Abstract
Background
The liver is the predominant site of metastases among patients with advanced neuroendocrine tumors (NETs). Prior retrospective studies have reported high response rates in patients treated with transarterial embolization (TAE). NETs are highly vascular and are known to express vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR). We hypothesized that administration of sunitinib, a VEGFR inhibitor, following TAE would extend progression-free survival (PFS).
Patients and methods
Patients with metastatic NETs to the liver underwent a series of selective TAEs followed by sunitinib (until disease progression or maximum of 12 months). Radiographic response (by RECIST), survival, and safety parameters were monitored.
Results
Thirty-nine patients were enrolled. The overall response rate was 72% [95% confidence interval (CI), 0.58–0.86]. Median PFS was 15.2 months. Rates of overall survival (OS) at 1 and 4 years were 95% (95% CI, 0.88–1.00) and 59% (95% CI, 0.38–0.80), respectively. A significant 34% rise in serum VEGF was observed following the initial TAE (P = 0.03).
Conclusions
Hepatic TAE is a highly active treatment option for patients with metastatic NETs to the liver. Embolization stimulates release of VEGF into the circulation. Sunitinib, an oral VEGFR inhibitor, can be safely administered following embolization. The high rates of PFS and OS associated with this sequence of therapies are encouraging.
Keywords: hepatic TAE, NET, sunitinib, VEGF
introduction
The liver is the predominant site of metastases among patients with neuroendocrine tumors (NETs) of the aerodigestive tract [1–4]. Patients with liver metastases may experience symptoms such as weight loss, anorexia, and pain due to tumor burden. Hormonally functioning NETs may also cause symptoms such as flushing and diarrhea due to secretion of vasoactive substances directly into the systemic circulation. Surgical referral is generally advocated for patients with limited hepatic metastases which can be resected with curative or near-curative intent [5–8]. Various ablative techniques have also been described, including alcohol ablation, cryoablation, and radiofrequency ablation [9–11]. However, for the majority of patients with widespread metastases, surgical or ablative therapies are rarely applicable.
In recent years, nonsurgical treatment options have expanded for patients with metastatic NETs. Systemic therapies include somatostatin analogs such as octreotide and lanreotide. These agents were initially developed to palliate hormonal symptoms associated with the carcinoid syndrome [12, 13]. In a recent randomized phase III trial of patients with metastatic midgut carcinoid tumors, octreotide long-acting repeatable (LAR) was associated with a significant increase in time to progression, thereby expanding its use to patients with nonfunctioning tumors [14]. Other novel systemic therapies have been found to be effective specifically for pancreatic NETs. For example, a recent placebo-controlled phase III trial studying the mammalian target of rapamycin inhibitor everolimus demonstrated a significant improvement in progression-free survival (PFS) [15]. Sunitinib, an inhibitor of the vascular endothelial growth factor receptor (VEGFR), was also found to prolong PFS among patients with advanced pancreatic NETs [16].
Despite advances in systemic therapy, hepatic transarterial embolization (TAE) remains an important treatment modality for patients with progressive or symptomatic liver metastases. Metastatic NETs, which are highly vascular, derive their blood supply primarily from the hepatic arterial circulation [17]. Thus, occlusion of hepatic artery branches leads to selective tumor ischemia, relatively sparing the normal liver parenchyma which derives the bulk of its blood supply from the portal vein. In patients with bilobar hepatic metastases, lobar embolizations are typically carried out at 4- to 6-week intervals with the entire liver treated in two or three stages.
The embolization procedure begins with a celiac angiogram designed to identify the hepatic vasculature, patency of the portal vein, and location of liver metastases. Selective catheterization of the left or right hepatic artery is then carried out under fluoroscopy. Various embolic materials have been used including Gelfoam (Pharmacia and Upjohn Co, Kalamazoo, MI), polyvinyl alcohol, and trisacryl gelatin microspheres (Embospheres; BiosSphere Medical Inc., Rockland, MA). Embolization can be carried out with the addition of intra-arterial cytotoxic drugs (transarterial chemoembolization; TACE) or without (bland embolization; TAE). There are no published randomized studies comparing TAE with TACE and no consensus favoring a particular approach.
Nearly all data on TAEs for NET patients derive from retrospective institutional series. Objective radiographic response rates (ORRs) have varied widely in some studies; however, the majority of institutions report partial response rates of ∼50% [18–23]. Symptomatic responses (e.g. improvement in hormonal syndromes or pain) as well as major biochemical responses (>50% reductions in hormone or tumor marker levels) are achieved in the majority of cases. Due to lack of prospective trials, there is little reliable data on time to disease progression following hepatic artery embolization; however, the largest retrospective series of 122 patients reported a median PFS of 10 months [24]. The main mechanism of disease progression in the liver is the revascularization of tumors from collateral vessels via angiogenesis. The process of embolization itself is thought to stimulate brisk angiogenesis by releasing vascular endothelial growth factor (VEGF) into the circulation [25].
Inhibition of the VEGF pathway has proven to be an effective treatment strategy for metastatic NETs which express both VEGF and VEGFR [26–28]. The most widely studied VEGFR-inhibiting agent in NETs is sunitinib, a multi-targeted inhibitor of VEGFR-1, -2, and -3 among other tyrosine kinase receptors [29, 30]. We hypothesized that administration of sunitinib following TAE, to coincide with the postembolization VEGF spike, would delay the process of neoangiogenesis and prolong time to tumor progression. We therefore conducted a phase II clinical trial of sunitinib combined with hepatic artery embolization, measuring levels of serum VEGF before and after embolization and assessing response rates and PFS using standard RECIST criteria.
patients and methods
patient selection
This study was an open-label, single-arm, phase II prospective clinical trial. Subjects were adults (age ≥ 18) with stage IV well- or moderately differentiated carcinoid tumors or pancreatic NETs who had measurable liver metastases. Patients with limited extrahepatic metastases were eligible as long as the liver was the predominant site of tumor spread. Prior systemic and surgical/ablative treatments were allowed; however, prior hepatic embolizations or angiogenesis inhibitors were prohibited. Other key eligibility criteria included Eastern Cooperative Oncology Group performance status ≤2, absolute neutrophil count ≥1500 cells/μl, platelets ≥100 000 cells/μl, total bilirubin ≤1.5 × upper limit of normal (ULN), aspartate aminotransferase and alanine aminotransferase ≤2.5 × ULN (≤5 × ULN if attributable to liver metastases), and serum creatinine ≤1.5 × ULN. Key exclusion criteria included brain or leptomeningial metastases, prolonged QTc interval on baseline electrocardiogram, symptomatic congestive heart failure, myocardial infarction or angina (within 6 months), and uncontrolled hypertension (>150/100 mmHg).
All patients were required to provide written informed consent before study enrollment. The study was approved by the Institutional Review Board and complied with the provisions of the Good Clinical Practice guidelines and the Declaration of Helsinki.
study design
Patients underwent a series of one to three bland hepatic artery embolizations using trisacryl gelatin microspheres (Embospheres®) scheduled at 6-week intervals. After obtaining arterial access, a diagnostic celiac arteriogram was carried out using a 5-F catheter to identify arterial supply to tumors and to confirm patency of the portal vein. A microcatheter was then advanced selectively into the right or left hepatic artery, distal to the gastroduodenal artery. Embolization was carried out using microspheres mixed with contrast until achievement of near-stasis. Patients were admitted to the hospital after each embolization in order to palliate the postembolization syndrome consisting of abdominal pain, nausea/vomiting, and fevers. Discharge generally occurred after liver transaminases peaked, resulting in a median hospitalization of 3 days.
The total number of embolizations was determined by the treating interventional radiologist based on the extent of hepatic involvement with metastases. Sunitinib was administered orally once daily on days 1–28 in a 42-day cycle, starting 7 days after each embolization and ending 7 days before subsequent embolizations. After completion of embolizations, treatment with sunitinib was continued at the same dose and schedule until disease progression or a maximum of eight cycles (whichever occurred earlier). The initial dose of sunitinib was 50 mg, with two dose reductions permitted at increments of 12.5 mg. The protocol was subsequently amended to reduce the starting dose to 37.5 mg.
outcomes assessment
Multiphasic computed tomography or magnetic resonance imaging scans of the abdomen (along with any other relevant body part with evidence of disease) were carried out at baseline and every 3 months subsequently. A radiologist independently assessed the baseline burden of disease in the liver (percentage of liver involvement). Extrahepatic metastases were included for assessment of response and progression by RECIST version 1.0. Among patients with elevated baseline hormones or tumor markers [e.g. urine 5-HIAA, serum chromogranin A (CgA), gastrin, glucagon, etc.], repeat levels were obtained every 3 months. A >50% reduction or normalization of levels was defined as a biochemical response.
Safety was assessed according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 3.0. Patients were evaluated every 6 weeks and at the end of treatment for assessment of safety and for the determination of the occurrence and severity of adverse events.
A correlative end point of this study was the serum concentration of circulating VEGF immediately before the initial embolization and 48 h later. The mean difference and natural log relative difference between baseline and postembolization serum VEGF were calculated.
sample size calculation
The primary end point was PFS. Secondary end points included ORR, overall survival (OS), and toxicity. For sample size calculation, the null hypothesis was a PFS rate of 50% at 12 months with embolization alone. The alternative hypothesis was that addition of sunitinib would increase the rate of PFS to 70% at 12 months. A sample size of 39 patients achieved a 90% power to detect this difference with a statistical significance (α-level) of 10%.
statistical analysis
Kaplan–Meier survival analysis was used to estimate all time-to-event functions. PFS was defined as time from start of treatment until disease progression or death as a result of any cause. OS was defined as time from start of treatment until death as a result of any cause, with patients censored at the date of last follow-up if still alive. Exact 95% confidence intervals (CIs) were calculated for each proportion of interest. Statistical analysis was carried out using Stata IC 10.0 software and SAS 9.2 software.
results
patient population
Thirty-nine patients were enrolled from January 2007 to April 2010. Primary tumor sites included the small intestine (26), pancreas (9), rectum (2), lung (1), and unknown (1). Twenty-six patients had hormonally functioning tumors, including 23 patients with the carcinoid syndrome and 3 patients with functional pancreatic NETs (an insulinoma, glucagonoma, and gastrinoma). Among the 26 patients with primary small intestinal NETs, 22 (85%) had received prior octreotide LAR as their only previous line of systemic treatment, 1 patient had received prior octreotide and interferon-α, and 2 patients had no prior therapy. Patients with primary pancreatic NETs were more heavily pretreated: in addition to octreotide LAR, six patients (66%) received prior temozolomide-based chemotherapy and one patient received prior everolimus. Seventy-two percent of patients had documented disease progression at time of enrollment. The remainder was selected for embolization based on symptomatology or high tumor burden. Demographic variables and tumor characteristics are listed in Table 1.
Table 1.
Baseline demographic and clinical characteristics
| Characteristic | No. | % |
|---|---|---|
| Gender | ||
| Male | 18 | 46 |
| Female | 21 | 54 |
| Age (years) | ||
| Median | 61 | |
| Range | 40–75 | |
| Race | ||
| White | 34 | 87 |
| Black or African ancestry | 4 | 10 |
| Native American | 1 | 3 |
| Primary site | ||
| Small bowel | 26 | 67 |
| Pancreas | 9 | 23 |
| Rectal | 2 | 5 |
| Lung | 1 | 3 |
| Unknown | 1 | 3 |
| Hormonal syndrome | ||
| None | 13 | 33 |
| Carcinoid syndrome | 23 | 60 |
| Zollinger–Ellison syndrome | 1 | 3 |
| Glucagonoma syndrome | 1 | 3 |
| Insulinoma syndrome | 1 | 3 |
| Grade | ||
| Low | 28 | 72 |
| Intermediate | 4 | 10 |
| Unspecified | 7 | 18 |
| Liver tumor burden | ||
| <25% | 13 | 33 |
| 25%–50% | 12 | 31 |
| 50%–75% | 8 | 21 |
| >75% | 6 | 15 |
| Disease at entry | ||
| Stable | 7 | 18 |
| Progressive | 28 | 72 |
| Unknown | 4 | 10 |
| Prior therapy | ||
| Octreotide LAR | 31 | 79 |
| Capecitabine/temozolomide | 6 | 15 |
| Everolimus | 1 | 3 |
| None | 5 | 13 |
| Concurrent octreotide LAR | ||
| No | 11 | 28 |
| Yes | 28 | 72 |
| Primary tumor resected | ||
| No | 23 | 59 |
| Yes | 16 | 41 |
| Extrahepatic metastases | ||
| No | 26 | 67 |
| Yes | 13 | 33 |
| Mesenteric | 7 | 54 |
| Peritoneal | 2 | 15 |
| Retroperitoneal | 2 | 15 |
| Ovarian | 1 | 8 |
| Skeletal | 1 | 8 |
| Pulmonary | 1 | 8 |
During the course of the study, 84 TAEs were performed; the median number of TAE treatments was two per patient (range 1–3). The initial starting dose of sunitinib was 50 mg (4 weeks on, 2 weeks off) with a maximum of two dose reductions permitted for toxicity (to 37.5 and 25 mg); however, the first five patients enrolled at the 50 mg dose all required at least one dose reduction (two due to nausea/vomiting, two due to diarrhea, and one due to poorly controlled hypertension). Subsequently, an amendment to the study lowered the starting dose of sunitinib to 37.5 mg, permitting a single dose reduction.
In total, 21 patients (54%) completed the maximum eight cycles of sunitinib. Among the remaining 18 patients, nine discontinued sunitinib due to disease progression, five discontinued sunitinib during their first cycle due to side effects, and one withdrew from the study after six cycles for personal reasons. Three patients underwent embolization but did not receive sunitinib: two due to postembolization pain and fatigue and one because of worsening carcinoid heart disease following initial embolization. A total of 16 patients required dose reductions of sunitinib to 25 mg due to side effects.
radiologic response, PFS, and OS
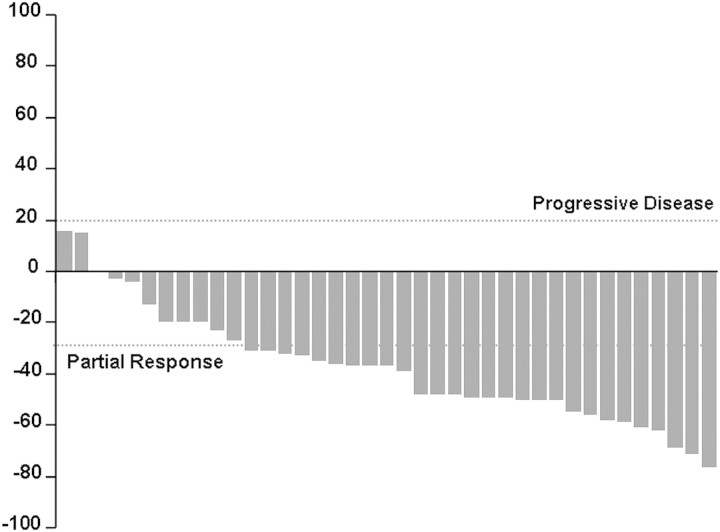
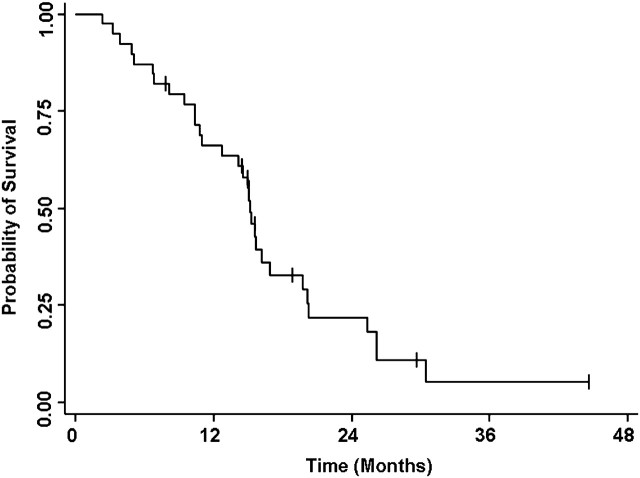
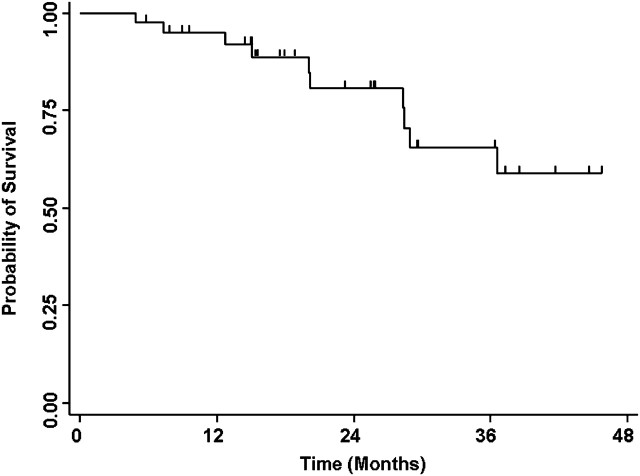
Twenty-eight patients (72%; 95% CI, 0.58–0.86) experienced a partial radiographic response, eight patients (20%) had stable disease, and three patients (8%) had progressive disease as their best response. Figure 1 summarizes the maximum percent change from baseline in the sum of the longest diameters of target lesions. Median PFS was 15 months and the PFS rate at 12 months was 66% (95% CI, 0.51–0.81; Figure 2). Rates of OS at 1 and 4 years were 95% (95% CI, 0.88–1.00) and 59% (95% CI, 0.38–0.80; Figure 3), respectively. Median OS has not been reached.
Figure 1.
Percentage change from baseline in sum of diameters of target lesions.
Figure 2.
Kaplan–Meier analysis of progression-free survival.
Figure 3.
Kaplan–Meier analysis of overall survival.
biochemical response
Nineteen patients had elevations in baseline urine 5-HIAA. All but one experienced reductions in urine 5-HIAA levels following embolization, including 16 patients (84%) who experienced a major response (defined as ≥50% decrease or normalization). Twenty-five patients had baseline elevations in serum CgAamong whom 18 patients (72%) experienced a major reduction following embolization. In total, 22 of 27 patients (81%) with an elevated baseline biomarker experienced a major biochemical response.
multivariate analysis of risk
Cox proportional hazards regression analysis was performed evaluating primary tumor site, tumor grade, liver tumor burden, progression prior to enrollment, and age. Statistically significant risk factors for disease progression included pancreatic versus small intestinal primary site (P = 0.04), age (P = 0.03), and progression before enrollment (P = 0.03; Table 2). Liver tumor burden was not a statistically significant prognostic factor for progression on multivariate analysis.
Table 2.
Univariate and multivariate analysis of prognostic factors
| Parameter | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Primary site | |||
| Small intestine | |||
| Pancreas | 5.05 | 1.12–22.8 | 0.04 |
| Other | 2.67 | 0.27–26.2 | 0.40 |
| Grade | |||
| Well | |||
| Moderate | 2.07 | 0.25–17.0 | 0.50 |
| Unspecified | 0.97 | 0.32–2.97 | 0.96 |
| Hepatic tumor burden | |||
| <50% | |||
| >50% | 1.32 | 0.44–3.96 | 0.62 |
| Prior progression | |||
| No | |||
| Yes | 6.05 | 1.45–25.2 | 0.03 |
| Age | 0.91 | 0.84–0.99 | 0.03 |
CI, confidence interval.
serum VEGF concentration
Measurements of serum VEGF were carried out immediately prior to the initial embolization and 48 h later in 19 patients who consented to blood draws. Fourteen patients experienced an increase and five patients had a decrease in their serum VEGF. A Wilcoxon signed-ranks test demonstrated a significant increase in postembolization serum VEGF compared with baseline (P = 0.03). The mean increase in serum VEGF at 48 h was 34%.
safety profile
Safety was monitored during study visits occurring a week after each embolization and every 6 weeks thereafter. The most common treatment-related adverse events were grades 1 and 2 and included fatigue, hypertension, abdominal pain, alterations in taste, and palmar–plantar erythrodysesthesia (Table 3). The most common grade 2 toxic effects were fatigue and nausea, both experienced by 23% of patients. The most common grade 3 toxicity was hypertension. There were no grade 4 events. The majority of sunitinib dose reductions occurred subsequent to embolizations during the first and second cycles. Acute TAE-related symptoms (fever, nausea, and transaminitis) transpiring during postembolization hospital admissions (before initiation of sunitinib) were not recorded.
Table 3.
Drug-related toxic effects occurring in ≥5% of patients
| Toxicity | Grade 1 | % | Grade 2 | % | Grade 3 | % |
|---|---|---|---|---|---|---|
| Hematological | ||||||
| Hemoglobin | 17 | 44 | 5 | 13 | 0 | 0 |
| Neutrophils/granulocytes | 6 | 15 | 7 | 18 | 6 | 15 |
| Platelets | 10 | 26 | 4 | 10 | 0 | 0 |
| Nonhematological | ||||||
| Abdominal pain | 10 | 26 | 5 | 13 | 3 | 8 |
| Anorexia | 6 | 15 | 4 | 10 | 1 | 3 |
| Arthralgia | 5 | 13 | 2 | 5 | 0 | 0 |
| Back pain | 2 | 5 | 1 | 3 | 0 | 0 |
| Constipation | 4 | 10 | 2 | 5 | 0 | 0 |
| Cough | 4 | 10 | 0 | 0 | 0 | 0 |
| Dehydration | 0 | 0 | 3 | 8 | 0 | 0 |
| Dermatological | 2 | 5 | 0 | 0 | 0 | 0 |
| Diarrhea | 4 | 10 | 2 | 5 | 2 | 5 |
| Dry skin | 2 | 5 | 0 | 0 | 0 | 0 |
| Dyspnea | 2 | 5 | 1 | 3 | 0 | 0 |
| Edema | 8 | 21 | 2 | 5 | 1 | 3 |
| Epistaxis | 2 | 5 | 0 | 0 | 0 | 0 |
| Fatigue | 13 | 33 | 9 | 23 | 2 | 5 |
| Fever | 11 | 28 | 1 | 3 | 0 | 0 |
| Hair loss | 5 | 13 | 0 | 0 | 0 | 0 |
| Headache | 7 | 18 | 2 | 5 | 0 | 0 |
| Hemorrhoids | 1 | 3 | 1 | 3 | 0 | 0 |
| Hyperpigmentation | 4 | 10 | 0 | 0 | 0 | 0 |
| Hypertension | 3 | 8 | 3 | 8 | 3 | 8 |
| Mood alterations | 2 | 5 | 0 | 0 | 0 | 0 |
| Mucositis/stomatitis | 6 | 15 | 0 | 0 | 0 | 0 |
| Nausea | 4 | 10 | 9 | 23 | 1 | 3 |
| Neuropathy | 3 | 8 | 0 | 0 | 0 | 0 |
| Potassium | 2 | 5 | 0 | 0 | 2 | 5 |
| Palmar-plantar erythrodysesthesia | 14 | 36 | 4 | 10 | 1 | 3 |
| Pulmonary | 1 | 3 | 1 | 3 | 0 | 0 |
| Rhinitis | 2 | 5 | 1 | 3 | 0 | 0 |
| Taste alterations | 13 | 33 | 1 | 3 | 0 | 0 |
| Weight loss | 1 | 3 | 1 | 3 | 0 | 0 |
| Vomiting | 4 | 10 | 5 | 13 | 1 | 3 |
discussion
This is the first phase II trial to investigate the combination of transarterial hepatic embolization with an angiogenesis-inhibiting agent and the first prospective evaluation of TAE in NETs. The study confirms that TAE is a safe and effective therapy for liver-predominant metastatic NETs, producing high radiographic and biochemical response rates. The data also confirm that embolization stimulates release of VEGF into the circulation and that sunitinib can be safely administered after embolization in order to counter effects of the VEGF spike. Most patients, however, have difficulty tolerating standard doses of sunitinib in the early postembolization period and require dose reductions.
The rates of PFS (66% at 1 year) and OS (59% at 4 years) observed in this study exceed those observed in prior retrospective studies of TACE or TAE alone and are highly encouraging. The safety data offer lessons which can be applied to future studies combining embolization with a systemic angiogenesis inhibitor. Because the highest incidence of adverse events occurs during the early postembolization period, it may be advisable to begin systemic treatment at a low dose and allow for dose escalation after recovery from the effects of embolization. Starting at a low dose may also allow for uninterrupted drug administration in the peri-embolization period.
The optimal duration of postembolization sunitinib is uncertain. In this trial, patients who had not progressed were treated for a maximum of 12 months (eight cycles). However, the relatively high number of progression events occurring shortly after discontinuation of sunitinib suggests that maintenance of treatment until progression may be a preferable strategy.
In summary, transarterial hepatic embolization is a highly active treatment option for patients with metastatic NETs to the liver. Embolization stimulates release of VEGF into the circulation. Sunitinib can be administered following hepatic artery embolization; however, most patients have difficulty tolerating standard dosing in the early postembolization period. The high rates of PFS and OS associated with this sequence of therapies are encouraging. Future multicenter randomized clinical trials investigating treatment with angiogenesis inhibitors versus placebo following hepatic artery embolization are warranted.
funding
Pfizer Inc.
disclosure
JRS has received research funding from Pfizer. LKK has received honoraria from Pfizer. All remaining authors have declared no conflicts of interest. Dr LKK: advisory board participation during study (total compensation <$10 000).
acknowledgements
Study was carried out at the H. Lee Moffitt Center and Research Institute.
references
- 1.Moertel CG. Karnofsky memorial lecture. An odyssey in the land of small tumors. J Clin Oncol. 1987;5(10):1502–1522. doi: 10.1200/JCO.1987.5.10.1502. [DOI] [PubMed] [Google Scholar]
- 2.Strosberg JR, Nasir A, Hodul P, Kvols L. Biology and treatment of metastatic gastrointestinal neuroendocrine tumors. Gastrointest Cancer Res. 2008;2(3):113–125. [PMC free article] [PubMed] [Google Scholar]
- 3.Norheim I, Oberg K, Theodorsson-Norheim E, et al. Malignant carcinoid tumors. An analysis of 103 patients with regard to tumor localization, hormone production, and survival. Ann Surg. 1987;206(2):115–125. doi: 10.1097/00000658-198708000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strosberg J, Gardner N, Kvols L. Survival and prognostic factor analysis of 146 metastatic neuroendocrine tumors of the mid-gut. Neuroendocrinology. 2009;89(4):471–476. doi: 10.1159/000197899. [DOI] [PubMed] [Google Scholar]
- 5.Sarmiento JM, Heywood G, Rubin J, et al. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003;197(1):29–37. doi: 10.1016/S1072-7515(03)00230-8. [DOI] [PubMed] [Google Scholar]
- 6.Norton JA, Warren RS, Kelly MG, et al. Aggressive surgery for metastatic liver neuroendocrine tumors. Surgery. 2003;134(6):1057–1063. doi: 10.1016/j.surg.2003.07.025. discussion 1063–1055. [DOI] [PubMed] [Google Scholar]
- 7.McEntee GP, Nagorney DM, Kvols LK, et al. Cytoreductive hepatic surgery for neuroendocrine tumors. Surgery. 1990;108(6):1091–1096. [PubMed] [Google Scholar]
- 8.Que FG, Nagorney DM, Batts KP, et al. Hepatic resection for metastatic neuroendocrine carcinomas. Am J Surg. 1995;169(1):36–42. doi: 10.1016/s0002-9610(99)80107-x. discussion 42–33. [DOI] [PubMed] [Google Scholar]
- 9.Ruszniewski P, O'Toole D. Ablative therapies for liver metastases of gastroenteropancreatic endocrine tumors. Neuroendocrinology. 2004; 80(Suppl 1):74–78. doi: 10.1159/000080746. [DOI] [PubMed] [Google Scholar]
- 10.Kvols LK, Turaga KK, Strosberg J, Choi J. Role of interventional radiology in the treatment of patients with neuroendocrine metastases in the liver. J Natl Compr Canc Netw. 2009;7(7):765–772. doi: 10.6004/jnccn.2009.0053. [DOI] [PubMed] [Google Scholar]
- 11.Hellman P, Ladjevardi S, Skogseid B, et al. Radiofrequency tissue ablation using cooled tip for liver metastases of endocrine tumors. World J Surg. 2002;26(8):1052–1056. doi: 10.1007/s00268-002-6663-3. [DOI] [PubMed] [Google Scholar]
- 12.Kvols LK, Moertel CG, O'Connell MJ, et al. Treatment of the malignant carcinoid syndrome. Evaluation of a long-acting somatostatin analogue. N Engl J Med. 1986;315(11):663–666. doi: 10.1056/NEJM198609113151102. [DOI] [PubMed] [Google Scholar]
- 13.Oberg K, Kvols L, Caplin M, et al. Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol. 2004;15(6):966–973. doi: 10.1093/annonc/mdh216. [DOI] [PubMed] [Google Scholar]
- 14.Rinke A, Muller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27(28):4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 15.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 17.Proye C. Natural history of liver metastasis of gastroenteropancreatic neuroendocrine tumors: place for chemoembolization. World J Surg. 2001;25(6):685–688. doi: 10.1007/s00268-001-0013-8. [DOI] [PubMed] [Google Scholar]
- 18.Gupta S, Yao JC, Ahrar K, et al. Hepatic artery embolization and chemoembolization for treatment of patients with metastatic carcinoid tumors: the M.D. Anderson experience. Cancer J. 2003;9(4):261–267. doi: 10.1097/00130404-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Eriksson BK, Larsson EG, Skogseid BM, et al. Liver embolizations of patients with malignant neuroendocrine gastrointestinal tumors. Cancer. 1998;83(11):2293–2301. [PubMed] [Google Scholar]
- 20.Strosberg JR, Choi J, Cantor AB, Kvols LK. Selective hepatic artery embolization for treatment of patients with metastatic carcinoid and pancreatic endocrine tumors. Cancer Control. 2006;13(1):72–78. doi: 10.1177/107327480601300110. [DOI] [PubMed] [Google Scholar]
- 21.Ruszniewski P, Rougier P, Roche A, et al. Hepatic arterial chemoembolization in patients with liver metastases of endocrine tumors. A prospective phase II study in 24 patients. Cancer. 1993;71(8):2624–2630. doi: 10.1002/1097-0142(19930415)71:8<2624::aid-cncr2820710830>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 22.Loewe C, Schindl M, Cejna M, et al. Permanent transarterial embolization of neuroendocrine metastases of the liver using cyanoacrylate and lipiodol: assessment of mid- and long-term results. AJR Am J Roentgenol. 2003;180(5):1379–1384. doi: 10.2214/ajr.180.5.1801379. [DOI] [PubMed] [Google Scholar]
- 23.Therasse E, Breittmayer F, Roche A, et al. Transcatheter chemoembolization of progressive carcinoid liver metastasis. Radiology. 1993;189(2):541–547. doi: 10.1148/radiology.189.2.7692465. [DOI] [PubMed] [Google Scholar]
- 24.Bloomston M, Al-Saif O, Klemanski D, et al. Hepatic artery chemoembolization in 122 patients with metastatic carcinoid tumor: lessons learned. J Gastrointest Surg. 2007;11(3):264–271. doi: 10.1007/s11605-007-0089-z. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Feng GS, Zheng CS, et al. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol. 2004;10(19):2878–2882. doi: 10.3748/wjg.v10.i19.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terris B, Scoazec JY, Rubbia L, et al. Expression of vascular endothelial growth factor in digestive neuroendocrine tumours. Histopathology. 1998;32(2):133–138. doi: 10.1046/j.1365-2559.1998.00321.x. [DOI] [PubMed] [Google Scholar]
- 27.Yao JC, Phan A, Hoff PM, et al. Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol. 2008;26(8):1316–1323. doi: 10.1200/JCO.2007.13.6374. [DOI] [PubMed] [Google Scholar]
- 28.Hansel DE, Rahman A, Hermans J, et al. Liver metastases arising from well-differentiated pancreatic endocrine neoplasms demonstrate increased VEGF-C expression. Mod Pathol. 2003;16(7):652–659. doi: 10.1097/01.MP.0000077416.68489.50. [DOI] [PubMed] [Google Scholar]
- 29.Faivre S, Demetri G, Sargent W, Raymond E. Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov. 2007;6(9):734–745. doi: 10.1038/nrd2380. [DOI] [PubMed] [Google Scholar]
- 30.Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9(1):327–337. [PubMed] [Google Scholar]