risch HA, Yu H, Doherty JA, Chang-Claude J, Hein R, Nickels S, Wang-Gohrke S, Goodman MT, Carney ME, Matsuno RK, Lurie G, Moysich K, Kjaer SK, Jensen A, Hogdall E, Goode EL, Fridley BL, Vierkant RA, Larson MC, Schildkraut J, Hoyo C, Moorman P, Weber RP, Cramer DW, Vitonis AF, Bandera EV, Olson SH, Rodriguez-Rodriguez L, King M, Brinton LA, Yang H, Garcia-Closas M, Lissowska J, Anton-Culver H, Ziogas A, Gayther SA, Ramus SJ, Menon U, Gentry-Maharaj A, Webb PM. Obesity and risk of ovarian cancer subtypes: evidence from the Ovarian Cancer Association Consortium. Endocr Relat Cancer. 2013;20 (2:251–262. doi: 10.1530/ERC-12-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal T, Permuth-Wey J, Betts JA, Krischer JP, Fiorica J, Arango H, LaPolla J, Hoffman M, Martino MA, Wakeley K, Wilbanks G, Nicosia S, Cantor A, Sutphen R. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104 (12:2807–2816. doi: 10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- Pal T, Permuth-Wey J, Kapoor R, Cantor A, Sutphen R. Improved survival in BRCA2 carriers with ovarian cancer. Fam Cancer. 2007;6 (1:113–119. doi: 10.1007/s10689-006-9112-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh N, Chandran U, Bandera EV. Obesity in cancer survival. Annu Rev Nutr. 2012;32:311–342. doi: 10.1146/annurev-nutr-071811-150713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavelka JC, Brown RS, Karlan BY, Cass I, Leuchter RS, Lagasse LO, Li AJ. Effect of obesity on survival in epithelial ovarian cancer. Cancer. 2006;107 (7:1520–1524. doi: 10.1002/cncr.22194. [DOI] [PubMed] [Google Scholar]
- Pike MC, Pearce CL, Peters R, Cozen W, Wan P, Wu AH. Hormonal factors and the risk of invasive ovarian cancer: a population-based case-control study. Fertil Steril. 2004;82 (1:186–195. doi: 10.1016/j.fertnstert.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Poniewierski MS, Crawford J, Dale DC, Culakova E, Kuderer NM, Wolff DA, Lyman GH. Reduced chemotherapy dose intensity in patients with ovarian cancer: Results from a prospective nationwide study. J Clin Oncol. 2008;26 (Suppl:abstr 16508. [Google Scholar]
- Protani MM, Nagle CM, Webb PM. Obesity and ovarian cancer survival: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2012;5 (7:901–910. doi: 10.1158/1940-6207.CAPR-12-0048. [DOI] [PubMed] [Google Scholar]
- Ptak A, Kolaczkowska E, Gregoraszczuk EL. Leptin stimulation of cell cycle and inhibition of apoptosis gene and protein expression in OVCAR-3 ovarian cancer cells. Endocrine. 2013;43 (2:394–403. doi: 10.1007/s12020-012-9788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramus SJ, Vierkant RA, Johnatty SE, Pike MC, Van Den Berg DJ, Wu AH, Pearce CL, Menon U, Gentry-Maharaj A, Gayther SA, Dicioccio RA, McGuire V, Whittemore AS, Song H, Easton DF, Pharoah PD, Garcia-Closas M, Chanock S, Lissowska J, Brinton L, Terry KL, Cramer DW, Tworoger SS, Hankinson SE, Berchuck A, Moorman PG, Schildkraut JM, Cunningham JM, Liebow M, Kjaer SK, Hogdall E, Hogdall C, Blaakaer J, Ness RB, Moysich KB, Edwards RP, Carney ME, Lurie G, Goodman MT, Wang-Gohrke S, Kropp S, Chang-Claude J, Webb PM, Chen X, Beesley J, Chenevix-Trench G, Goode EL. Consortium analysis of 7 candidate SNPs for ovarian cancer. Int J Cancer. 2008;123 (2:380–388. doi: 10.1002/ijc.23448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D, Million Women Study C Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335 (7630:1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch HA, Bale AE, Beck PA, Zheng W. PGR +331A/G and increased risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2006;15 (9:1738–1741. doi: 10.1158/1055-9965.EPI-06-0272. [DOI] [PubMed] [Google Scholar]
- Risum S, Loft A, Hogdall C, Berthelsen AK, Hogdall E, Lundvall L, Nedergaard L, Engelholm SA. Standardized FDG uptake as a prognostic variable and as a predictor of incomplete cytoreduction in primary advanced ovarian cancer. Acta Oncol. 2011;50 (3:415–419. doi: 10.3109/0284186X.2010.500296. [DOI] [PubMed] [Google Scholar]
- Rossing MA, Cushing-Haugen KL, Wicklund KG, Doherty JA, Weiss NS. Menopausal hormone therapy and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2007;16 (12:2548–2556. doi: 10.1158/1055-9965.EPI-07-0550. [DOI] [PubMed] [Google Scholar]
- Royar J, Becher H, Chang-Claude J. Low-dose oral contraceptives: protective effect on ovarian cancer risk. Int J Cancer. 2001;95 (6:370–374. doi: 10.1002/1097-0215(20011120)95:6<370::aid-ijc1065>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17 (4:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- Schildkraut JM, Iversen ES, Wilson MA, Clyde MA, Moorman PG, Palmieri RT, Whitaker R, Bentley RC, Marks JR, Berchuck A. Association between DNA damage response and repair genes and risk of invasive serous ovarian cancer. PLoS One. 2010;5 (4:e10061. doi: 10.1371/journal.pone.0010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildkraut JM, Moorman PG, Bland AE, Halabi S, Calingaert B, Whitaker R, Lee PS, Elkins-Williams T, Bentley RC, Marks JR, Berchuck A. Cyclin E overexpression in epithelial ovarian cancer characterizes an etiologic subgroup. Cancer Epidemiol Biomarkers Prev. 2008;17 (3:585–593. doi: 10.1158/1055-9965.EPI-07-0596. [DOI] [PubMed] [Google Scholar]
- Sieh W, Kobel M, Longacre TA, Bowtell DD, deFazio A, Goodman MT, Hogdall E, Deen S, Wentzensen N, Moysich KB, Brenton JD, Clarke BA, Menon U, Gilks CB, Kim A, Madore J, Fereday S, George J, Galletta L, Lurie G, Wilkens LR, Carney ME, Thompson PJ, Matsuno RK, Kjaer SK, Jensen A, Hogdall C, Kalli KR, Fridley BL, Keeney GL, Vierkant RA, Cunningham JM, Brinton LA, Yang HP, Sherman ME, Garcia-Closas M, Lissowska J, Odunsi K, Morrison C, Lele S, Bshara W, Sucheston L, Jimenez-Linan M, Driver K, Alsop J, Mack M, McGuire V, Rothstein JH, Rosen BP, Bernardini MQ, Mackay H, Oza A, Wozniak EL, Benjamin E, Gentry-Maharaj A, Gayther SA, Tinker AV, Prentice LM, Chow C, Anglesio MS, Johnatty SE, Chenevix-Trench G, Whittemore AS, Pharoah PD, Goode EL, Huntsman DG, Ramus SJ. Hormone-receptor expression and ovarian cancer survival: an Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol. 2013;14 (9:853–862. doi: 10.1016/S1470-2045(13)70253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soegaard M, Jensen A, Hogdall E, Christensen L, Hogdall C, Blaakaer J, Kjaer SK. Different risk factor profiles for mucinous and nonmucinous ovarian cancer: results from the Danish MALOVA study. Cancer Epidemiol Biomarkers Prev. 2007;16 (6:1160–1166. doi: 10.1158/1055-9965.EPI-07-0089. [DOI] [PubMed] [Google Scholar]
- Song H, Ramus SJ, Tyrer J, Bolton KL, Gentry-Maharaj A, Wozniak E, Anton-Culver H, Chang-Claude J, Cramer DW, DiCioccio R, Dork T, Goode EL, Goodman MT, Schildkraut JM, Sellers T, Baglietto L, Beckmann MW, Beesley J, Blaakaer J, Carney ME, Chanock S, Chen Z, Cunningham JM, Dicks E, Doherty JA, Durst M, Ekici AB, Fenstermacher D, Fridley BL, Giles G, Gore ME, De Vivo I, Hillemanns P, Hogdall C, Hogdall E, Iversen ES, Jacobs IJ, Jakubowska A, Li D, Lissowska J, Lubinski J, Lurie G, McGuire V, McLaughlin J, Medrek K, Moorman PG, Moysich K, Narod S, Phelan C, Pye C, Risch H, Runnebaum IB, Severi G, Southey M, Stram DO, Thiel FC, Terry KL, Tsai YY, Tworoger SS, Van Den Berg DJ, Vierkant RA, Wang-Gohrke S, Webb PM, Wilkens LR, Wu AH, Yang H, Brewster W, Ziogas A, Australian Cancer S, Australian Ovarian Cancer Study G, Ovarian Cancer Association C. Houlston R, Tomlinson I, Whittemore AS, Rossing MA, Ponder BA, Pearce CL, Ness RB, Menon U, Kjaer SK, Gronwald J, Garcia-Closas M, Fasching PA, Easton DF, Chenevix-Trench G, Berchuck A, Pharoah PD, Gayther SA. A genome-wide association study identifies a new ovarian cancer susceptibility locus on 9p22.2. Nat Genet. 2009;41 (9:996–1000. doi: 10.1038/ng.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry KL, De Vivo I, Titus-Ernstoff L, Shih MC, Cramer DW. Androgen receptor cytosine, adenine, guanine repeats, and haplotypes in relation to ovarian cancer risk. Cancer Res. 2005;65 (13:5974–5981. doi: 10.1158/0008-5472.CAN-04-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation 1995. Physical status: the use and interpretation of anthropometry. Report of a World Health Organisation Expert Committee. [PubMed]
- Wu AH, Pearce CL, Tseng CC, Templeman C, Pike MC. Markers of inflammation and risk of ovarian cancer in Los Angeles County. Int J Cancer. 2009;124 (6:1409–1415. doi: 10.1002/ijc.24091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Lu X, Hua KQ, Sun H, Yu YH, Feng YJ. Oestrogen receptor alpha mediates 17beta-estradiol enhancement of ovarian cancer cell motility through up-regulation of survivin expression. Arch Gynecol Obstet. 2012;286 (3:729–737. doi: 10.1007/s00404-012-2368-5. [DOI] [PubMed] [Google Scholar]
- Ziogas A, Gildea M, Cohen P, Bringman D, Taylor TH, Seminara D, Barker D, Casey G, Haile R, Liao SY, Thomas D, Noble B, Kurosaki T, Anton-Culver H. Cancer risk estimates for family members of a population-based family registry for breast and ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2000;9 (1:103–111. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.