Abstract
Context:
Patellofemoral pain (PFP) is the most common injury in running and jumping athletes. Randomized controlled trials suggest that incorporating hip and core strengthening (HIP) with knee-focused rehabilitation (KNEE) improves PFP outcomes. However, no randomized controlled trials have, to our knowledge, directly compared HIP and KNEE programs.
Objective:
To compare PFP pain, function, hip- and knee-muscle strength, and core endurance between KNEE and HIP protocols after 6 weeks of rehabilitation. We hypothesized greater improvements in (1) pain and function, (2) hip strength and core endurance for patients with PFP involved in the HIP protocol, and (3) knee strength for patients involved in the KNEE protocol.
Design:
Randomized controlled clinical trial.
Setting:
Four clinical research laboratories in Calgary, Alberta; Chicago, Illinois; Milwaukee, Wisconsin; and Augusta, Georgia.
Patients or Other Participants:
Of 721 patients with PFP screened, 199 (27.6%) met the inclusion criteria (66 men [31.2%], 133 women [66.8%], age = 29.0 ± 7.1 years, height = 170.4 ± 9.4 cm, weight = 67.6 ± 13.5 kg).
Intervention(s):
Patients with PFP were randomly assigned to a 6-week KNEE or HIP protocol.
Main Outcome Measure(s):
Primary variables were self-reported visual analog scale and Anterior Knee Pain Scale measures, which were conducted weekly. Secondary variables were muscle strength and core endurance measured at baseline and at 6 weeks.
Results:
Compared with baseline, both the visual analog scale and the Anterior Knee Pain Scale improved for patients with PFP in both the HIP and KNEE protocols (P < .001), but the visual analog scale scores for those in the HIP protocol were reduced 1 week earlier than in the KNEE group. Both groups increased in strength (P < .001), but those in the HIP protocol gained more in hip-abductor (P = .01) and -extensor (P = .01) strength and posterior core endurance (P = .05) compared with the KNEE group.
Conclusions:
Both the HIP and KNEE rehabilitation protocols produced improvements in PFP, function, and strength over 6 weeks. Although outcomes were similar, the HIP protocol resulted in earlier resolution of pain and greater overall gains in strength compared with the KNEE protocol.
Key Words: anterior knee pain, clinical trial, outcomes assessment, knee rehabilitation, patella
Key Points
To our knowledge, this was the first randomized controlled clinical trial to directly compare rehabilitation protocols focused on the hip and core versus the knee for patients with patellofemoral pain.
Over 6 weeks, both the hip and core and the knee rehabilitation protocols produced improvements in patellofemoral pain, function, and strength.
Compared with the knee protocol, the hip and core protocol resulted in earlier resolution of pain and greater overall gains in strength.
Patellofemoral pain (PFP) is an idiopathic condition characterized by aching pain in the peripatellar area, which is exacerbated by physical activities, such as climbing stairs, squatting, jumping, running, and prolonged sitting.1 Patellofemoral pain is the most common musculoskeletal overuse injury in physically active individuals, including runners, military recruits, triathletes, and other athletes, regardless of sex or age.2–8 Traditionally, research and clinical practice have focused on muscle function of the quadriceps, based on the theory that an imbalance between the vastus medialis oblique and the vastus lateralis can lead to increased lateral stress in the patellofemoral joint.9,10 More recently, PFP was proposed to be related to reduced hip strength and core endurance.11–17 However, few randomized controlled trials (RCTs) have been performed to determine whether increases in hip-muscle strength and core endurance improve rehabilitation outcomes for patients with PFP.
Fukuda et al18 conducted an RCT on 54 females with PFP to determine whether the addition of hip-muscle strengthening to a more traditional program of knee-muscle stretching and strengthening resulted in better outcomes than the knee program alone. They reported that the addition of the hip-muscle–strengthening exercises resulted in better improvements in pain and function than did a knee-focused rehabilitation program. However, that study involved sedentary females, and the rehabilitation protocol lasted only 4 weeks, whereas at least 6 weeks of rehabilitation may be necessary to gain the greatest treatment effect.19–21 Ismail et al22 conducted an RCT to investigate the effect of adding hip-muscle strengthening to a squatting, step-up, and knee-extension protocol for 32 patients with PFP. At the end of the 6-week protocol, the group that performed the additional hip strengthening reported greater improvements in pain control during functional activities than did the control group. Thus, although the findings of previous studies suggest that including hip- and core–muscle strengthening is beneficial to PFP outcomes, no authors have directly compared a hip-core–focused rehabilitation program with a knee-focused rehabilitation program for PFP.
Therefore, the purpose of our RCT was to compare pain, function, hip- and knee-muscle strength, and core endurance for patients with PFP assigned to either a hip- and core-focused (HIP) or a knee-focused (KNEE) 6-week rehabilitation protocol. We hypothesized that improvements in self-reported outcome measures for patients with PFP involved in the HIP protocol would be greater than those involved in the KNEE protocol. We also hypothesized that improvements in hip-muscle strength and core-endurance measures would be greater for patients with PFP involved in the HIP protocol compared with those in the KNEE protocol, and improvements in knee-muscle strength would be greater for patients involved in the KNEE protocol compared with those in the HIP protocol.
METHODS
This study was a single-blind, multicentered RCT across 4 clinical research laboratories: Calgary, Alberta; Chicago, Illinois; Milwaukee, Wisconsin; and Augusta, Georgia. Volunteers from these areas responded to print, radio, and television advertisements; media releases; notice boards; word of mouth; and referrals from sports medicine practitioners. Inclusion criteria were based on Boling et al12 and can be found in Table 1. The most affected lower extremity was used for participants exhibiting bilateral symptoms. We chose participants having an insidious onset of pain with no discernable cause, other than overuse, because they represent most patients diagnosed with PFP.2,3,12,14,15,23,24
Table 1.
Inclusion and Exclusion Criteriaa
| Inclusion Criteria |
| 1. Visual analog score rating of pain during activities of daily living during the previous week at a minimum of 3 cm on a 10-cm scale |
| 2. Insidious onset of symptoms unrelated to trauma and persistent for at least 4 wk |
| 3. Pain in the anterior knee associated with at least 3 of the following: |
| a. During or after activity |
| b. Prolonged sitting |
| c. Stair ascent or descent |
| d. Squatting |
| 4. Pain with palpation of the patellar facets or pain during step down from a 20-cm box or during a double-legged squat |
| 5. Recreationally active (≥30 min/d, 3–4 d/wk for the past 6 mo and exclusive of pain) |
| Exclusion Criteria |
| 1. Meniscal or other intra-articular injury |
| 2. Cruciate or collateral ligament laxity or tenderness |
| 3. Patellar tendon, iliotibial band, or pes anserine tenderness |
| 4. Positive patellar-apprehension sign |
| 5. Osgood-Schlatter or Sinding-Larsen-Johansson syndrome |
| 6. Evidence of effusion |
| 7. Hip or lumbar referred pain |
| 8. History of recurrent patellar subluxation or dislocation |
| 9. History of surgery to the knee joint |
| 10. Nonsteroidal anti-inflammatory drug or corticosteroid use within 24 hours before testing |
| 11. History of head injury or vestibular disorder within the last 6 months |
| 12. Pregnancy |
Adapted from Boling et al.12
Protocol
When a potential patient contacted 1 of the 4 investigators, a telephone interview was conducted, and the investigator determined eligibility based on the inclusion and exclusion criteria (Table 1). If the patient passed the telephone screening, he or she was invited to the appropriate clinical laboratory for physical inspection. During that visit, once the investigator determined that the patient met all of the inclusion criteria and none of the exclusion criteria, the patient read, agreed to, and signed the informed consent, which was approved by the institutional review boards at each of the 4 centers. The institutional review boards also approved the study.
After the patient provided informed consent, we obtained baseline demographic, outcomes, and clinical measures. Next, the patients with PFP were randomly assigned to receive 1 of 2 treatment protocols (HIP or KNEE). The randomization sequence was developed and kept at the University of Calgary (Alberta, Canada) by the research coordinator, using a random-number generator, and the same sequence of randomization was used at each site. Only the athletic trainer (AT) at each site communicated with the research coordinator to ensure that the investigators, who were responsible for outcomes measurement and data analysis, remained blinded to group allocation. No placebo treatments were used, and there was no control group. However, the KNEE protocol served as the “gold standard” rehabilitation program because it was deemed to be the most widely used and considered the standard-of-care protocol for PFP. A summary of the participant's demographic information across each of the 4 centers and between treatment groups appears in Table 2.
Table 2.
Baseline Characteristics of Study Participants Across 4 Research Centers and Between 2 Treatment Groups
| Characteristic |
Mean ± SD |
|||||||
| HIP |
KNEE |
|||||||
| Research Center | ||||||||
| Calgary, AB |
Chicago, IL |
Milwaukee, WI |
Augusta, GA |
Calgary, AB |
Chicago, IL |
Milwaukee, WI |
Augusta, GA |
|
| Sex, men/women | 10/19 | 6/23 | 10/17 | 8/18 | 8/15 | 5/16 | 12/11 | 7/14 |
| Age, y | 31.28 ± 8.74 | 28.62 ± 4.88 | 30.11 ± 8.13 | 27.50 ± 5.44 | 30.83 ± 8.46 | 27.10 ± 6.53 | 31.39 ± 7.94 | 25.86 ± 442 |
| Height, cm | 169.56 ± 8.59 | 168.47 ± 8.30 | 173.26 ± 9.90 | 170.14 ± 10.63 | 170.32 ± 9.67 | 169.52 ± 8.74 | 172.74 ± 8.41 | 174.78 ± 10.19 |
| Weight, kg | 65.18 ± 9.95 | 64.01 ± 11.63 | 72.24 ± 17.52 | 69.54 ± 12.88 | 67.67 ± 10.49 | 67.50 ± 10.49 | 76.58 ± 16.07 | 76.48 ± 16.40 |
| Activity, h/wk | 3.89 ± 2.64 | 2.64 ± 1.55 | 5.07 ± 4.67 | 2.88 ± 1.37 | 4.80 ± 3.93 | 3.66 ± 2.32 | 4.87 ± 2.90 | 3.45 ± 2.58 |
| Patellofemoral pain duration, mo | 4.71 ± 27.18 | 29.02 ± 35.91 | 47.65 ± 61.02 | 20.04 ± 22.21 | 12.17 ± 15.42 | 30.19 ± 38.88 | 48.63 ± 49.64 | 31.48 ± 35.44 |
| Anterior Knee Pain Scale score, of 100 points | 74.48 ± 8.76 | 75.52 ± 8.29 | 75.04 ± 7.21 | 74.58 ± 13.06 | 76.09 ± 10.76 | 75.10 ± 10.34 | 76.13 ± 7.23 | 72.24 ± 9.60 |
| Visual analog scale (10 cm) score | 5.15 ± 1.98 | 5.10 ± 1.39 | 5.37 ± 1.48 | 4.64 ± 1.74 | 4.48 ± 1.97 | 5.58 ± 1.52 | 5.40 ± 1.35 | 4.37 ± 1.44 |
Abbreviations: HIP, hip- and core-strengthening protocol; KNEE, knee-focused protocol.
Variables of Interest
The primary variables of interest were self-reported worst pain in the previous week as measured using the visual analog scale (VAS; maximum score = 10 cm) and self-reported physical function as measured using the Anterior Knee Pain Scale (AKPS; maximum score = 100). The VAS is a self-reporting tool used to assess the level of pain patients with PFP experience.25 Patients are asked to draw a mark along a 10-cm line that indicates the amount of pain they are experiencing relative to a score of 0, indicating no pain, and a score of 10, indicating the most pain (worst). On the VAS, we chose to measure “worst” pain because Crossley et al25 suggested that it was more reliable than measuring “usual” pain. The AKPS is also a self-reporting tool used to assess the functional activity level of patients with PFP.25 It is a self-administered questionnaire with 13 weighted questions regarding knee function. A score of 100 indicates no disability, and an increase in the score indicates improved function. Both the VAS measuring usual pain and the AKPS have been reported to be valid and reliable outcome measures.25 These outcomes data were collected at baseline, during each week of rehabilitation, and after the 6-week protocol.
The secondary variables of interest were muscle-strength measures, and the methods for each measure have been described elsewhere.12–18,22 Specifically, for strength variables, we measured the hip-abductor (HABD),14–17,22,26 hip-extensor,26 hip–external- and internal-rotator,15,17,26 and knee-extensor27 output of the maximum voluntary isometric contraction force against a force dynamometer (percentage of body weight), along with the front-plank and side-bridge exercises and horizontal-extension test to assess anterior, lateral (affected side), and posterior core endurance (in seconds),15,28 respectively. These secondary variables were assessed at baseline and after the 6-week protocol.
Rehabilitation Protocol
For rehabilitation progression, each patient with PFP visited the AT up to 3 times/wk during the 6-week period. The AT was responsible for administering the rehabilitation protocol at each center, and he or she demonstrated all the exercises, explained the home-exercise booklet provided to each patient, and made clinic-based decisions about the weekly progression of exercises. Progression of exercises, increases or decreases in sets and repetitions or duration of exercises, and changes in TheraBand (Hygenic Corp, Akron, OH) resistance were at the discretion of the AT, based on patient feedback, PFP, swelling, and symptoms during rehabilitation progression. The AT asked all patients with PFP to perform their prescribed exercises a minimum of 6 d/wk (including the visits with the AT) for 6 weeks. Compliance was monitored and recorded within the home-exercise rehabilitation booklet.
Patients with PFP in the HIP treatment group initially performed non–weight-bearing, muscle-strengthening exercises that focused on activating the hip musculature (Table 3). Those exercises progressed to weight-bearing exercises, including core-strengthening and balance exercises that were designed to target the core musculature, with specific emphasis placed on stabilizing the core musculature before initiating any of the movements. The AT chose the TheraBand resistance based on the patient's ability to complete 10 repetitions of the exercise; the final 3 repetitions were challenging in intensity but the patient was required to maintain good form. All exercises were performed bilaterally. Although some exercises involved the quadriceps musculature, most of the exercises involved hip- and core-muscle strengthening, and many of these exercises have been described in the literature for PFP rehabilitation.10,24,29–33
Table 3.
Six-Week Hip- and Core-Strengthening Protocol
| Week |
Exercise |
Sets, No. |
Repetitions or Seconds, s |
| 1 | Hip abduction—standing | 3 | 10 |
| Hip external rotator—standing | 3 | 10 | |
| Hip external rotator—seated | 3 | 10 | |
| 2 | Hip abduction—standing | 3 | 10 |
| Hip internal rotator—standing | 3 | 10 | |
| Hip external rotator—standing | 3 | 10 | |
| 3 | Hip abduction—standing | 3 | 10 (w/ stronger band) |
| Hip internal rotator—standing | 3 | 10 (w/ stronger band) | |
| Hip external rotator—standing | 3 | 10 (w/ stronger band) | |
| Balancing 2 feet–Airexa pad | 3 | 30–45 s | |
| 4–6 | Hip extension at 45°—standing | 3 | 10–15 |
| Hip internal rotator—standing | 3 | 10–15 | |
| Hip external rotator—standing | 3 | 10–15 | |
| Balancing 1 foot—Airexa pad | 3 | 45–60 s |
Abbreviation: w/, with.
Airex AG, Sins, Switzerland.
Patients in the KNEE treatment group initially performed non–weight-bearing quadriceps strengthening and then progressed to weight-bearing quadriceps-strengthening exercises (Table 4). No emphasis was placed on stabilizing the core musculature before initiating any of the movements. Similar to the HIP protocol, the TheraBand resistance allowed the patient to complete 10 repetitions, with the final 3 repetitions being challenging in intensity but with the patient still able to maintain good form. All exercises were performed bilaterally. Although some exercises involved the hip musculature, most of the exercises involved quadriceps-muscle strengthening and, as with the hip exercises, those exercises have been used previously.12,14,15,20,34
Table 4.
Six-Week Knee-Focused Protocol
| Week |
Exercise |
Sets, No. |
Repetitions or Seconds, s |
| 1 | Isometric quadriceps setting | 3 | 10 |
| Knee extensions—standing | 3 | 10 | |
| Double-legged, one-quarter squats | 3 | 10 | |
| 2 | Isometric quadriceps setting | 3 | 15 |
| Double-legged, one-half squats | 3 | 15 | |
| Terminal knee extension w/ TheraBanda | 3 | 15 | |
| Double-legged, one-quarter squats | 3 | 30 s | |
| 3 | Double-legged, one-half squats | 3 | 10 |
| Single-legged, one-quarter squats | 3 | 10 | |
| Double-legged, one-quarter wall squats | 3 | 10 | |
| Terminal-knee extension w/ TheraBand | 3 | 10 (w/ stronger band) | |
| 4 | Single-legged, one-half squats | 3 | 10 |
| Forward, one-quarter lunges | 3 | 10 | |
| Lateral step-down (4-in [3.6-cm] step), No. | 3 | 10 | |
| Forward step-down (4-in [3.6-cm] step), No. | 3 | 10 | |
| Double-legged, one-half wall squats, s | 3 | 30 | |
| 5–6 | Double-legged wall squats (to max 90° knee flexion), s | 3 | 45–60 s |
| Lateral step-downs (6–10 in [5.6–9.6-cm] step) | 3 | 15 | |
| Forward step-downs (6–10 in [5.6–9.6-cm] step) | 3 | 15 | |
| Forward one-half full lunges (to maximum 90° of knee flexion) | 3 | 15 | |
| Single-legged one-half full squats (to maximum 90° of knee flexion) | 3 | 15 |
Abbreviation: w/, with.
TheraBand, Hygenic Corp, Akron, OH.
Our a priori definition of treatment success was a minimum 2-cm decrease in VAS and a minimum 8-point increase in AKPS score by the end of the 6-week protocol compared with baseline measures.25 However, we recognized that, for some patients, the VAS score might decrease by >2 cm, whereas the AKPS score might not increase by ≥8 points. Thus, patients who were in that category were classified as “successful” using 2 methods: (1) those whose VAS score decreased >2 cm and whose AKPS score increased by ≥8 points or (2) those whose VAS score decreased >2 cm or whose AKPS score increased by ≥8 points. This method of determining treatment success was based on recommendations from Crossley et al.25
After the 6-week protocol, patients who did not meet the a priori definition of self-reported improvement in pain or function were asked to continue with the rehabilitation protocol for an additional 2 weeks and were advised to see a physician to investigate potential confounding disorders (eg, degenerative joint disease, plica, focal chondral injury) that would help to explain their lack of treatment success. If treatment success had not been achieved after the additional 2 weeks of rehabilitation, further therapeutic treatment (eg, ultrasound, interferential modalities) was recommended. Those patients were no longer enrolled in this investigation, but their data were still included in the final analysis.
Sample Size
We conducted an a priori power analysis, and the sample-size calculations were based on a clinically meaningful improvement of 2 cm on a 10-cm VAS or an 8-point increase in the AKPS score or both.25 Assuming a standard deviation of 2 cm, power of 0.80, and an α level of .05, we required 25 participants per group at each site (200 total patients).
Statistical Analysis
Statistical analysis was done on a blinded, intention-to-treat basis using SPSS software (version 19; formerly SPSS Inc, now IBM Corporation, Armonk, NY). Missing data were replaced using a conservative method with the last score carried forward. The VAS and AKPS outcomes data were collected each week, but the primary endpoint was 6 weeks (POST) after baseline (PRE) testing. Descriptive statistics included mean, standard deviation, and 95% confidence interval values, and we calculated 2 × 2 analyses of variance (ANOVAs; group × time; P ≤ .05) for each primary and secondary variable of interest. A secondary analysis was performed using a repeated-measures ANOVA (group × time; P ≤ .05) to determine time to success based on the weekly VAS and AKPS scores. Significant differences revealed by the ANOVA were further examined using Bonferroni post hoc analysis. Finally, we performed an intracluster correlation analysis to determine sample homogeneity across the 4 testing centers for baseline measures of VAS and AKPS using design-effect and effective–sample-size calculations as well as comparing patient demographics using a 1-way ANOVA (P ≤ .05).
RESULTS
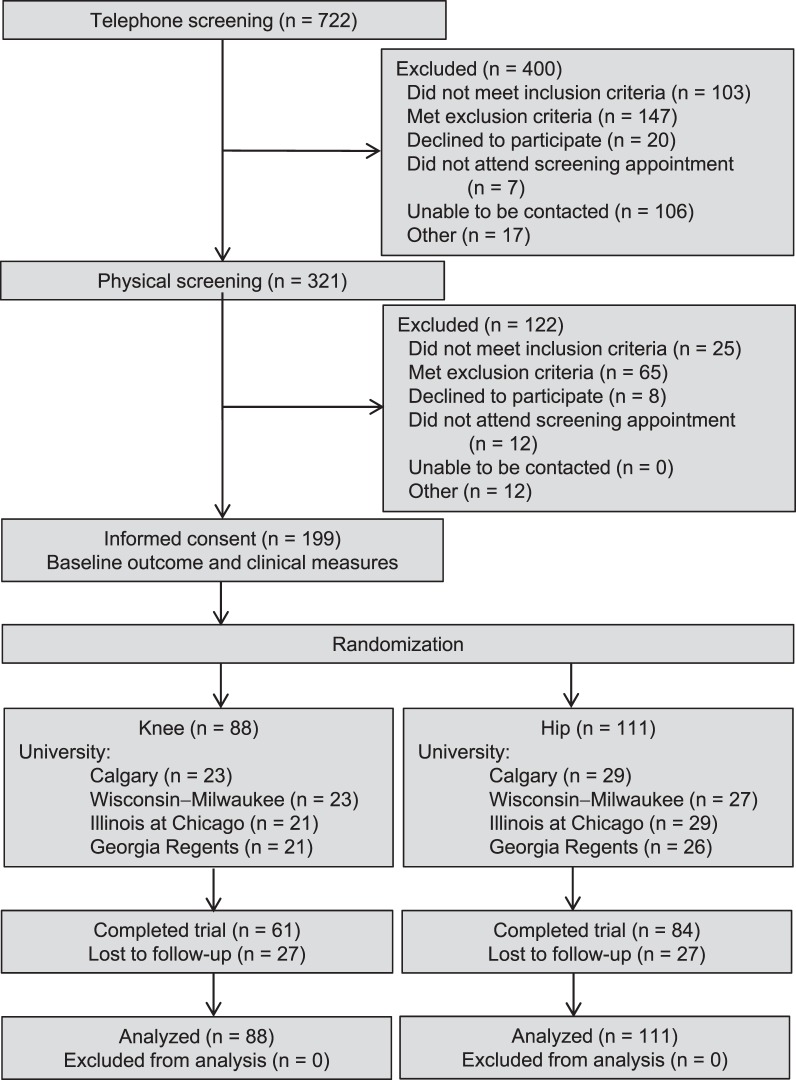
Volunteers were recruited from May 2009 until November 2012. Of 721 patients with PFP screened, 199 (27.6%) met the inclusion criteria and agreed to participate (Figure 1). Overall, 66 men (33.2%) and 133 women (66.8%) were enrolled (age = 29.0 ± 7.1 years, height = 170.4 ± 9.4 cm, and mass = 67.6 ± 13.5 kg). At the postrehabilitation follow-up, we collected outcomes and clinical data from 146 of the 199 patients (73.4%) with PFP.
Figure 1.
Flow of participants through the study protocol.
Compliance
Based on participant documentation in the exercise log, patients involved in the HIP protocol were 80.3% compliant (mean ± SD = 4.82 ± 1.90 d/wk), and patients involved in the KNEE protocol were 81.7% compliant (mean ± SD = 4.90 ± 1.82 d/wk) with the rehabilitation program.
Outcomes
Of the 199 patients with PFP, 157 patients (78.9%) reported treatment success and resolution of symptoms based on our a priori definition, and 42 (21.1%) were unsuccessful. Specifically, 89 of the 111 patients (80.2%) involved in the HIP protocol were successful, and 68 of the 88 patients (77%) involved in the KNEE protocol were successful. No significant group-by-time interactions were present (F1,199 = 0.20, P = .66), and VAS scores decreased from PRE to POST testing for patients involved in both the HIP and KNEE protocols (F1,199 = 274.57, P < .001; Table 5; Figure 2). For AKPS, no significant group-by-time interactions were present (F1,199 = 0.02, P = .90), and scores increased from PRE to POST testing for patients involved in both the HIP and KNEE protocols (F1,199 = 230.03, P < .001; Table 5; Figure 3).
Table 5.
Baseline and 6-Week Visual Analog Scale and Anterior Knee Pain Scale Scores by Rehabilitation Protocol
| Variable |
Protocol |
Score, Mean ± SD (95% Confidence Interval) |
Difference |
|
| Baseline |
6 Wk |
|||
| Visual analog scale, cm | ||||
| Hip- and core-strengthening | 5.12 ± 1.66 (4.76, 5.38) | 1.96 ± 1.92a (1.60, 2.32) | 3.11 ± 2.22 (2.69, 3.52) | |
| Knee-focused | 4.96 ± 1.66 (4.61, 5.30) | 1.99 ± 2.05a (1.60, 2.45) | 2.98 ± 2.08 (2.50, 3.36) | |
| Anterior Knee Pain Scale, points | ||||
| Hip- and core-strengthening | 75.00 ± 9.74 (72.72, 77.28) | 87.95 ± 11.26a (84.95, 90.22) | 12.58 ± 11.93 (9.79, 15.38) | |
| Knee-focused | 75.62 ± 9.81 (73.34, 77.91) | 87.67 ± 10.53a (85.22, 90.12) | 12.90 ± 13.55 (9.75, 16.05) | |
Denotes P ≤ .05 compared with baseline measures.
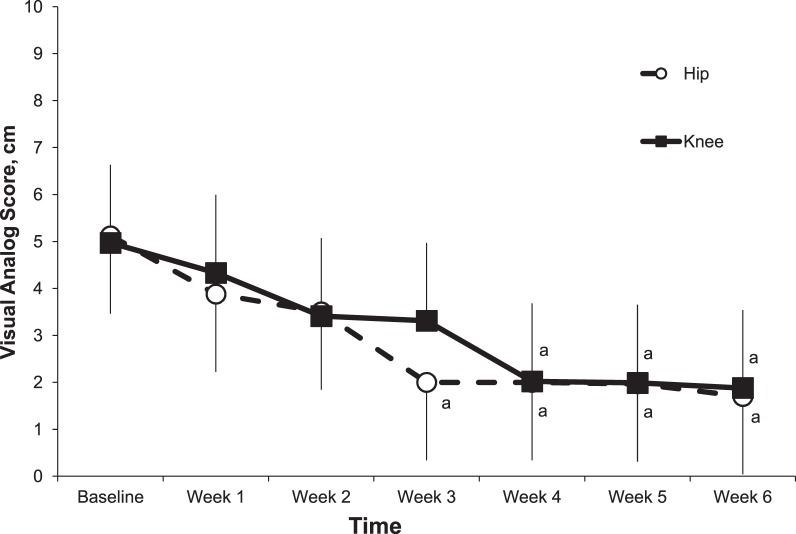
Figure 2.
Mean visual analog scores measures for patients with patellofemoral pain each week during the 6-week hip- and core- and knee-focused rehabilitation protocols. a Different than baseline score.
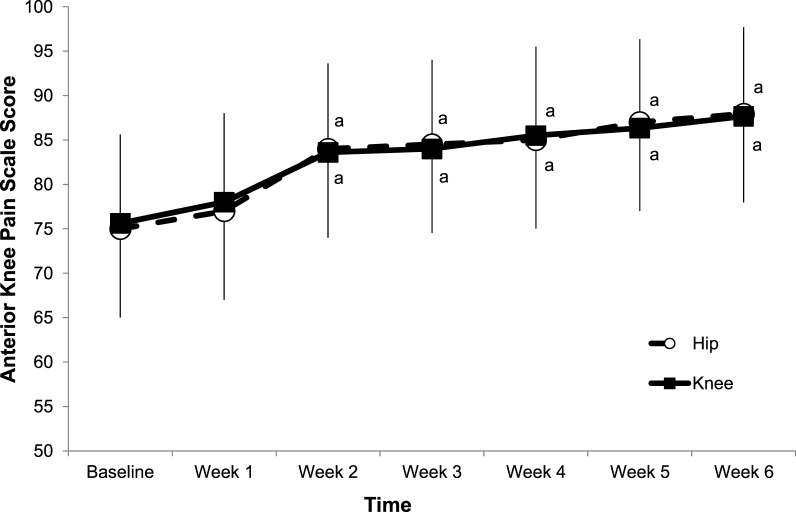
Figure 3.
Mean Anterior Knee Pain Scale measures for patients with patellofemoral pain each week during the 6-week hip- and core- and knee-focused rehabilitation protocols. a Different than baseline score.
Time to Success
A significant effect was present for test-by-group interactions for the VAS scores (F6,6 = 12.69, P = .01). Post hoc analysis showed that the patients involved in the HIP protocol had a significant reduction in self-reported pain, based on our a priori definition of success,25 starting at week 3 of the rehabilitation program, and those involved in the KNEE protocol had a significant reduction starting in week 4 (Figure 2). No significant test-by-group interaction effects were present for self-reported function measured by the AKPS (F6,6 = 1.23, P = .21). Post hoc analysis revealed a significant increase in AKPS scores for patients involved in both the HIP and KNEE protocols, from baseline, starting at week 2 (Figure 3).
Strength
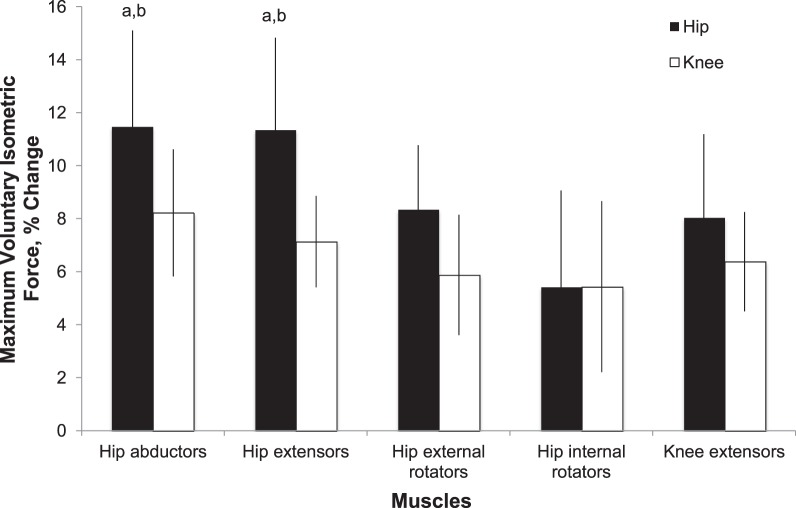
For patients involved in either the HIP or KNEE rehabilitation protocols, HABD (F1,199 = 23.19, P < .001), hip–external-rotator (F1,199 = 15.27, P < .001), hip–internal-rotator (F1,199 = 8.42, P < .001), hip-extensor (F1,199 = 20.04, P < .001), and knee-extensor (F1,199 = 14.39, P < .001) strength significantly increased after the 6-week intervention (Figure 4; Table 6). When we compared the percentage change in strength, we found a group-by-time interaction: patients who performed the HIP program exhibited overall greater, but nonsignificant, increases in muscular strength for all muscle groups tested than did those who performed the KNEE program (Figure 4; Table 6). However, post hoc analysis revealed that those patients involved in the HIP protocol exhibited greater changes in HABD (P = .01) and hip-extensor (P = .01) strength than did those involved in the KNEE protocol (Figure 4).
Figure 4.
Percentage of change in output of maximum voluntary isometric force from baseline for hip abductors, extensors, external rotators, internal rotators, and knee extensors. a Different than baseline score (P ≤ .05) . b Different than patients with patellofemoral pain who followed the knee-focused protocol (P ≤ .05).
Table 6.
Baseline and 6-Week Hip- and Knee-Muscle Maximum Voluntary Isometric Contraction by Rehabilitation Protocol
| Muscles |
Protocol |
Maximum Voluntary Isometric Contraction, Nm/kg (Mean ± SD [95% Confidence Interval]) |
Difference, % Change |
|
| Baseline |
6 Wk |
|||
| Hip abductors | ||||
| HIP | 3.21 ± 1.14 (3.00, 3.43) | 3.58 ± 1.08 (3.38, 3.78) | 11.46a,b | |
| KNEE | 3.15 ± 1.19 (2.90, 3.40) | 3.41 ± 1.28 (3.14, 3.68) | 8.21a | |
| Hip extensors | ||||
| HIP | 2.39 ± 1.01 (2.20, 2.58) | 2.66 ± 1.15 (2.45, 2.88) | 11.34a,b | |
| KNEE | 2.44 ± 1.09 (2.21, 2.67) | 2.61 ± 1.18 (2.37, 2.86) | 7.13a | |
| Hip external rotators | ||||
| HIP | 1.19 ± 0.42 (1.11, 1.27) | 1.29 ± 0.41 (1.21, 1.37) | 8.33a | |
| KNEE | 1.18 ± 0.45 (1.09, 1.28) | 1.25 ± 0.44 (1.16, 1.35) | 5.87a | |
| Hip internal rotators | ||||
| HIP | 1.48 ± 0.55 (1.38, 1.58) | 1.56 ± 0.59 (1.45, 1.67) | 5.42a | |
| KNEE | 1.42 ± 0.64 (1.29, 1.55) | 1.49 ± 0.62 (1.36, 1.63) | 5.43a | |
| Knee extensors | ||||
| HIP | 3.88 ± 1.59 (3.58, 4.17) | 4.19 ± 1.50 (3.91, 4.47) | 8.04a | |
| KNEE | 3.93 ± 1.47 (3.62, 4.23) | 4.18 ± 1.60 (3.84, 4.51) | 6.37a | |
Abbreviations: HIP, hip- and core-strengthening protocol; KNEE, knee-focused protocol.
a Greater than baseline (P < .05).
Greater than results for KNEE group (P < .05).
Core Endurance
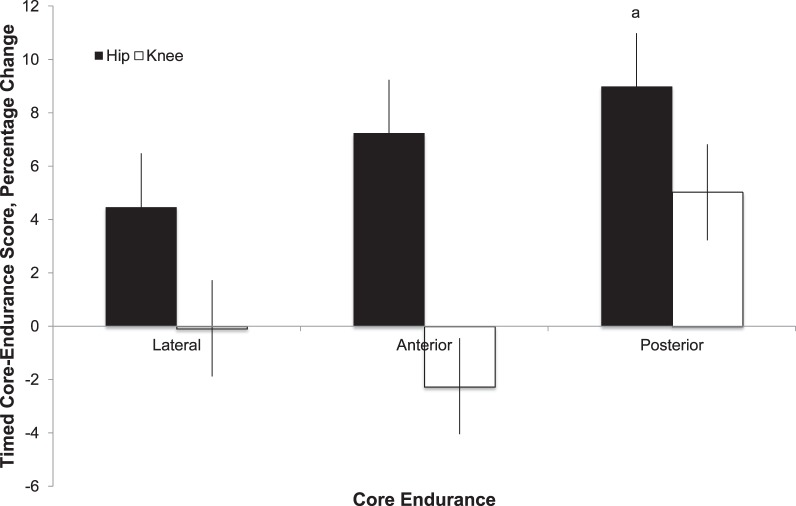
From PRE to POST, anterior (F1,199 = 0.87, P = .35) and lateral (F1,199 = 0.66, P = .42) endurance scores did not increase (Figure 5; Table 7), whereas POST core endurance did increase (F1,199 = 3.90, P = .05) for patients involved in both the HIP and KNEE rehabilitation protocols. Descriptively, patients who performed the HIP program exhibited greater, but nonsignificant, changes in endurance for all muscle groups tested than did those in the KNEE group (Figure 5; Table 7).
Figure 5.
Percentage of change in timed core-endurance scores from baseline for side-bridge and front-plank exercises and horizontal-extension test to assess lateral, anterior, and posterior core endurance, respectively. a Different than baseline score (P ≤ .05).
Table 7.
Baseline and 6-Week Core-Endurance Values by Hip- and Core-Strengthening (HIP) and Knee-Focused (KNEE) Rehabilitation Protocol
| Core Muscles |
Protocol |
Core Endurance, s (Mean ± SD [95% Confidence Interval]) |
Difference, % Change |
|
| Baseline |
6-Wk |
|||
| Anterior | ||||
| HIP | 83.76 ± 45.13 (75.37, 92.16) | 89.82 ± 47.98 (80.89, 98.74) | 7.23 | |
| KNEE | 93.56 ± 55.24 (83.28, 103.83) | 91.45 ± 55.76 (81.08, 101.82) | −2.25 | |
| Posterior | ||||
| HIP | 114.04 ± 60.15 (102.85, 125.23) | 124.29 ± 63.41 (112.49, 136.08) | 8.98a | |
| KNEE | 96.97 ± 48.77 (87.90, 106.05) | 101.84 ± 52.37 (92.10, 111.59) | 5.02 | |
| Lateral | ||||
| HIP | 53.03 ± 30.46 (47.37, 58.70) | 55.41 ± 28.40 (50.12, 60.69) | 4.48 | |
| KNEE | 54.18 ± 35.24 (47.62, 60.73) | 54.13 ± 32.07 (48.17, 60.10) | −0.08 | |
Greater than baseline (P < .05)
Between-Centers Homogeneity
The intracluster correlation analysis revealed no differences in baseline measures of VAS or AKPS (ρ = .004, design effect = 1.24, effective sample size = 194). Moreover, with the exception of sex, all groups were well matched at baseline for demographics (age: P = .38; height: P = .86; mass: P = .10), bilateral symptoms (P = .12), and duration of symptoms (P = .51; Table 2).
DISCUSSION
The primary purpose of this single-blind, multicenter RCT study was to compare self-reported pain and function outcome measures for patients with PFP who were randomized into either a hip and core- or quadriceps-focused rehabilitation protocol. We hypothesized greater improvements in pain and function for patients involved in the HIP protocol than for those involved in the KNEE protocol. However, in contrast to the hypothesis, patients with PFP involved in both rehabilitation protocols reported similar improvements in pain and function. Those results were in contrast to recent RCT studies18,22 that showed the addition of hip strengthening to a knee-focused rehabilitation program resulted in better outcomes than the knee program alone. However, those authors investigated combined hip and knee rehabilitation programs, so comparisons with our study are difficult.
In partial support of the hypothesis, patients with PFP involved in the HIP protocol reported an earlier time to success, based on self-reported VAS scores. Specifically, patients in the HIP protocol reported a clinically meaningful decrease in VAS score at the 3-week mark of the rehabilitation protocol, whereas those involved in the KNEE protocol reported that decrease during the fourth week (Figure 4). These time-to-success results were similar to those found by Boling et al,12 who noted a reduction in self-reported pain starting at week 4 of a 6-week hip-based rehabilitation program. Our results were also similar to those involving females with PFP who performed 4 weeks of either hip- or quadriceps-muscle strengthening before a 4-week functional exercise protocol.35 Those authors reported reduced pain scores for patients with PFP in a hip-focused rehabilitation program after the first 4 weeks. However, participants in both groups reported similar improvements in function after 8 weeks. Thus, the results of the present study suggest that a HIP-based rehabilitation protocol resulted in earlier time to PFP reduction than did a KNEE-based protocol, but after 6 weeks, similar amounts of pain and functional improvement can be expected from both rehabilitation protocols.
The secondary purpose of our study was to compare changes in muscle strength and core endurance between rehabilitation protocols, and we hypothesized greater improvements in those measures for patients with PFP involved in the HIP protocol as compared with those involved in the KNEE protocol. All participants, regardless of group assignment, increased strength. However, those involved in the HIP program had greater increases in HABD and hip-extensor force output than did those involved in the KNEE program, a finding in partial support of the hypothesis. Patients with PFP in the HIP protocol also exhibited greater improvements in POST core-endurance measures during the 6-week rehabilitation protocol, as compared with those in the KNEE protocol. These results were in partial agreement with Dolak et al,35 who reported that 4 weeks of hip-muscle strengthening resulted in a 21% increase in HABD force output, whereas strength remained unchanged in the quadriceps group. Therefore, based on our results, a hip-focused rehabilitation program resulted in greater overall gains in lower extremity muscle-strength and core-endurance scores, along with an earlier time to clinically relevant reductions in pain, when compared with a knee-focused protocol.
Differences in results for the present study versus previous RCT investigations may be explained by the rehabilitation protocols. We used a combination of standing, open and closed chain, hip-muscle strengthening, and balance exercises for the HIP-rehabilitation protocol, and the KNEE protocol incorporated several open and closed chain exercises. Therefore, both protocols could be considered comprehensive approaches to therapeutic exercise and were designed to target, not isolate, those muscle groups. In contrast, Fukuda et al18 added 4 open chain strengthening exercises for the hip abductors, lateral rotators, and extensors to a traditional knee-stretching and -strengthening program consisting of seated-knee extensions, squats, leg presses, and calf raises. In a similar manner, Ismail et al22 used miniwall squats, forward and lateral step-ups, and terminal knee extensions with TheraBand, and added side-lying hip abduction and seated hip external-rotation exercises using ankle weights. Thus, those researchers used relatively simple muscle-strengthening protocols compared with those used in the present study. Moreover, both the HIP and KNEE protocols in the current study increased the exercise progression, the number of sets, the repetitions, and the intensity (the color of the TheraBand) each week based on changes in pain and swelling, patient feedback, and rehabilitation progression and as decided by the AT. In contrast, progression for the aforementioned RCT studies18,22 was based on the ability to perform 10 repetitions and was implemented on a weekly basis; progression was not based on changes in PFP symptoms. Moreover, those studies did not involve ATs or other health care professionals. Similar to the present study, Dolak et al35 involved a physical therapist, used similar week-to-week decisions regarding treatment progression, and based rehabilitation progression for their patients with PFP on the goal of performing exercises against a resistance equal to 7% of their body weight. However, and in contrast to our study, the KNEE protocol used by Dolak et al35 involved all open chain exercises, and the HIP protocol involved combinations of side-lying and standing, open chain hip abduction and external rotation as well as combinations of those 2 movements for weeks 3 and 4. Thus, other hip- and quadriceps-focused protocols were dissimilar to the protocol used in the current study, so comparisons with previous investigations are difficult.
Patients reported similar outcomes in pain and function regardless of whether they were involved in the HIP or KNEE rehabilitation protocol, which may be explained by the similarities between the protocols. Although we designed the protocols to target specific muscle groups, we acknowledge that some exercises involved similar muscle groups. Most noticeably, the squat, lunge, step-down, and wall-slide exercises in the KNEE protocol all involve the gluteal musculature, a key component of the HIP protocol. Similarly, the standing hip-flexor exercises within the HIP protocol most likely involved the quadriceps musculature. Thus, it was possible that the exercises were not specific enough to completely isolate the hip and knee musculature. However, considering that many muscles crossing the hip and knee joints are biarticular, complete isolation does not seem possible or functionally relevant. Moreover, the HIP and KNEE protocols were dissimilar because most of the exercises in the KNEE protocol involved both limbs performing the same exercise and could be considered symmetric. In contrast, the HIP protocol consisted of exercises that were generally asymmetric with 1 limb supporting or balancing the patient, while the opposite limb performed the exercise. Previous authors36–39 have shown an asymmetric level of electromyographic activation for the stance (involved) limb during many of the HIP exercises. Therefore, the earlier time to success and greater gains in muscle strength observed in the HIP group may be the result of the asymmetric component of the rehabilitation protocol. Considering the overall successful outcomes despite the notable differences in rehabilitation protocols between the previous and current RCT studies,18,22,35 future investigators should focus on understanding why seemingly dissimilar protocols resulted in similar outcomes for patients with PFP.
Another possibility was that the exercises performed in both the HIP and KNEE protocols caused enough muscle activation to produce strength gains, even if the exercises did not target 1 particular muscle. Clinically, both the hip and core- and quadriceps-focused strengthening protocols seem to have effectively produced strength gains and improved PFP and function. However, the HIP protocol resulted in greater overall improvements in isometric–force-output and core-endurance measures than did the KNEE protocol. Future research on other exercise-therapy protocols, which better isolate the individual muscle groups, and on clinical-decision rules will help guide clinicians to a protocol that may be optimal for their patients with PFP.
To our knowledge, this investigation was the first multicenter RCT for PFP. Therefore, we measured the intracluster correlation coefficient to determine between-centers and within-center variance for our primary variables of interest. The small ρ value suggested that the within-cluster variance was greater than the between-clusters variance, and our values were within the typical range for human clinical RCT studies.40 Moreover, clinical investigators can use these data to determine sample sizes for future RCT studies using the reported design-effect and effective–sample-size values. Our results revealed that a sample size of 185 to 194 across 4 centers (23–24 participants/group per site) was necessary to achieve 80% power to detect differences in VAS and AKPS for patients with PFP. Thus, based on the effective sample size, our study was appropriately powered for 4 centers and 2 intervention groups.
Limitations
Limitations in our study are acknowledged. First, we did not include a control group for the course of treatment, so we cannot conclude that hip- or knee-based muscle strengthening was better than no treatment for the 6 weeks. Regardless, a case can be made for intervening with either a HIP or KNEE rehabilitation protocol considering that our treatment success rate was 78.8%, a rate consistent with previous RCT studies.18,19,22,35 Second, the use of other, self-reported functional and pain measures, such as the Functional Index Questionnaire, Quality of Life Scale, or Global Improvement Scale, may have provided further insight into differences between the rehabilitation protocols. However, we chose to use the VAS and AKPS scales based on recommendations from Crossely et al25 and to provide an a priori definition of treatment success. Finally, the present study lacked follow-up, unlike Collins et al19 in their RCT investigation of foot orthoses and physiotherapy for the treatment of PFP. Those authors had a primary endpoint of 6 weeks after treatment and then followed up with patients at 12 and 52 weeks to gather outcomes data for global improvement, severity of usual and worst VAS scores, the Functional Index Questionnaire, and the AKPS. However, we are currently conducting a similar follow-up investigation at 6, 12, and 24 months postrehabilitation to better understand PFP and whether the HIP or KNEE protocol can reduce the relatively high recurrence rate reported for PFP.41,42
This is the first RCT study, to our knowledge, directly comparing HIP and KNEE protocols for patients with PFP. We provide level I evidence regarding 2 kinds of rehabilitative therapy for one of the most common musculoskeletal injuries in running- and jumping-based sports, regardless of age or sex. We conclude that both 6-week HIP and KNEE rehabilitation protocols resulted in significant improvements in pain, function, and muscle strength in patients with PFP. Although both hip- and quadriceps-focused strengthening were equally effective for the short-term treatment of PFP, a hip and core-focused rehabilitation protocol provided earlier resolution of PFP and greater overall gains in muscle strength and core endurance than did a knee-focused protocol.
ACKNOWLEDGMENTS
This study was funded by the National Athletic Trainers' Association Research and Education Foundation through the Outcomes Grant program (808OUT003R) and Alberta Innovates: Health Solutions through a Population Health New Investigator Award to Dr Ferber. Additional funding was provided by the Wisconsin Athletic Trainers' Association and the University of Wisconsin, Milwaukee, College of Health Sciences. We thank Jill Baxter for serving as the research coordinator for this multicenter study.
REFERENCES
- 1.Evidence based management of acute musculoskeletal pain. Australian Acute Musculoskeletal Pain Guidelines Group Web site. 2014 https://www.nhmrc.gov.au/guidelines/publications/cp94-cp95. Accessed September 11, [Google Scholar]
- 2.van Middelkoop M, van Linschoten R, Berger MY, Koes BW, Bierma-Zeinstra SM. Knee complaints seen in general practice: active sport participants versus non-sport participants. BMC Musculoskelet Disord. 2008;9:36. doi: 10.1186/1471-2474-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clement DB, Tauton JE, Smart GW, McNicol KL. A survey of overuse running injuries. Phys Sportsmed. 1981;9(1):47–58. doi: 10.1080/00913847.1981.11711077. [DOI] [PubMed] [Google Scholar]
- 4.Taunton JE, Ryan MB, Clement DB, McKenzie DC, Lloyd-Smith DR, Zumbo BD. A retrospective case-control analysis of 2002 running injuries. Br J Sports Med. 2002;36(2):95–101. doi: 10.1136/bjsm.36.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bovens AM, Janssen GM, Vermeer HG. Hoeberigs JH, Janssen MP, Verstappen FT. Occurrence of running injuries in adults following a supervised training program. Int J Sports Med. 1989;10((suppl 3)):S186–S190. doi: 10.1055/s-2007-1024970. [DOI] [PubMed] [Google Scholar]
- 6.Marti B, Vader JP, Minder CE, Abelin T. On the epidemiology of running injuries: the 1984 Bern Grand-Prix study. Am J Sports Med. 1988;16(3):285–294. doi: 10.1177/036354658801600316. [DOI] [PubMed] [Google Scholar]
- 7.Jordaan G, Schwellnus MP. The incidence of overuse injuries in military recruits during basic military training. Mil Med. 1994;159(6):421–426. [PubMed] [Google Scholar]
- 8.Taanila H, Suni J, Pihlajamäki H, et al. Musculoskeletal disorders in physically active conscripts: a one-year follow-up study in the Finnish Defense Forces. BMC Musculoskelet Disord. 2009;10:89. doi: 10.1186/1471-2474-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chester R, Smith TO, Sweeting D, Dixon J, Wood S, Song F. The relative timing of VMO and VL in the aetiology of anterior knee pain: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2008;9:64. doi: 10.1186/1471-2474-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowan SM, Bennell KL, Crossley KM, Hodges PW, McConnell J. Physical therapy alters recruitment of the vasti in patellofemoral pain syndrome. Med Sci Sports Exerc. 2002;34(12):1879–1885. doi: 10.1097/00005768-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Powers CM. The influence of abnormal hip mechanics on knee injury: a biomechanical perspective. J Orthop Sports Phys Ther. 2010;40(2):42–51. doi: 10.2519/jospt.2010.3337. [DOI] [PubMed] [Google Scholar]
- 12.Boling MC, Bolgla LA, Mattacola CG, Uhl TL, Hosey RG. Outcomes of a weight-bearing rehabilitation program for patients diagnosed with patellofemoral pain syndrome. Arch Phys Med Rehabil. 2006;87(11):1428–1435. doi: 10.1016/j.apmr.2006.07.264. [DOI] [PubMed] [Google Scholar]
- 13.Magalhães E, Fukuda TY, Sacramento SN, Forgas A, Cohen M, Abdalla RJ. A comparison of hip strength between sedentary females with and without patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2010;40(10):641–647. doi: 10.2519/jospt.2010.3120. [DOI] [PubMed] [Google Scholar]
- 14.Ferber R, Kendall KD, Farr L. Changes in knee biomechanics after a hip-abductor strengthening protocol for runners with patellofemoral pain syndrome. J Athl Train. 2011;46(2):142–149. doi: 10.4085/1062-6050-46.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Earl JE, Hoch AZ. A proximal strengthening program improves pain, function, and biomechanics in women with patellofemoral pain syndrome. Am J Sports Med. 2011;39(1):154–163. doi: 10.1177/0363546510379967. [DOI] [PubMed] [Google Scholar]
- 16.Ireland ML, Willson JD, Ballantyne BT, Davis IM. Hip strength in females with and without patellofemoral pain. J Orthop Sports Phys Ther. 2003;33(11):671–676. doi: 10.2519/jospt.2003.33.11.671. [DOI] [PubMed] [Google Scholar]
- 17.Bolgla LA, Malone TR, Umberger BR, Uhl TL. Hip strength and hip and knee kinematics during stair descent in females with and without patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2008;38(1):12–18. doi: 10.2519/jospt.2008.2462. [DOI] [PubMed] [Google Scholar]
- 18.Fukuda TY, Rossetto FM, Magalhães E, et al. Short-term effects of hip abductors and lateral rotators strengthening in females with patellofemoral pain syndrome: a randomized controlled clinical trial. J Orthop Sports Phys Ther. 2010;40(11):736–742. doi: 10.2519/jospt.2010.3246. [DOI] [PubMed] [Google Scholar]
- 19.Collins N, Crossley K, Beller E, Darnell R, McPoil T, Vicenzino B. Foot orthoses and physiotherapy in the treatment of patellofemoral pain syndrome: randomised clinical trial. Br J Sports Med. 2009;43(3):169–171. doi: 10.1136/bmj.a1735. [DOI] [PubMed] [Google Scholar]
- 20.Witvrouw E, Lysens R, Bellemans J, Peers K, Vanderstraeten G. Open versus closed kinetic chain exercises for patellofemoral pain: a prospective, randomized study. Am J Sports Med. 2000;28(5):687–694. doi: 10.1177/03635465000280051201. [DOI] [PubMed] [Google Scholar]
- 21.Harrison EL, Sheppard MS, McQuarrie AM. A randomized controlled trial of physical therapy treatment programs in patellofemoral pain syndrome. Physiother Can. 1999;51:93–106. [Google Scholar]
- 22.Ismail MM, Gamaleldein MH, Hassa KA. Closed kinetic chain exercises with or without additional hip strengthening exercises in management of patellofemoral pain syndrome: a randomized controlled trial. Eur J Phys Rehabil Med. 2013;49(5):687–698. [PubMed] [Google Scholar]
- 23.Dye SF. Patellofemoral pain current concepts: an overview. Sports Med Arthrosc Rev. 2001;9(4):264–272. [Google Scholar]
- 24.Fulkerson JP. Diagnosis and treatment of patients with patellofemoral pain. Am J Sports Med. 2002;30(3):447–456. doi: 10.1177/03635465020300032501. [DOI] [PubMed] [Google Scholar]
- 25.Crossley KM, Bennell KL, Cowan SM, Green S. Analysis of outcome measures for persons with patellofemoral pain: which are reliable and valid? Arch Phys Med Rehabil. 2004;85(5):815–822. doi: 10.1016/s0003-9993(03)00613-0. [DOI] [PubMed] [Google Scholar]
- 26.Robinson RL, Nee RJ. Analysis of hip strength in females seeking physical therapy treatment for unilateral patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2007;37(5):232–238. doi: 10.2519/jospt.2007.2439. [DOI] [PubMed] [Google Scholar]
- 27.Bohannon RW. Reference values for extremity muscle strength obtained by hand-held dynamometry from adults aged 20 to 79 years. Arch Phys Med Rehabil. 1997;78(1):26–32. doi: 10.1016/s0003-9993(97)90005-8. [DOI] [PubMed] [Google Scholar]
- 28.McGill SM, Childs A, Lieberman C. Endurance times for low back stabilization exercises: clinical targets for testing and training from a normal database. Arch Phys Med Rehabil. 1999;80(8):941–944. doi: 10.1016/s0003-9993(99)90087-4. [DOI] [PubMed] [Google Scholar]
- 29.Cowan SM, Bennell KL, Hodges PW, Crossley KM, McConnell J. Simultaneous feedforward recruitment of the vasti in untrained postural tasks can be restored by physical therapy. J Orthop Res. 2003;21(3):553–558. doi: 10.1016/S0736-0266(02)00191-2. [DOI] [PubMed] [Google Scholar]
- 30.Thomee R. A comprehensive treatment approach for patellofemoral pain syndrome in young women. Phys Ther. 1997;77(12):1690–1703. doi: 10.1093/ptj/77.12.1690. [DOI] [PubMed] [Google Scholar]
- 31.Powers CM. Rehabilitation of patellofemoral joint disorders: a critical review. J Orthop Sports Phys Ther. 1998;28(5):345–354. doi: 10.2519/jospt.1998.28.5.345. [DOI] [PubMed] [Google Scholar]
- 32.Malone T, Davies G, Walsh WM. Muscular control of the patella. Clin Sports Med. 2002;21(3):349–362. doi: 10.1016/s0278-5919(02)00014-5. [DOI] [PubMed] [Google Scholar]
- 33.Davies GJ, Manske RC, Cooley K, Fletcher-Kios D, Johnson-Stuhr P. Selective activation of the vastus medialis oblique: what does the literature really tell us? Physiother Can. 2001;53:136–151. [Google Scholar]
- 34.Mascal CL, Landel R, Powers C. Management of patellofemoral pain targeting hip, pelvis, and trunk muscle function: 2 case reports. J Orthop Sports Phys Ther. 2003;33(11):647–660. doi: 10.2519/jospt.2003.33.11.647. [DOI] [PubMed] [Google Scholar]
- 35.Dolak KL, Silkman C, Medina McKeon J, Hosey RG, Lattermann C, Uhl TL. Hip strengthening prior to functional exercises reduces pain sooner than quadriceps strengthening in females with patellofemoral pain syndrome: a randomized clinical trial. J Orthop Sports Phys Ther. 2011;41(8):560–570. doi: 10.2519/jospt.2011.3499. [DOI] [PubMed] [Google Scholar]
- 36.Boren K, Conrey C, Le Coguic J, Paprocki L, Voight M, Robinson TK. Electromyographic analysis of gluteus medius and gluteus maximus during rehabilitation exercises. Int J Sports Phys Ther. 2011;6(3):206–223. [PMC free article] [PubMed] [Google Scholar]
- 37.Distefano LJ, Blackburn JT, Marshall SW, Padua DA. Gluteal muscle activation during common therapeutic exercises. J Orthop Sports Phys Ther. 2009;39(7):532–540. doi: 10.2519/jospt.2009.2796. [DOI] [PubMed] [Google Scholar]
- 38.Bolgla LA, Uhl TL. Electromyographic analysis of hip rehabilitation exercises in a group of healthy subjects. J Orthop Sports Phys Ther. 2005;35(8):487–494. doi: 10.2519/jospt.2005.35.8.487. [DOI] [PubMed] [Google Scholar]
- 39.Krause DA, Jacobs RS, Pilger KE, Sather BR, Sibunka SP, Hollman JH. Electromyographic analysis of the gluteus medius in five weight-bearing exercises. J Strength Cond Res. 2009;23(9):2689–2694. doi: 10.1519/JSC.0b013e3181bbe861. [DOI] [PubMed] [Google Scholar]
- 40.Killip S, Mahfoud Z, Pearce K. What is an intracluster correlation coefficient? Crucial concepts for primary care researchers. Ann Fam Med. 2004;2(3):204–208. doi: 10.1370/afm.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stathopulu E, Baildam E. Anterior knee pain: a long-term follow-up. Rheumatology (Oxford) 2003;42(2):380–382. doi: 10.1093/rheumatology/keg093. [DOI] [PubMed] [Google Scholar]
- 42.Nimon G, Murray D, Sandow M, Goodfellow J. Natural history of anterior knee pain: a 14- to 20-year follow-up of nonoperative management. J Pediatr Orthop. 1998;18(1):118–122. [PubMed] [Google Scholar]