Abstract
Context:
Chronic ankle instability (CAI) occurs in some people after a lateral ankle sprain and often results in residual feelings of instability and episodes of the ankle's giving way. Compared with healthy people, patients with CAI demonstrated poor postural control and used a more anteriorly and laterally positioned center of pressure (COP) during a single-limb static-balance task on a force plate. Balance training is an effective means of altering traditional COP measures; however, whether the overall location of the COP distribution under the foot also changes is unknown.
Objective:
To determine if the spatial locations of COP data points in participants with CAI change after a 4-week balance-training program.
Design:
Randomized controlled trial.
Setting:
Laboratory.
Patients or Other Participants:
Thirty-one persons with self-reported CAI.
Intervention(s):
Participants were randomly assigned to a 4-week balance-training program or no balance training.
Main Outcome Measure(s):
We collected a total of 500 COP data points while participants balanced using a single limb on a force plate during a 10-second trial. The location of each COP data point relative to the geometric center of the foot was determined, and the frequency count in 4 sections (anteromedial, anterolateral, posteromedial, posterolateral) was analyzed for differences between groups.
Results:
Overall, COP position in the balance-training group shifted from being more anterior to less anterior in both eyes-open trials (before trial = 319.1 ± 165.4, after trial = 160.5 ± 149.5; P = .006) and eyes-closed trials (before trial = 387.9 ± 123.8, after trial = 189.4 ± 102.9; P < .001). The COP for the group that did not perform balance training remained the same in the eyes-open trials (before trial = 214.1 ± 193.3, after trial = 230.0 ± 176.3; P = .54) and eyes-closed trials (before trial = 326.9 ± 134.3, after trial = 338.2 ± 126.1; P = .69).
Conclusions:
In participants with CAI, the balance-training program shifted the COP location from anterolateral to posterolateral. The program may have repaired some of the damaged sensorimotor system pathways, resulting in a more optimally functioning and less constrained system.
Key Words: sprains, rehabilitation, postural control
Key Points
A 4-week progressive balance-training program effectively altered the spatial locations of center-of-pressure data points in participants with chronic ankle instability.
The alteration in the spatial locations of center-of-pressure data points may indicate a more optimally functioning sensorimotor system.
Lateral ankle ligament injuries are among the most common injuries in the general population, active-duty military service members, and athletes.1–4 Although the initial symptoms associated with lateral ankle sprains generally resolve in a short time, many patients continue to report residual problems, such as pain, instability, and feelings of the ankle's giving way.5 These residual symptoms have been reported to last 6 to 18 months after initial injury and have been observed in 55% to 72% of patients.6–8 Repeated incidences of lateral ankle instability, recurrent sprains with persistent symptoms, and diminished self-reported function have been termed chronic ankle instability (CAI).9,10
Postural control requires integration of visual, vestibular, and somatosensory input.11 Somatosensory input combines contributions from cutaneous, articular, and musculotendinous receptors. Afferent information gathered from these 3 sources is processed within the central nervous system and used to control motor commands.12 Deficient contributions from any of the afferent receptors can lead to diminished postural control.12–15 Postural-control deficits have been found repeatedly in patients with CAI.9,10,16–26 These deficits have been identified using a variety of outcome measures, including time to stabilization,18,22 Star Excursion Balance Test (SEBT) reach distances,19 center-of-pressure (COP) excursion measures, and time-to-boundary (TTB) measures.23,27
The COP measures from force-plate data can be analyzed to determine the instantaneous point of application of ground reaction forces, and they are useful in evaluating the stability and function of the foot.28 Recently, Pope et al27 evaluated a novel measure, COP location, which involves examining the location of COP data points in relation to the plantar aspect of the foot. Identifying the COP location indicates where the forces are distributed on the foot and provides insight into the postural-control strategy being used. Traditional COP measures and TTB measures cannot elicit the mechanism a person is using to improve balance. The COP location provides information about the spatial distributions of force application under the foot. To determine the COP location, the foot was modeled into a rectangle and divided into 16 equal sections. While the participant performed a single-limb balance task on a force plate, the location of each COP data point was mapped into 1 of the 16 sections. Differences were found between uninjured participants and those with CAI.27 Specifically, COP was more anteriorly and laterally positioned in participants with CAI compared with uninjured persons during eyes-open and eyes-closed single-legged standing.27 The authors hypothesized that this spatial difference may represent a more constrained sensorimotor system and compensatory postural-control mechanisms in participants with CAI.27 Having a more anterolateral COP is likely associated with the foot being more supinated, placing the ankle and subtalar joints in a closed-packed and more stable position that decreases feelings of instability in those with CAI.27 However, this positioning moves the COP closer to the lateral border of support and consequently decreases the amount of time available for postural corrections in this direction.27 Plantar-pressure studies have also demonstrated increased force and pressure concentration under the lateral midfoot and forefoot in participants with CAI during gait, which supports the notion of an anterior and lateral COP shift.29–31
Balance-training programs have been effective in improving postural control in participants with CAI.28,32–37 McKeon et al28 showed static and dynamic postural-control improvements in a sample of participants with CAI after 4 weeks of balance training that was intended to challenge their sensorimotor systems. Significant improvement occurred in self-reported function, magnitude and variability of TTB measures, and SEBT reach distances compared with pretest and control values.28
Although balance improvements have been found after rehabilitation in those with CAI, the neuromechanical mechanism driving these changes is unknown. Determining how the COP location in patients with CAI is affected by a balance-training program may be useful beyond simply evaluating traditional COP measures and also provide insight into how to restore normal function. Understanding how a balance-training rehabilitation program changes COP location offers information on the adaptation strategies participants with CAI use and may enable clinicians to more specifically target each person's deficits. The purpose of our study, therefore, was to determine how balance training affected COP location. We hypothesized that COP location would be more posteriorly and medially positioned, similar to that of the uninjured participants, after balance training.
METHODS
This randomized controlled study was performed in a laboratory setting. The independent variables were group (balance training, no balance training) and time (pretraining, posttraining). The dependent variable was the frequency of COP data points in each section of the foot, which was modeled as a rectangle and divided into 16 equal sections.
Participants
The participants in this investigation were also studied by McKeon et al.28 In total, 31 physically active persons with self-reported CAI participated in the study (Table 1). Inclusion criteria were more than 1 ankle sprain and residual symptoms that included episodes of the ankle's giving way (quantified by 4 or more yes responses on the Ankle Instability Instrument), self-reported symptoms of disability of ≤90% on the Foot and Ankle Disability Index, and scoring ≤75% on the Foot and Ankle Disability Index–Sport. Exclusion criteria were a history of lower extremity injury in the previous 6 weeks, lower extremity surgery, balance disorder, neuropathy, diabetes, or any other condition known to affect balance. In cases of reported bilateral CAI, the self-reported worse limb was used. Before the study, all participants signed an informed consent form. The study methods were approved by the university's institutional review board.
Table 1.
Participants' Information
| Characteristic |
Group |
|
| Balance Training (n = 16) |
No Balance Training (n = 15) |
|
| Age, y | 22.2 ± 4.5 | 19.6 ± 1.3 |
| Height, cm | 168.9 ± 7.7 | 173.3 ± 9.8 |
| Weight, kg | 63.0 ± 8.1 | 67.7 ± 13.7 |
| No. of sprains | 6.3 ± 7.1 | 4.6 ± 2.5 |
| Months since last sprain | 26.4 ± 46.4 | 5.5 ± 3.9 |
| Foot and Ankle Disability Index, % | 85.5 ± 8.4 | 82.9 ± 7.4 |
| Foot and Ankle Disability Index–Sport, % | 69.9 ± 12.1 | 66.5 ± 9.8 |
| Sex, men:women | 6:10 | 6:9 |
Instruments
We assessed postural control using an AccuSway force platform (AMTI Corp, Watertown, MA), which has a detailed grid on its surface to allow for exact foot placement between trials. Translational forces (Fx, Fy, Fz) and moments of force (Mx, My, Mz) were recorded at 50 Hz, producing a time series of 500 COP data points for a 10-second trial.
Testing Procedures
Participants were pretested and randomly allocated to the balance-training group or the no–balance-training group. Those in the no–balance-training group were instructed not to perform any rehabilitation activities. Those in the balance-training group completed the 4-week progressive program described by McKeon et al (three 20-minute sessions per week, 12 supervised sessions total).28 This program was intended to challenge their ability to maintain a single-limb stance during various balance activities that included hop to stabilization, hop to stabilization and reach, hop-to-stabilization box drill, progressive single-limb–stance balancing with eyes open, and progressive single-limb–stance balancing with eyes closed. Over the course of 4 weeks, participants progressed through 7 difficulty levels for each activity.
We collected the COP data before and after the balance-training program.27,28 To achieve this, length and width measurements of each person's foot were used to center the foot on the force-plate grid so that the foot was bisected by the anteroposterior and mediolateral midlines of the grid. All participants performed 3 successful 10-second trials of eyes-open and eyes-closed barefoot, single-limb stance. Three successful trials of the eyes-open stance were achieved before proceeding to the eyes-closed trials. Participants were instructed to stand as still as possible with the hands folded across the chest and to position the opposite limb at approximately 45° of knee flexion and 30° of hip flexion. They were allowed 1 practice trial per condition to become familiar with the task. A trial was a failure if at any time the individual touched down with the opposite limb, made contact with the stance limb, or was unable to maintain the position for 10 seconds. If a failed trial occurred, the trial was terminated and repeated.
Data Processing
We filtered the COP data with a fourth-order, zero-lag, low-pass filter with a cutoff frequency of 5 Hz. Balance Clinic software (version 2.01.01; AMTI Corp) was used to calculate COP signal derivatives from the previously filtered data with first-order finite difference equations. These data were then processed using the custom software program MATLAB (version 2008; The MathWorks, Inc, Natick, MA) to determine the location of COP relative to the dimensions of each participant's foot.
For each person, the plantar surface of the foot was modeled as a rectangle divided into 4 equal columns and 4 equal rows, which created 16 sections of equal proportion (Figure). The spatial location of each COP data point was recorded relative to 1 of the 16 sections. The frequency of COP data points in each section was counted and used for analysis.
Figure.
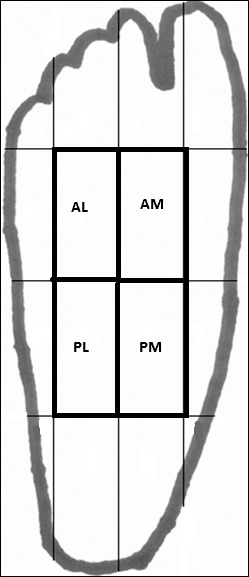
Quadrants. Abbreviations: AM, anteromedial; AL, anterolateral; PM, posteromedial; PL, posterolateral.
After data processing, we discovered, similar to findings of previous researchers,27 that all of our COP data points were located in the 4 innermost sections. We named these 4 sections anteromedial, anterolateral, posteromedial, and posterolateral (Figure).
Statistical Analysis
The eyes-open and eyes-closed trials were analyzed separately. For each condition, a 2 × 2 mixed-model multivariate analysis of variance (MANOVA) was run between group (balance training or no balance training) and time (pretraining and posttraining), with the dependent variables being the number of COP data points recorded in the anteromedial, anterolateral, posteromedial, and posterolateral sections. When a MANOVA revealed significant findings, we examined the individual 2 × 2 analysis of variance for each section to identify a significant interaction or main effect; if this occurred, additional pairwise comparisons were performed to detect specific differences.
A planned secondary analysis was performed between the rows and between the columns. To do this, we separated the foot into 2 columns, medial (anteromedial and posteromedial sections) and lateral (anterolateral and posterolateral sections), and 2 rows, anterior (anteromedial and anterolateral sections) and posterior (posteromedial and posterolateral sections). For the secondary analyses, separate 2 × 2 mixed-model MANOVAs were run for the medial-lateral column and anterior-posterior row comparisons. Post hoc analyses were performed in the same manner as described previously. The α value was set a priori at P ≤ .05 for all analyses. Consistent with contemporary statistical recommendations, we elected to not adjust the P value for multiple comparisons.38
RESULTS
The groups were similar in regard to the reported total number of sprains, number of months since the last sprain, and self-reported disability.28 Demographics can be seen in Table 1.
Eyes-Open Results
In the eyes-open trials, the group × time interaction was significant (Wilks λ [4,26] = 2.75, P = .05). For the individual sections, significant interactions were identified for the anterolateral and posterolateral sections (Table 2). Post hoc pairwise comparisons revealed fewer COP data points in the anterolateral section after balance training, whereas the locations of the COP data points in the no–balance-training group did not shift. In the posterolateral section, the balance-training group had significantly more COP data points after the program, whereas the no–balance-training group did not have a shift in COP data-point locations.
Table 2.
Center-of-Pressure Data Points for the Eyes-Open Trials
| Group, No. of Center-of-Pressure Data Points, Mean ± SD (% of Totala) |
P Values |
||||||
| Variable |
Balance Training (n = 16) |
No Balance Training (n = 15) |
Interaction |
Group Main Effect |
Time Main Effect |
||
| Pretraining |
Posttraining |
Pretraining |
Posttraining |
||||
| Section | |||||||
| Anteromedial | 88.1 ± 79.2 (18) | 42.2 ± 45.0 (8) | 64.2 ± 54.9 (13) | 71.2 ± 98.0 (14) | .08 | .91 | .19 |
| Anterolateral | 231.0 ± 149.6 (46) | 118.3 ± 134.4 (24) | 150.0 ± 161.4 (30) | 158.8 ± 155.1 (32) | .05b,c | .66 | .09 |
| Posteromedial | 51.8 ± 62.0 (10) | 104.0 ± 96.0 (21) | 115.9 ± 97.2 (34) | 109.7 ± 106.7 (22) | .08 | .23 | .17 |
| Posterolateral | 130.4 ± 146.8 (26) | 236.8 ± 130.9 (47) | 171.0 ± 120.0 (34) | 161.4 ± 120.2 (32) | .02b,d | .67 | .05 |
| Vertical columns | |||||||
| Medial | 139.9 ± 106.3 (28) | 146.2 ± 108.8 (29) | 180.1 ± 85.9 (36) | 180.9 ± 135.7 (36) | .91 | .25 | .88 |
| Lateral | 361.4 ± 105.7 (72) | 355.0 ± 108.6 (71) | 321.0 ± 85.6 (64) | 320.3 ± 135.5 (64) | .91 | .25 | .88 |
| Horizontal rows | |||||||
| Anterior | 319.1 ± 165.4 (64) | 160.5 ± 149.5 (32) | 214.1 ± 193.3 (43) | 230.0 ± 176.3 (46) | .004b,c | .75 | .02 |
| Posterior | 182.1 ± 165.4 (36) | 340.8 ± 149.6 (68) | 287.0 ± 193.6 (57) | 271.1 ± 176.1 (54) | .004b,d | .75 | .02 |
Percentage of center-of-pressure data points that fell in each section across the 10-second trial (500 total data points per trial).
Significant at P ≤ .05.
Greater frequency for pretraining versus posttraining in the balance-training group.
Lower frequency for pretraining versus posttraining in the balance-training group.
Our secondary analyses demonstrated no medial-lateral shifts for either group after the intervention period (MANOVA group × time interaction: Wilks λ [2,28] = 1.0, P = .991; group main effect: Wilks λ [2,28] = .90, P = .21; time main effect: Wilks λ [2,28] = 1.0, P = .986). However, for the rows, the MANOVA revealed a group × time interaction (Wilks λ [2,28] = .75, P = .018). Both the anterior and posterior rows had interactions (see Table 2). Post hoc tests revealed that the balance-training group shifted from a more anteriorly located COP to a less anteriorly located COP. The control group did not show an anterior-posterior shift.
Eyes-Closed Results
In the eyes-closed trials, the group × time interaction was significant (Wilks λ [4,26] = .56, P = .004). All 4 sections had group × time interactions (Table 3). Compared with the pretest, the balance-training group had fewer COP data points in the anteromedial and anterolateral sections after training. Correspondingly, the balance-training group also had more COP data points in the posteromedial and posterolateral sections after training compared with before. Conversely, the position of the COP data points did not change in the no–balance-training group over time for any section (P > .26 for all sections).
Table 3.
Center-of-Pressure Data Points for the Eyes-Closed Trials
| Group, No. of Center-of-Pressure Data Points, Mean ± SD (% of Totala) |
P Values |
||||||
| Variable |
Balance Training (n = 16) |
No Balance Training (n = 15) |
Interaction |
Group Main Effect |
Time Main Effect |
||
| Pretraining |
Posttraining |
Pretraining |
Posttraining |
||||
| Section | |||||||
| Anteromedial | 183.2 ± 98.7 (37) | 76.8 ± 53.9 (15) | 143.2 ± 76.7 (29) | 170.2 ± 82.2 (34) | .001b,c | .22 | .05 |
| Anterolateral | 204.7 ± 75.7 (41) | 112.6 ± 68.4 (23) | 183.7 ± 98.4 (37) | 168.0 ± 92.0 (33) | .002b,c | .54 | <.001 |
| Posteromedial | 46.4 ± 57.0 (9) | 123.6 ± 59.5 (24) | 71.5 ± 58.9 (14) | 79.7 ± 65.1 (16) | .008b,d | .60 | .002 |
| Posterolateral | 66.5 ± 75.7 (13) | 187.6 ± 73.8 (38) | 102.2 ± 103.9 (20) | 82.7 ± 71.4 (17) | <.001b,d | .15 | .008 |
| Vertical columns | |||||||
| Medial | 229.5 ± 75.8 (46) | 200.3 ± 69.8 (40) | 214.7 ± 81.7 (43) | 249.9 ± 71.3 (50) | .041b | .44 | .84 |
| Lateral | 271.2 ± 75.8 (54) | 300.2 ± 69.7 (60) | 285.9 ± 81.6 (57) | 250.7 ± 71.0 (50) | .041b | .44 | .84 |
| Horizontal rows | |||||||
| Anterior | 387.9 ± 123.8 (78) | 189.4 ± 102.9 (38) | 326.9 ± 134.3 (65) | 338.2 ± 126.1 (68) | <.001b,c | .24 | .001 |
| Posterior | 112.8 ± 124.0 (22) | 311.2 ± 102.9 (62) | 173.7 ± 134.4 (35) | 162.4 ± 126.2 (32) | <.001b,d | .24 | .001 |
Percentage of center-of-pressure data points that fell in each section across the 10-second trial (500 total data points per trial).
Significant at P ≤ .05.
Greater frequency for pretraining versus posttraining in the balance-training group.
Lower frequency for pretraining versus posttraining in the balance-training group.
In the secondary analysis, no medial-lateral shifts occurred for either group after the intervention period (MANOVA group × time interaction: Wilks λ [2,28] = .84, P = .09; group main effect: Wilks λ [2,28] = .98, P = .74; time main effect: Wilks λ [2,28] = .99, P = .89). Conversely, for the anterior-posterior comparison, the group × time interaction was significant (Wilks λ [2,28] = .58, P < .001). Both the anterior and posterior rows had significant interactions (Table 3). Post hoc tests revealed that the balance-training group shifted from a more anteriorly located COP to a less anteriorly located COP. The control group did not demonstrate an anterior-posterior shift.
DISCUSSION
After 4 weeks of balance training, participants with CAI shifted the frequency of COP data points from a more anterior location to a more posterior location. Specifically, in both the eyes-open and eyes-closed trials, they shifted posteriorly while also maintaining a more laterally placed COP. After balance training, during the more difficult task of unipedal balance with eyes closed, participants presented with a greater distribution of the COP data points in the lateral half of the foot. Our results provide insight into the mechanistic changes that occurred after the balance-training protocol. Compared with uninjured participants, those with CAI have a more anteriorly and laterally positioned COP27; our study showed that a balance intervention changed the COP location. Our hypothesis, that after completing the balance-training program, participants with CAI would demonstrate COP locations more similar to those of the uninjured participants, was partially supported. Although COP location shifted posteriorly after supervised rehabilitation, no changes occurred in the frequency of COP data points in the mediolateral direction.
Our participants were studied previously by McKeon et al,28 who noted improved self-reported function, traditional force-plate, and SEBT measures after the balance-training intervention. Other authors33,35,39 have also shown improvements in postural control and self-reported function assessments after balance training in patients with CAI. Our results provide unique insight into the improved postural control because they demonstrate the behavioral differences indicated by the shift in spatial distribution of the COP excursions during single-legged balancing. Although we did not measure lower extremity joint kinematics, achieving a more posterior COP would theoretically require less ankle dorsiflexion, less knee flexion, or a less anterior trunk lean (or a combination of these). Earlier researchers27 hypothesized that the anterolateral positioning associated with CAI before balance training was due to a more dorsiflexed talocrural joint and a more supinated subtalar joint27; both of these represent the joints' closed-packed positions and would be associated with the most mechanically stable joint congruency. Pope et al27 proposed that persons with CAI adopt the closed-packed position as a protective compensation to provide more postural stability by limiting (or “freezing”) movement degrees of freedom at the ankle and subtalar joints in the presence of sensorimotor deficits associated with CAI.
We found that the frequency of the COP data points shifted posteriorly, while remaining more lateral, after balance training, which suggests that participants may have been able to free some movement degrees of freedom at the ankle. However, a limitation of our study is that we knew only the total frequency of COP data points in each section and not the actual location within the section or the distance of the shift of the data points. Yet more time spent posteriorly during the balance trials may reflect an improved sensorimotor system. In accordance with dynamical systems theory, more degrees of freedom offer more options for movement execution and a less constrained sensorimotor system.40,41 Constraining the task by asking participants to perform balance trials with their eyes closed further supports this theory. All participants initially used an anterolaterally distributed COP. By increasing the task difficulty, we believe they had to limit their degrees of freedom to accomplish the task. However, those who completed the balance-training program were able to free some of their degrees of freedom, as revealed in the posttraining trials. Interestingly, participants who completed balance training were able to shift the frequency of COP data points in the sagittal plane, but there were no frontal-plane changes. Thus, although the balance-training program was beneficial, it may not have fully restored the postural-control system to that of an uninjured person.27 The balance strategies of participants who did not perform the intervention did not change.
Pursuing a balance-training program that required participants to gradually explore their limits of stability by implementing progressive and changing tasks resulted in their increasing degrees of freedom at the ankle. Evaluating more traditional force-plate measures in the same participants, McKeon et al28 reported an increase in standard deviations in the TTB minima in the anteroposterior direction, indicating either more variability or a greater number of available strategies to achieve single-legged balance. This may be advantageous as more options for movement at the ankle give a person more ways to react or adapt to challenging and changing environments and tasks. It is also important to note that along with an increase in degrees of freedom, balance improved according to traditional COP and TTB measures.28 Future researchers should measure the long-term effects of a rehabilitation program on the frequency of COP data points in various plantar locations to determine if our observed changes persist for an extended period after balance training has ceased.
Although we did not measure kinematics, a shift from anterior to posterior is hypothesized to affect ankle sagittal-plane motion. An anteriorly placed COP would occur because of a more dorsiflexed ankle, whereas a posteriorly placed COP would correspond to a less dorsiflexed ankle. A more dorsiflexed position presented by participants with CAI before balance training could also have been a means of compensating for the lack of sensory input from the damaged lateral ligaments by stretching the soleus muscle and thus increasing muscle-spindle sensitivity. By increasing the sensitivity of the soleus muscle spindle, those with CAI may be able to better adjust to postural perturbations. Increased Ia afferent activation occurs in the soleus when sensory information is altered through vibration.42 Studies of tendon vibration have also shown that changes in proprioceptive information from the soleus influence COP location during quiet-standing balance tasks.42,43 Thompson et al43 found that Achilles tendon vibration resulted in a posterior shift, whereas rear-foot vibration caused an anterior shift. Increased sensitivity of the soleus and Achilles tendon to input from the γ motor-neuron system associated with CAI may affect COP location in a similar manner. The balance-training program may have altered the sensorimotor system by sensitizing the soleus spindles, which decreased the need to stretch the soleus to activate these spindles.
Another potential reason for the kinematic alterations in joint position may be neuromuscular adaptations. Anticipatory postural adjustments are intended to counteract anticipated perturbations.44 The magnitude of postural adjustment is thought to be proportional to the action causing a postural perturbation.44 A posteriorly situated COP could be beneficial because it provides increased time and space for a person to make the appropriate postural adjustments to an anterior perturbation in order to maintain balance. Training effects have been seen such that postural perturbations caused by rehearsed actions decrease in proportion to an increase in postural adjustments.44 Perhaps the participants with CAI developed postural strategies after injury that caused their COP location to deviate from that of healthy people,27 and the balance training restored an appropriate amount of neuromuscular control to counteract perturbations during single-legged balancing, as reflected by the COP location change after balance training.
Our study used a specific, progressive balance-training program, and the results cannot be generalized to all balance-training programs.28 The balance-training program in this study possessed distinct characteristics that may be responsible for the observed changes. The program was designed to be challenging and progressive, while stressing the entire postural-control system in an individualized manner. Exercises included single-legged balancing tasks and dynamic activities such as hopping with an emphasis on speed-of-movement execution. No cues or instructions to decrease dorsiflexion or alter balance strategies were given that could have altered COP location.
In conclusion, the balance-training program influenced the COP location for participants with CAI. Specifically, the number of COP data points shifted posteriorly, while remaining laterally placed in participants who underwent the balance-training program. A more posteriorly located COP may result from a more optimally functioning and less constrained sensorimotor system. The balance-training program that was implemented may have repaired some of the damaged sensorimotor system pathways, increasing movement degrees of freedom.
REFERENCES
- 1.Waterman BR, Owens BD, Davey S, Zacchilli MA, Belmont PJ., Jr The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92(13):2279–2284. doi: 10.2106/JBJS.I.01537. [DOI] [PubMed] [Google Scholar]
- 2.Waterman BR, Belmont PJ, Cameron KL, Deberardino TM, Owens BD. Epidemiology of ankle sprain at the United States Military Academy. Am J Sports Med. 2010;38(4):797–803. doi: 10.1177/0363546509350757. [DOI] [PubMed] [Google Scholar]
- 3.Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311–319. [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez WG, Yard EE, Comstock RD. Epidemiology of lower extremity injuries among U.S. high school athletes. Acad Emerg Med. 2007;14(7):641–645. doi: 10.1197/j.aem.2007.03.1354. [DOI] [PubMed] [Google Scholar]
- 5.van Rijn RM, van Os AG, Bernsen RM, Luijsterburg PA, Koes BW, Bierma-Zeinstra SM. What is the clinical course of acute ankle sprains? A systematic literature review. Am J Med. 2008;121(4):324–331. doi: 10.1016/j.amjmed.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 6.Gerber JP, Williams GN, Scoville CR, Arciero RA, Taylor DC. Persistent disability associated with ankle sprains: a prospective examination of an athletic population. Foot Ankle Int. 1998;19(10):653–660. doi: 10.1177/107110079801901002. [DOI] [PubMed] [Google Scholar]
- 7.Braun BL. Effects of ankle sprain in a general clinic population 6 to 18 months after medical evaluation. Arch Fam Med. 1999;8(2):143–148. doi: 10.1001/archfami.8.2.143. [DOI] [PubMed] [Google Scholar]
- 8.Hiller C, Nightingale E, Raymond J, et al. Prevalence and impact of chronic musculoskeletal ankle disorders in the community. Arch Phys Med Rehabil. 2012;93(10):1801–1807. doi: 10.1016/j.apmr.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Hertel J. Functional anatomy, pathomechanics, and pathophysiology of lateral ankle instability. J Athl Train. 2002;37(4):364–375. [PMC free article] [PubMed] [Google Scholar]
- 10.Hiller CE, Kilbreath SL, Refshauge KM. Chronic ankle instability: evolution of the model. J Athl Train. 2011;46(2):133–141. doi: 10.4085/1062-6050-46.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKeon PO, Hertel J. Plantar hypoesthesia alters time-to-boundary measures of postural control. Somatosens Mot Res. 2007;24(4):171–177. doi: 10.1080/08990220701637224. [DOI] [PubMed] [Google Scholar]
- 12.Riemann BL. Is there a link between chronic ankle instability and postural instability? J Athl Train. 2002;37(4):386–393. [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman MA, Wyke B. The innervation of the ankle joint: an anatomical and histological study in the cat. Acta Anat (Basel) 1967;68(3):321–333. doi: 10.1159/000143037. [DOI] [PubMed] [Google Scholar]
- 14.Freeman MA, Wyke B. Articular reflexes at the ankle joint: an electromyographic study of normal and abnormal influences of ankle-joint mechanoreceptors upon reflex activity in the leg muscles. Br J Surg. 1967;54(12):990–1001. doi: 10.1002/bjs.1800541204. [DOI] [PubMed] [Google Scholar]
- 15.Freeman M, Wyke B. Articular contributions to limb muscle reflexes: the effects of partial neurectomy of the knee-joint on postural reflexes. Br J Surg. 1966;53(1):61–68. doi: 10.1002/bjs.1800530116. [DOI] [PubMed] [Google Scholar]
- 16.de Vries J, Kingma I, Blankevoort L, van Dijk C. Difference in balance measures between patients with chronic ankle instability and patients after an acute ankle inversion trauma. Knee Surg Sports Traumatol Arthrosc. 2010;18(5):601–606. doi: 10.1007/s00167-010-1097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gribble PA, Hertel J, Denegar CR. Chronic ankle instability and fatigue create proximal joint alterations during performance of the Star Excursion Balance Test. Int J Sports Med. 2007;28(3):236–242. doi: 10.1055/s-2006-924289. [DOI] [PubMed] [Google Scholar]
- 18.Gribble PA, Robinson RH. Alterations in knee kinematics and dynamic stability associated with chronic ankle instability. J Athl Train. 2009;44(4):350–355. doi: 10.4085/1062-6050-44.4.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gribble PA, Hertel J, Denegar CR, Buckley WE. The effects of fatigue and chronic ankle instability on dynamic postural control. J Athl Train. 2004;39(4):321–329. [PMC free article] [PubMed] [Google Scholar]
- 20.Hubbard TJ, Kramer LC, Denegar CR, Hertel J. Contributing factors to chronic ankle instability. Foot Ankle Int. 2007;28(3):343–354. doi: 10.3113/FAI.2007.0343. [DOI] [PubMed] [Google Scholar]
- 21.Konradsen L. Factors contributing to chronic ankle instability: kinesthesia and joint position sense. J Athl Train. 2002;37(4):381–385. [PMC free article] [PubMed] [Google Scholar]
- 22.Brown C, Mynark R. Balance deficits in recreational athletes with chronic ankle instability. J Athl Train. 2007;42(3):367–373. [PMC free article] [PubMed] [Google Scholar]
- 23.McKeon PO, Hertel J. Spatiotemporal postural control deficits are present in those with chronic ankle instability. BMC Musculoskelet Disord. 2008;9:76. doi: 10.1186/1471-2474-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tropp H, Odenrick P. Postural control in single-limb stance. J Orthop Res. 1988;6(6):833–839. doi: 10.1002/jor.1100060607. [DOI] [PubMed] [Google Scholar]
- 25.Wikstrom EA, Fournier KA, McKeon PO. Postural control differs between those with and without chronic ankle instability. Gait Posture. 2010;32(1):82–86. doi: 10.1016/j.gaitpost.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Docherty CL, Arnold BL, Gansneder BM, Hurwitz S, Gieck J. Functional-performance deficits in volunteers with functional ankle instability. J Athl Train. 2005;40(1):30–34. [PMC free article] [PubMed] [Google Scholar]
- 27.Pope M, Chinn L, Mullineaux D, McKeon PO, Drewes L, Hertel J. Spatial postural control alterations with chronic ankle instability. Gait Posture. 2011;34(2):154–158. doi: 10.1016/j.gaitpost.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 28.McKeon PO, Ingersoll CD, Kerrigan DC, Saliba E, Bennett BC, Hertel J. Balance training improves function and postural control in those with chronic ankle instability. Med Sci Sports Exerc. 2008;40(10):1810–1819. doi: 10.1249/MSS.0b013e31817e0f92. [DOI] [PubMed] [Google Scholar]
- 29.Nyska M, Shabat S, Simkin A, Neeb M, Matan Y, Mann G. Dynamic force distribution during level walking under the feet of patients with chronic ankle instability. Br J Sports Med. 2003;37(6):495–497. doi: 10.1136/bjsm.37.6.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrison KE, Hudson DJ, Davis IS, et al. Plantar pressure during running in subjects with chronic ankle instability. Foot Ankle Int. 2010;31(11):994–1000. doi: 10.3113/FAI.2010.0994. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt H, Sauer L, Lee S, Saliba S, Hertel J. Increased in-shoe lateral plantar pressures with chronic ankle instability. Foot Ankle Int. 2011;32(11):1075–1080. doi: 10.3113/FAI.2011.1075. [DOI] [PubMed] [Google Scholar]
- 32.Eils E, Schroter R, Schroder M, Gerss J, Rosenbaum D. Multistation proprioceptive exercise program prevents ankle injuries in basketball. Med Sci Sports Exerc. 2010;42(11):2098–2105. doi: 10.1249/MSS.0b013e3181e03667. [DOI] [PubMed] [Google Scholar]
- 33.Hale SA, Hertel J, Olmsted-Kramer LC. The effect of a 4-week comprehensive rehabilitation program on postural control and lower extremity function in individuals with chronic ankle instability. J Orthop Sports Phys Ther. 2007;37(6):303–311. doi: 10.2519/jospt.2007.2322. [DOI] [PubMed] [Google Scholar]
- 34.McKeon PO, Hertel J. Systematic review of postural control and lateral ankle instability, part II: is balance training clinically effective? J Athl Train. 2008;43(3):305–315. doi: 10.4085/1062-6050-43.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rozzi SL, Lephart SM, Sterner R, Kuligowski L. Balance training for persons with functionally unstable ankles. J Orthop Sports Phys Ther. 1999;29(8):478–486. doi: 10.2519/jospt.1999.29.8.478. [DOI] [PubMed] [Google Scholar]
- 36.Webster KA, Gribble PA. Functional rehabilitation interventions for chronic ankle instability: a systematic review. J Sport Rehabil. 2010;19(1):98–114. doi: 10.1123/jsr.19.1.98. [DOI] [PubMed] [Google Scholar]
- 37.Buchanan A, Docherty C, Schrader J. Functional performance testing in participants with functional ankle instability and in a healthy control group. J Athl Train. 2008;43(4):342–346. doi: 10.4085/1062-6050-43.4.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hopkins WG, Marshall SW, Batterham AM, Hanin J. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc. 2009;41(1):3–13. doi: 10.1249/MSS.0b013e31818cb278. [DOI] [PubMed] [Google Scholar]
- 39.Bernier J, Perrin DH. Effect of coordination training on proprioception of the functionally unstable ankle. J Orthop Sports Phys Ther. 1998;27(4):264–275. doi: 10.2519/jospt.1998.27.4.264. [DOI] [PubMed] [Google Scholar]
- 40.Davids K, Glazier P, Araujo D, Bartlett R. Movement systems as dynamical systems: the functional role of variability and its implications for sports medicine. Sports Med. 2003;33(4):245–260. doi: 10.2165/00007256-200333040-00001. [DOI] [PubMed] [Google Scholar]
- 41.Van Emmerik R, Hamill J, McDermott W. Variability and coordinative function in human gait. Quest. 2005;57(1):102–123. [Google Scholar]
- 42.Thompson C, Bélanger M, Fung J. Effects of bilateral Achilles tendon vibration on postural orientation and balance during standing. Clin Neurophysiol. 2007;118(11):2456–2467. doi: 10.1016/j.clinph.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 43.Thompson C, Bélanger M, Fung J. Effects of plantar cutaneo-muscular and tendon vibration on posture and balance during quiet and perturbed stance. Hum Mov Sci. 2011;30(2):153–171. doi: 10.1016/j.humov.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Aruin A, Latash ML. The role of motor action in anticipatory postural adjustments studied with self-induced and externally triggered perturbations. Exp Brain Res. 1995;106(2):291–300. doi: 10.1007/BF00241125. [DOI] [PubMed] [Google Scholar]


