Abstract
Context:
The most common modality used to address acute inflammation is cryotherapy. Whereas pain decreases with cryotherapy, evidence that changes occur in perfusion of skeletal muscle is limited. We do not know whether ice attenuates the increases in perfusion associated with acute inflammation.
Objective:
To examine the effects of repeated applications of ice bags on perfusion of the gastrocnemius muscle after an eccentric exercise protocol.
Design:
Controlled laboratory study.
Setting:
Laboratory.
Patients or Other Participants:
Eighteen healthy participants (3 men, 15 women; age = 22.2 ± 2.2 years, height = 166.0 ± 11.9 cm, mass = 69.4 ± 25.0 kg).
Intervention(s):
To induce eccentric muscle damage, participants performed 100 unilateral heel-lowering exercises off a step to the beat of a metronome. A randomized intervention (cryotherapy, sham, control) was applied to the exercised lower extremity immediately after the protocol and again at 10, 24, and 34 hours after the protocol.
Main Outcome Measure(s):
Baseline perfusion measurements (blood volume, blood flow, and blood flow velocity) were taken using contrast-enhanced ultrasound of the exercised leg. Perfusion was reassessed after the first intervention and 48 hours after the protocol as percentage change scores. Pain was measured with a visual analog scale at baseline and at 10, 24, 34, and 48 hours after the protocol. Separate repeated-measures analyses of variance were used to assess each dependent variable.
Results:
We found no interactions among interventions for microvascular perfusion. Blood volume and blood flow, however, increased in all conditions at 48 hours after exercise (P < .001), and blood flow velocity decreased postintervention from baseline (P = .041). We found a time-by-intervention interaction for pain (P = .009). Visual analog scale scores were lower for the cryotherapy group than for the control group at 34 and 48 hours after exercise.
Conclusions:
Whereas eccentric muscle damage resulted in increased blood flow, ice did not decrease muscle perfusion 48 hours after exercise. Therefore, ice does not seem to decrease muscle perfusion when blood flow is elevated, as it would be during inflammation.
Key Words: contrast-enhanced ultrasound, delayed-onset muscle soreness, eccentric exercise, muscle cooling
Key Points
Although cryotherapy helped to control pain, it did not decrease muscle blood flow 48 hours after eccentric exercise.
Although skeletal muscle was cooled, ice did not seem to decrease muscle blood flow immediately after either initial application or sequential applications.
Researchers may need to re-examine the proposed benefits of the clinical application of ice.
Cryotherapy, or cold therapy, is the standard of care for acute inflammation. It is often applied immediately after musculoskeletal injury, as well as throughout the initial inflammatory phase of healing. Cold is used in conjunction with rest, elevation, and compression to decrease pain and attenuate swelling and commonly is acknowledged to decrease skin blood flow and pain.1 Cooling the skin, which has numerous cutaneous thermoreceptors, profoundly affects the vasculature of the skin that aids in thermoregulation,2 but skeletal muscle has no thermoreceptors. Recent evidence3 has suggested that cooling the muscle may not affect skeletal muscle capillary perfusion; thus cryotherapy may not influence the vascular effects of acute inflammation in this area.
Distinguishing the perfusion of the skin from that of the capillaries of skeletal muscle has been difficult.4–6 A novel technique has permitted better assessment of perfusion characteristics and has allowed more thorough investigation of the physiologic factors associated with cryotherapy. Contrast-enhanced ultrasound (CEUS) allows the visualization of microvascular perfusion in a specific region, such as muscle. Skeletal muscle perfusion can be measured without factoring in skin perfusion. Cryotherapy and eccentric exercise have been examined with CEUS in isolation,3,7 but the effect of cryotherapy on inflammation caused by eccentric contractions has not been studied.
Unaccustomed eccentric exercise is used to create delayed-onset muscle soreness (DOMS), which is a common model for studying trauma to skeletal muscle,8 particularly acute inflammation.9 Using eccentric exercise to induce DOMS results in reversible muscle damage with a predictable sequence of events.10 Tenderness to palpation or movement is associated with DOMS,11 as it results from a strain to type I muscle fibers.11,12 This tenderness originates from the myotendinous junction13 and progresses through the entire muscle over a 24- to 48-hour period postexercise.13–17 Eccentric exercise has been shown to increase muscle blood flow,7 which also occurs after acute injury during vasodilation.18 Whereas ice decreases blood flow in the skin,19 it is unclear what happens at the muscular level. Researchers20 have shown that, with the exception of pain, cryotherapy does not reduce signs and symptoms of muscle damage after eccentric exercise, but changes in muscle blood flow have not been examined. Therefore, the purpose of our study was to examine the effects of repeated cryotherapy treatments on microvascular muscle perfusion after eccentric exercise. We hypothesized that microvascular perfusion would decrease in the cryotherapy condition compared with the sham and control conditions at 48 hours after eccentric exercise. We also expected that the subjective reporting of pain in the cryotherapy condition would be lower than in the sham and control conditions over a 48-hour period.
METHODS
Design
This single-blinded randomized controlled laboratory study consisted of 3 intervention groups (cryotherapy, sham, and control). Muscle blood volume, blood flow, and blood flow velocity were measured at baseline, postintervention, and 48 hours postexercise. We used the visual analog scale (VAS) to measure pain at baseline and at 10, 24, 34, and 48 hours postexercise.
Participants
Eighteen healthy volunteers (3 men, 15 women; age = 22.2 ± 2.2 years, height = 166.0 ± 11.9 cm, mass = 69.4 ± 25.0 kg, medial gastrocnemius subcutaneous tissue thickness = 5.74 ± 1.81 mm) participated in the study. They had no lower extremity injuries in the 6 months before the study or lower extremity surgeries in the year before the study. Exclusion criteria were performance of eccentric exercise in the 6 months before the study; having a history of cardiovascular disease, subcutaneous thickness of the gastrocnemius greater than 20 mm as measured with diagnostic ultrasound, an abnormal electrocardiogram (ECG), or heart murmur; abnormal blood draw findings, which were contraindications for the contrast agent, at the time of the study; and being pregnant. All participants provided written informed consent, and the study was approved by the Institutional Review Board of Health Sciences Research at the University of Virginia.
Instruments
We recorded the CEUS measurements using the SONOS 7500 ultrasound machine (Philips Medical Systems, Andover, MA). The S3 adult echo transducer was used to image the muscle. The mechanical index was set at 1.5. The transducer was a linear array with a frequency ratio of 1.3:3.6 MHz.
The contrast agent (microbubbles) (Definity; Lantheus Medical Imaging, North Billerica, MA) has been approved by the Federal Drug Administration (NDA 021064) and contains octafluoropropane gas-filled albumin. The microbubbles were supplied in 1.5-mL vials and were mixed with 0.9% sodium chloride in a ratio of 0.3-mL microbubbles per 7-mL saline. A maximum of 2 vials (3.0 mL) was allowed to be infused per day. The solution was infused intravenously at a rate of 1.5 mL/min.
The VAS was used as the primary tool for pain quantification. A 100-mm line with markings only for no pain at the left end and worst pain at the right end of the continuum was used. We instructed participants to mark a vertical dash on the horizontal line indicating their level of pain relative to the continuum. A line without numerical markings was used so that participants would be less apt to remember previous markings. The reliability of the VAS for acute pain is 0.90.21 A change of 10 mm or more has been used to define clinically relevant changes in pain.21
Eccentric Exercise Protocol
We chose a modified method described by Tegeder et al22 because it used a unilateral exercise procedure for the calf. We modified the protocol, using an exercise step instead of a sloped box and having participants perform eccentric-only contractions with the involved lower extremity. Participants lowered the heel of a randomized limb slowly for 3 seconds using a metronome (Metronome app by MarketWall.com; Apple Inc, Cupertino, CA) set at 60 beats per minute. The control limb was used to shift the weight distribution off the exercised limb so that it could be returned to the starting position without performing a concentric contraction. A random-number generator was used to determine which limb would be exercised. The exercise protocol consisted of 2 sets of 50 contractions separated by a 5-minute rest interval.
Interventions
For the cryotherapy condition, a 750-g bag of crushed ice was placed over the medial gastrocnemius. For the sham condition, we applied a 750-g bag of room-temperature candy corn in the same location. For the control condition, a towel was placed over the medial gastrocnemius. We applied the interventions immediately and at 10, 24, and 34 hours postexercise. Intervention duration was determined from the amount of subcutaneous tissue over the medial gastrocnemius as suggested by Otte et al,23 using musculoskeletal ultrasound instead of skinfold calipers to determine skinfold thickness. The interventions ranged from 15 to 60 minutes. Musculoskeletal ultrasound imaging is more reliable than skinfold thickness in measuring subcutaneous tissue.24
Procedures
Participants met with the lead investigator (N.M.S.) and a physician (D.C.H.) for the initial screening. Height, mass, subcutaneous tissue thickness, blood pressure, pulse, and respirations were recorded. They completed general and lower extremity health history questionnaires. If no exclusion criteria were discovered, participants reported to the General Clinical Research Center (GCRC) within a week to have a diagnostic 12-lead ECG (PageWriter Trim III; Philips Medical Systems) and metabolic blood draw. We used the ECG to rule out heart abnormalities and the blood draw to check the function of the liver and kidneys. Abnormal ECG or blood draw findings were contraindications for the contrast agent and excluded participants from the study. Female participants were administered a serum pregnancy test. Results of all laboratory measurements were obtained within 48 hours and were reviewed by a physician. After the physician cleared individuals to participate, they were enrolled in the intervention portion of the study.
To control for exercise before the vascular assessments, all participants checked in at 8 pm at the GCRC and stayed overnight. Given that a week or more may have passed from the time participants were cleared (metabolic blood draw, ECG, health history, etc) to reporting to the GCRC, female participants were administered a urine pregnancy test to ensure they were not pregnant. Participants were instructed to stay in bed except to use the bathroom to limit the amount of physical activity before the muscle-perfusion measurements. Lights were turned off at 11 pm to encourage participants to sleep. Between 5 and 6 am the next morning, we instructed participants to mark the VAS for current pain in the limb that would perform the exercise. Next, a registered nurse, who was not an author, prepared the contralateral upper extremity of the limb to be exercised for placement of an intravenous catheter in the antecubital vein using sterile techniques. A 3-lead ECG was attached to participants for continuous monitoring of heart rhythm and pulse oximetry until they were discharged. Next, they were positioned prone, and the lower extremity randomized to perform the exercise was marked with a 2.5- × 2.5-cm square, which was the size of the transducer head, over the medial gastrocnemius where the muscle belly had the greatest girth. The nurse then began the infusion of microbubbles. After steady state was reached (about 2 minutes), CEUS measurements began.25 We applied an ample amount of ultrasound gel to the ultrasound transducer and placed it over the mark on the gastrocnemius. Images were recorded automatically at pulse intervals triggered to the ECG.26 An image was captured immediately after heartbeats 1, 2, 3, 4, 5, 8, 12, 16, and 20. Three images were captured at every pulse interval, resulting in 27 images, which took between 3 and 5 minutes. After the CEUS measurements were taken, participants turned supine and rested for 5 minutes.
Next, participants got up, used the restroom if needed, and performed the eccentric exercise. After the protocol was finished, participants lay prone, and we administered a randomized intervention of the cryotherapy, sham, or control condition within 1 minute postexercise. The treatment was determined using concealed envelopes and was single blinded; the investigator (N.M.S.) performing the CEUS measurements and data reduction left the room when the intervention was applied, but participants were aware of the intervention when the limb was or was not cooled. Two minutes before the intervention was removed, infusion of the contrast agent began so that steady state could be reached before removal. This allowed CEUS measurements to begin within 30 seconds of intervention removal. After the designated time, the intervention was removed, and the investigator returned to the room. Participants were monitored for 30 minutes before being discharged from the GCRC.
Participants reported to the sports medicine laboratory 10, 24, and 34 hours postexercise to mark the VAS and to receive the designated intervention. The VAS was always completed first so the intervention would not affect the score. Again, the CEUS examiner was blinded to the intervention. We chose these time points because they allowed for 2 interventions each day and replicated when a person might go to a clinic for rehabilitation. At 48 hours, participants returned to the GCRC and lay quietly for 2 hours before the VAS and CEUS measurements to allow perfusion to stabilize after they walked into the GCRC. Again, participants indicated their levels of pain on the VAS before the nurse placed the intravenous catheter in the upper extremity and repeated the CEUS procedure. Participants were monitored for 30 minutes before being discharged, and the study was concluded. A flow chart of the study timeline is presented in Figure 1. Throughout the study, participants were not instructed to limit their physical activity but were advised to not begin a new activity.
Figure 1.
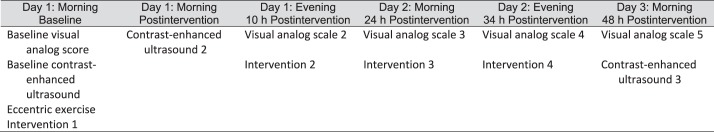
Timeline of study over 48 hours.
Data Analysis
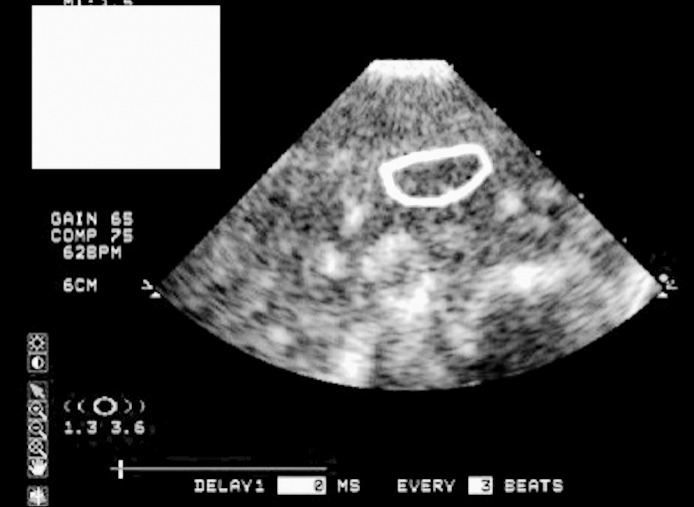
We used specialized software (QLAB; Philips Healthcare, Andover, MA) capable of analyzing CEUS images to assess muscle blood volume and blood flow velocity with replenishment kinetics for a specific region of interest. A separate investigator (S.A.S.) labeled the images so that the person measuring the images was blinded to group assignment. An examiner (N.M.S.) manually drew a circle around the region of interest, avoiding large blood vessels (bright white areas in Figure 2), around the 2-cm mark on the first image. We chose this depth to correspond to imaging 1 cm into the muscle belly after accounting for subcutaneous tissue. The circle was transposed automatically in the same spot on each of the remaining 26 images, so maintaining the transducer in the same location was crucial. The software created a graph comprising 27 points (1 for each frame) that indicated muscle blood volume in the region of interest with the equation y = A(1 − exp−βt), where y equals video intensity (VI) at pulse interval t, A is VI plateau (muscle blood volume), and β is the rate of rise in VI (muscle blood flow velocity).26 Only muscle blood volume and flow velocity were provided in the graph, so we manually calculated muscle blood flow by multiplying blood volume by blood flow velocity. For each participant, the average muscle blood volume and flow velocity from the 27 points were used, resulting in a single value for all 3 variables. Given that the microvascular-perfusion measurements were given in decibels, we used log transformations to calculate percentage change values for easier interpretation.
Figure 2.
Contrast-enhanced ultrasound measurement. A total of 27 images were collected for each participant during this measurement. The circle indicates the region of interest where measurements were calculated.
Statistical Analysis
Sample size was calculated for 24 participants using the minimal detectable change for muscle blood flow, which is 0.026 dB/s, when power equals 0.80 and the α level is set at .05.7 However, we measured only 18 participants because 2 people had abnormal ECGs, 1 person did not complete the study, and we did not have the resources in the GCRC to fully recruit 24 participants due to a decrease in funding.
Separate 3 × 3 repeated-measures analyses of variance were used to assess microvascular muscle-perfusion characteristics across the interventions. We used a 3 × 5 repeated-measures analysis of variance to assess pain values across the interventions. Pairwise comparisons were used to compare findings that were different. Effect sizes were calculated using Cohen d for pooled standard deviations for findings that were different. We used SPSS software (version 17; SPSS Inc, Chicago, IL) to analyze the data.
RESULTS
No differences were found among groups at baseline (P > .05), and no interactions were found among interventions for muscle blood volume (F4,13 = 1.341, P = .28), muscle blood flow (F4,13 = 1.329, P = .28), or muscle blood flow velocity (F4,13 = 1.270, P = .30). We noted a time main effect for muscle blood volume (F2,15 = 40.174, P < .001) and muscle blood flow (F2,15 = 42.532, P < .001). Those values increased postintervention and remained elevated at 48 hours postexercise compared with baseline. Effect sizes were strong for the main time effect of blood volume (Cohen d = 2.24; 95% confidence interval [CI] = 1.41, 3.07) and blood flow (Cohen d = 2.60; 95% CI = 1.71, 3.48) at postintervention from baseline. We observed a main effect of time for muscle blood flow velocity (F2,15 = 3.869, P = .041) and a moderate effect size (Cohen d = 0.72; 95% CI = 0.05, 1.40). Blood flow velocity decreased at postintervention from baseline (Table). A difference of approximately 15% existed between the control and cryotherapy groups (Table); however, before the log transformation, we found only a 0.05-dB difference among the groups, which was not statistically different.
Table.
Percentage Change Scores for Microvascular Perfusion Over Time
| Variable |
Intervention |
Baseline to Postintervention |
Postintervention to 48 h Postexercise |
Baseline to 48 h Postexercise |
| Blood volume | Cryotherapy | 27.99 ± 2.94a | 4.59 ± 0.88 | 33.86 ± 3.55a |
| Sham | 54.62 ± 4.20a | 2.98 ± 0.58 | 59.24 ± 1.90a | |
| Control | 40.43 ± 3.55a | −6.72 ± 1.59 | 30.99 ± 2.53a | |
| Blood flow | Cryotherapy | 54.52 ± 1.08a | 9.00 ± 0.32a | 68.43 ± 1.06a |
| Sham | 88.19 ± 0.63a | 10.84 ± 0.31a | 108.58 ± 0.80a | |
| Control | 66.81 ± 0.88a | −6.51 ± 0.54a | 55.94 ± 0.80a | |
| Blood flow velocity | Cryotherapy | 5.60 ± 0.04 | 5.59 ± 0.06 | 11.50 ± 0.04 |
| Sham | 11.50 ± 0.08 | −10.31 ± 0.08 | 0.00 ± 0.04 | |
| Control | 21.05 ± 0.04a | −5.29 ± 0.03 | 14.65 ± 0.03a |
Indicates change (P < .05).
We demonstrated a time × intervention interaction for pain (F5,12 = 5.639, P = .009). The VAS scores were lower for the cryotherapy group than the control group at 34 and 48 hours postexercise. The VAS also was lower in the cryotherapy group than in the sham group at 34 hours (Figure 3). Compared with the control group, the effect sizes for the cryotherapy group at 34 (Cohen d = −1.17; 95% CI = −2.45, 0.01) and 48 (Cohen d = −1.7; 95% CI = −2.97, −0.43) hours postexercise were strong, with wide 95% CIs. Compared with the sham group at 34 hours, the effect size for the cryotherapy group was strong, with a wide 95% CI (Cohen d = −1.74; 95% CI = −3.08, −0.40).
Figure 3.
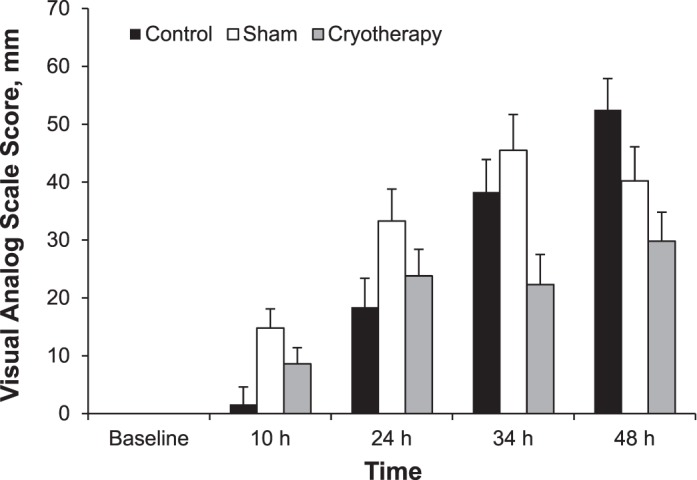
Measurements of the visual analog scale across interventions.
DISCUSSION
Cryotherapy did not decrease microvascular muscle perfusion associated with increased muscle blood flow after an eccentric exercise model. A primary rationale for the clinical use of cryotherapy is the suggestion that cold decreases blood flow, helping with clotting and controlling acute inflammation in the injured tissue.19 However, this decrease has not been shown through research. The influence of cold on pain27,28 and other sensory-motor effects29 seems certain. Cryotherapy has an important role in treating musculoskeletal injuries, particularly acute injuries, but the physiologic effects require further investigation. We did not measure muscle temperature during the cryotherapy intervention because we did not want to cause inflammation by inserting a thermocouple in the muscle. Still, we are confident the muscle was cooled. Researchers7 using the same measures have found muscle temperature decreased by an average of 7°C.
Assessing skeletal muscle-perfusion characteristics is difficult. Authors4–6 investigating cryotherapy have used different techniques to assess muscle blood flow, which have focused more on arterial blood flow than on intramuscular perfusion. Our results contradict their findings, but the disagreement may be due to the different measurement methods used. In general, limited research is available on therapeutic agents and muscle blood flow changes and often involves girth measures, plethysmography, and Doppler. Any measure that assesses arterial function or whole-limb analysis would not distinguish the skin and muscle factors, which are mediated by different physiologic factors. The skin has a powerful thermoregulatory mechanism, and vasoconstriction is consistent when the skin is cooled. When the skin is exposed to cold, vasoconstriction occurs to limit the loss of heat from the body and prevent hypothermia.30 When whole-body temperature decreases below 33°C, a reflex cutaneous vasoconstriction by the adrenergic system preserves heat within the core.31 The muscle, however, is under metabolic control and vasodilates according to the demand for oxygen. A clear association of decreased blood flow with cold in the muscle has not been established.
Using CEUS, researchers7 from our laboratory have demonstrated that microvascular perfusion was not altered in healthy people after an ice bag was applied to the calf. Similarly, Fiscus et al32 used plethysmography and observed no changes in muscle blood flow after cryotherapy on the leg. A limitation of those investigations is that healthy volunteers were studied at rest when cryotherapy was applied.
In our study, eccentric exercise was used as a model to increase the microvascular perfusion that might be seen with acute inflammation.3 We hypothesized that ice would attenuate the increases in muscle blood flow associated with acute inflammation, thereby helping with clotting and potentially reducing the adverse vascular effects of the inflammatory process as seen at the skin. Cryotherapy did not seem to affect the increases in muscle blood flow observed at 48 hours postexercise, but we do not know what happened to blood flow throughout those 48 hours. In the eccentric exercise model, increased blood volume and flow may have resulted from increased surface area for the exchange of substrates across the plasma membrane,25 allowing macrophages, neutrophils, and other repair cytokines to accumulate around the injured area. Whereas the muscle was cool, the vascular response in the muscle was unchanged.
Cryotherapy is beneficial in decreasing secondary hypoxia, which revolves more around the muscle being cool than decreasing muscle blood flow. Secondary hypoxia results when lack of oxygen due to decreased blood flow from increased blood viscosity, muscle spasm, increased extravascular pressure, hemorrhage, and the clotting cascade maintaining homeostasis causes uninjured, healthy cells to die after initial injury.1 Hypoxia can last for minutes or up to 6 hours, depending on the injury.33,34 After this period of decreased blood flow, reperfusion occurs; however, free radicals are produced, resulting in further injury.35 Cryotherapy decreases metabolic demand by slowing the demand for oxygen by mitochondria,36 so decreased muscle blood flow is not necessary. The reported benefits of cryotherapy as a treatment in acute inflammation may be either as a sensory mechanism to reduce pain or as a local cooling response to reduce local metabolism, thus reducing the potential for secondary cell hypoxia and enzymatic injury. The Q10 effect, in which decreases in temperature of 10°C decrease biological processes by one-half to two-thirds,37 may reduce the local need for oxygen and diminish the chemical or enzymatic activity of the mediators of inflammation, so that decreased blood flow is not necessary. A limitation to this study is that we did not take CEUS measurements each time ice was applied; therefore, changes in perfusion may have been present over the 48 hours postexercise but not observed.
In our study, DOMS symptoms were greatest at 48 hours postexercise for the control condition, supporting previous research13–17; however, we do not know how pain would have responded after 48 hours. Pain results from the activation of type III and type IV nerve fibers by histamine, acetylcholine, bradykinin, potassium, serotonin, and prostaglandin E.38 The delay in pain sensation may result from the delay of neutrophils entering the injury site 24 hours after injury.39 When examining the effect sizes for cryotherapy, the negative effect sizes indicate that the participants in the sham and control conditions had more pain. We expected this finding because excessive eccentric exercise is meant to cause pain. The finding at 34 hours postexercise that the cryotherapy condition decreased pain compared with the control condition should be taken with caution because the effect size CI crossed zero. Cryotherapy may or may not decrease pain at 34 hours postexercise compared with a control intervention. However, when compared with the sham intervention, the effect sizes range from moderate to strong, indicating that cryotherapy was better at decreasing pain. Therefore, at 48 hours postexercise, cryotherapy decreased pain compared with the control condition. This decreased pain with cryotherapy seems to result more from a cutaneous effect and gate control of nociceptors.40
Our study had some limitations. We could not measure microvascular perfusion at the time points when the intervention was applied. Funding and exposure to the microbubbles were factors, and we could not collect these measurements at all time points. At 48 hours postexercise, participants lay quietly for 2 hours before the measurements were taken. This may not have been enough time for perfusion to stabilize, so another overnight visit before the measurement would have been optimal. Participants also were able to perform activities of daily living, which varied from person to person. No activity logs were maintained to compare what participants did between visits.
The proposed benefits of the clinical application of ice may need to be re-examined. Although the muscle is cool, muscle blood flow does not seem to decrease immediately postintervention or after sequential ice treatments. Ice is one of the best modalities we have, but it is not the ultimate treatment. With other modalities, clinicians use a prescription model, taking into account the stage of the injury, signs and symptoms, functional outcomes, and other factors, to determine what modality to apply. The same approach should be considered with cryotherapy so it can be used properly.
CONCLUSIONS
Microvascular perfusion was elevated at 48 hours after eccentric exercise. Whereas we do not know what is happening to muscle blood flow over that time period, we question whether cooling skeletal muscle actually decreased intramuscular blood flow. Cryotherapy, however, did help control pain at 48 hours postexercise. During acute inflammation, cryotherapy may not affect microvascular perfusion as previously thought, but its use is warranted for pain control.
REFERENCES
- 1.Swenson C, Sward L, Karlsson J. Cryotherapy in sports medicine. Scand J Med Sci Sports. 1996;6(4):193–200. doi: 10.1111/j.1600-0838.1996.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 2.Hodges GJ, Johnson JM. Adrenergic control of the human cutaneous circulation. Appl Physiol Nutr Metab. 2009;34(5):829–839. doi: 10.1139/H09-076. [DOI] [PubMed] [Google Scholar]
- 3.Selkow NM, Herman DC, Liu Z, Hertel J, Hart JM, Saliba SA. Microvascular perfusion increases following eccentric exercise of the gastrocnemius. J Ultrasound Med. 2013;32(4):653–658. doi: 10.7863/jum.2013.32.4.653. [DOI] [PubMed] [Google Scholar]
- 4.Frank LR, Wong EC, Haseler LJ, Buxton RB. Dynamic imaging of perfusion in human skeletal muscle during exercise with arterial spin labeling. Magn Reson Med. 1999;42(2):258–267. doi: 10.1002/(sici)1522-2594(199908)42:2<258::aid-mrm7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 5.Saumet JL, Kellogg DL, Jr, Taylor WF, Johnson JM. Cutaneous laser-Doppler flowmetry: influence of underlying muscle blood flow. J Appl Physiol (1985) 1988;65(1):478–481. doi: 10.1152/jappl.1988.65.1.478. [DOI] [PubMed] [Google Scholar]
- 6.Kuznetsova LV, Tomasek N, Sigurdsson GH, Banic A, Erni D, Wheatley AM. Dissociation between volume blood flow and laser-Doppler signal from rat muscle during changes in vascular tone. Am J Physiol. 1998;274((4 pt 2)):H1248–H1254. doi: 10.1152/ajpheart.1998.274.4.H1248. [DOI] [PubMed] [Google Scholar]
- 7.Selkow NM, Day C, Liu Z, Hart JM, Hertel J, Saliba SA. Microvascular perfusion and intramuscular temperature of the calf during cooling. Med Sci Sports Exerc. 2012;44(5):850–856. doi: 10.1249/MSS.0b013e31823bced9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buford TW, Cooke MB, Shelmadine BD, Hudson GM, Redd L, Willoughby DS. Effects of eccentric treadmill exercise on inflammatory gene expression in human skeletal muscle. Appl Physiol Nutr Metab. 2009;34(4):745–753. doi: 10.1139/H09-067. [DOI] [PubMed] [Google Scholar]
- 9.Miles MP, Clarkson PM. Exercise-induced muscle pain, soreness, and cramps. J Sports Med Phys Fitness. 1994;34(3):203–216. [PubMed] [Google Scholar]
- 10.Newham DJ, Jones DA, Edwards RH. Large delayed plasma creatine kinase changes after stepping exercise. Muscle Nerve. 1983;6(5):380–385. doi: 10.1002/mus.880060507. [DOI] [PubMed] [Google Scholar]
- 11.Gulick DT, Kimura IF, Sitler M, Paolone A, Kelly JD. Various treatment techniques on signs and symptoms of delayed onset muscle soreness. J Athl Train. 1996;31(2):145–152. [PMC free article] [PubMed] [Google Scholar]
- 12.Safran MR, Seaber AV, Garrett WE., Jr Warm-up and muscular injury prevention: an update. Sports Med. 1989;8(4):239–249. doi: 10.2165/00007256-198908040-00004. [DOI] [PubMed] [Google Scholar]
- 13.Brewer BJ. Athletic injuries; musculotendinous unit. Clin Orthop. 1962;23:30–38. [PubMed] [Google Scholar]
- 14.MacIntyre DL, Reid WD, McKenzie DC. Delayed muscle soreness: the inflammatory response to muscle injury and its clinical implications. Sports Med. 1995;20(1):24–40. doi: 10.2165/00007256-199520010-00003. [DOI] [PubMed] [Google Scholar]
- 15.Ebbeling CB, Clarkson PM. Exercise-induced muscle damage and adaptation. Sports Med. 1989;7(4):207–234. doi: 10.2165/00007256-198907040-00001. [DOI] [PubMed] [Google Scholar]
- 16.Clarkson PM, Nosaka K, Braun B. Muscle function after exercise-induced muscle damage and rapid adaptation. Med Sci Sports Exerc. 1992;24(5):512–520. [PubMed] [Google Scholar]
- 17.Armstrong RB. Mechanisms of exercise-induced delayed onset muscular soreness: a brief review. Med Sci Sports Exerc. 1984;16(6):529–538. [PubMed] [Google Scholar]
- 18.Houglum PA. Soft tissue healing and its impact on rehabilitation. J Sport Rehabil. 1992;1(1):19–39. [Google Scholar]
- 19.Knight KL. Cryotherapy in Sports Injury Management. Champaign, IL: Human Kinetics;; 1995. [Google Scholar]
- 20.Eston R, Peters D. Effects of cold water immersion on the symptoms of exercise-induced muscle damage. J Sports Sci. 1999;17(3):231–238. doi: 10.1080/026404199366136. [DOI] [PubMed] [Google Scholar]
- 21.Bijur PE, Silver W, Gallagher EJ. Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med. 2001;8(12):1153–1157. doi: 10.1111/j.1553-2712.2001.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 22.Tegeder L, Zimmermann J, Meller ST, Geisslinger G. Release of algesic substances in human experimental muscle pain. Inflamm Res. 2002;51(8):393–402. doi: 10.1007/pl00000320. [DOI] [PubMed] [Google Scholar]
- 23.Otte JW, Merrick MA, Ingersoll CD, Cordova ML. Subcutaneous adipose tissue thickness alters cooling time during cryotherapy. Arch Phys Med Rehabil. 2002;83(11):1501–1505. doi: 10.1053/apmr.2002.34833. [DOI] [PubMed] [Google Scholar]
- 24.Selkow NM, Pietrosimone BG, Saliba SA. Subcutaneous thigh fat assessment: a comparison of skinfold calipers and ultrasound imaging. J Athl Train. 2011;46(1):50–54. doi: 10.4085/1062-6050-46.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vincent MA, Clerk LH, Lindner JR, et al. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab. 2006;290(6):E1191–E1197. doi: 10.1152/ajpendo.00497.2005. [DOI] [PubMed] [Google Scholar]
- 26.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998;97(5):473–483. doi: 10.1161/01.cir.97.5.473. [DOI] [PubMed] [Google Scholar]
- 27.Ingram J, Dawson B, Goodman C, Wallman K, Beilby J. Effect of water immersion methods on post-exercise recovery from simulated team sport exercise. J Sci Med Sport. 2009;12(3):417–421. doi: 10.1016/j.jsams.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 28.King M, Duffield R. The effects of recovery interventions on consecutive days of intermittent sprint exercise. J Strength Cond Res. 2009;23(6):1795–1802. doi: 10.1519/JSC.0b013e3181b3f81f. [DOI] [PubMed] [Google Scholar]
- 29.Pietrosimone BG, Hart JM, Saliba SA, Hertel J, Ingersoll CD. Immediate effects of transcutaneous electrical nerve stimulation and focal knee joint cooling on quadriceps activation. Med Sci Sports Exerc. 2009;41(6):1175–1181. doi: 10.1249/MSS.0b013e3181982557. [DOI] [PubMed] [Google Scholar]
- 30.Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc. 2003;78(5):603–612. doi: 10.4065/78.5.603. [DOI] [PubMed] [Google Scholar]
- 31.Hodges GJ, Johnson JM. Adrenergic control of the human cutaneous circulation. Appl Physiol Nutr Metab. 2009;34(5):829–839. doi: 10.1139/H09-076. [DOI] [PubMed] [Google Scholar]
- 32.Fiscus KA, Kaminski TW, Powers ME. Changes in lower-leg blood flow during warm-, cold-, and contrast-water therapy. Arch Phys Med Rehabil. 2005;86(7):1404–1410. doi: 10.1016/j.apmr.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 33.Belkin M, Brown RD, Wright JG, LaMorte WW, Hobson RW., II A new quantitative spectrophotometric assay of ischemia-reperfusion injury in skeletal muscle. Am J Surg. 1988;156(2):83–86. doi: 10.1016/s0002-9610(88)80360-x. [DOI] [PubMed] [Google Scholar]
- 34.Block ER, Patel JM, Edwards D. Mechanism of hypoxic injury to pulmonary artery endothelial cell plasma membranes. Am J Physiol. 1989;257((2 pt 1)):C223–C231. doi: 10.1152/ajpcell.1989.257.2.C223. [DOI] [PubMed] [Google Scholar]
- 35.Sabido F, Milazzo VJ, Hobson RW, II, Duran WN. Skeletal muscle ischemia-reperfusion injury: a review of endothelial cell-leukocyte interactions. J Invest Surg. 1994;7(1):39–47. doi: 10.3109/08941939409018281. [DOI] [PubMed] [Google Scholar]
- 36.Merrick MA, Rankin JM, Andres FA, Hinman CL. A preliminary examination of cryotherapy and secondary injury in skeletal muscle. Med Sci Sports Exerc. 1999;31(11):1516–1521. doi: 10.1097/00005768-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Prosser C. Comparative Animal Physiology. Philadelphia, PA: Saunders;; 1973. [Google Scholar]
- 38.Symons JD, Theodossy SJ, Longhurst JC, Stebbins CL. Intramuscular accumulation of prostaglandins during static contraction of the cat triceps surae. J Appl Physiol (1985) 1991;71(5):1837–1842. doi: 10.1152/jappl.1991.71.5.1837. [DOI] [PubMed] [Google Scholar]
- 39.Wall PD. On the relation of injury to pain: the John J. Bonica Lecture. Pain. 1979;6(3):253–264. doi: 10.1016/0304-3959(79)90047-2. [DOI] [PubMed] [Google Scholar]
- 40.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]