Abstract
Context:
Researchers have postulated that reduced hip-abductor muscle strength may have a role in the progression of knee osteoarthritis by increasing the external knee-adduction moment. However, the relationship between hip-abductor strength and frontal-plane biomechanics remains unclear.
Objective:
To experimentally reduce hip-abduction strength and observe the subsequent changes in frontal-plane biomechanics.
Design:
Descriptive laboratory study.
Setting:
Research laboratory.
Patients or Other Participants:
Eight healthy, recreationally active men (age = 27 ± 6 years, height = 1.75 ± 0.11 m, mass = 76.1 ± 10.0 kg).
Intervention(s):
All participants underwent a superior gluteal nerve block injection to reduce the force output of the hip-abductor muscle group.
Main Outcome Measure(s):
Maximal isometric hip-abduction strength and gait biomechanical data were collected before and after the injections. Gait biomechanical variables collected during walking consisted of knee- and hip-adduction moments and impulses and the peak angles of contralateral pelvic drop, hip adduction, and ipsilateral trunk lean.
Results:
Hip-abduction strength was reduced after the injection (P = .001) and remained lower than baseline values at the completion of the postinjection gait data collection (P = .02). No alterations in hip- or knee-adduction moments (hip: P = .11; knee: P = .52) or impulses (hip: P = .16; knee: P = .41) were found after the nerve block. Similarly, no changes in angular kinematics were observed for contralateral pelvic drop (P = .53), ipsilateral trunk lean (P = .78), or hip adduction (P = .48).
Conclusions
A short-term reduction in hip-abductor strength was not associated with alterations in the frontal-plane gait biomechanics of young, healthy men. Further research is needed to determine whether a similar relationship is true in older adults with knee osteoarthritis.
Key Words: gait analysis, knee, moment, kinematics, pelvic drop
Key Points
A 26% reduction in force output of the hip-abductor muscle group did not alter the external knee-adduction moment in young, healthy men.
No alterations in hip-adduction moment, hip adduction, or contralateral pelvic drop were associated with a reduction in hip-abductor strength.
More research is needed to determine whether a similar relationship between hip-abductor strength and frontal-plane gait biomechanics exists in older adults with knee osteoarthritis.
Biomechanical changes in gait have been observed in patients with medial compartment knee osteoarthritis (MC-KOA). In particular, external knee-adduction moment (EKAM) has been linked with the incidence, severity, and progression of MC-KOA.1–4 Discrete EKAM variables, including the first peak (during the first 50% stance), second peak (during the second 50% stance), and knee-adduction angular impulse have been shown to discriminate between individuals with and without MC-KOA.1,3,4 Moreover, the risk of MC-KOA progression has been reported2 to increase 6.5 times with a 1% increase in EKAM.
The external hip-adduction moment (EHAM) during gait has also been associated with progression of MC-KOA. Mundermann et al3 reported that patients with severe osteoarthritis (OA) had lower first- and second-peak EHAMs than did patients serving as controls. Chang et al5 provided further evidence linking frontal-plane hip moments and MC-KOA. They concluded that a greater EHAM at baseline reduced the likelihood of disease progression during the next 18 months.5 Both of these research groups determined that, given that the EHAM is balanced by the hip-abductor muscles, those muscles may have an important role in preventing OA progression. Indeed, Chang et al5 postulated that weak hip-abductor muscles allow greater contralateral pelvic drop, which could shift the center of mass away from the stance limb, resulting in elevated loading across the medial knee compartment. This postulation was based on the assumption that the frontal-plane lever arm of the ground reaction force passing medial to the knee-joint center would be increased, resulting in a greater EKAM. However, if the center of mass was shifted away from the stance limb because of pelvic drop, then the frontal-plane lever arm of the ground reaction force about the hip-joint center also should be increased. Logically, this would result in increased EHAM (Figure 1), suggesting that weakness of the hip abductors would result in an increase in EHAM, rather than the decrease that Chang et al5 postulated. Therefore, the relationship among hip-abductor strength, frontal-plane hip-joint biomechanics, and knee-joint loading remains unclear and requires further attention.
Figure 1.
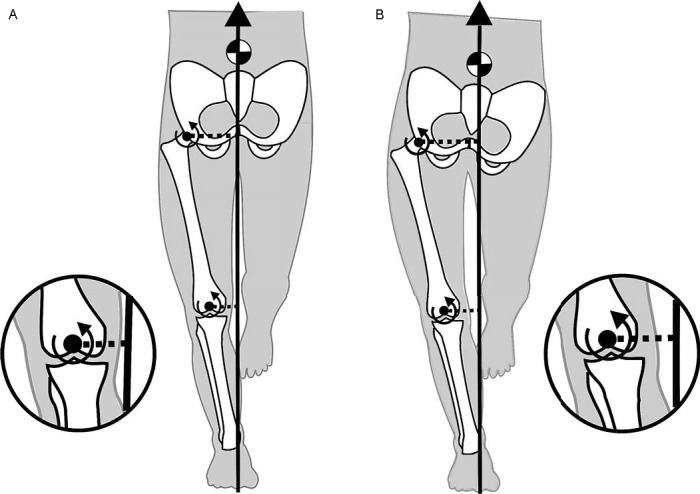
The external hip- and knee-adduction moments are influenced by the lever-arm distance from which the ground reaction force passes medial to the joint center. A, Neutral pelvic alignment during gait is illustrated. The free-body diagram is provided for simplicity using a quasistatic approach. B, However, increasing pelvic drop potentially could increase the length of the ground reaction force lever arm at the hip and knee, subsequently increasing the external-adduction moments at both joints.
Henriksen et al6 explored the link between reduced hip-abductor function and knee-joint loading during gait by injecting a pain-inducing solution into the gluteus medius. Whereas the injection successfully reduced hip-abductor muscular activation, it resulted in unexpected reductions in both EKAM and EHAM.6 However, whether the gait adaptations observed postinjection were simply an antalgic gait response to pain remains unclear. An alternative method to experimentally reduce the force output of muscles is to perform a nerve block injection. For example, blocking the tibial nerve transmission to the intrinsic foot muscles has been used to explore their role in supporting the medial longitudinal arch of the foot.7 To our knowledge, this approach has not been used to investigate the relationship between reduced hip-abductor muscle function and knee- and hip-joint loading.
Therefore, the aim of this study was to experimentally reduce hip-abductor muscle function in healthy participants via a superior gluteal nerve block injection and observe the subsequent alterations in gait. Specifically, we hypothesized that the EKAM during gait would be greater after an experimentally induced reduction in the output force of the hip-abductor muscle. We also hypothesized that we would observe greater EHAM, contralateral pelvic-drop angles, and hip-adduction angles postinjection. Finally, we expected that the magnitude of the alteration in hip-abductor muscle force would be related to the magnitude of any observed changes in both the EKAM and EHAM.
METHODS
Experimental Protocol
Participants visited the laboratory on 1 occasion and underwent hip-abductor muscle-strength testing and a biomechanical gait analysis before and after a unilateral superior gluteal nerve block procedure (preinjection and postinjection condition, respectively). Strength testing was conducted before gait analysis in the preinjection and postinjection conditions. A third set of hip-abductor maximal voluntary contractions was collected immediately after analysis of the postinjection gait (postcollection condition) to determine the level of recovery during the testing session.
Participants
Eight healthy, recreationally active men (age = 27 ± 6 years, height = 1.75 ± 0.11 m, mass = 76.1 ± 10.0 kg) volunteered to participate in the study. Volunteers were male, were more than 18 years old, were engaged in physical activity on a regular basis, had no previous surgery to the spine or lower extremity, demonstrated 1.5% or more body weight for hip-abductor strength bilaterally, and demonstrated neutral pelvic alignment as assessed by a certified athletic therapist using standard clinical methods.8 Only male participants were included because they generally have less adipose tissue around the injection site, increasing the odds of an accurate injection and successful nerve block procedure. Further, a minimal hip-abductor strength threshold was selected to ensure the potential for a large decrease in strength postinjection, thus avoiding a ceiling effect. Exclusion criteria were an injury to the spine or lower extremity within the 12 months before the study; history of surgery to the lumbar spine, pelvis, or hip; discrepancy in lower extremity lengths greater than 1.5 cm; or any physical or medical problems for which strength testing or walking would be contraindicated. All participants provided written informed consent, and the study was reviewed and approved by the Conjoint Health Research Ethics Board at the University of Calgary.
Hip-Abductor Strength-Testing Procedures
Hip-abductor strength was assessed using maximal voluntary isometric contractions. Participants were positioned in a side-lying pose with the hip abducted against a fixed strap so that the thigh was horizontal to the surface.9 A digital protractor (PRO 360; SmartTool, Oklahoma City, OK) was used to verify the horizontal position. A force dynamometer (Manual Muscle Tester; Lafayette Instruments, Lafayette, IN) was placed 1.5 cm above the lateral knee-joint line and beneath the strap to measure force output. Participants were given 2 practice trials, followed by 3 experimental trials, with 30 seconds of rest between trials.10 Practice trials were conducted for all 3 time points during which strength data were collected. We provided standardized oral encouragement, instructing the participants to exert themselves maximally. The mean of the 3 trials was converted to torque by multiplying by the resistance lever arm and was normalized as a percentage of body weight.11 The lever arm was defined as the distance between the greater trochanter and the location at which the dynamometer was positioned. All strength testing was performed by a single tester (K.D.K.). Within-day reliability of the tester was assessed on a separate sample of 5 participants who underwent 2 testing sessions for hip-abduction strength approximately 1 hour apart. Participants remained seated in the laboratory between the 2 testing sessions. Reliability values were 0.06 Nm/kg for mean absolute difference, 0.09 Nm/kg for SEM, and 0.90 for intraclass correlation coefficient (3,1).
Gait Biomechanics Procedures
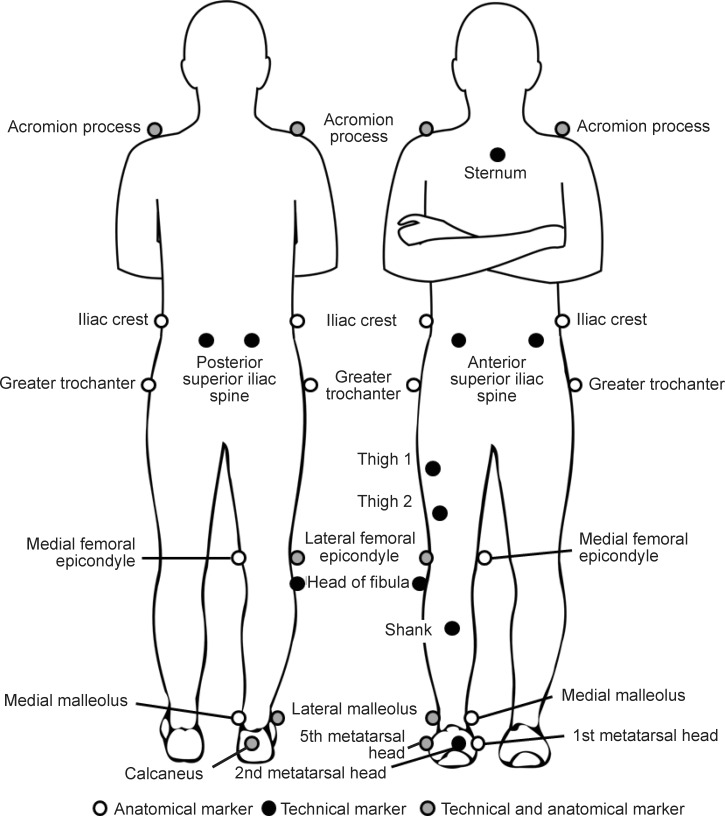
We collected 3-dimensional gait-analysis data using 6 motion-analysis cameras (model Falcon; Motion Analysis Corp, Santa Rosa, CA) and a force plate (model OR6-6; Advanced Mechanical Technology, Inc, Watertown, MA) positioned in the middle of a 15-m walkway. A customized set of reflective markers was placed on the lower extremity (Figure 2). Given that the markers were removed during the injection procedure, the exact location of each marker was ink stamped on the skin to enable precise replacement for the postinjection condition. After a standing trial, kinematic and kinetic gait data were collected for 5 over-ground, barefoot walking trials of the right limb. Walking velocity was monitored using photoelectronic cells (model TC Timing System; Brower Timing Systems, Draper, UT) and was maintained within ±5% of the pace that the participant self-selected. Kinematic and kinetic data were collected at 120 and 1200 Hz, respectively.
Figure 2.
Placement of the anatomical and technical markers used to define and track segments, respectively: acromion processes, sternum, iliac crests, anterior- and posterior-superior iliac spines, greater trochanters, thigh 1 and 2, lateral and medial femoral epicondyles, head of the fibula, shank, lateral and medial malleoli, calcaneus, and first, second, and fifth metatarsal heads.
All kinematic and kinetic data were processed using Visual3D software (version 4; C-Motion, Inc, Germantown, MD). Raw marker-trajectory and force data were filtered at 8 and 50 Hz, respectively, using a fourth-order Butterworth filter. Segmental coordinate systems were constructed for the trunk, pelvis, thigh, shank, and foot using the regression techniques described by Pohl et al.12 All 3-dimensional joint angles were referenced as the distal segment relative to the proximal segment with the Cardan sequence of rotations using a Z-X-Y (mediolateral, anteroposterior, vertical) convention. External joint moments were resolved into the distal coordinate system and were expressed as a percentage of body weight by height.2,3 Joint kinematics and moments were analyzed for the stance phase and normalized to 101 data points. To determine the stance phase, we identified initial contact and toe-off using a force threshold of 20 N.
Nerve Block Intervention
The superior gluteal nerve block was performed by an interventional radiologist (C.P.) with the assistance of a sports medicine physician (J.P.W.). The participant was placed in a side-lying position on the left hip while an ACUSON Sequoia 512 ultrasound system (Siemens Medical Solutions USA Inc, Mountain View, CA) was used to guide a spinal needle and inject 10 mL of a 1% lidocaine solution along the fascias between the right gluteus medius and minimus muscles.
Gait Biomechanical Outcomes
Discrete kinematic and kinetic variables were identified and averaged for the 5 over-ground walking trials for each participant using custom LabVIEW software (National Instruments, Austin, TX). The kinetic variables of interest were the first and second peaks of the EKAM (EKAM1 and EKAM2, respectively). Specifically, EKAM1 and EKAM2 were defined as the peak magnitudes during the first and second halves of the stance phase, respectively. Other variables of interest were knee-adduction angular impulse, EHAM at the instance of EKAM1 (EHAM1), EHAM at the instant of EKAM2 (EHAM2), hip-adduction angular impulse, and lateral ground reaction force at the instance of EKAM1. Kinematic variables of interest were calculated at the instance of EKAM1 and consisted of contralateral pelvic-drop, trunk-lean, hip-adduction, and knee-adduction angles.
Statistical Analysis
The mean of the peak values of the biomechanical variables for each participant during each condition was derived from his 5 trials. Next, the ensemble mean and standard deviation values for the preinjection and postinjection conditions were calculated using the mean values of each of the 8 participants. Because evaluating normality on small sample sizes is problematic, we used nonparametric statistics. Wilcoxon signed-rank tests were performed for between-conditions (preinjection versus postinjection) statistical comparisons for all discrete variables of interest, with an α level set at .05. Given the small sample size, the α level was not adjusted for multiple comparisons to prevent statistical power from being reduced further. Effect sizes were calculated using the Cohen d (mean difference divided by pooled standard deviation [SD]). We explored the relationship of the percentage drop in hip-abductor strength between postinjection and postcollection with the corresponding changes in EKAM and EHAM external-adduction moments using the Spearman correlation coefficient. The Wilcoxon signed-rank test was conducted for a post hoc comparison of the first and fifth postinjection gait trials for all biomechanical variables of interest. We used SPSS software (version 17.0; SPSS Inc, Chicago, IL) for all statistical analyses.
RESULTS
The mean ± SD hip-abductor torque values for the preinjection, postinjection, and postcollection conditions were 1.51 ± 0.38, 0.81 ± 0.30, and 1.12 ± 0.31 Nm/kg, respectively. Compared with the preinjection values, hip strength (torque) was lower for both postinjection (P = .001) and postcollection (P = .02) conditions. This equated to hip-abductor strength reductions of 46% and 26% in the postinjection and postcollection values, respectively. Although hip strength increased between postinjection and postcollection, this finding was not different (P = .08). The elapsed time between the postinjection and postcollection strength testing was 38 ± 10 minutes. The elapsed time between the injection time and postcollection strength testing was 61 ± 9 minutes. We found no differences postinjection in hip- and knee-joint moments and impulses or lateral ground reaction forces (Table; Figure 3). Similarly, we observed no alterations in kinematic variables, including contralateral pelvic drop, hip adduction, and knee adduction. Given the slight recovery in hip-abductor strength between postinjection and postcollection, we conducted a post hoc comparison of the first and fifth postinjection gait trials for all biomechanical variables of interest. However, no statistical differences were present for any variables. The correlation analysis revealed a poor relationship between the percentage drop in hip-abductor strength and the corresponding change in EKAM (ρ = 0.289, P = .49) and EHAM (ρ = 0.35, P = .40) postinjection.
Table.
Strength and Kinetic and Kinematic Variables of Interest Preinjection and Postinjectiona
| Variable |
Preinjection, Mean ± SD |
Postinjection, Mean ± SD |
Mean Difference |
95% Confidence Interval |
P |
Effect Size |
Post Hoc Power Estimate |
| Hip-abduction strength, Nm/kg | 1.5 ± 0.4 | 0.8 ± 0.3 | −0.7 | −1.0, −0.4 | .001b | 2.04 | Not applicable |
| First peak of external knee-adduction moment, % body weight × height | 3.0 ± 1.1 | 2.9 ± 1.0 | −0.1 | −0.3, 0.1 | .52 | 0.10 | 0.06 |
| Second peak of external knee-adduction moment, % body weight × height | 2.7 ± 0.6 | 2.7 ± 0.5 | 0.0 | −0.2, 0.2 | .89 | 0.00 | 0.05 |
| Knee-adduction angular impulse, % body weight × height × s | 1.2 ± 0.3 | 1.1 ± 0.3 | −0.1 | −0.1, 0.1 | .41 | 0.33 | 0.13 |
| Hip external-adduction moment at the instance of the first peak of external knee-adduction moment, % body weight × height | 4.7 ± 1.0 | 4.5 ± 1.0 | −0.2 | −0.4, 0.1 | .11 | 0.20 | 0.08 |
| Hip external-adduction moment at the instance of the second peak of external knee-adduction moment, % body weight × height | 5.3 ± 1.1 | 5.4 ± 1.2 | 0.1 | −0.2, 0.3 | .62 | 0.09 | 0.06 |
| Hip-adduction angular impulse, % body weight × height × s | 2.1 ± 0.6 | 2.0 ± 0.5 | −0.1 | −0.2, 0.1 | .16 | 0.18 | 0.07 |
| Lateral ground reaction force, body weight | 0.05 ± 0.02 | 0.05 ± 0.02 | 0.00 | −0.01, 0.01 | .43 | 0.00 | 0.05 |
| Contralateral pelvic-drop angle, ° | 1.7 ± 2.7 | 1.3 ± 2.5 | 0.4 | −0.6, 1.4 | .53 | 0.15 | 0.07 |
| Hip-adduction angle, ° | 3.5 ± 3.7 | 3.2 ± 3.4 | −0.3 | −1.6, 0.9 | .48 | 0.08 | 0.05 |
| Knee-adduction angle, ° | 3.7 ± 3.3 | 4.0 ± 2.7 | 0.3 | −0.6, 1.1 | .24 | 0.10 | 0.06 |
| Ipsilateral trunk-lean angle, ° | 1.9 ± 1.9 | 2.0 ± 2.2 | 0.1 | −0.5, 0.6 | .78 | 0.00 | 0.05 |
| Gait speed, m/s | 1.34 ± 0.15 | 1.36 ± 0.11 | 0.02 | −0.02, 0.07 | .26 | 0.15 | 0.07 |
We calculated the 95% confidence intervals and effect sizes using the formulas presented by Portney and Watkins.13
Indicates difference (P < .05).
Figure 3.
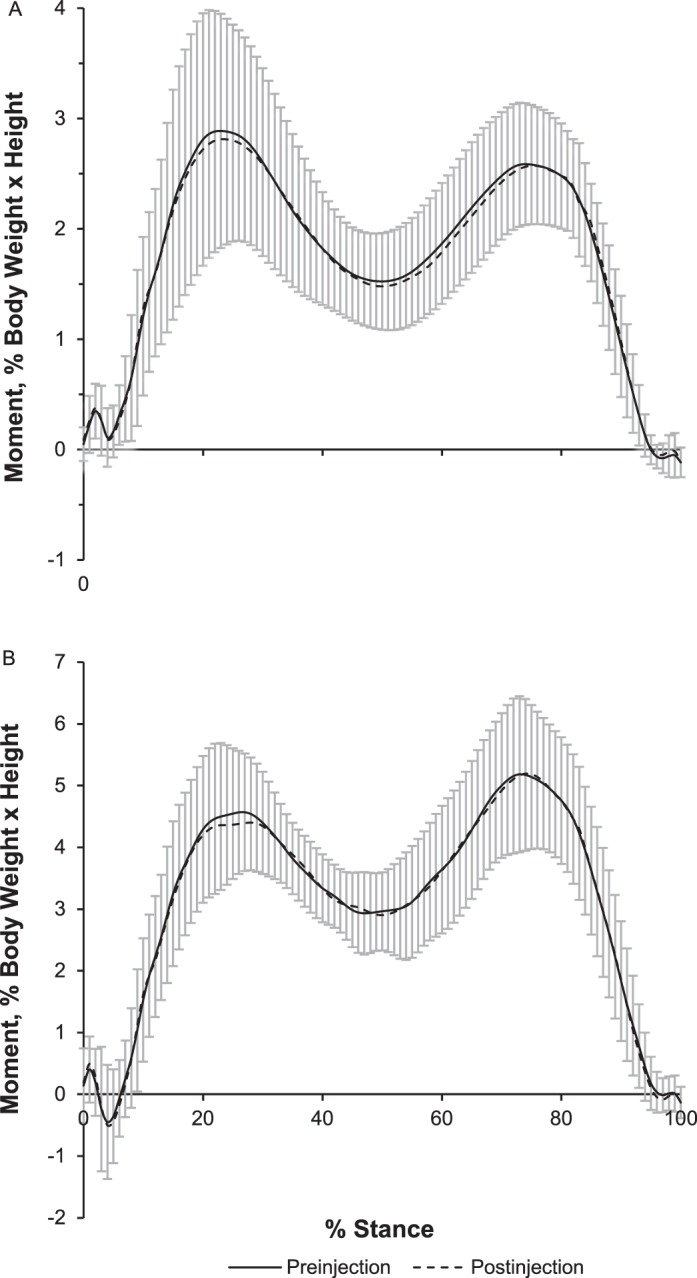
Mean ensemble curves for, A, external hip-adduction moments and, B, knee-adduction moments before and after the nerve block injection.
DISCUSSION
The purpose of our study was to explore the effect of experimentally reduced hip-abductor muscle strength on gait biomechanics. We hypothesized that a reduction in hip-abductor strength would result in greater EKAM, EHAM, and impulses along with greater contralateral pelvic-drop and hip-adduction angles. Contrary to the hypotheses, we did not observe alterations in any of the aforementioned gait biomechanical variables, despite achieving a mean reduction in hip-abductor force output of 46%.
Our findings suggest that reducing the force output of the hip-abductor muscles does not result in a subsequent increase in EKAM. The results are in contrast to those of Henriksen et al,6 who reported reduced hip-abductor function was accompanied by a decrease in peak EKAM during gait. However, given that hip-abductor function was reduced in that study by inducing a pain response, it is unclear whether the gait adaptations they observed postinjection were due to an antalgic gait pattern. We postulate that the nerve block procedure is a promising alternative method of reducing hip-abductor force output because it can be achieved without inducing pain.
After the experimental reduction in hip-abductor strength, we found no associated alterations in EHAM. Thus, our results indicate that maximal isometric hip-abductor strength was not related to EHAM during gait. This conclusion supports the regression analysis results of Rutherford and Hubley-Kozey,14 who found that hip-abductor strength did not explain the variability in EHAM.
In contrast to our results, Henriksen et al6 reported a reduction in EHAM after the medial gluteal saline injection. They noted greater ipsilateral trunk lean postinjection, whereas we found no change between the preinjection and postinjection conditions. Greater ipsilateral trunk lean has been shown to reduce both EHAM and EKAM15 by shifting the center of mass toward the joint centers of the stance limb. The difference between the 2 studies may be attributable to participants in the Henriksen et al5 study having to reduce the demand placed on the hip abductors because of pain. This finding provides preliminary evidence that simple reductions in hip-abductor strength without a pain response may not result in a compensatory trunk lean. Rather, pain, instead of weakness, may lead to the adoption of a trunk-lean strategy.
Our results bring into question the possibility that reduced hip-abductor strength leads to alterations in EKAM and EHAM that might be detrimental to the progression of MC-KOA. Patients with lower EHAMs have faster radiographic progression of knee OA than do patients with greater moments at baseline.5 Chang et al5 postulated that the lower EHAM was indicative of weak hip-abductor muscles and that the weakness would lead to greater contralateral pelvic drop and an associated increase in the EKAM. However, they did not present data on contralateral pelvic drop or hip-abductor strength. Our data suggest that a reduction in hip-abductor strength may not result in greater external-adduction moments at the knee and hip. Moreover, no alterations in contralateral pelvic-drop or hip-adduction angles were observed after the nerve block procedure. This finding was surprising because impaired gluteal nerve function traditionally has been associated with a Trendelenburg gait pattern, which is characterized by excessive pelvic drop, hip adduction, and trunk lean.16
One possible explanation for the lack of gait alterations is that the magnitude of the induced hip-abductor strength deficit was insufficient to evoke changes in walking biomechanics. For instance, Rutherford and Hubley-Kozey14 showed that gluteus medius electromyographic activation during walking reached only 70% of the value recorded during a maximal voluntary isometric contraction for hip-abductor strength. Taking a conservative estimate of induced strength decrements and using the postcollection strength values, we found that the isometric hip-abductor strength of our participants was still 76% of the baseline value. If walking requires less hip-abductor strength than the maximal voluntary isometric contraction, perhaps participants still had sufficient strength postinjection to maintain a normal gait pattern. However, a 26% reduction in hip-abductor strength may have biomechanical implications for more dynamic movement tasks, such as running and jumping, which place the muscles under greater demand.
Another explanation for our findings is that we studied young, asymptomatic men. Thus, the results may not be directly transferable to patients with MC-KOA. Given that the participants were young and healthy, we expected that they would have greater hip-abduction strength than would older individuals with OA.11 Therefore, the hip-abductor muscular force required during walking may constitute a greater percentage of the maximal available strength in older individuals than it does in their younger counterparts. This would increase the odds that any experimentally induced strength reduction would cause alterations in the gait patterns of older individuals. Further research is needed to determine whether a similar reduction in hip-abductor strength would have a stronger effect on gait biomechanics in an older population. In addition, the young, healthy men in our study may have had sufficient overall lower extremity strength to successfully compensate for reduced hip-abduction strength. A population with knee OA often has weakness in other lower extremity muscle groups, which may contribute to the altered gait patterns typically seen in that population.
Gauging the time that the lidocaine injection would keep the gluteal muscles in a weakened state was difficult. Whereas lidocaine has a serum half-life of 90 to 120 minutes, this value would be shorter for nerves. The duration of the effect would depend on the proximity of the injection to the superior gluteal nerve and could vary among participants. We conducted the postinjection testing procedures as quickly as possible to minimize this as a confounding factor. Moreover, we reported our strength decrements after the second gait analysis to document any recovery in strength that may have occurred.
Given the transient, acute nature of the nerve block procedure, we could only observe the short-term gait adaptations associated with reduced hip-abductor strength. It remains unclear whether prolonged hip-abductor weakness would lead to compensatory gait alterations over time. Finally, the conclusions of our study may have been limited by the small sample size. The number of participants was restricted because of the invasive nature of the protocol and the availability of the medical staff, equipment, and laboratory space required for this project. A post hoc power analysis revealed values of 0.08, 0.06, and 0.07 for the hip-adduction moment, knee-adduction moment, and pelvic-drop angle, respectively. Furthermore, we determined that sample sizes of 867, 198, and 334 would be required to find differences in the aforementioned variables of interest (α = .05, β = 0.2). Moreover, the low effect sizes observed for all the independent variables suggest the mean differences found were too small to be of clinical relevance. Indeed, many of the preinjection-to-postinjection differences were smaller than the precision of the measurement system.
Within the limitations of this preliminary study, we concluded that a 26% reduction in force output of the hip-abductor muscle group did not alter the EKAM in young, healthy men. Furthermore, no alterations in hip-adduction moment, hip adduction, or contralateral pelvic drop were associated with reduced hip-abductor strength. However, the magnitude of the induced weakness was small, and our conclusions should be considered preliminary because of the limited statistical power. A more extensive study involving a larger sample size is needed to fully validate the findings of this study. Further research is also required to determine whether a similar relationship between hip-abductor strength and frontal-plane gait biomechanics exists in older adults with knee OA.
ACKNOWLEDGMENTS
Funding for this study was provided by Alberta Innovates–Health Solutions and the Workers' Compensation Board–Alberta. We thank Craig Mathison, Janet Ronsky, and Roy Park for their assistance with the project.
REFERENCES
- 1.Huang SC, Wei IP, Chien HL, et al. Effects of severity of degeneration on gait patterns in patients with medial knee osteoarthritis. Med Eng Phys. 2008;30(8):997–1003. doi: 10.1016/j.medengphy.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Miyazaki T, Wada M, Kawahara H, Sato M, Baba H, Shimada S. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann Rheum Dis. 2002;61(7):617–622. doi: 10.1136/ard.61.7.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mundermann A, Dyrby CO, Andriacchi TP. Secondary gait changes in patients with medial compartment knee osteoarthritis: increased load at the ankle, knee, and hip during walking. Arthritis Rheum. 2005;52(9):2835–2844. doi: 10.1002/art.21262. [DOI] [PubMed] [Google Scholar]
- 4.Thorp LE, Sumner DR, Block JA, Moisio KC, Shott S, Wimmer MA. Knee joint loading differs in individuals with mild compared with moderate medial knee osteoarthritis. Arthritis Rheum. 2006;54(12):3842–3849. doi: 10.1002/art.22247. [DOI] [PubMed] [Google Scholar]
- 5.Chang A, Hayes K, Dunlop D, et al. Hip abduction moment and protection against medial tibiofemoral osteoarthritis progression. Arthritis Rheum. 2005;52(11):3515–3519. doi: 10.1002/art.21406. [DOI] [PubMed] [Google Scholar]
- 6.Henriksen M, Aaboe J, Simonsen EB, Alkjaer T, Bliddal H. Experimentally reduced hip abductor function during walking: implications for knee joint loads. J Biomech. 2009;42(9):1236–1240. doi: 10.1016/j.jbiomech.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Fiolkowski P, Brunt D, Bishop M, Woo R, Horodyski M. Intrinsic pedal musculature support of the medial longitudinal arch: an electromyography study. J Foot Ankle Surg. 2003;42(6):327–333. doi: 10.1053/j.jfas.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Magee DJ. Orthopedic Physical Assessment. 5th ed. St Louis, MO: Saunders Elsevier;; 2008. [Google Scholar]
- 9.Earl JE, Hoch AZ. A proximal strengthening program improves pain, function, and biomechanics in women with patellofemoral pain syndrome. Am J Sports Med. 2011;39(1):154–163. doi: 10.1177/0363546510379967. [DOI] [PubMed] [Google Scholar]
- 10.Kendall KD, Schmidt C, Ferber R. The relationship between hip-abductor strength and the magnitude of pelvic drop in patients with low back pain. J Sport Rehabil. 2010;19(4):422–435. doi: 10.1123/jsr.19.4.422. [DOI] [PubMed] [Google Scholar]
- 11.Bohannon RW. Reference values for extremity muscle strength obtained by hand-held dynamometry from adults aged 20 to 79 years. Arch Phys Med Rehabil. 1997;78(1):26–32. doi: 10.1016/s0003-9993(97)90005-8. [DOI] [PubMed] [Google Scholar]
- 12.Pohl MB, Lloyd CH, Ferber R. Can the reliability of three-dimensional running kinematics be improved using functional joint methodology. Gait Posture. 2010;32(4):559–563. doi: 10.1016/j.gaitpost.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 13.Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. 2nd ed. Upper Saddle River, NJ: Prentice-Hall Inc;; 2000. pp. 425–708. [Google Scholar]
- 14.Rutherford DJ, Hubley-Kozey C. Explaining the hip adduction moment variability during gait: implications for hip abductor strengthening. Clin Biomech (Bristol, Avon) 2009;24(3):267–273. doi: 10.1016/j.clinbiomech.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Mundermann A, Asay JL, Mundermann L, Andriacchi TP. Implications of increased medio-lateral trunk sway for ambulatory mechanics. J Biomech. 2008;41(1):165–170. doi: 10.1016/j.jbiomech.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Petrofsky JS. The use of electromyogram biofeedback to reduce Trendelenburg gait. Eur J App Physiol. 2001;85(5):491–495. doi: 10.1007/s004210100466. [DOI] [PubMed] [Google Scholar]