Summary
Recent advances have clarified how the brain detects CO2 to regulate breathing (central respiratory chemoreception). These mechanisms are reviewed and their significance is presented in the general context of CO2/pH homeostasis through breathing. At rest, respiratory chemoreflexes initiated at peripheral and central sites mediate rapid stabilization of arterial PCO2 and pH. Specific brainstem neurons (e.g., retrotrapezoid nucleus, RTN; serotonergic) are activated by PCO2 and stimulate breathing. RTN neurons detect CO2 via intrinsic proton receptors (TASK-2, GPR4), synaptic input from peripheral chemoreceptors and signals from astrocytes. Respiratory chemoreflexes are arousal state-dependent whereas chemoreceptor stimulation produces arousal. When abnormal, these interactions lead to sleep-disordered breathing. During exercise, “central command” and reflexes from exercising muscles produce the breathing stimulation required to maintain arterial PCO2 and pH despite elevated metabolic activity. The neural circuits underlying central command and muscle afferent control of breathing remain elusive and represent a fertile area for future investigation.
Introduction
All cellular functions of the brain and body are influenced by the prevailing pH and only small pH variations are compatible with life. Because metabolically-produced CO2 is in rapid equilibrium with H+, and can be removed via lung ventilation, dynamic control of breathing by CO2 provides a major homeostatic mechanism for acute regulation of acid-base status. The molecular, cellular, and neural bases for this critical interoceptive chemosensory control system have been greatly clarified in recent years.
Three classes of neural mechanisms are implicated in matching the metabolic production of CO2 to its elimination by the lungs: the chemoreflexes, central command and neural feedback from muscles (Forster et al., 2012). The central respiratory chemoreflex is the breathing stimulation elicited by elevated brain PCO2 (CNS hypercapnia); the peripheral chemoreflex is the breathing stimulation elicited by activation of the carotid bodies and related organelles (aortic bodies)(Dempsey et al., 2012; Kumar and Prabhakar, 2012). The carotid bodies are activated by arterial hypoxemia in a pH-dependent manner (i.e., blood acidification enhances the stimulatory effect of reduced PaO2), by blood flow reduction and by increased blood concentration of lactate, potassium and catecholamine (Kumar and Prabhakar, 2012). The chemoreflexes minimize PaCO2 fluctuations by making corrective changes in lung ventilation and thus CO2 elimination. This regulation operates continuously because chemoreceptors provide a tonic stimulus to breathe (e.g.(Blain et al., 2009; Dempsey et al., 2012)). The chemoreflexes are state-dependent and, conversely, chemoreceptor stimulation produces arousal. The neural mechanisms that underlie these reciprocal interactions are important because many sleep-related pathologies are manifest as breathing disorders (Javaheri and Dempsey, 2013). In this review we focus on the cellular sensors and molecular detectors underlying central respiratory chemosensitivity and the neuronal networks they activate to stimulate breathing or to cause arousal. The central pathways that integrate information from carotid bodies and central respiratory chemoreceptors will also be considered but the reader is directed to more extensive reviews on the carotid bodies and oxygen sensing (e.g., (Nurse, 2014; Prabhakar, 2013)).
PaCO2 and PO2 do not change significantly during light to moderate aerobic exercise (Forster et al., 2012) ruling out chemoreceptor stimulation as the cause of the increased breathing (hyperpnea). Instead, exercise hyperpnea and PaCO2 stability depend primarily on feedback from skeletal muscle afferents and on central command (Forster et al., 2012; Kaufman, 2012; Waldrop and Iwamoto, 2006). Central command refers to the influence of brain structures involved in locomotion on the respiratory network during physical exercise (Eldridge et al., 1981; Forster et al., 2012). We will also briefly summarize current understanding of central command and muscle afferent mechanisms for exercise hyperpnea.
Respiratory chemoreflexes: general considerations
During normal unlabored breathing (eupnea), PaCO2 is maintained within a few mmHg of a physiological set-point (~35 mmHg) (Duffin et al., 1980); small fluctuations around this set-point are not consciously perceived and have no impact on the state of vigilance. By contrast, large acute increases in PaCO2 (e.g., from airway blockade, diving, sleep apnea, bronchial disease and accidental or experimental exposure to CO2) produce noxious sensations in awake subjects (dyspnea, urge to breathe, panic) and arousal from sleep (Kaur et al., 2013; Parshall et al., 2012). Some of the responses to high PCO2 are adaptive, e.g. CO2-induced arousal protects against accidental asphyxia by enabling postural changes that alleviate airway obstruction. Arousal, negative emotions and, in rodents olfactory sensation, can, in turn, stimulate breathing and contribute to the ventilatory response to CO2 (Hu et al., 2007; Kaur et al., 2013; Taugher et al., 2014).
The high gain of the hypercapnic ventilatory chemoreflex (breathing stimulation caused by a rise in PaCO2, Figure 1A) requires a sensitive CO2/H+ detection mechanism and a specialized neural circuit capable of converting changes in sensor activation into a powerful breathing response. The fundamental, open questions related to respiratory chemoreception are as follows: Does the process rely on specialized CO2 or proton detectors or on protonation of broadly distributed CNS channels, receptors or enzymes? If specialized CO2 or proton detectors exist, where are they located (neurons, glia, vasculature)? Are they expressed throughout the respiratory pattern generator (RPG) or is this circuitry CO2-insensitive and regulated by specialized clusters of CO2-responsive neurons? Finally, given that respiratory chemoreflexes rely on sensory information from both peripheral and central chemoreceptors, how is that information integrated?
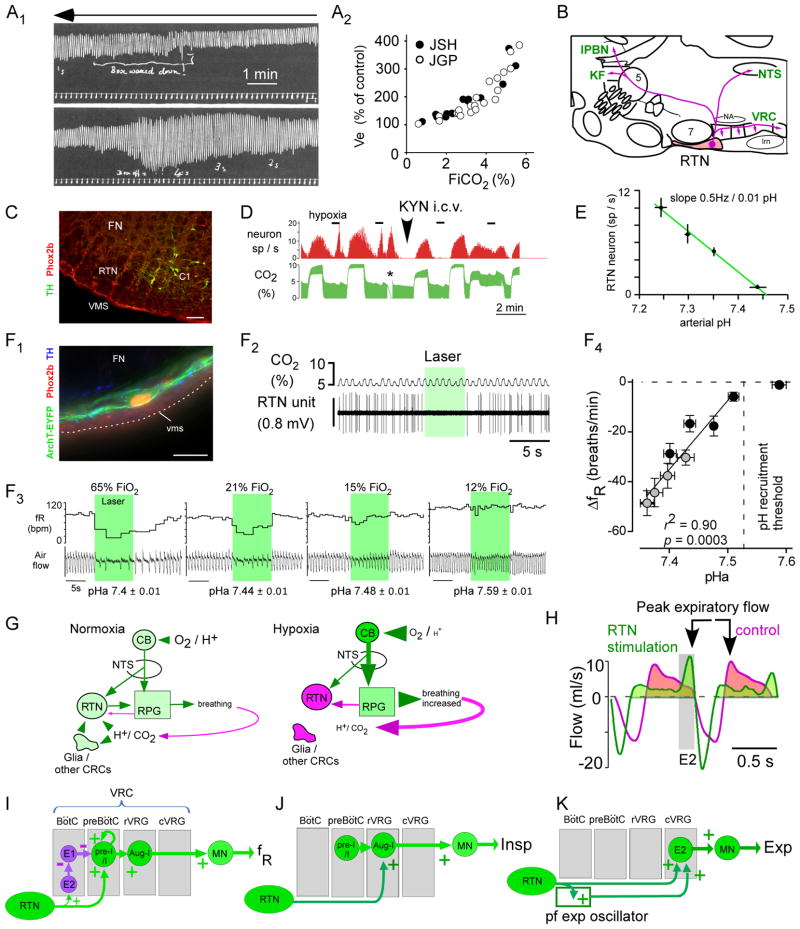
Figure 1. the retrotrapezoid nucleus, RTN.
(A1) the hypercapnic ventilatory reflex in humans (smoked drum recording to be read from right to left, top to bottom). Fraction inspired CO2 (FiCO2) was gradually increased by rebreathing air from box placed around head (reproduced from (Haldane and Priestley, 1905)). Amplitude of signal represents Vt (tidal volume). (A2) Plot of % increase in Ve (Vt x fR) vs. FiCO2 from Table on page 249 of (Haldane and Priestley, 1905)(JSH, Haldane; JGP, Priestly). (B) Location and projections of the rodent RTN (parasagittal section). Abbreviations: KF, Kölliker-Fuse nucleus; lPBN, lateral parabrachial nuc.; lrn, lateral reticular nuc.; NTS, solitary tract nuc.; VRC, ventral respiratory column; 5, trigeminal motor nuc.; 7, facial nuc.; (C) Transverse section at bregma level −11.5mm of an adult rat (left side) showing RTN neurons (FN: facial motor nucleus). The Phox2b+:tyrosine-hydroxylase (TH)− neurons are RTN neurons. The Phox2b+:TH+ are C1 adrenergic neurons. Cal: 0.1 mm. From (Guyenet, 2008) (D) single RTN neuron recorded in an anesthetized rat. The firing frequency of the neuron (upper trace) is increased both by raising FiCO2 in a background of hyperoxia (selective central chemoreceptor activation), by brief hypoxia (selective carotid body stimulation) or by short asphyxia (asterisk); the top of the CO2 trace (CO2 concentration at end-expiration) approximates arterial PCO2 measured in % of atmospheric pressure. Blocking excitatory transmission with kynurenic acid i.c.v. eliminates the input from the carotid body but the effect of hypercapnia is unchanged (adapted from (Mulkey et al., 2004). (E) Relationship between discharge rate of RTN neurons (N=11) and arterial pH in anesthetized rats (glutamatergic transmission blocked with kynurenate as in D; adapted from (Guyenet et al., 2005)). (F1) Rat RTN neuron transduced with archaerhodopsin-eYFP (ArchT). Cal: 20 μm. (F2) Optogenetic inhibition of one ArchT-transduced RTN neuron (anesthetized rat). (F3) Effects of bilateral optogenetic inhibition of RTN neurons on respiratory frequency (fR) and air flow in a conscious rat exposed to four FiO2. (F4) The breathing reduction elicited by inhibiting RTN is a linear function of arterial pH (pHa); RTN is silent above pHa 7.53. Open symbols: pHa changed via respiratory alkalosis (graded hypoxia); filled circles, pHa changed by administration of acetazolamide (F1-4 reproduced from (Basting et al., 2015)). (G) Contribution of the carotid bodies and RTN to respiratory homeostasis in normoxia vs. hypoxia. Magenta denotes reduced activity (neurons, glia), shades of green depict increasing activity and font sizes symbolize changes in plasma or brain [H+], PCO2 or PO2. From (Basting et al., 2015). (H) Optogenetic gain of function experiment. ChR2-mediated activation of RTN increases inspiration amplitude (inspiration downward), produces active expiration (E2 phase), and delays peak expiratory flow suggesting brief glottis closure after inspiration (modified from (Burke et al., 2015). E1: early (passive) expiratory phase. (I–K) Possible connections through which RTN increases breathing frequency, inspiratory amplitude and active expiration (see text for details).
Is central respiratory chemoreception an emergent property of the CNS?
Since the 1980s, the dominant view has been that the central respiratory chemoreflex derives from direct effects of [H+] distributed throughout the CNS (Nattie, 2011). One supportive argument is the huge variety and ubiquitous presence of pH-sensitive proteins (channels included) in the brain (Holzer, 2009). Two types of experimental results are also invoked. First, acidification commonly excites or inhibits neurons in vitro and dialyzing CO2-enriched artificial CSF in many brain regions alters breathing, albeit weakly (Nattie, 2011). Second, the hypercapnic ventilatory chemoreflex is attenuated by lesioning structures that are clearly not part of the respiratory pattern generator (e.g. serotonergic neurons, orexinergic neurons, locus coeruleus) (Nattie, 2011). Neither line of evidence, singly or in combination, is conclusive (Guyenet, 2014). The level of acidification applied in such experiments (up to 0.5pH) may occur only during severe hypercapnia, asphyxia or brain ischemia and the in vitro evidence typically does not demonstrate that the neurons under study regulate breathing. Interpreting effects of brain lesions on the chemoreflex is also problematic (reviewed in (Guyenet, 2014; Nattie, 2011)). In such experiments, the chemoreflex is measured by exposing animals acutely to CO2 levels that produce arousal and elicit behavioral effects (Kaur et al., 2013; Taugher et al., 2014). The effective lesions could have reduced ventilatory responses by any of three mechanisms: the lesioned neurons could indeed be respiratory chemoreceptors, they could mediate the emotional, behavioral or arousal effects of hypercapnia (Kaur et al., 2013; Taugher et al., 2014) or they could simply bias the chemoreflexes at multiple brain sites.
Intense carotid body stimulation is aversive in intact mammals (Marshall, 1994) and hypoxia causes arousal, supporting a role of these particular chemoreceptors in eliciting emotional and other non-cardiorespiratory effects of CO2 and/or hypoxia. Central respiratory chemoreceptors likely trigger non-respiratory effects of CO2 because hypercapnia evokes no adverse sensation, emotional or otherwise in patients with congenital central hypoventilation syndrome (CCHS) who lack a respiratory chemoreflex but have normal intellect and sensory perception (Shea et al., 1993; Weese-Mayer et al., 2010). Yet, supporting a more widespread effect of high PCO2, freezing and conditioned place avoidance in mice elicited by exposure to 10% CO2 requires expression of acid-sensitive ASIC channels in specific forebrain nuclei (e.g.(Taugher et al., 2014)). Thus, these aversive, behavioral and arousal effects elicited by large acute increases in PCO2 must recruit broad regions of the brain, which may be either directly sensitive to severe acidification or recruited synaptically when lower brainstem chemoreceptors and the carotid bodies are strongly stimulated.
For central respiratory chemoreception, the most compelling argument against the notion of a highly distributed brain property is that genetic lesion of a very small cluster of lower brainstem neurons, the retrotrapezoid nucleus (RTN), or mere deletion of two proton detectors expressed by RTN neurons (TASK-2 and GPR4) nearly eliminates the hypercapnic ventilatory reflex (Guyenet, 2014; Kumar et al., 2015; Ruffault et al., 2015). This review’s leitmotif will be that, contrary to prevalent opinion, the breathing stimulation elicited by low level hypercapnia is probably largely mediated by a direct effect of protons on the carotid bodies and very few brainstem structures, among which the RTN and a subset of serotonergic neurons are preeminent.
The retrotrapezoid nucleus, RTN
The RTN moniker was coined to describe neurons located under the facial nucleus in a region suspected since the 1960s to contain respiratory chemoreceptors ((Smith et al., 1989) and (Guyenet, 2014) for review)(Figure 1B). Early experiments (1989–2003) revealed that this region contains neurons that innervate caudal portions of the ventral respiratory column, are activated by hypercapnia and increase breathing when stimulated with glutamate or bicuculline ((Guyenet, 2014) for review). The relevant neurons were later shown to express VGlut2 and to encode PaCO2 linearly in vivo with a 0–10Hz dynamic range of action potential discharge between 35 and 76 mmHg PaCO2 (Figure 1D–E)(Mulkey et al., 2004). Unlike other respiratory neurons, RTN neurons are still activated by hypercapnia after glutamate receptor blockade in vivo suggesting that they respond to changes of local PCO2 (Fig. 1D)(Mulkey et al., 2004). Consistent with this hypothesis, RTN neurons are activated by hypercapnia or acidification ex vivo, including after isolation (Figure 2B) (Mulkey et al., 2004; Wang et al., 2013b). In 2003, mutations of homeodomain transcription factor Phox2b, were shown to cause CCHS (Amiel et al., 2003) and, in 2006, Phox2b was identified in all RTN CO2-responsive neurons (Figure 1C–F1)(Stornetta et al., 2006). Finally, these particular Phox2b-positive neurons are selectively missing in newborn mice that express a CCHS-causing Phox2b mutation (Phox2b27ala/+) (Dubreuil et al., 2008); these mice respond poorly to CO2 and suffer fatal central apneas at birth, recapitulating key features of this disease.
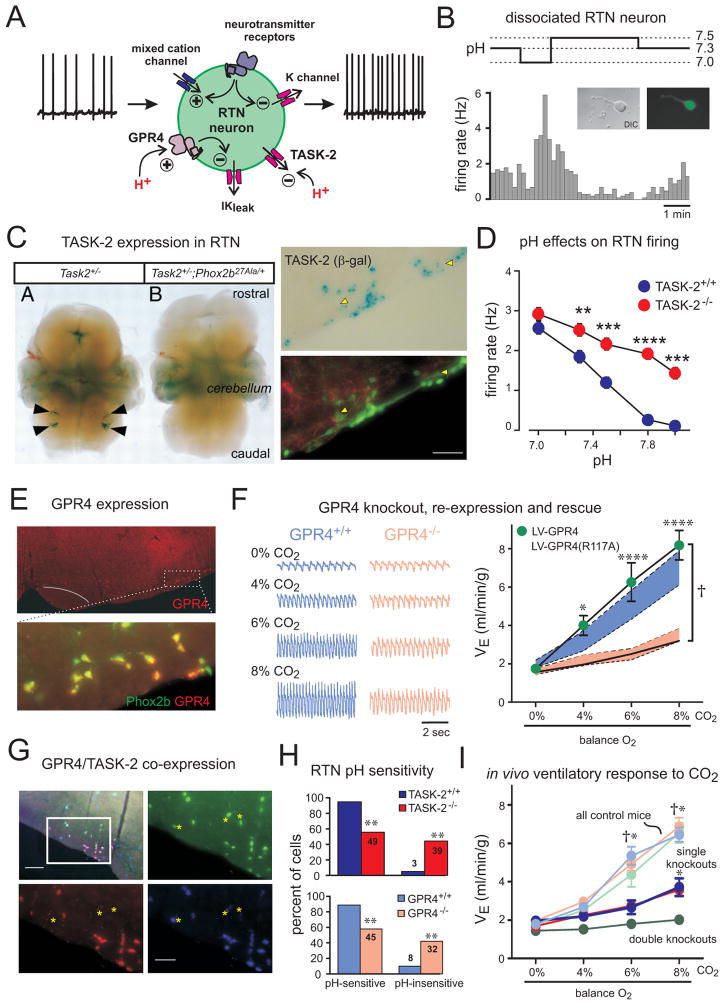
Figure 2. TASK-2 and GPR4 are proton detectors in RTN neurons required for CO2 stimulation of breathing.
(A) Schematic of RTN neuron showing ionic mechanisms for intrinsic pH sensitivity and transmitter modulation. (B) Firing rate histogram from GFP-expressing, dissociated RTN neuron. (C) Left: Staining for β-galactosidase (β-gal; from the TASK-2 locus) in embryo whole mounts from the indicated genotypes; arrowheads indicate RTN region. Right: β-gal staining for TASK-2 (upper) and GFP and TH (lower) in Phox2b::GFP;TASK-2+/− mice; white arrowheads indicate Phox2b-expressing RTN neurons that also express TASK-2. (D) Averaged firing rates at different bath pH for RTN neurons from TASK-2+/+ and TASK-2−/− mice. (E) GPR4 and Phox2b expression detected by in situ hybridization in transverse mouse brainstem section. (F) Left: Respiratory flow recording from GPR4+/+ and GPR4−/− mice with increased inspired CO2 concentrations (balance O2). Right: Lentiviral-mediated, PRSx8-driven re-expression in the RTN of GPR4, but not a non-functional mutant GPR4(R117A), fully rescued ventilatory response to CO2 in GPR4-deleted mice. Shaded areas are 95% confidence intervals for GPR4+/+ (blue) or GPR4−/− mice before lentiviral injection (pink). (G) Multiplex in situ hybridization illustrates differential, but overlapping, expression of GPR4 and TASK-2 in Phox2b-expressing RTN neurons; TASK-2-expressing Phox2b+ neurons without GPR4 are indicated (asterisks). (H) Percent of pH-sensitive and pH-insensitive RTN neurons recorded from mice of the indicated genotypes. (I) Ventilation during incremental CO2 challenge for the indicated genotypes. †, all controls (TASK-2+/+, light blue; GPR4+/+, light pink; and TASK-2+/+:GPR4+/+, light green) greater than single (TASK-2−/−, blue; and GPR4−/−, red) or double knockouts (TASK-2−/−:GPR4−/−, green); *, both single knockouts greater than double knockouts. Panel B adapted from (Wang et al., 2013b); panel C (left) from (Gestreau et al., 2010); panels C (right) & D from (Wang et al., 2013a), and panels E–I from (Kumar et al., 2015).
Within the medullary reticular formation “nuclei” can only be defined as collections of neurons with closely related biochemical characteristics, connectivity, function and, ideally, genetic lineage. CO2/H+-sensitive RTN neurons can be defined histochemically as a bilateral cluster of ~2000 Phox2b-immunoreactive (in rats, ~800 in mice) located under the facial nucleus (Lazarenko et al., 2009). These neurons have a shared genetic lineage (i.e., derived from neurons that express Phox2b, Atoh-1, and Egr-2) and unique phenotype (express Phox2b, NK1 receptors, VGlut2, TASK-2, GPR4, galanin, but not GABA, glycine, ACh or catecholamines) (Goridis et al., 2010; Guyenet, 2014; Lazarenko et al., 2009; Ruffault et al., 2015; Wang et al., 2013b). Differential expression of galanin, TASK-2 and GPR4 defines subsets of RTN neurons (Kumar et al., 2015). A separate terminology, either RTN/pfRG (parafacial respiratory group) or “parafacial nuclei”, includes RTN and additional unidentified neurons that contribute to active expiration (see section on RTN development) (Feldman et al., 2013; Huckstepp et al., 2015). Active expiration refers to the recruitment of expiratory muscles for breathing. This occurs only when high levels of lung ventilation are required (exercise, hypercapnia); at rest, expiration is passive and relies purely on lung recoil.
RTN neurons can be transduced to express excitatory or inhibitory actuators by using viral vectors that employ a powerful Phox2b-activated artificial promoter, PRSx8 (Abbott et al., 2009; Hwang et al., 2001)(Figure 1F1). In conscious rats, optogenetic stimulation of RTN neurons increases the rate (fR) and amplitude of breathing (tidal volume, VT) in a manner that mimics effects of hypercapnia (Abbott et al., 2011). RTN stimulation also increases lung ventilation by transiently reducing airflow during early expiration and by triggering active expiration (Fig. 1H)(Burke et al., 2015). Conversely, opto- or pharmacogenetic inhibition of RTN neurons reduces fR and VT and eliminates CO2-induced active expiration (Basting et al., 2015; Marina et al., 2010). In conscious rats, opto-inhibition of RTN neurons reduces fR and VT in direct proportion to arterial blood pH below a threshold of 7.5; above this level, opto-inhibition is ineffective suggesting that RTN neurons are silent (Figure 1F1–4) (Basting et al., 2015).
The RTN region receives input from brain areas ranging from spinal cord to insular cortex (Craig, 1995; Lazarenko et al., 2009; Otake et al., 1992; Rosin et al., 2006; Song et al., 2012; Tan et al., 2010) but ultrastructural confirmation of defined synaptic contacts with the chemoreceptors is largely lacking. Next, we describe inputs whose existence and function are supported by physiological data. Inputs from the spinal cord and the raphe are considered later.
RTN neurons regulate breathing in concert with the carotid bodies. The dorsal medullary neurons that receive carotid body afferent input (CB second-order neurons) are glutamatergic and target the same pontomedullary regions as RTN, where final integration between central and peripheral chemoreflexes presumably occurs (Guyenet, 2014; Song et al., 2012). However, CB second-order neurons also powerfully activate RTN (Takakura et al., 2006) and this connection has important functional implications. Usually, arterial PO2 and arterial PCO2 vary in opposite direction, so that central and peripheral chemoreceptors are either co-activated (during hypoventilation) or co-inhibited (with hyperventilation). Under such conditions, the excitatory input from the carotid bodies to RTN neurons contributes importantly to the breathing changes, and the carotid bodies and central chemoreceptors (RTN) act in concert to regulate breathing (Figure 1D,G)(Blain et al., 2010; Mulkey et al., 2004). By contrast, in hypoxia (e.g., at altitude), carotid body-mediated hyperventilation alkalizes the plasma, which inhibits RTN and renders it unresponsive to carotid body input (Fig. 1F3,G)(Basting et al., 2015). Thus, under hypocapnic hypoxia, the RTN fails to support the breathing stimulation elicited by the carotid bodies. The potential consequences are insufficient alveolar ventilation and, at night, periodic breathing (Dempsey et al., 2012). Conversely, if the normally tonic input from the carotid body is reduced (e.g., by hyperoxia), RTN neurons provide a proportionally larger drive to breathe to minimize the change in CO2 (Figure 1F) (Basting et al., 2015).
Glycinergic/GABAergic neurons located in the NTS mediate inhibitory effects of lung inflation on RTN neurons (Takakura et al., 2007), reducing inspiratory drive during lung overinflation. RTN neurons also receive inhibitory inputs during specific periods of the respiratory cycle (early inspiration, post-inspiration, late expiration) (Guyenet et al., 2005). These inputs likely arise from the ventral respiratory column and the pontine respiratory group (Song et al., 2012; Tan et al., 2010). Their role may be to reduce RTN neuron activity when the respiratory pattern generator is already highly active for reasons other than a blood gas imbalance (e.g. emotional or voluntary control of breathing).
In brief, RTN neurons are highly sensitive to changes of CO2/H+ in vivo, they activate breathing in proportion to CNS acidification in conscious animals and their genetic elimination in mice reduces the central chemoreflex and increases apneas. RTN neurons regulate breathing in concert with the carotid bodies, and they are controlled by feedback from the respiratory pattern generator and lung afferents.
Pathways mediating the effects of RTN on breathing
RTN neurons target only four brainstem regions, all of which are essential for breathing (Figure 1B): the ventral respiratory column (VRC), the Kölliker-Fuse nucleus, the lateral parabrachial nucleus (lPBN), and the nucleus of the solitary tract (NTS) (Bochorishvili et al., 2012; Smith et al., 2013). The VRC consists of four rostrocaudally stacked modules all of which receive RTN input (Fig. 1B,I–K). The rostral-most module is the Bötzinger region, followed by the preBötzinger complex. The latter contains a bilateral cluster of glutamatergic bursters that operate as rhythm generator for breathing (Bouvier et al., 2010; Feldman et al., 2013; Feldman and Kam, 2015). The rostral ventral respiratory group (rVRG) and the caudal VRG (cVRG) reside more caudally in the VRC and harbor inspiratory and expiratory premotor neurons, respectively (Smith et al., 2013). The pump (e.g. phrenic, lumbar) and airway motoneurons (hypoglossal, facial) have no direct input from RTN (Bochorishvili et al., 2012). Therefore, RTN neurons must increase breathing amplitude via inputs to either premotor neurons or to neurons further up the network. Finally, most RTN neurons innervate both the VRC and the dorsolateral pons, controlling several components of the respiratory pattern generator simultaneously (Mulkey et al., 2004).
RTN neurons could accelerate breathing frequency via monosynaptic projections to the rhythm-generating neurons of the pre-Bötzinger complex (Figure 1I)(Bochorishvili et al., 2012). They could also disinhibit these neurons as shown in Fig. 1I (Bochorishvili et al., 2012; Potts et al., 2005) or via more complex pathways involving the dorsal pons (Mizusawa et al., 1995).
RTN effects on inspiratory amplitude may derive from direct projections to inspiratory premotor neurons located in the rVRG (Figure 1J), the Kölliker-Fuse nucleus (not illustrated) (Bochorishvili et al., 2012; Damasceno et al., 2014; Mizusawa et al., 1995; Smith et al., 2013; Yokota et al., 2007) and the lateral parabrachial nucleus (Yokota et al., 2015). Control of expiration by the RTN (Abbott et al., 2011; Marina et al., 2010) may involve projections to bulbospinal expiratory premotor neurons located in the cVRG (Bochorishvili et al., 2012; Gerrits and Holstege, 1996) and to the nearby parafacial oscillator for active expiration (Figure 1K) (Feldman et al., 2013; Huckstepp et al., 2015). Finally, RTN regulation of airway resistance (e.g. laryngeal adductors) likely occurs by way of the Kölliker-Fuse nucleus (Dutschmann and Herbert, 2006; Song et al., 2012).
In sum, RTN neurons regulate alveolar ventilation by controlling breathing rate, inspiratory amplitude, active expiration and airway patency. These effects are mediated via axonal projections to unidentified neurons located within four respiratory-related lower brainstem regions.
Proton detection by RTN neurons
Via carbonic anhydrase, molecular CO2 is in equilibrium with protons, hydroxyl radicals and bicarbonate. The effects of CO2 on breathing are presumably mediated via changes in [H+] but additional mechanisms are being considered such as carbamylation reactions (e.g., to activate connexin-26) or via bicarbonate-regulated adenylyl cyclase (Huckstepp and Dale, 2011; Meigh et al., 2013). As reviewed below, RTN neurons respond to changes in local tissue PCO2 at least partially in a cell autonomous manner (Figure 2A) via at least two molecular proton detectors: TASK-2 and GPR4 (Gestreau et al., 2010; Kumar et al., 2015; Wang et al., 2013a).
In brain slices, RTN neurons maintain a regular tonic discharge that is dynamically modulated by an intrinsic sensitivity to extracellular [H+]. The actions of CO2 on RTN neurons in vitro are also mediated via changes in [H+] (Mulkey et al., 2004). The pH sensitivity of RTN neurons persists in vitro during blockade of fast synaptic transmission (Lazarenko et al., 2010; Mulkey et al., 2004; Mulkey et al., 2007b) and, after acute RTN neuron isolation, it is indistinguishable from that observed in slice preparations (Wang et al., 2013b) (Figure 2B).
Initial characterization of pH sensitive membrane currents in RTN neurons identified a pH-sensitive background K+ current (Mulkey et al., 2004), suggesting possible contributions from the K2P family of background K channels. Despite evidence for widespread expression of acid-sensitive TASK-1 (K2P3) and TASK-3 (K2P9) channels in brainstem respiratory neurons, genetic deletion of those channels had no effect on RTN neuronal pH-sensitivity or the ventilatory response to CO2 (Bayliss et al., 2015; Mulkey et al., 2007b). On the other hand, strong evidence implicates TASK-2 (K2P5), a member of the alkaline-activated subgroup of K2P channels, both in RTN neuronal pH sensitivity and the central respiratory chemoreflex (Bayliss et al., 2015; Gestreau et al., 2010; Wang et al., 2013b). By using a gene-trap mouse line, TASK-2 expression was revealed in the RTN and very few other brainstem regions (Figure 2C) (Gestreau et al., 2010; Kumar et al., 2015). Notably, TASK-2 is undetectable in the RTN of Phox2b27Ala/+ mice, in which the chemoreceptors do not develop and TASK-2 co-localizes with Phox2b and VGlut2 in most RTN neurons, as expected for expression within the pH-sensitive cell population (Figure 2C,G) (Gestreau et al., 2010; Wang et al., 2013a). Consistent with this, genetic elimination of TASK-2 yields a subgroup of RTN neurons (~44%) that are pH-insensitive (Figure 2D,H), and which lack a pH-sensitive background K+ current; in the remaining pH-sensitive RTN neurons the effect of pH on firing rate is blunted (Wang et al., 2013a). The aggregate firing rate is higher in TASK-2-deleted RTN neurons through the physiological pH range, as expected for elimination of a background K+ channel (Figure 2D) (Bayliss et al., 2015; Wang et al., 2013a). Importantly, the ventilatory response to CO2 is significantly reduced in mice lacking TASK-2 channels (Gestreau et al., 2010; Kumar et al., 2015).
A proton-activated G protein-coupled receptor, GPR4, also accounts for pH-sensitivity in a subset of RTN neurons (Kumar et al., 2015; Ludwig et al., 2003). In the brainstem, GPR4 expression is very high in Phox2b-expressing RTN neurons (Figure 2E,G), low in raphe neurons and undetectable elsewhere (Kumar et al., 2015). A subgroup of pH-insensitive RTN neurons (~40%) is found in mice deleted for GPR4 (Figure 2H) or following exposure to a small molecule GPR4 antagonist, and RTN neuronal pH sensitivity is disrupted by interfering with intracellular G protein signaling (Kumar et al., 2015). In GPR4−/− mice, CO2-evoked RTN neuronal activation (i.e., cFos activation) and CO2-stimulated breathing are diminished (Figure 2F) (Kumar et al., 2015). Crucially, virally-mediated re-expression of GPR4 selectively in RTN neurons of GPR4−/− mice restores CO2-evoked neuronal activation in vivo (i.e., cFos expression), and rescues the ventilatory phenotype (Figure 2F) (Kumar et al., 2015).
GPR4 does not affect pH-sensitive TASK-2 currents in recombinant expression systems, and GPR4 and TASK-2 are expressed in distinct, but overlapping, subsets of Phox2b-expressing RTN neurons (Figure 2G) (Kumar et al., 2015). Consistent with independent cellular actions in RTN neurons, the ventilatory response to CO2 is reduced by more than 85% in double GPR4−/−:TASK-2−/− mice but by only ~60% in single knock-out mice (Figure 2I) (Kumar et al., 2015). These results implicate both GPR4 and TASK-2 as molecular proton detectors for RTN neuronal pH sensitivity, and for RTN-mediated central respiratory chemosensitivity.
The ongoing activity of other ion channels in RTN neurons, and their modulation by neurotransmitter systems, may also alter RTN cellular activity and transduction of chemoreceptor stimuli into respiratory output (Figure 2A). This may be true whether or not the cellular source of the transmitters, or the channels themselves, have any intrinsic pH sensitivity. In this respect, the effect of serotonin on RTN neurons and breathing is instructive. Hypercapnia enhances the respiratory activation induced by optogenetic stimulation of raphe obscurus neurons, even though that particular group of serotonergic neurons is not CO2-responsive (Brust et al., 2014; Depuy et al., 2011). Serotonin excites RTN neurons, in part, via 5-HT2-mediated inhibition of a KV7 channel current and 5-HT7-mediated activation of HCN channels (Figures 2A, 3A–C) (Hawkins et al., 2015; Hawryluk et al., 2012), even though the pH-sensitivity of RTN neurons is unchanged by serotonin (Figure 3A,B) or by pharmacologically modulating KV7 and/or HCN channels (Hawryluk et al., 2012; Mulkey et al., 2007a). However, both 5-HT exposure and direct KV7/HCN channel modulation in the RTN can shift the CO2 threshold for respiration and enhance respiratory output at physiological pH or PCO2 levels (Hawryluk et al., 2012; Mulkey et al., 2007a). Thus, changes in RTN neuron excitability elicited by serotonin, independent of pH sensing per se, can be manifest as altered threshold or gain of the ventilatory response to CO2; this may also be true for other neurons that contribute, directly or indirectly, to the respiratory chemoreflex.
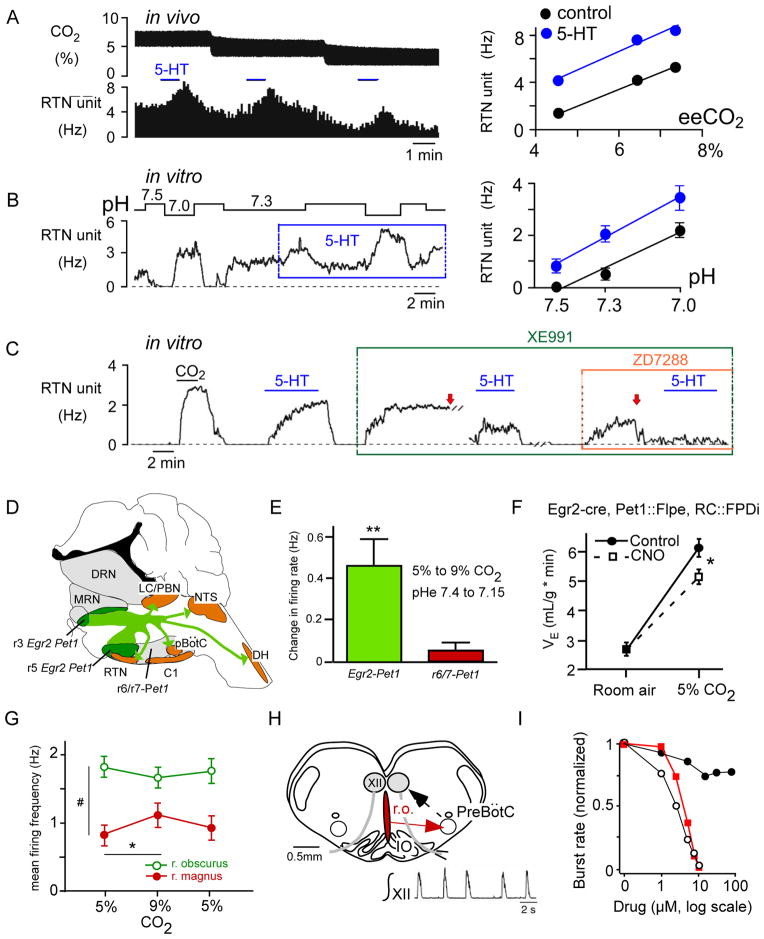
Figure 3. lower brainstem serotonergic neurons and chemoreflexes.
(A) CO2 sensitivity of RTN neurons in vivo ( Hz/arterialPCO2) is unchanged by iontophoretic application of serotonin (5-HT; raw data for a single neuron at left) (from (Mulkey et al., 2007a)). (B) pH sensitivity of RTN neurons in vivo ( Hz/ pH) is unchanged by bath application of 5 μM 5-HT (raw data for one neuron at left) (from (Mulkey et al., 2007a)). (C) Blocking KV7 channels (with 10 μM XE991) and HCN channels (with 50 μM ZD7288) essentially eliminated 5-HT effects on firing rate in a CO2-sensitive rat RTN neuron in vitro; arrows indicate current injection to reset baseline firing (from (Hawkins et al., 2015)). (D) Serotonergic neurons of egr-2 lineage innervate most lower brainstem and spinal cord regions implicated in respiratory control (C1, C1 adrenergic neurons; DRN, dorsal raphe; MRN, median raphe; LC, locus coeruleus; DH, dorsal horn; see Figure 1 for other abbreviations) (redrawn from (Brust et al., 2014)). (E) Serotonergic neurons of egr-2 lineage are significantly activated by hypercapnia in mouse brain slices unlike serotonergic neurons of rhombomere 6/7 origin (redrawn from (Brust et al., 2014)). (F) Pharmacogenetic inhibition of Egr-2 –derived serotonergic neurons attenuates the hypercapnic ventilatory reflex in mice (redrawn from (Brust et al., 2014)). Clozapine-N-oxide (CNO) was administered to activate an inhibitory DREAAD expressed selectively in Egr-2-derived serotonergic neurons. (G) Mild activation of raphe magnus serotonergic neurons by hypercapnia in an arterially perfused rat (redrawn from (Iceman et al., 2013)). (H) Rat “breathing slice” preparation. Inset shows integrated respiratory-like activity of hypoglossal nerve rootlet. (I) Dose-dependent inhibition of the respiratory burst rate by bath application of methysergide (broad spectrum serotonin antagonist; open circles), a substance P receptor antagonist (SR140333; red squares) and RS102221, an inactive serotonin receptor antagonist (black circles). The antagonists blocked the excitatory effects of serotonin and substance P presumably released by raphe obscurus (ro) neurons (H, I redrawn from (Ptak et al., 2009). Abbrs: IO, inferior olive; r.o., raphe obscurus; XII, hypoglossal motoneurons.
CO2-sensitivity of RTN neurons: the role of astrocytes
RTN neurons display greater CO2 sensitivity in vivo than in vitro (5 Hz/0.1 pH, mean dynamic range 0–10 Hz vs. ~0.6 Hz/0.1 pH, 0–3 Hz) (Guyenet et al., 2005; Lazarenko et al., 2009; Mulkey et al., 2004). These differences may be technical and trivial (e.g., for slices or isolated cells: low temperature recordings, tissue immaturity, neuronal damage; for unit recordings in vivo: effects of anesthesia), but they might also reflect other important mechanisms at play in vivo. For example, the RTN response to CO2 may be facilitated in vivo by neuromodulators (Hawryluk et al., 2012) or inputs from additional CO2-activated CNS neurons. These quantitative differences may also reflect the role of astrocytes (Figure 4) (Erlichman and Leiter, 2010; Gourine et al., 2010; Wenker et al., 2010).
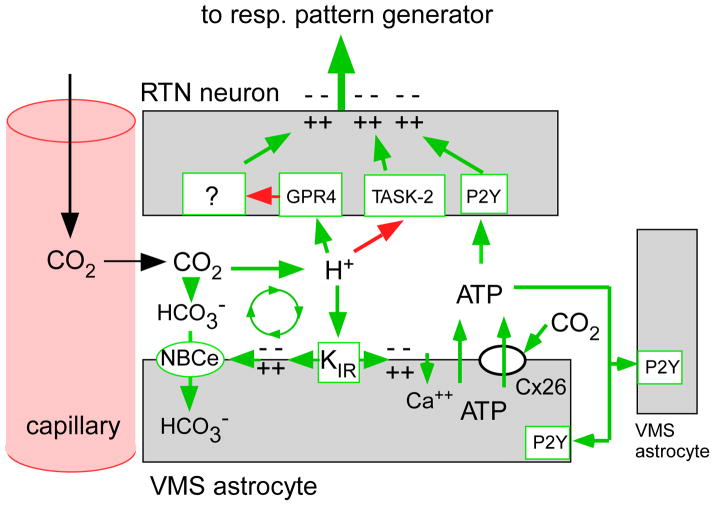
Figure 4. hypothetical contribution of astrocytes to the CO2 sensitivity of RTN neurons.
The activation of RTN neurons by CO2 requires the expression of two proton receptors (TASK-2 and GPR4) but this response may be facilitated or potentiated by surrounding astrocytes in several ways. Extracellular acidification depolarizes RTN astrocytes by closing an inwardly rectifying potassium channel (KIR). This depolarization, which can also be mimicked optogenetically by introducing ChR2 into these astrocytes, elicits the release ATP and, possibly, other gliotransmitters (Gourine et al., 2010; Kasymov et al., 2013). ATP then contributes to the activation of RTN neurons via P2Y receptors and recruits more astrocytes. Astrocyte depolarization may also activate an electrogenic sodium-bicarbonate transporter (NBCe) which moves bicarbonate into the cells, thereby further acidifying the extracellular space and enhancing the depolarization of RTN neurons (Erlichman and Leiter, 2010). Finally, CO2 may also trigger ATP release through Cx-26 hemichannels (Huckstepp et al., 2010).
CO2-induced ATP release and acid-depolarized astrocytes are present throughout the ventral surface of the medulla oblongata (Fukuda and Honda, 1975; Gourine et al., 2005; Kasymov et al., 2013; Wenker et al., 2010). Injection into RTN of fluorocitrate, which selectively depolarizes astrocytes by blocking their metabolism, produces local acidification and activates breathing (Erlichman et al., 1998). Most famously, ChR2-mediated depolarization of nearby astrocytes activates RTN neurons and increases breathing in anesthetized rats (Gourine et al., 2010). The mechanisms of astrocytic ATP release may include depolarization-induced exocytosis (Kasymov et al., 2013), or CO2-evoked carbamylation and activation of ATP-permeant connexin-26 channels (Huckstepp et al., 2010; Meigh et al., 2013). Because effects of astrocytic opto-depolarization were blocked by MRS 2179, a P2Y1 purinergic receptor antagonist, it was proposed that astrocytes, not neurons, are the cellular proton/CO2 sensors responsible for respiratory chemoreception, with ATP providing an obligate excitatory signal from astrocytes to neurons via P2Y1 receptors (Gourine et al., 2010). However, later work revealed that RTN neurons are intrinsically chemosensitive (Wang et al., 2013b), and that various P2 receptor antagonists, including MRS 2179, reduced the activation of RTN neurons by CO2 modestly, at best (Mulkey et al., 2006; Onimaru et al., 2012; Wenker et al., 2012).
Can the initial suggestion of a primary sensory role for astrocytes be reconciled with new data showing that [H+] detectors in RTN neurons are required for CO2-stimulated breathing? As usual, there are potential technical issues to consider: ChR2 is permeable to protons and acidifies the cytoplasm of glial cells causing the release of lactate and glutamate, which can depolarize surrounding neurons (Beppu et al., 2014; Tang et al., 2014). Also, the enhanced GFAP promoter used to transduce astrocytes with ChR2 may not be totally selective for glia. A more interesting possibility is that the acid-depolarized glia enhances the apparent pH sensitivity of RTN neurons by augmenting local extracellular acidification relative to arteriolar pH. Astrocytes are capable of dynamic and bidirectional regulation of local blood flow (Gordon et al., 2011). If CO2 (or ChR2)-mediated depolarization of astrocytes in vivo were to cause vasoconstriction, the consequence would be tissue CO2 retention and breathing activation via local acidification. Yet another possibility is astrocytic depolarization-induced alkalization (DIA), which causes simultaneous extrusion of protons (Figure 4)(Erlichman et al., 2004). These hypotheses are appealing given that specific proton detectors, selectively expressed by RTN neurons but absent from glia, are required for the ventilatory response to CO2.
In conclusion, pH-sensitive astrocytes may contribute to the CO2-response of RTN neurons by releasing ATP, lactate or glutamate, or by exaggerating local changes in extracellular pH. However, the importance of these mechanisms to central respiratory chemoreception is not clearly established.
RTN development
RTN originates from an Egr-2-dependent embryonic domain (rhombomeres 3/5) and co-expresses Atoh-1 and Phox2b during late embryogenesis (Dubreuil et al., 2009; Ramanantsoa et al., 2011). Deletion of any one of these three transcription factors prevents RTN from developing (Ruffault et al., 2015) or, in the case of Atoh-1, from establishing proper connections (Huang et al., 2012).
RTN or the RTN region has been described as an inspiratory rhythm generator, an oscillator for active expiration or a central respiratory chemoreceptor (Guyenet, 2008; Marina et al., 2010; Pagliardini et al., 2011; Wittmeier et al., 2008). These interpretations should be considered in a proper developmental and physiological context.
Prenatally, RTN neurons, a.k.a. embryonic parafacial oscillator (e-pF) exhibit group pacemaker properties that rely on persistent Na current (INaP), hyperpolarization-activated cationic current (Ih) and gap junctions (Fortin and Thoby-Brisson, 2009; Ruffault et al., 2015; Thoby-Brisson et al., 2009). RTN is pH-modulated and activates the preBötzinger complex. In vitro the embryonic RTN and the preBötzinger complex operate as coupled oscillators (Fortin and Thoby-Brisson, 2009; Thoby-Brisson et al., 2009).
Around birth (P0-P2), RTN neurons seem to retain many of their embryonic characteristics. Originally described as the “parafacial respiratory group” (pfRG), many neonatal RTN neurons (that are pH-sensitive and express Phox2b, VGlut2 and NK1 receptors) retain intrinsic burst-generating properties in vitro (Onimaru and Homma, 2003; Onimaru et al., 2008; Onimaru et al., 2014). Like the e-pF, neonatal RTN is pH-modulated and operates as an oscillator coupled to the preBötzinger complex (Onimaru and Homma, 2003).
In certain preparations of neonatal rats the abdominal muscles or abdominal nerve rootlets display a double burst that resembles the discharge pattern of pfRG neurons (Janczewski and Feldman, 2006; Janczewski et al., 2002; Onimaru et al., 1987). In neonatal anesthetized midbrain transected rats (7–12 days), opiate agonists produce quantal slowing of the inspiratory air flow but have little effect on the respiratory-synchronous contractions of expiratory abdominal muscles (Feldman et al., 2013; Janczewski and Feldman, 2006). A second brain transection at mid-facial nucleus level eliminates the respiratory-like abdominal contractions, suggesting that the transected region (RTN/pfRG) contains an opiate-resistant expiratory “oscillator” (Feldman et al., 2013; Janczewski and Feldman, 2006). Consistent with this interpretation, respiratory-synchronous discharges of lumbar nerves can be enabled or silenced in anesthetized rats by activating or inhibiting neurons located near the facial motor nucleus (Huckstepp et al., 2015; Pagliardini et al., 2011). Neurons other than RTN have been implicated in these effects (Huckstepp et al., 2015) but selective activation of RTN neurons in adult conscious rats does elicit active expiration and active expiration is suppressed when these neurons are inhibited (Abbott et al., 2011; Marina et al., 2010). Thus, as summarized in Figure 1K, RTN neurons may either gate or enable a nearby parafacial network that drives active expiration.
In conclusion, the use of preparations varying in age from embryos to adults has contributed to the divergent views regarding non-chemosensory roles of RTN neurons. During the late embryonic and early postnatal period, RTN neurons have intrinsic bursting properties and operate together with the preBötzinger complex as coupled oscillators, at least in vitro. There is no evidence that such pacemaker properties persist in the adult RTN. By contrast, this nucleus clearly functions as a central chemoreceptor in adults and controls multiple aspects of breathing including frequency, depth of inspiration, active expiration and airway patency.
RTN, Phox2b and congenital central hypoventilation syndrome (CCHS)
As already mentioned, mice with a Phox2b mutation that cause a severe form of CCHS (Phox2b27ala/+) die at birth of respiratory failure and are born without RTN neurons (Amiel et al., 2003; Dubreuil et al., 2008); however, these mice have normal numbers of other neurons that also depend on Phox2b for their development e.g. locus coeruleus, type-I glomus cells (the oxygen sensors of the carotid bodies) and serotonergic neurons (Dubreuil et al., 2008). Thus, for unknown reasons, RTN development is especially vulnerable to this Phox2b mutation. When the Phox2b27ala mutation is restricted to neurons of r3/r5 lineage, RTN neurons and the ventilatory response to CO2 are again largely absent at birth but these mice survive and their chemoreflex is partially (~35%) restored by two weeks after birth (Ramanantsoa et al., 2011). An even more selective genetic lesion of RTN produces a similar respiratory phenotype (Ruffault et al., 2015). These mice may survive because of an incomplete loss of RTN neurons and a compensatory increase in contributions from other central chemoreceptors or carotid bodies (Ramanantsoa et al., 2011).
An RTN-like structure has been identified in humans (Rudzinski and Kapur, 2010). However, the crucial evidence that these neurons are actually missing in patients with CCHS-causing Phox2b mutations is yet to be produced.
Serotonergic neurons, breathing and CO2 homeostasis
Several lines of evidence suggest that subsets of serotonergic neurons may have central respiratory chemoreceptor properties. CO2-induced ventilation in mice is reduced when serotonergic neuron development is impaired (Buchanan and Richerson, 2010; Hodges et al., 2008; Hodges et al., 2009). Global acute pharmacogenetic inhibition of serotonergic neurons or selective inhibition of r3/5-derived serotonergic neurons (Figure 3F) attenuates CO2-stimulated ventilation (Brust et al., 2014; Ray et al., 2011). Also, optogenetic stimulation of medullary raphe serotonergic neurons activates breathing in conscious or anesthetized mice (Depuy et al., 2011). Thus, two facts are clearly established: activation of serotonergic raphe neurons stimulates ventilation and full effects of CO2 on breathing require ongoing activity of raphe neurons.
In the mature brain, classically defined serotonergic raphe neuronal clusters (B1-B9) contain neurons derived from several embryonic domains or rhombomeres, with the pontine and medullary raphe nuclei providing most of serotonergic innervation to the lower brainstem and spinal cord (Bang et al., 2012; Brust et al., 2014; Jensen et al., 2008). In conscious cats, midbrain and lower brainstem raphe cells display a slow, regular discharge rate with a prominent state-dependence (i.e. highest during active waking, silent during REM) and about 20% of these neurons are activated by elevated CO2 (Jacobs et al., 2002; Martin-Cora et al., 2005; Martin-Cora et al., 2000; Veasey et al., 1995). In rodents, serotonergic neurons located in raphe magnus are usually mildly activated by CO2 in slices or in an arterially perfused preparation (<0.5 Hz, on average) (Brust et al., 2014; Iceman et al., 2013), whereas those in raphe obscurus are typically unresponsive (Figure 3E,G) (Depuy et al., 2011; Iceman et al., 2013) and parapyramidal serotonergic cells usually inhibited (Mulkey et al., 2004). A much higher proportion of serotonergic neurons from various raphe nuclei (73–100%) are CO2-responsive when recorded in slices or in culture (Severson et al., 2003; Wang et al., 2001). Thus, CO2/H+-activated serotonergic neurons have been repeatedly identified within a variety of classically-defined raphe neuron subgroups, albeit in highly variable proportion depending on the preparation. These discrepancies may be related to the intermingling of CO2-sensitive and insensitive serotonergic neurons within a given brain region. Indeed, when distinct serotonergic neuron subtypes are identified based both on anatomic location and intersection of select genetic markers, those Egr2- and Pet1-expressing lower brainstem serotonergic neurons located primarily in raphe magnus are nearly all CO2 sensitive ex vivo, and inhibition of that genetically-identified population reduces CO2 effects on breathing in vivo (Brust et al., 2014). This particular group of genetically-defined, CO2-sensitive serotonergic neurons may therefore function as central respiratory chemoreceptors (Teran et al., 2014). However, it is important to note that serotonergic neurons can facilitate the respiratory chemoreflex in other ways. For example, the ventilatory deficits caused by inhibition or lesion of serotonergic neurons are completely reversed by intracerebral administration of serotonin (Hodges et al., 2008); this observation suggests that serotonin facilitates a respiratory reflex initiated by CO2 sensors located elsewhere. Also, serotonergic neurons recently judged to be insensitive to CO2, in vivo or ex vivo (e.g., raphe obscurus) are clearly able to activate breathing in reduced preparations or in conscious mice (Brust et al., 2014; Depuy et al., 2011; Ptak et al., 2009)(Figure 3H,I).
In summary, although several medullary raphe subdivisions seem capable of activating breathing, intrinsic pH-sensitivity may be restricted to a subset of raphe magnus serotonergic neurons that serve as bona fide respiratory chemoreceptors (Brust et al., 2014; Teran et al., 2014). However, as recently achieved for RTN, a critical test of this hypothesis will require identification of the molecular bases for their pH sensitivity, and demonstration that selective elimination of that sensing mechanism (rather than wholesale inhibition or destruction of the neurons) attenuates the respiratory chemoreflex. The most obvious changes in the activity of serotonergic neurons in vivo are state-related (Jacobs and Azmitia, 1992) and serotonin loss-of-function experiments may partially reproduce the generally depressant effects of REM sleep on muscle tone, breathing, autonomic functions and thermogenesis (Berthon-Jones and Sullivan, 1984; Horner et al., 2002; Lovering et al., 2003; Teran et al., 2014). Thus, lower brainstem serotonergic neurons likely contribute to arousal state-dependent modulation of multiple systems, including breathing.
Serotonergic neurons and sudden infant death syndrome (SIDS)
A triple threat hypothesis posits that SIDS requires: 1) a genetic predisposition; 2) an immature and inherently unstable respiratory network; and 3) precipitating environmental factors (Becker, 1990; Kinney and Thach, 2009). Many SIDS cases may be caused by a defect in asphyxia-induced arousal or auto-resuscitation (Darnall, 2013). Severe brain hypoxia can cause the glottis to constrict during inspiration as opposed to immediately after, with potentially dire consequences on air flow (Dutschmann and Paton, 2005). Auto-resuscitation is a powerful stimulation of breathing that is probably triggered by severe CNS hypoxia. Its main respiratory manifestation, gasping, is a brief and intense series of inspiratory efforts which, if unsuccessful in restoring normal breathing and oxygenation, precedes death. The failure to arouse may also prevent a life-saving shift in body position that would otherwise free obstructed airways (Garcia et al., 2013).
The brainstem cholinergic system, the RTN, peripheral chemoreceptors and brainstem serotonergic neurons have been judged abnormal in some cases of SIDS (Duncan et al., 2010; Lavezzi et al., 2012; Pena et al., 2004; Porzionato et al., 2013) but defects of the serotonergic system may be most critical. Transgenic mice lacking serotonin neurons have high mortality during development, severe neonatal apneas (Hodges et al., 2009) and fail to arouse when exposed to CO2 (Buchanan and Richerson, 2010). Also, lesioning serotonergic neurons in neonate rodents weakens hypoxia-induced gasping (Cummings et al., 2011). Gasping is attributed to increased INaP in lower brainstem respiratory neurons (Del Negro et al., 2002; Paton et al., 2006; Ramirez et al., 1998) with possible contribution of ATP release from astrocytes (Marina et al., 2013). An INaP-dependent gasp-like inspiratory pattern is also observed in hypoxic “breathing” slices, which is facilitated by serotonin (Pena et al., 2004; Tryba et al., 2006).
In summary, in rodents, the serotonergic system is critical to breathing during the neonatal period. Severe deficits of CNS serotonin neurons impair two mechanisms considered essential to survive central apneas or accidental airway obstruction during sleep: asphyxia-induced arousal, and hypoxia-induced gasping and resuscitation. Abnormalities of the lower brainstem serotonergic system and many other areas, including RTN, have been reported in SIDS. The proximate cause of SIDS, however, remains unknown.
Chemoreflexes and sleep
The chemoreflexes are depressed during sleep whereas chemoreceptor stimulation produces arousal from sleep. These reciprocal interactions have important implications for sleep medicine (Javaheri and Dempsey, 2013).
During non-REM sleep the pontomedullary respiratory pattern generator is presumed to be autorhythmic and its activity is highly dependent on inputs from central and peripheral chemoreceptors (Janczewski et al., 2013; Javaheri and Dempsey, 2013). Consistent with this, opto-inhibition of RTN reduces breathing considerably during non-REM sleep and this inhibition is much greater when the carotid bodies are silenced by hyperoxia (Burke et al., 2015). The low level of breathing present during non-REM sleep and its heavy dependence on chemoreceptors explains why minor fluctuations of PCO2 can cause apneas or periodic breathing and CNS-damaging hypoxemia (Dempsey et al., 2012; Javaheri and Dempsey, 2013). Periodic breathing, commonly present during sleep at altitude (i.e., in hypobaric hypoxia) and in advanced heart failure, is attributed to an increase in peripheral chemoreflex gain and/or to a longer time constant of the central chemoreflex (Dempsey et al., 2012; Marcus et al., 2014). The apneas are probably caused by recurring episodes of CNS hypocapnia and the ensuing inactivity of central respiratory chemoreceptors such as RTN (Basting et al., 2015).
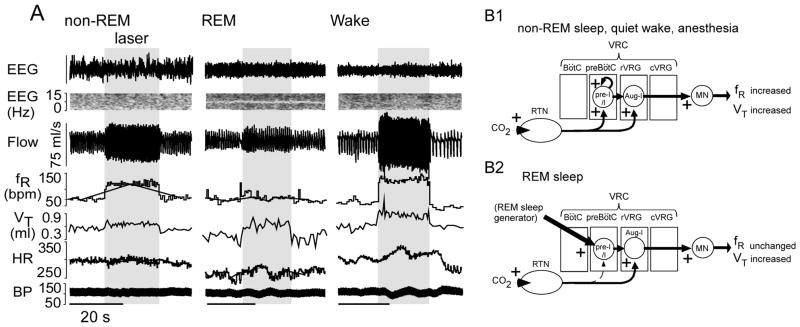
During REM sleep, the chemoreflexes are greatly attenuated because breathing frequency is no longer under the control of chemoreceptors ((Burke et al., 2015) and refs. therein). Despite the presumed loss of the stimulatory effects of wake-on modulators (e.g., orexin, noradrenaline and serotonin), the relative atonia of many respiratory muscles in REM (e.g., abdominals, airways) and a reduced tidal volume, overall ventilation is actually well maintained during REM sleep; this is due to an net increase in mean breathing frequency, with highly variable inspiratory burst intervals, of unknown origin (Orem et al., 2005) and no longer under RTN control (Figure 5)(Burke et al., 2015). This evidence suggests that the preBötzinger complex is no longer autorhythmic in REM sleep and that the frequency of inspiratory bursts is governed by inputs from other brain regions, conceivably the cortex (analogous to the voluntary control of breathing) or brainstem structures that contribute to other aspects of REM sleep (Fraigne and Orem, 2011). Serotonergic and pontine noradrenergic neurons are generally silent during REM sleep and those serotonergic neurons that remain active during REM sleep no longer respond to CO2 (Veasey et al., 1995). Therefore these aminergic neuron groups are unlikely to operate as central chemoreceptors during REM sleep. The only central chemoreceptors known to exert any influence on breathing during REM sleep are the RTN neurons, and they influence breathing amplitude but not frequency.
Figure 5. state-dependent control of breathing by RTN.
(A) Unilateral optogenetic activation of RTN (ChR2) increases breathing frequency during non-REM sleep and quiet waking but has no effect during REM sleep (identified by ~7Hz theta rhythm in EEG). By contrast inspiratory (tidal) volume (VT) is increased regardless of the state of vigilance (reproduced from (Burke et al., 2015)). (B) Speculative interpretation. (B1) During quiet waking, non-REM sleep or anesthesia, the breathing rhythm is generated autonomously by the synchronized bursts of preI/I neurons located in the pre-Bötzinger complex (Janczewski et al., 2013; Koshiya and Smith, 1999; St-John et al., 2009). The pacemaker depolarization of these neurons is accelerated by RTN thereby increasing breathing frequency. The RTN input to the premotor neurons (rVRG) increases the burst amplitude, and thus VT. (B2) During REM sleep, we speculate that the burst frequency of the pre-Bötzinger complex is controlled by inputs that originate outside the respiratory pattern generator (hypothetical REM sleep generator) and prevent RTN from modulating the respiratory frequency. The excitatory input from RTN to the inspiratory premotor neurons is still operating hence the control of inspiratory amplitude by RTN persists.
Chemoreceptor stimuli produce arousal from sleep (Ayas et al., 2000; Berry and Gleeson, 1997; Guyenet and Abbott, 2013). Either hypoxia or hypercapnia alone can produce arousal but, because severe hypercapnia is normally paired with hypoxemia during sleep (hypoventilation), arousal typically occurs under the combined effect of central and peripheral chemoreceptors. The lateral parabrachial nucleus contributes to the arousal produced by chemoreceptor stimulation in mice (Kaur et al., 2013). This region receives convergent input from RTN, from caudal NTS neurons that relay carotid body inputs, from serotonergic neurons and from the C1 adrenergic cells (Bochorishvili et al., 2012; Burke et al., 2014; Song et al., 2011). The C1 neurons are highly responsive to carotid body stimulation and brain hypoxemia (Guyenet, 2014; Koganezawa and Paton, 2014) and arousal from NREM sleep is reliably elicited by C1 cell activation (Burke et al., 2014).
In brief, RTN generates a considerable portion of the drive to breathe during non-REM sleep. During REM sleep, the contribution of RTN is reduced but still present, REM-off aminergic systems are generally silent, and breathing frequency is regulated by unknown mechanisms. Drugs that selectively activate RTN neurons could, in principle, be useful to treat central sleep apnea syndromes and periodic breathing that are prevalent during nREM sleep.
The hyperpnea of exercise
Breathing increases instantly at the beginning of exercise (phase 1 of hyperpnea), and then more slowly until a steady-state is reached (phase 2, t1/2 ~1 min in humans). For mild to moderate aerobic exercise, PaCO2 is invariant throughout (or even drops a little in non-human mammals) and the gain of the hypercapnic ventilatory reflex is unchanged (Forster et al., 2012). Therefore the hyperpnea of moderate exercise is not driven by an increase in PaCO2 or proton concentration caused by the sudden increase in metabolism. This evidence has been taken to rule out the participation of “central chemoreceptors” in exercise hyperpnea, but such an interpretation should be qualified. First, the chemoreflex can attenuate the ventilatory overshoot that occurs at the initiation of exercise (Figure 6 of (Forster et al., 2012)). Secondly, RTN and lower brainstem serotonergic neurons are activated during dynamic exercise (Barna et al., 2012; Veasey et al., 1995). Thus, central chemoreceptor neurons may well contribute to exercise hyperpnea but by mechanisms that are independent of a change in brain PCO2. Finally, during intense anaerobic exercise, when blood lactate accumulation occurs, breathing increases even further. The fall in PaCO2 associated with this hyperventilation moderates lactic acid-induced acidosis, with additional benefits for blood oxygenation and thermoregulation ((Forster et al., 2012) for review). The chemoreceptors, in particular the carotid bodies, contribute to this hyperventilation.
Exercise hyperpnea and the concomitant increase in sympathetic tone (exercise pressor reflex) presumably rely, in part, on activation of group III (thinly myelinated) and group IV (unmyelinated) muscle afferents (Forster et al., 2012; Kaufman, 2012). These afferents are activated by mechanical distortion of their receptive field, metabolic by-products of muscle contraction (H+, K+, and lactate), local inflammation, a rise in tissue temperature and factors that cause muscle pain (Haouzi et al., 1995; Jankowski et al., 2013; Kaufman, 2012). Some of these fibers also respond to venous distension, a variable that could conceivably encode the overall metabolic activity of the muscles via the proxy of muscle blood flow (Haouzi et al., 1995). Groups III and IV muscle afferents innervate and activate lamina I of the dorsal horn (Craig and Mense, 1983; Jankowski et al., 2013; Wilson et al., 2002). In turn, lamina I neurons directly innervate the intermediolateral cell column and several lower brainstem regions involved in breathing and blood pressure control (Craig, 2002, 2013). Therefore, lamina I neurons are the likely initial relay for cardiorespiratory effects produced by unmyelinated muscle afferents.
The degree to which muscle afferents contribute to the hyperpnea of exercise is somewhat controversial. The sufficiency criterion has been satisfied by showing that activation of muscle afferents increases breathing and blood pressure, albeit with the important caveat that these responses could be related to deep muscle pain rather than aerobic exercise. The necessity criterion would require showing that selectively silencing small caliber muscle afferents reduces exercise hyperpnea without impacting muscle work or the chemoreflexes. Toward this end, Amann et al. found that a very low dose of fentanyl given intrathecally to attenuate synaptic transmission between muscle afferents and lamina I neurons blunts exercise hyperpnea (Amann et al., 2010; Dempsey, 2012).
The central command theory posits that motor pathways for locomotion and respiration are driven in parallel by a central feed-forward mechanism (reviewed in (Forster et al., 2012; Paterson, 2014)). A seminal observation was that electrical and chemical stimulation of the caudal hypothalamus (subthalamic locomotor region) produces parallel and proportional activation of locomotion and breathing in decorticated cats (Eldridge et al., 1981). The interpretation of this experiment depends on the assumption, still unverified, that the increase in locomotion and breathing were not caused by the simultaneous stimulation of two functionally independent pathways. Similar interpretative problems exist concerning the role of the other “locomotor centers”: the spinal cord (Le Gal et al., 2014), the periaqueductal gray matter (Paterson, 2014) and the mesencephalic locomotor region (MLR) (Gariepy et al., 2012; Karachi et al., 2010; Le Ray et al., 2011).
Conclusions
The neural control of CO2 homeostasis relies on three processes: the chemoreflexes, “central command” and somatic afferent feedback. The last decade has witnessed rapid progress in understanding the cellular, molecular, and integrative mechanisms underlying the chemoreflex regulation of breathing. Comparable insights into the neural substrates and processes underlying the central command and muscle feedback that drive exercise hyperpnea are lagging.
The RTN is the most thoroughly characterized cluster of central respiratory chemoreceptor neurons. Genetic elimination of these neurons reduces the central chemoreflex to a very large extent. Two proton receptors (TASK-2 and GPR4), with sparse representation elsewhere in the brain are required for RTN neurons to detect changes in brain pH; in the absence of these proton detectors, the central respiratory chemoreflex is nearly abolished. Accordingly, the stimulatory effect of brain PCO2 on breathing is ultimately mediated by changes in brain [H+] that are detected, directly and predominantly, by RTN neurons. This conclusion is at variance with the view that the central respiratory chemoreflex results from actions of H+ or CO2 distributed throughout the respiratory pattern generator and the rest of the brain but it is consistent with earlier ideas hypothesizing specific chemoreceptors in the rostral medulla (Loeschcke, 1982).
Although proton receptors (TASK-2 and GPR4) are required for RTN neurons to respond to elevated brain PCO2, the CO2-sensitivity of these neurons may be boosted by specialized astrocytes that respond to pH and/or to molecular CO2 by releasing ATP locally, enhancing extracellular acidification relative to intravascular pH changes, and modifying local blood flow. The relative importance of these mechanisms requires further evaluation.
RTN neurons regulate alveolar ventilation by adjusting the breathing rate, inspiratory and expiratory muscle activity and airway resistance. These effects occur via excitatory projections to multiple segments of the lower brainstem respiratory pattern generator but the targeted respiratory neurons have yet to be identified. The effect of RTN on breathing is state-dependent; it is most prominent when the brainstem respiratory network is auto-rhythmic (e.g. during non-REM sleep) and breathing frequency is presumably defined by the group pacemaker properties of the preBötzinger complex. During REM sleep, RTN regulates tidal volume but not breathing frequency.
RTN development depends on expression of at least three transcription factors: Atoh-1, Egr-2 and Phox2b and is particularly vulnerable to a Phox2b mutation that causes CCHS in humans (Phox2b27ala/+). This mutation recapitulates in mice the cardinal respiratory signs of the human disease. The congenital absence of RTN neurons could therefore underlie the respiratory deficits observed in CCHS but, in the absence of histopathological evidence from patients, this interpretation remains tentative.
RTN neurons and the carotid bodies normally work in concert to stimulate or reduce breathing in response to hypo- or hyperventilation. During hypoxia, the ventilatory stimulation elicited by carotid body hyperactivity is opposed by a reduction in RTN neuronal activity caused by the concomitant alkalosis. This phenomenon limits the increase in breathing elicited by hypobaric hypoxia and likely contributes to altitude sickness. Conversely, when respiratory drive from the carotid bodies is reduced or eliminated (e.g., hyperoxia), the contribution of RTN neurons to breathing increases, minimizing the respiratory deficit.
Serotonergic, orexinergic and noradrenergic neurons increase breathing and can facilitate the chemoreflex by multiple mechanisms, including RTN stimulation. A subset of serotonergic neurons located in raphe magnus likely has central chemoreceptor properties but their response to CO2 in vivo is small and evidence that actions on the respiratory chemoreflex reflect a specific effect of pH in those cells, via an intrinsic molecular proton detector, has not yet been obtained. Major perturbations in serotonergic systems exacerbate effects of asphyxia in rodents by attenuating CO2-induced arousal and hypoxia-induced auto-resuscitation, and developmental defects in serotonergic transmission may contribute to SIDS.
The stability of PCO2 during exercise is based on coincident activation of the respiratory pattern generator by circuits involved in locomotion (central command) and by small caliber muscle afferents that relay via lamina I of the dorsal horn. Ultimately, a more detailed phenotypic and functional characterization of these afferents and their central connections will require genetic approaches similar to those recently implemented for the study of cutaneous and vagal afferents (Abraira and Ginty, 2013; Chang et al., 2015). The projections from lamina I to the brainstem convey multiple modalities of interoceptive and exteroceptive information, and the challenge remains to identify which particular neurons relay inputs from muscle afferents that are relevant to exercise hyperpnea.
Central command is the least understood of the three mechanisms involved in CO2 homeostasis. The role of the various candidate locomotor centers to exercise hyperpnea should be thoroughly reinvestigated, with a goal of identifying specific roles and precise connectivity of chemically-defined neuronal populations present within the broadly-defined locomotor regions. This recalcitrant research area is ready to yield to the ever-expanding toolkit of contemporary integrative neuroscience.
Acknowledgments
Funding sources: HL074011, HL28785 (PGG); HL108609 (DAB)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott SB, Stornetta RL, Coates MB, Guyenet PG. Phox2b-expressing neurons of the parafacial region regulate breathing rate, inspiration, and expiration in conscious rats. J Neurosci. 2011;31:16410–16422. doi: 10.1523/JNEUROSCI.3280-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott SB, Stornetta RL, Fortuna MG, Depuy SD, West GH, Harris TE, Guyenet PG. Photostimulation of retrotrapezoid nucleus phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J Neurosci. 2009;29:5806–5819. doi: 10.1523/JNEUROSCI.1106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraira VE, Ginty DD. The sensory neurons of touch. Neuron. 2013;79:618–639. doi: 10.1016/j.neuron.2013.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol. 2010;109:966–976. doi: 10.1152/japplphysiol.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiel J, Laudier B, Attie-Bitach T, Trang H, de PL, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet. 2003;33:459–461. doi: 10.1038/ng1130. [DOI] [PubMed] [Google Scholar]
- Ayas NT, Brown R, Shea SA. Hypercapnia can induce arousal from sleep in the absence of altered respiratory mechanoreception. Am J Respir Crit Care Med. 2000;162:1004–1008. doi: 10.1164/ajrccm.162.3.9908040. [DOI] [PubMed] [Google Scholar]
- Bang SJ, Jensen P, Dymecki SM, Commons KG. Projections and interconnections of genetically defined serotonin neurons in mice. Eur J Neurosci. 2012;35:85–96. doi: 10.1111/j.1460-9568.2011.07936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barna BF, Takakura AC, Moreira TS. Pontomedullary and hypothalamic distribution of Fos-like immunoreactive neurons after acute exercise in rats. Neuroscience. 2012;212:120–130. doi: 10.1016/j.neuroscience.2012.03.039. [DOI] [PubMed] [Google Scholar]
- Basting TM, Burke PG, Kanbar R, Viar KE, Stornetta DS, Stornetta RL, Guyenet PG. Hypoxia Silences Retrotrapezoid Nucleus Respiratory Chemoreceptors via Alkalosis. J Neurosci. 2015;35:527–543. doi: 10.1523/JNEUROSCI.2923-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss DA, Barhanin J, Gestreau C, Guyenet PG. The role of pH-sensitive TASK channels in central respiratory chemoreception. Pflugers Arch. 2015;467:917–929. doi: 10.1007/s00424-014-1633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker LE. Neural maturational delay as a link in the chain of events leading to SIDS. Can J Neurol Sci. 1990;17:361–371. doi: 10.1017/s0317167100030894. [DOI] [PubMed] [Google Scholar]
- Beppu K, Sasaki T, Tanaka KF, Yamanaka A, Fukazawa Y, Shigemoto R, Matsui K. Optogenetic countering of glial acidosis suppresses glial glutamate release and ischemic brain damage. Neuron. 2014;81:314–320. doi: 10.1016/j.neuron.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Berry RB, Gleeson K. Respiratory arousal from sleep: mechanisms and significance. Sleep. 1997;20:654–675. doi: 10.1093/sleep/20.8.654. [DOI] [PubMed] [Google Scholar]
- Berthon-Jones M, Sullivan CE. Ventilation and arousal responses to hypercapnia in normal sleeping humans. J Appl Physiol. 1984;57:59–67. doi: 10.1152/jappl.1984.57.1.59. [DOI] [PubMed] [Google Scholar]
- Blain GM, Smith CA, Henderson KS, Dempsey JA. Contribution of the carotid body chemoreceptors to eupneic ventilation in the intact, unanesthetized dog. J Appl Physiol. 2009;106:1564–1573. doi: 10.1152/japplphysiol.91590.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blain GM, Smith CA, Henderson KS, Dempsey JA. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO(2) J Physiol. 2010;588:2455–2471. doi: 10.1113/jphysiol.2010.187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochorishvili G, Stornetta RL, Coates MB, Guyenet PG. Pre-Botzinger complex receives glutamatergic innervation from galaninergic and other retrotrapezoid nucleus neurons. J Comp Neurol. 2012;520:1047–1061. doi: 10.1002/cne.22769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier J, Thoby-Brisson M, Renier N, Dubreuil V, Ericson J, Champagnat J, Pierani A, Chedotal A, Fortin G. Hindbrain interneurons and axon guidance signaling critical for breathing. Nat Neurosci. 2010;13:1066–1074. doi: 10.1038/nn.2622. [DOI] [PubMed] [Google Scholar]
- Brust RD, Corcoran AE, Richerson GB, Nattie E, Dymecki SM. Functional and developmental identification of a molecular subtype of brain serotonergic neuron specialized to regulate breathing dynamics. Cell Rep. 2014;9:2152–2165. doi: 10.1016/j.celrep.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci USA. 2010;107:16354–16359. doi: 10.1073/pnas.1004587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke PG, Abbott SB, Coates MB, Viar KE, Stornetta RL, Guyenet PG. Optogenetic stimulation of adrenergic C1 neurons causes sleep state-dependent cardiorespiratory stimulation and arousal with sighs in rats. Am J Respir Crit Care Med. 2014;190:1301–1310. doi: 10.1164/rccm.201407-1262OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke PG, Kanbar R, Basting TM, Hodges WM, Viar KE, Stornetta RL, Guyenet PG. State-dependent control of breathing by the retrotrapezoid nucleus. J Physiol. 2015 Mar 27; doi: 10.1113/JP270053. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang RB, Strochlic DE, Williams EK, Umans BD, Liberles SD. Vagal Sensory Neuron Subtypes that Differentially Control Breathing. Cell. 2015;161:622–633. doi: 10.1016/j.cell.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. Distribution of brainstem projections from spinal lamina I neurons in the cat and the monkey. J Comp Neurol. 1995;361:225–248. doi: 10.1002/cne.903610204. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Cooling, pain, and other feelings from the body in relation to the autonomic nervous system. Handb Clin Neurol. 2013;117:103–109. doi: 10.1016/B978-0-444-53491-0.00009-2. [DOI] [PubMed] [Google Scholar]
- Craig AD, Mense S. The distribution of afferent fibers from the gastrocnemius-soleus muscle in the dorsal horn of the cat, as revealed by the transport of horseradish peroxidase. Neurosci Lett. 1983;41:233–238. doi: 10.1016/0304-3940(83)90456-1. [DOI] [PubMed] [Google Scholar]
- Cummings KJ, Hewitt JC, Li A, Daubenspeck JA, Nattie EE. Postnatal loss of brainstem serotonin neurones compromises the ability of neonatal rats to survive episodic severe hypoxia. J Physiol. 2011;589:5247–5256. doi: 10.1113/jphysiol.2011.214445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasceno RS, Takakura AC, Moreira TS. Regulation of the chemosensory control of breathing by Kolliker-Fuse neurons. Am J Physiol Regul Integr Comp Physiol. 2014;307:R57–67. doi: 10.1152/ajpregu.00024.2014. [DOI] [PubMed] [Google Scholar]
- Darnall RA. The carotid body and arousal in the fetus and neonate. RespirPhysiol Neurobiol. 2013;185:132–143. doi: 10.1016/j.resp.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Koshiya N, Butera RJ, Jr, Smith JC. Persistent sodium current, membrane properties and bursting behavior of pre-Botzinger complex inspiratory neurons in vitro. J Neurophysiol. 2002;88:2242–2250. doi: 10.1152/jn.00081.2002. [DOI] [PubMed] [Google Scholar]
- Dempsey JA. New perspectives concerning feedback influences on cardiorespiratory control during rhythmic exercise and on exercise performance. J Physiol. 2012;590:4129–4144. doi: 10.1113/jphysiol.2012.233908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JA, Smith CA, Blain GM, Xie A, Gong Y, Teodorescu M. Role of central/peripheral chemoreceptors and their interdependence in the pathophysiology of sleep apnea. Adv Exp Med Biol. 2012;758:343–349. doi: 10.1007/978-94-007-4584-1_46. [DOI] [PubMed] [Google Scholar]
- Depuy SD, Kanbar R, Coates MB, Stornetta RL, Guyenet PG. Control of breathing by raphe obscurus serotonergic neurons in mice. J Neurosci. 2011;31:1981–1990. doi: 10.1523/JNEUROSCI.4639-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF, Goridis C. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnoea and specific loss of parafacial neurons. Proc Natl Acad Sci USA. 2008;105:1067–1072. doi: 10.1073/pnas.0709115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil V, Thoby-Brisson M, Rallu M, Persson K, Pattyn A, Birchmeier C, Brunet JF, Fortin G, Goridis C. Defective respiratory rhythmogenesis and loss of central chemosensitivity in phox2b mutants targeting retrotrapezoid nucleus neurons. J Neurosci. 2009;29:14836–14846. doi: 10.1523/JNEUROSCI.2623-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffin J, Bechbache RR, Goode RC, Chung SA. The ventilatory response to carbon dioxide in hyperoxic exercise. Respir Physiol. 1980;40:93–105. doi: 10.1016/0034-5687(80)90007-9. [DOI] [PubMed] [Google Scholar]
- Duncan JR, Paterson DS, Hoffman JM, Mokler DJ, Borenstein NS, Belliveau RA, Krous HF, Haas EA, Stanley C, Nattie EE, Trachtenberg FL, Kinney HC. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA. 2010;303:430–437. doi: 10.1001/jama.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Herbert H. The Kolliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur J Neurosci. 2006;24:1071–1084. doi: 10.1111/j.1460-9568.2006.04981.x. [DOI] [PubMed] [Google Scholar]
- Dutschmann MA, Paton JFR. Dynamic Changes in Glottal Resistance during Exposure to Severe Hypoxia in Neonatal Rats In Situ. Pediatr Res. 2005;58:193–198. doi: 10.1203/01.PDR.0000169968.07488.AD. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE, Waldrop TG. Exercise hyperpnea and locomotion: parallel activation from the hypothalamus. Science. 1981;211:844–846. doi: 10.1126/science.7466362. [DOI] [PubMed] [Google Scholar]
- Erlichman JS, Cook A, Schwab MC, Budd TW, Leiter JC. Heterogeneous patterns of pH regulation in glial cells in the dorsal and ventral medulla. Am J Physiol Regul Integr Comp Physiol. 2004;286:R289–R302. doi: 10.1152/ajpregu.00245.2003. [DOI] [PubMed] [Google Scholar]
- Erlichman JS, Leiter JC. Glia modulation of the extracellular milieu as a factor in central CO2 chemosensitivity and respiratory control. J Appl Physiol (1985) 2010;108:1803–1811. doi: 10.1152/japplphysiol.01321.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlichman JS, Li A, Nattie EE. Ventilatory effects of glial dysfunction in a rat brain stem chemoreceptor region. J Appl Physiol. 1998;85:1599–1604. doi: 10.1152/jappl.1998.85.5.1599. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol. 2013;75:423–452. doi: 10.1146/annurev-physiol-040510-130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Kam K. Facing the challenge of mammalian neural microcircuits: taking a few breaths may help. J Physiol. 2015;593:3–23. doi: 10.1113/jphysiol.2014.277632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster HV, Haouzi P, Dempsey JA. Control of breathing during exercise. Compr Physiol. 2012;2:743–777. doi: 10.1002/cphy.c100045. [DOI] [PubMed] [Google Scholar]
- Fortin G, Thoby-Brisson M. Embryonic emergence of the respiratory rhythm generator. Respir Physiol Neurobiol. 2009;168:86–91. doi: 10.1016/j.resp.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Fraigne JJ, Orem JM. Phasic motor activity of respiratory and non-respiratory muscles in REM sleep. Sleep. 2011;34:425–434. doi: 10.1093/sleep/34.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y, Honda Y. pH-sensitive cells at ventro--lateral surface of rat medulla oblongata. Nature. 1975;256:317–318. doi: 10.1038/256317a0. [DOI] [PubMed] [Google Scholar]
- Garcia AJ, III, Koschnitzky JE, Ramirez JM. The physiological determinants of Sudden Infant Death Syndrome. Respir Physiol Neurobiol. 2013;189:288–300. doi: 10.1016/j.resp.2013.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariepy JF, Missaghi K, Chevallier S, Chartre S, Robert M, Auclair F, Lund JP, Dubuc R. Specific neural substrate linking respiration to locomotion. Proc Natl Acad Sci U S A. 2012;109:E84–92. doi: 10.1073/pnas.1113002109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits PO, Holstege G. Pontine and medullary projections to the nucleus retroambiguus: A wheat germ agglutinin horseradish peroxidase and autoradiographic tracing study in the cat. J Comp Neurol. 1996;373:173–185. doi: 10.1002/(SICI)1096-9861(19960916)373:2<173::AID-CNE2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Gestreau C, Heitzmann D, Thomas J, Dubreuil V, Bandulik S, Reichold M, Bendahhou S, Pierson P, Sterner C, Peyronnet-Roux J, Benfriha C, Tegtmeier I, Ehnes H, Georgieff M, Lesage F, Brunet JF, Goridis C, Warth R, Barhanin J. Task2 potassium channels set central respiratory CO2 and O2 sensitivity. Proc Natl Acad Sci USA. 2010;107:2325–2330. doi: 10.1073/pnas.0910059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Howarth C, MacVicar BA. Bidirectional control of arteriole diameter by astrocytes. Exp Physiol. 2011;96:393–399. doi: 10.1113/expphysiol.2010.053132. [DOI] [PubMed] [Google Scholar]
- Goridis C, Dubreuil V, Thoby-Brisson M, Fortin G, Brunet JF. Phox2b, congenital central hypoventilation syndrome and the control of respiration. Semin Cell Dev Biol. 2010;21:814–822. doi: 10.1016/j.semcdb.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436:108–111. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The 2008 Carl Ludwig Lecture: retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J Appl Physiol. 2008;105:404–416. doi: 10.1152/japplphysiol.90452.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG. Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol. 2014;4:1511–1562. doi: 10.1002/cphy.c140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Abbott SB. Chemoreception and asphyxia-induced arousal. Respir Physiol Neurobiol. 2013;188:333–343. doi: 10.1016/j.resp.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci. 2005;25:8938–8947. doi: 10.1523/JNEUROSCI.2415-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldane JS, Priestley JG. The regulation of the lung-ventilation. J Physiol. 1905;32:225–266. doi: 10.1113/jphysiol.1905.sp001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haouzi P, Huszczuk A, Gille JP, Chalon B, Marchal F, Crance JP, Whipp BJ. Vascular distension in muscles contributes to respiratory control in sheep. Respir Physiol. 1995;99:41–50. doi: 10.1016/0034-5687(94)00083-c. [DOI] [PubMed] [Google Scholar]
- Hawkins VE, Hawryluk JM, Takakura AC, Tzingounis AV, Moreira TS, Mulkey DK. HCN channels contribute to serotonergic modulation of ventral surface chemosensitive neurons and respiratory activity. J Neurophysiol. 2015;113:1195–1205. doi: 10.1152/jn.00487.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawryluk JM, Moreira TS, Takakura AC, Wenker IC, Tzingounis AV, Mulkey DK. KCNQ channels determine serotonergic modulation of ventral surface chemoreceptors and respiratory drive. J Neurosci. 2012;32:16943–16952. doi: 10.1523/JNEUROSCI.3043-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Wehner M, Aungst J, Smith JC, Richerson GB. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J Neurosci. 2009;29:10341–10349. doi: 10.1523/JNEUROSCI.1963-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. Acid-sensitive ion channels and receptors. In: Canning BJ, Spina D, editors. Sensory Nerves. Springer; Berlin Heidelberg: 2009. pp. 283–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner RL, Liu X, Gill H, Nolan P, Liu H, Sood S. Effects of sleep-wake state on the genioglossus vs.diaphragm muscle response to CO(2) in rats. J Appl Physiol. 2002;92:878–887. doi: 10.1152/japplphysiol.00855.2001. [DOI] [PubMed] [Google Scholar]
- Hu J, Zhong C, Ding C, Chi Q, Walz A, Mombaerts P, Matsunami H, Luo M. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317:953–957. doi: 10.1126/science.1144233. [DOI] [PubMed] [Google Scholar]
- Huang WH, Tupal S, Huang TW, Ward CS, Neul JL, Klisch TJ, Gray PA, Zoghbi HY. Atoh1 governs the migration of postmitotic neurons that shape respiratory effectiveness at birth and chemoresponsiveness in adulthood. Neuron. 2012;75:799–809. doi: 10.1016/j.neuron.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RT, Cardoza KP, Henderson LE, Feldman JL. Role of parafacial nuclei in control of breathing in adult rats. J Neurosci. 2015;35:1052–1067. doi: 10.1523/JNEUROSCI.2953-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RT, Dale N. Redefining the components of central CO2 chemosensitivity--towards a better understanding of mechanism. J Physiol. 2011;589:5561–5579. doi: 10.1113/jphysiol.2011.214759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RT, Eason R, Sachdev A, Dale N. CO2-dependent opening of connexin 26 and related beta connexins. J Physiol. 2010;588:3921–3931. doi: 10.1113/jphysiol.2010.192096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang DY, Carlezon WA, Jr, Isacson O, Kim KS. A high-efficiency synthetic promoter that drives transgene expression selectively in noradrenergic neurons. Hum Gene Ther. 2001;12:1731–1740. doi: 10.1089/104303401750476230. [DOI] [PubMed] [Google Scholar]
- Iceman KE, Richerson GB, Harris MB. Medullary serotonin neurons are CO2-sensitive in situ. J Neurophysiol. 2013;110:2536–2544. doi: 10.1152/jn.00288.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. [Review] Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Martín-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Rev. 2002;40:45–52. doi: 10.1016/s0165-0173(02)00187-x. [DOI] [PubMed] [Google Scholar]
- Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol. 2006;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski WA, Onimaru H, Homma I, Feldman JL. Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: in vivo and in vitro study in the newborn rat. J Physiol. 2002;545:1017–1026. doi: 10.1113/jphysiol.2002.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski WA, Tashima A, Hsu P, Cui Y, Feldman JL. Role of inhibition in respiratory pattern generation. J Neurosci. 2013;33:5454–5465. doi: 10.1523/JNEUROSCI.1595-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, Rau KK, Ekmann KM, Anderson CE, Koerber HR. Comprehensive phenotyping of group III and IV muscle afferents in mouse. J Neurophysiol. 2013;109:2374–2381. doi: 10.1152/jn.01067.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaheri S, Dempsey JA. Central sleep apnea. Compr Physiol. 2013;3:141–163. doi: 10.1002/cphy.c110057. [DOI] [PubMed] [Google Scholar]
- Jensen P, Farago AF, Awatramani RB, Scott MM, Deneris ES, Dymecki SM. Redefining the serotonergic system by genetic lineage. Nat Neurosci. 2008;11:417–419. doi: 10.1038/nn2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karachi C, Grabli D, Bernard FA, Tande D, Wattiez N, Belaid H, Bardinet E, Prigent A, Nothacker HP, Hunot S, Hartmann A, Lehéricy S, Hirsch EC, François C. Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J Clin Invest. 2010;120:2745–2754. doi: 10.1172/JCI42642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasymov V, Larina O, Castaldo C, Marina N, Patrushev M, Kasparov S, Gourine AV. Differential sensitivity of brainstem versus cortical astrocytes to changes in pH reveals functional regional specialization of astroglia. J Neurosci. 2013;33:435–441. doi: 10.1523/JNEUROSCI.2813-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MP. The exercise pressor reflex in animals. Exp Physiol. 2012;97:51–58. doi: 10.1113/expphysiol.2011.057539. [DOI] [PubMed] [Google Scholar]
- Kaur S, Pedersen NP, Yokota S, Hur EE, Fuller PM, Lazarus M, Chamberlin NL, Saper CB. Glutamatergic signaling from the parabrachial nucleus plays a critical role in hypercapnic arousal. J Neurosci. 2013;33:7627–7640. doi: 10.1523/JNEUROSCI.0173-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Thach BT. The sudden infant death syndrome. N Engl J Med. 2009;361:795–805. doi: 10.1056/NEJMra0803836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koganezawa T, Paton JF. Intrinsic chemosensitivity of rostral ventrolateral medullary sympathetic premotor neurons in the in situ arterially perfused preparation of rats. Exp Physiol. 2014;99:1453–1466. doi: 10.1113/expphysiol.2014.080069. [DOI] [PubMed] [Google Scholar]
- Koshiya N, Smith JC. Neuronal pacemaker for breathing visualized in vitro. Nature. 1999;400:360–363. doi: 10.1038/22540. [DOI] [PubMed] [Google Scholar]
- Kumar NN, Velic A, Soliz J, Shi Y, Li K, Wang S, Weaver JL, Sen J, Abbott SB, Lazarenko RM, Ludwig MG, Perez-Reyes E, Mohebbi N, Bettoni C, Gassmann M, Suply T, Seuwen K, Guyenet PG, Wagner CA, Bayliss DA. Regulation of breathing by CO2 requires the proton-activated receptor GPR4 in retrotrapezoid nucleus neurons. Science. 2015;348:1255–1260. doi: 10.1126/science.aaa0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Prabhakar NR. Peripheral chemoreceptors: function and plasticity of the carotid body. Compr Physiol. 2012;2:141–219. doi: 10.1002/cphy.c100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzi AM, Weese-Mayer DE, Yu MY, Jennings LJ, Corna MF, Casale V, Oneda R, Matturri L. Developmental alterations of the respiratory human retrotrapezoid nucleus in sudden unexplained fetal and infant death. Auton Neurosci. 2012;170:12–19. doi: 10.1016/j.autneu.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Lazarenko RM, Fortuna MG, Shi Y, Mulkey DK, Takakura AC, Moreira TS, Guyenet PG, Bayliss DA. Anesthetic activation of central respiratory chemoreceptor neurons involves inhibition of a THIK-1-like background K(+) current. J Neurosci. 2010;30:9324–9334. doi: 10.1523/JNEUROSCI.1956-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarenko RM, Milner TA, Depuy SD, Stornetta RL, West GH, Kievits JA, Bayliss DA, Guyenet PG. Acid sensitivity and ultrastructure of the retrotrapezoid nucleus in Phox2b-EGFP transgenic mice. J Comp Neurol. 2009;517:69–86. doi: 10.1002/cne.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gal JP, Juvin L, Cardoit L, Thoby-Brisson M, Morin D. Remote control of respiratory neural network by spinal locomotor generators. PLoS One. 2014;9:e89670. doi: 10.1371/journal.pone.0089670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Ray D, Juvin L, Ryczko D, Dubuc R. Chapter 4--supraspinal control of locomotion: the mesencephalic locomotor region. Prog Brain Res. 2011;188:51–70. doi: 10.1016/B978-0-444-53825-3.00009-7. [DOI] [PubMed] [Google Scholar]
- Loeschcke HH. Central chemosensitivity and the reaction theory. J Physiol. 1982;332:1–24. doi: 10.1113/jphysiol.1982.sp014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering AT, Dunin-Barkowski WL, Vidruk EH, Orem JM. Ventilatory response of the cat to hypoxia in sleep and wakefulness. J Appl Physiol. 2003;95:545–554. doi: 10.1152/japplphysiol.01051.2002. [DOI] [PubMed] [Google Scholar]
- Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, Hofstetter H, Wolf RM, Seuwen K. Proton-sensing G-protein-coupled receptors. Nature. 2003;425:93–98. doi: 10.1038/nature01905. [DOI] [PubMed] [Google Scholar]
- Marcus NJ, RDR, Schultz EP, Xia XH, Schultz HD. Carotid body denervation improves autonomic and cardiac function and attenuates disordered breathing in congestive heart failure. J Physiol. 2014;592:391–408. doi: 10.1113/jphysiol.2013.266221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina N, Abdala AP, Trapp S, Li A, Nattie EE, Hewinson J, Smith JC, Paton JF, Gourine AV. Essential role of Phox2b-expressing ventrolateral brainstem neurons in the chemosensory control of inspiration and expiration. J Neurosci. 2010;30:12466–12473. doi: 10.1523/JNEUROSCI.3141-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina N, Tang F, Figueiredo M, Mastitskaya S, Kasimov V, Mohamed-Ali V, Roloff E, Teschemacher AG, Gourine AV, Kasparov S. Purinergic signalling in the rostral ventro-lateral medulla controls sympathetic drive and contributes to the progression of heart failure following myocardial infarction in rats. Basic Res Cardiol. 2013;108:317. doi: 10.1007/s00395-012-0317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JM. Peripheral chemoreceptors and cardiovascular regulation. Physiol Rev. 1994;74:543–594. doi: 10.1152/physrev.1994.74.3.543. [DOI] [PubMed] [Google Scholar]
- Martin-Cora FJ, Fornal CA, Jacobs BL. Single-unit responses of serotonergic medullary raphe neurons to cardiovascular challenges in freely moving cats. EurJ Neurosci. 2005;22:3195–3204. doi: 10.1111/j.1460-9568.2005.04519.x. [DOI] [PubMed] [Google Scholar]
- Martin-Cora FJ, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic medullary and pontine raphe neurons to environmental cooling in freely moving cats. Neuroscience. 2000;98:301–309. doi: 10.1016/s0306-4522(00)00133-0. [DOI] [PubMed] [Google Scholar]
- Meigh L, Greenhalgh SA, Rodgers TL, Cann MJ, Roper DI, Dale N. CO2 directly modulates connexin 26 by formation of carbamate bridges between subunits. Elife. 2013;22:e01213. doi: 10.7554/eLife.01213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizusawa A, Ogawa H, Kikuchi Y, Hida W, Shirato K. Role of the parabrachial nucleus in ventilatory responses of awake rats. J Physiol. 1995;489(Pt 3):877–884. doi: 10.1113/jphysiol.1995.sp021100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Mistry AM, Guyenet PG, Bayliss DA. Purinergic P2 receptors modulate excitability but do not mediate pH sensitivity of RTN respiratory chemoreceptors. J Neurosci. 2006;26:7230–7233. doi: 10.1523/JNEUROSCI.1696-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci. 2007a;27:14128–14138. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci. 2007b;27:14049–14058. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie E. Julius H. Comroe, Jr., distinguished lecture: central chemoreception: then ... and now. J Appl Physiol. 2011;110:1–8. doi: 10.1152/japplphysiol.01061.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse CA. Synaptic and paracrine mechanisms at carotid body arterial chemoreceptors. J Physiol. 2014;592:3419–3426. doi: 10.1113/jphysiol.2013.269829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Localization of respiratory rhythm-generating neurons in the medulla of brainstem-spinal cord preparations from newborn rats. Neurosci Lett. 1987;78:151–155. doi: 10.1016/0304-3940(87)90624-0. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K, Kawakami K. CO2-Sensitive Preinspiratory Neurons of the Parafacial Respiratory Group Express Phox2b in the Neonatal Rat. J Neurosci. 2008;28:12845–12850. doi: 10.1523/JNEUROSCI.3625-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K, Kawakami K. Postsynaptic mechanisms of CO(2) responses in parafacial respiratory neurons of newborn rats. J Physiol. 2012;590:1615–1624. doi: 10.1113/jphysiol.2011.222687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K, Mariho T, Kawakami K. Cytoarchitecture and CO(2) sensitivity of Phox2b-positive Parafacial neurons in the newborn rat medulla. Prog Brain Res. 2014;209:57–71. doi: 10.1016/B978-0-444-63274-6.00004-7. [DOI] [PubMed] [Google Scholar]
- Orem JM, Lovering AT, Vidruk EH. Excitation of medullary respiratory neurons in REM sleep. Sleep. 2005;28:801–807. doi: 10.1093/sleep/28.7.801. [DOI] [PubMed] [Google Scholar]
- Otake K, Ezure K, Lipski J, Wong She RB. Projections from the commissural subnucleus of the nucleus of the solitary tract: an anterograde tracing study in the cat. J Comp Neurol. 1992;324:365–378. doi: 10.1002/cne.903240307. [DOI] [PubMed] [Google Scholar]
- Pagliardini S, Janczewski WA, Tan W, Dickson CT, Deisseroth K, Feldman JL. Active expiration induced by excitation of ventral medulla in adult anesthetized rats. J Neurosci. 2011;31:2895–2905. doi: 10.1523/JNEUROSCI.5338-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, Calverley PM, Gift AG, Harver A, Lareau SC, Mahler DA, Meek PM, O’Donnell DE. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185:435–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson DJ. Defining the neurocircuitry of exercise hyperpnoea. J Physiol. 2014;592:433–444. doi: 10.1113/jphysiol.2013.261586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JF, Abdala AP, Koizumi H, Smith JC, St-John WM. Respiratory rhythm generation during gasping depends on persistent sodium current. Nat Neurosci. 2006;9:311–313. doi: 10.1038/nn1650. [DOI] [PubMed] [Google Scholar]
- Pena F, Parkis MA, Tryba AK, Ramirez JM. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron. 2004;43:105–117. doi: 10.1016/j.neuron.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Porzionato A, Macchi V, Stecco C, De Caro R. The carotid body in Sudden Infant Death Syndrome. Respir Physiol Neurobiol. 2013;185:194–201. doi: 10.1016/j.resp.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Potts JT, Rybak IA, Paton JF. Respiratory rhythm entrainment by somatic afferent stimulation. J Neurosci. 2005;25:1965–1978. doi: 10.1523/JNEUROSCI.3881-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR. Sensing hypoxia: physiology, genetics and epigenetics. J Physiol. 2013;591:2245–2257. doi: 10.1113/jphysiol.2012.247759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci. 2009;29:3720–3737. doi: 10.1523/JNEUROSCI.5271-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanantsoa N, Hirsch MR, Thoby-Brisson M, Dubreuil V, Bouvier J, Ruffault PL, Matrot B, Fortin G, Brunet JF, Gallego J, Goridis C. Breathing without CO2 chemosensitivity in conditional Phox2b mutants. J Neurosci. 2011;31:12880–12888. doi: 10.1523/JNEUROSCI.1721-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Quellmalz UJ, Wilken B, Richter DW. The hypoxic response of neurones within the in vitro mammalian respiratory network. J Physiol. 1998;507:571–582. doi: 10.1111/j.1469-7793.1998.571bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333:637–642. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. JCompNeurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- Rudzinski E, Kapur RP. PHOX2B immunolocalization of the candidate human retrotrapezoid nucleus. Pediatr Dev Pathol. 2010;13:291–299. doi: 10.2350/09-07-0682-OA.1. [DOI] [PubMed] [Google Scholar]
- Ruffault PL, D’Autreaux F, Hayes JA, Nomaksteinsky M, Autran S, Fujiyama T, Hoshino M, Hagglund M, Kiehn O, Brunet JF, Fortin G, Goridis C. The retrotrapezoid nucleus neurons expressing Phox2b and Atoh-1 are essential for the respiratory response to CO2. Elife. 2015;4 doi: 10.7554/eLife.07051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson CA, Wang W, Pieribone VA, Dohle CI, Richerson GB. Midbrain serotonergic neurons are central pH chemoreceptors. Nat Neurosci. 2003;6:1139–1140. doi: 10.1038/nn1130. [DOI] [PubMed] [Google Scholar]
- Shea SA, Andres LP, Shannon DC, Guz A, Banzett RB. Respiratory sensations in subjects who lack a ventilatory response to CO2. Respir Physiol. 1993;93:203–219. doi: 10.1016/0034-5687(93)90006-v. [DOI] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Borgmann A, Rybak IA, Paton JF. Brainstem respiratory networks: building blocks and microcircuits. Trends Neurosci. 2013;36:152–162. doi: 10.1016/j.tins.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. JCompNeurol. 1989;281:69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- Song G, Wang H, Xu H, Poon CS. Kolliker-Fuse neurons send collateral projections to multiple hypoxia-activated and nonactivated structures in rat brainstem and spinal cord. Brain Struct Funct. 2012;217:835–858. doi: 10.1007/s00429-012-0384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Xu H, Wang H, Macdonald SM, Poon CS. Hypoxia-excited neurons in NTS send axonal projections to Kolliker-Fuse/parabrachial complex in dorsolateral pons. Neuroscience. 2011;175:145–153. doi: 10.1016/j.neuroscience.2010.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-John WM, Stornetta RL, Guyenet PG, Paton JF. Location and properties of respiratory neurones with putative intrinsic bursting properties in the rat in situ. J Physiol. 2009;587:3175–3188. doi: 10.1113/jphysiol.2009.170308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, West GH, Gwilt JM, Colombari E, Stornetta RL, Guyenet PG. GABAergic pump cells of solitary tract nucleus innervate retrotrapezoid nucleus chemoreceptors. J Neurophysiol. 2007;98:374–381. doi: 10.1152/jn.00322.2007. [DOI] [PubMed] [Google Scholar]
- Tan W, Pagliardini S, Yang P, Janczewski WA, Feldman JL. Projections of preBotzinger complex neurons in adult rats. J Comp Neurol. 2010;518:1862–1878. doi: 10.1002/cne.22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Lane S, Korsak A, Paton JF, Gourine AV, Kasparov S, Teschemacher AG. Lactate-mediated glia-neuronal signalling in the mammalian brain. Nat Commun. 2014;5:3284. doi: 10.1038/ncomms4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taugher RJ, Lu Y, Wang Y, Kreple CJ, Ghobbeh A, Fan R, Sowers LP, Wemmie JA. The bed nucleus of the stria terminalis is critical for anxiety-related behavior evoked by CO2 and acidosis. J Neurosci. 2014;34:10247–10255. doi: 10.1523/JNEUROSCI.1680-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teran FA, Massey CA, Richerson GB. Serotonin neurons and central respiratory chemoreception: where are we now? Prog Brain Res. 2014;209:207–233. doi: 10.1016/B978-0-444-63274-6.00011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Karlen M, Wu N, Charnay P, Champagnat J, Fortin G. Genetic identification of an embryonic parafacial oscillator coupling to the preBotzinger complex. Nat Neurosci. 2009;12:1028–1035. doi: 10.1038/nn.2354. [DOI] [PubMed] [Google Scholar]
- Tryba AK, Pena F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci. 2006;26:2623–2634. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci. 1995;15:5346–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrop TG, Iwamoto GA. Point: supraspinal locomotor centers do contribute significantly to the hyperpnea of dynamic exercise. J Appl Physiol (1985) 2006;100:1077–1079. doi: 10.1152/japplphysiol.01528.2005. [DOI] [PubMed] [Google Scholar]
- Wang S, Benamer N, Zanella S, Kumar NN, Shi Y, Bevengut M, Penton D, Guyenet PG, Lesage F, Gestreau C, Barhanin J, Bayliss DA. TASK-2 channels contribute to pH sensitivity of retrotrapezoid nucleus chemoreceptor neurons. J Neurosci. 2013a;33:16033–16044. doi: 10.1523/JNEUROSCI.2451-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Shi Y, Shu S, Guyenet PG, Bayliss DA. Phox2b-expressing retrotrapezoid neurons are intrinsically responsive to acidification and CO2. J Neurosci. 2013b;33:7756–7761. doi: 10.1523/JNEUROSCI.5550-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WG, Tiwari JK, Bradley SR, Zaykin AV, Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J Neurophysiol. 2001;85:2224–2235. doi: 10.1152/jn.2001.85.5.2224. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravis EM, Ceccherini I, Keens TG, Loghmanee DA, Trang H. An official ATS clinical policy statement: Congenital central hypoventilation syndrome: genetic basis, diagnosis, and management. Am J Respir Crit Care Med. 2010;181:626–644. doi: 10.1164/rccm.200807-1069ST. [DOI] [PubMed] [Google Scholar]
- Wenker IC, Kreneisz O, Nishiyama A, Mulkey DK. Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1-Kir5.1-like current and may contribute to chemoreception by a purinergic mechanism. J Neurophysiol. 2010;104:3042–3052. doi: 10.1152/jn.00544.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenker IC, Sobrinho CR, Takakura AC, Moreira TS, Mulkey DK. Regulation of ventral surface CO2/H+-sensitive neurons by purinergic signalling. J Physiol. 2012;590:2137–2150. doi: 10.1113/jphysiol.2012.229666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LB, Andrew D, Craig AD. Activation of spinobulbar lamina I neurons by static muscle contraction. J Neurophysiol. 2002;87:1641–1645. doi: 10.1152/jn.00609.2001. [DOI] [PubMed] [Google Scholar]
- Wittmeier S, Song G, Duffin J, Poon CS. Pacemakers handshake synchronization mechanism of mammalian respiratory rhythmogenesis. Proc Natl Acad Sci USA. 2008;105:18000–18005. doi: 10.1073/pnas.0809377105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Kaur S, VanderHorst VG, Saper CB, Chamberlin NL. Respiratory-related outputs of glutamatergic, hypercapnia-responsive parabrachial neurons in mice. J Comp Neurol. 2015;523:907–920. doi: 10.1002/cne.23720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Oka T, Tsumori T, Nakamura S, Yasui Y. Glutamatergic neurons in the Kolliker-Fuse nucleus project to the rostral ventral respiratory group and phrenic nucleus: a combined retrograde tracing and in situ hybridization study in the rat. Neurosci Res. 2007;59:341–346. doi: 10.1016/j.neures.2007.08.004. [DOI] [PubMed] [Google Scholar]