Abstract
Context:
The use and popularity of whole-body vibration (WBV) has increased in recent years, but there is a lack of consensus in the literature about the effectiveness of the treatment.
Objective:
To quantitatively examine the effects of WBV on muscle oxygenation and peripheral blood flow in healthy adults.
Data Sources:
We searched Web of Science and PubMed databases and reference lists from relevant articles using the key terms whole body vibration, whole-body vibration, WBV, blood flow, peripheral blood flow, oxygenation, muscle oxygenation, circulation, circulatory, near infrared spectroscopy, NIRS, and power Doppler. Key terms were searched using single word and combination searches. No date range was specified.
Study Selection:
Criteria for inclusion were (1) use of a commercially available WBV device, (2) a human research model, (3) a pre-WBV condition and at least 1 WBV experimental condition, and (4) reporting of unstandardized means and standard deviations of muscle oxygenation or peripheral blood flow.
Data Extraction:
Means, standard deviations, and sample sizes were extracted from the text, tables, and figures of included studies. A total of 35 and 90 data points were extracted for the muscle-oxygenation and blood-flow meta-analyses, respectively. Data for each meta-analysis were combined and analyzed using meta-analysis software. Weighted, random-effects meta-analyses using the Hedges g metric were completed for muscle oxygenation and blood flow. We then conducted follow-up analyses using the moderator variables of vibration type, vibration time, vibration frequency, measurement location, and sample type.
Data Synthesis:
We found 18 potential articles. Further examination yielded 10 studies meeting the inclusion criteria. Whole-body vibration was shown to positively influence peripheral blood flow. Additionally, the moderators of vibration type and frequency altered the influence of WBV on blood flow. Overall, WBV did not alter muscle oxygenation; however, when the measurement site was considered, muscle oxygenation increased or decreased depending on the location.
Conclusions:
Acute bouts of WBV increase peripheral blood flow but do not alter skeletal muscle oxygenation. Vibration type appears to be the most important factor influencing both muscle oxygenation and peripheral blood flow.
Key Words: interventions, modalities, circulatory system
Key Points
An acute bout of therapeutic whole-body vibration (WBV) increases peripheral blood flow but does not alter muscle-oxygenation levels.
Vibration type and frequency affect peripheral blood flow and muscle oxygenation during an acute bout of WBV.
Additional work examining the effects of WBV on circulatory measures in injured populations is needed.
Although WBV is promising when used in healthy people, clinicians should be cautious in applying WBV as a therapeutic modality to improve vascular measures after injury or illness.
Over the past decade, the incorporation of whole-body vibration (WBV) into fitness and injury treatment programs has expanded rapidly.1,2 Researchers have demonstrated that WBV can improve aspects of physical performance, such as strength3–5 and flexibility,6,7 whereas evidence regarding the physiologic changes associated with therapeutic WBV remains unclear.8–12 The influence of WBV on peripheral blood flow and muscle oxygen use is of special interest, as these measures provide insight into the metabolic changes occurring in skeletal muscle. Blood flow and muscle oxygenation are closely related. During exercise, blood flow to the exercising muscle increases in response to increased demands for oxygen and fuel and increased carbon dioxide and hydrogen ion concentrations, among other factors.13 Changes in peripheral blood flow resulting from WBV application could indicate a possible mechanism of action for WBV treatment. A frequent clinical goal of applying therapeutic modalities is to increase circulation at the site of musculoskeletal injury during the fibroblastic-repair and maturation-remodeling phases of healing.14 To prevent secondary ischemic injury after musculoskeletal damage, athletic trainers must understand tissue oxygenation. A lack of oxygen (ischemia) results in decreased availability of energy at the cellular level because oxygen serves as the final electron acceptor in the electron transport chain; this lack of oxygen leads to cell death and eventually necrosis.15 If WBV increases blood flow or oxygenation, it could be used as a therapeutic intervention when the clinical goal is to increase blood flow or muscle oxygenation.
Results of WBV research on muscle oxygenation and peripheral blood flow have been mixed because of the variations in the type of vibration used, time, frequency, amplitude, and locations measured. Near-infrared spectroscopy (NIRS) is a tool commonly used to examine the effect of WBV on muscle oxygen use.8,9,12,16–18 The NIRS noninvasively and indirectly measures changes in oxygen-saturation levels by penetrating superficial structures (eg, skin) and being absorbed by hemoglobin or scattered into the tissue. The amount of near-infrared light scattered and absorbed indicates the level of oxygenated hemoglobin.19
Authors of WBV studies of peripheral blood flow have used NIRS8,9,16 and 2 other methods of assessment: laser Doppler10,20 and Doppler ultrasound.21–23 Laser Doppler uses light wavelength changes to calculate the number and velocity of blood cells in the region of interest.24 Similar to laser Doppler, Doppler ultrasound calculates the number and velocity of moving blood cells based on a Doppler shift. With Doppler ultrasound, the Doppler shift is measured using the changes in frequency of ultrasonic waves delivered to the region of interest.25 In addition to the range of methods used to collect data, investigators have used various vibration settings (which clinicians may change depending on the goal of treatment), so comparing studies of peripheral blood flow is difficult.
Whole-body vibration is a relatively new tool for treating and rehabilitating patients with musculoskeletal injuries. However, little is understood about its mechanism of action. Results of research to determine if WBV alters peripheral blood flow and muscle oxygenation have been conflicting, so making an informed decision on whether to implement WBV into their practices can be difficult for clinicians. A quantitative examination of the literature is needed to help clinicians understand this potentially important treatment modality and to determine where gaps in the research exist. Therefore, the purpose of our study was to use meta-analyses to quantitatively assess the research literature to date regarding the acute effects of WBV on muscle oxygenation and peripheral blood flow in healthy adults. A better understanding of the physiologic effects of WBV regarding blood flow and muscle oxygenation can potentially improve patient outcomes in clinical practice.
METHODS
Literature Search
The methods and presentation of results used in this meta-analysis conform to the PRISMA statement, with the exception that the review protocol is not registered.26 We searched the Web of Science and PubMed databases between January 2012 and March 2012 for relevant articles. We also used the reference lists of relevant articles to locate additional articles for review. No date range was specified, and searches were conducted to include all possible years of publication in each respective database. Key terms for the searches were whole body vibration, whole-body vibration, WBV, blood flow, peripheral blood flow, oxygenation, muscle oxygenation, circulation, circulatory, near infrared spectroscopy, NIRS, and power Doppler. We used single and combined key terms for our searches. The language was limited to English. The search yielded 18 potential studies for inclusion in the meta-analyses. On closer examination, 8 studies did not meet the inclusion criteria: (1) use of a commercially available WBV device, (2) a human research model, (3) provision of a pre-WBV condition and at least 1 WBV experimental condition, and (4) reporting of unstandardized means and standard deviations for muscle oxygenation or peripheral blood flow. We chose these inclusion criteria based on the purpose of our meta-analysis. A total of 10 studies met the inclusion criteria.
Data Extraction
Two separate meta-analyses were completed. For studies included in the muscle-oxygenation meta-analysis, investigators had examined the total oxygen index and the oxyhemoglobin levels measured by NIRS at multiple locations in the lower extremity. Both vertical and side-alternating WBV devices were included and amplitude ranged from 2 to 6 mm. Total WBV application time ranged from 30 seconds (0.5 minutes) to 300 seconds (5 minutes). All WBV application times were from a single session of WBV, which may have included a single bout of WBV exposure or multiple bouts within a single session. The WBV frequencies for the muscle-oxygenation data ranged from 16 to 50 Hz.
For studies included in the meta-analysis of peripheral blood flow, researchers had examined total hemoglobin levels in the lower extremity measured by NIRS, common femoral artery blood velocity assessed by color Doppler ultrasound, popliteal artery blood velocity measured with power Doppler ultrasound, and skin blood flow assessed by laser Doppler in both the upper and lower extremities. The WBV settings present in these data consisted of both side-alternating and vertical vibration at frequencies ranging from 5 to 50 Hz. Total WBV exposure times ranged from 30 seconds (0.5 minutes) to 900 seconds (15 minutes). Data on peripheral blood flow included WBV application times from a single session, which could reflect a single bout or multiple bouts of WBV exposure during a data-collection session.
Of the 10 studies included in the final analyses, muscle oxygenation was measured in 5 studies,8,9,16–18 and peripheral blood flow was measured in 8 studies.8–10,16,20–23 Authors of 3 studies8,9,16 examined both muscle-oxygenation changes and peripheral blood-flow changes. In the combined studies, the data were separated and included in each of the respective analyses. Authors of all studies used repeated-measures designs, exposed participants to multiple WBV settings, and examined 1 or more anatomic locations. Study variables included vibration type, vibration exposure time, vibration frequency, vibration amplitude (eg, males or females), measurement collection method, measurement location, and sample sex composition. Data were extracted from the text, tables, and graphs of each study and included the pre-WBV condition and WBV experimental condition mean, standard deviation, and sample size for each condition. Data presented in graphs were extracted using Scion Image (version 4.0.3.2; Scion Corp, Frederick, MD). All means, standard deviations, and sample sizes were recorded and organized into a custom spreadsheet (Excel 2010; Microsoft Corp, Redmond, WA). Moderator variables were extracted from each study and consisted of vibration type, vibration time, vibration frequency, vibration amplitude, measurement method, measurement location, and sample type. The meta-analyses for muscle oxygenation and peripheral blood flow consisted of 35 and 90 control-treatment comparisons, respectively.
Data Synthesis
To standardize comparisons across studies, we calculated an effect size using the Hedges g for muscle oxygenation and peripheral blood flow. The Hedges g metric is based on a standardized (pooled variation) difference of paired means between pre-WBV measures and measures during or immediately after WBV for each data point. The measures used in the original studies were not on the same scale, making the standardized mean difference metric (Hedges g) appropriate. Next, 95% confidence intervals (CIs) were calculated around the mean effect sizes. Mean effect sizes and 95% CIs that were above zero indicated that WBV increased muscle oxygenation and peripheral blood flow above the pre-WBV conditions. Mean effects and 95% CIs that were below zero indicated that WBV decreased muscle oxygenation and peripheral blood flow. Mean effect sizes and 95% CIs that crossed zero indicated that WBV had no effect. The studies used a range of measures and WBV settings, so a weighted, random-effects model was appropriate to analyze muscle oxygenation and peripheral blood flow. We created funnel plots for both analyses to check for publication bias at the outcome level. A fail-safe N was calculated for both overall analyses to assess the number of unpublished works needed to nullify that a finding was different. Random-effects meta-regressions were conducted after the overall analyses for both muscle oxygenation and blood flow using the continuous moderating variables of vibration application time and vibration frequency.
We conducted several subgroup meta-analyses on both sets of data: 6 muscle-oxygenation subgroup analyses for the moderator variable of data-collection location (gastrocnemius lateralis,18 gastrocnemius medialis,8,9,17 rectus femoris,16 vastus lateralis,8,16 upper leg [rectus femoris and vastus lateralis combined],8,16 and lower leg [gastrocnemius lateralis and medialis combined]8,9,17,18) and 5 subanalyses for the moderator of vibration frequency (50, 40, 30, 25, and 16 Hz). Meta-analyses for the peripheral blood-flow subgroup included 2 analyses for the moderator of vibration type (side alternating and vertical), 9 analyses for the moderator of vibration frequency (50, 45, 40, 30, 25, 20, 15, 10, and 5 Hz), 3 analyses for the moderator of measurement location (vascular tissue [eg, femoral artery], skeletal muscles [eg, vastus lateralis], cutaneous blood flow [eg, skin blood flow]), and 3 analyses for the moderator variable of data-collection method (NIRS total hemoglobin, ultrasound, laser Doppler). Although they were not included in our original plan, we completed additional subanalyses to examine the effect of loading on vibration frequency. Data for peripheral blood flow were from 8 studies, 7 of which applied WBV in a weight-bearing position; Maloney-Hinds et al20 applied WBV to a body segment at 2 vibration frequencies (30 Hz and 50 Hz). All statistical analyses were performed with Comprehensive Meta-Analysis Software (version 2.0; Biostat Inc, Englewood, NJ). The α level was set a priori at ≤.05 for all analyses.
RESULTS
The data points and source study information included in the meta-analyses for muscle oxygenation and peripheral blood flow and a summary forest plot of the Hedges g effect-size metrics and 95% CIs of each study are presented in Figures 1 and 2, respectively.
Figure 1.
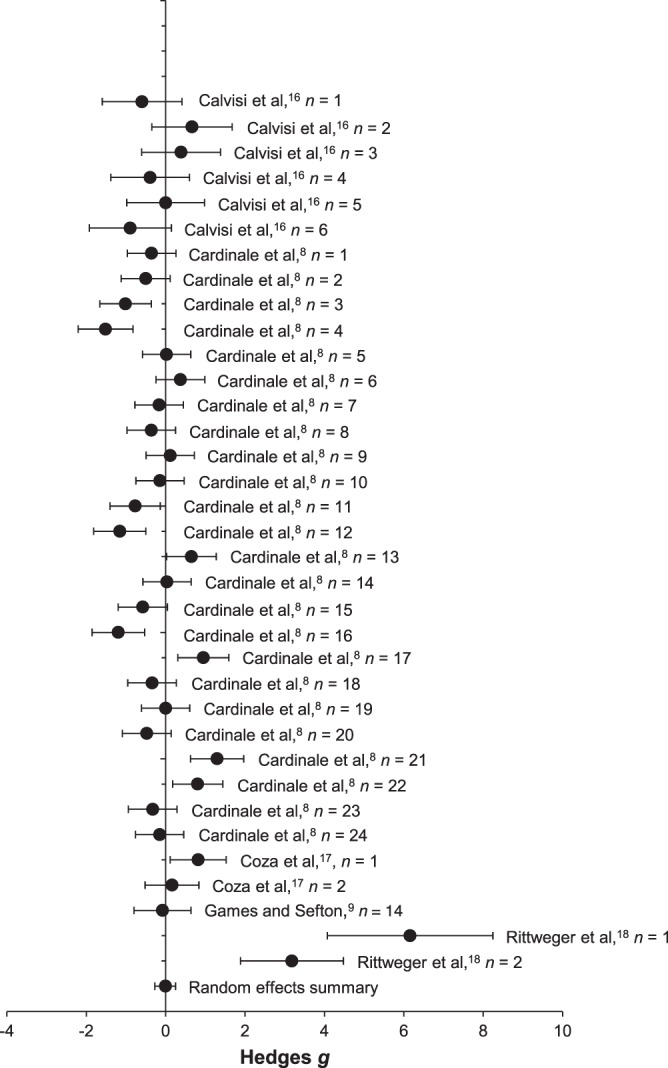
Forest plot and source study information for the muscle-oxygenation meta-analysis (mean ± 95% confidence interval).
Figure 2.
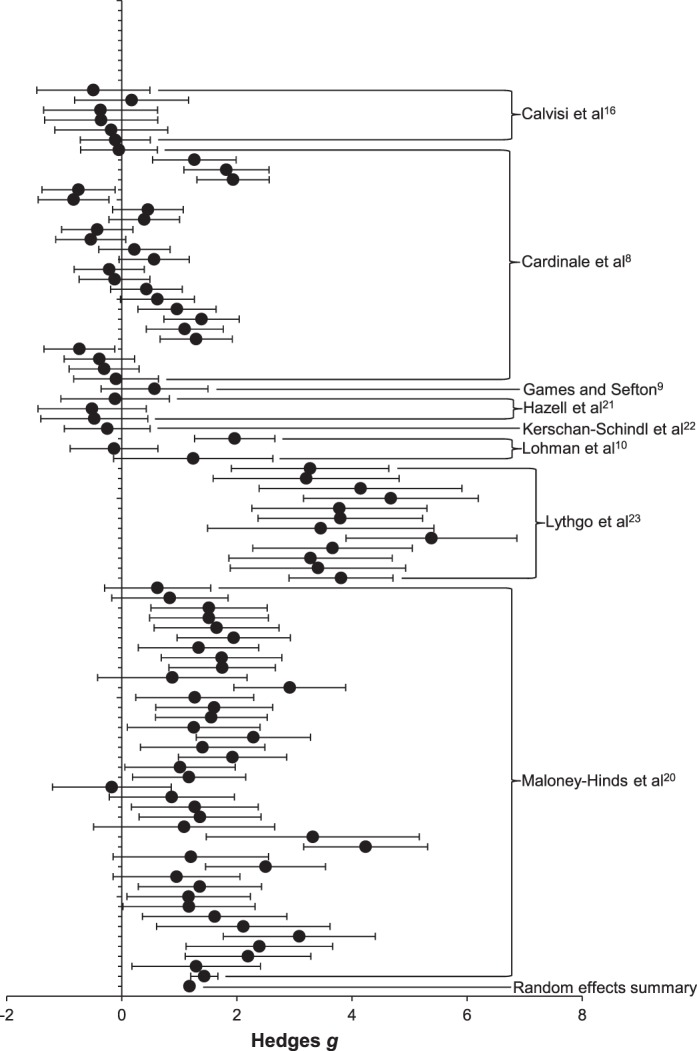
Forest plot and source study information for the peripheral blood-flow meta-analysis (mean ± 95% confidence interval).
Muscle Oxygenation
The funnel-plot analysis revealed a roughly symmetrical distribution about the mean effect for muscle oxygenation, indicating that publication bias did not influence our results. Overall, therapeutic WBV did not alter muscle oxygenation compared with control values (Hedges g = −0.005, n = 35, P = .98; 95% CI = −0.272, 0.261), suggesting that WBV does not alter muscle-oxygenation levels. Given the lack of effect, we did not calculate a fail-safe N. The I2 value of 0.80 suggested that true variance was observed in the meta-analysis for muscle oxygenation, and that subanalyses were warranted.27 The meta-regression for the effects of vibration time on muscle oxygenation revealed no clear linear relationship (Qmodel = 1.138, df = 1, P = .29), suggesting that WBV application time during an acute bout did not alter the effect of WBV on muscle oxygenation. Meta-regression also showed no clear effect of vibration frequency on muscle-oxygenation effect size (Qmodel = 0.993, df = 1, P = .32).
Compared with pre-WBV levels, measurements taken at the gastrocnemius lateralis muscle showed increased muscle-oxygenation levels (Hedges g = 4.553, n = 2, P = .002; 95% CI = 1.647, 7.459), whereas measurements taken at the gastrocnemius medialis muscle indicated decreased muscle-oxygenation levels (Hedges g = −0.302, n = 15, P = .046; 95% CI = −0.599, −0.005). Measurements of muscle oxygenation at the rectus femoris (Hedges g = 0.143, n = 3, P = .69; 95% CI = −0.593, 0.898), vastus lateralis (Hedges g = −0.021, n = 15, P = .91; 95% CI = −0.374, 0.332), upper leg (Hedges g = 0.003, n = 18, P = .98; 95% CI = −0.315, 0.322), and lower leg (Hedges g = 0.024, n = 17, P = .91; 95% CI = −0.410, 0.458) yielded no changes.
The mean effects, sample sizes, and 95% CIs for the moderator of vibration frequency are presented in Table 1. Compared with pre-WBV levels, a vibration frequency of 30 Hz decreased muscle-oxygenation levels, whereas a vibration frequency of 25 Hz increased muscle-oxygenation levels. The 30-Hz data were from studies using vertical vibration, whereas the 25-Hz data were from studies using side-alternating vibration. Vibration frequencies of 50, 40, and 16 Hz used vertical vibration and had no mean effect on muscle-oxygenation levels.
Table 1.
Results of Subanalyses of the Vibration-Frequency Moderator on Muscle Oxygenation
| Vibration Frequency, Hz |
No. of Data Points Included |
Hedges g |
95% Confidence Interval |
| 50 | 11 | −0.074 | −0.501, 0.352 |
| 40 | 10 | 0.035 | −0.251, 0.321 |
| 30 | 10 | −0.538 | −0.941, −0.134 |
| 25 | 2 | 4.553 | 1.647, 7.459 |
| 16 | 2 | 0.483 | −0.165, 1.131 |
Peripheral Blood Flow
Funnel-plot analysis showed that no publication bias existed for the peripheral blood-flow data set. Peripheral blood flow was positively influenced by WBV (Hedges g = 1.179, n = 90, P < .001; 95% CI = 0.942, 1.416). Calculation of a fail-safe N revealed that 9718 unpublished studies with results that were not different would be required to nullify the mean effects. This number is sufficiently high to conclude that these results are robust and not influenced by a possible publication preference for results that are different.
The I2 value of 0.84 suggested that true variance was observed in the meta-analysis for peripheral blood flow, and subanalyses were warranted.27 The meta-regression for the effects of vibration frequency on peripheral blood flow revealed a direct, negative linear relationship (Qmodel = 28.66, df = 1, P < .001), suggesting that lower frequencies may lead to greater changes in peripheral blood flow than higher frequencies (Figure 3). We found a linear relationship that was not different for the effects of vibration time on peripheral blood flow, suggesting that research to date does not indicate if short or long vibration times alter peripheral blood flow differently (Qmodel = 0.383, df = 1, P = .535). Analyses of the moderator vibration type indicated that vertical WBV produced no mean effect on peripheral blood flow (Hedges g = 0.182, n = 35, P = .15; 95% CI = −0.067, 0.431), whereas side-alternating vibration increased peripheral blood flow (Hedges g = 1.906, n = 55, P < .001; 95% CI = 1.628, 2.184). These results suggest that using side-alternating vibration could influence peripheral blood flow, whereas vertical vibration may not affect peripheral blood flow.
Figure 3.
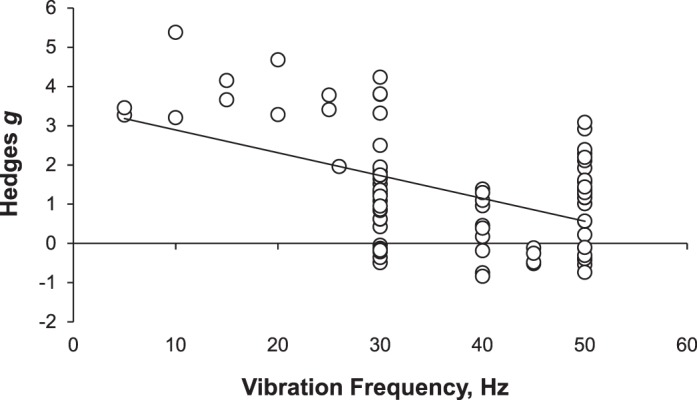
Meta-regression of the moderator of vibration frequency (Hz) on the mean effect of therapeutic whole-body vibration on peripheral blood flow.
We analyzed vibration-frequency moderators of 50, 45, 40, 30, 25, 20, 15, 10, and 5 Hz. The mean effects, sample sizes, and 95% CIs are summarized in Table 2. Vibration frequencies of 50, 30, 25, 20, 15, 10, and 5 Hz increased peripheral blood flow, whereas frequencies of 45 and 40 Hz did not have a mean effect. The 26-Hz vibration frequency was included in the overall analysis of peripheral blood flow, but a subanalysis was not completed because only 1 data point existed at this frequency.
Table 2.
Results of Subanalyses of the Vibration-Frequency Moderator on Blood Flow
| Vibration Frequency, Hz |
No. of Data Points Included |
Hedges g |
95% Confidence Interval |
| 50 | 31 | 0.943 | 0.584, 1.303 |
| 45 | 4 | −0.338 | −0.806, 0.13 |
| 40 | 10 | 0.407 | −0.112, 0.927 |
| 30 | 34 | 1.157 | 0.823, 1.489 |
| 25 | 2 | 3.581 | 2.545, 4.617 |
| 20 | 2 | 3.866 | 2.516, 5.217 |
| 15 | 2 | 3.884 | 2.791, 4.977 |
| 10 | 2 | 4.172 | 2.051, 6.293 |
| 5 | 2 | 3.36 | 2.365, 4.356 |
Follow-up analyses were completed for measurement location. A mean effect was revealed for measures collected from vascular structures (Hedges g = 2.641, n = 17, P < .001; 95% CI = 1.691, 3.592) and measures of skin blood flow (Hedges g = 1.48, n = 40, P < .001; 95% CI = 1.27, 1.69). No effect was revealed for measures collected from skeletal muscles (Hedges g = 0.25, n = 33, P = .052; 95% CI = −0.002, 0.51). These results suggest that alterations in peripheral blood flow from WBV can be quantified in vascular and muscular tissues.
No mean effect of WBV on peripheral blood flow was evident from NIRS total hemoglobin (Hedges g = 0.23, n = 31, P = .08; 95% CI = −0.029, 0.506). However, assessment by ultrasound (Hedges g = 2.641, n = 17, P < .001; 95% CI = 1.691, 3.592) and skin blood flow measured with laser Doppler (Hedges g = 1.436, n = 42, P < .001; 95% CI = 1.214, 1.659) showed that WBV increased peripheral blood flow.
The supplemental subanalyses revealed that both weight-bearing (loaded) WBV (Hedges g = 0.84, n = 14, P = .002; 95% CI = 0.30, 1.38) and body-segment (unloaded) WBV (Hedges g = 1.37, n = 20, P < .001; 95% CI = 1.04, 1.70) at 30 Hz increased peripheral blood flow. Conversely, at 50 Hz, we found that WBV applied in a weight-bearing (loaded) position did not alter peripheral blood flow (Hedges g = −0.15, n = 11, P = .25; 95% CI = −0.41, 0.11); however, when it was applied in an unloaded position (body-segment vibration), WBV again increased peripheral blood flow (Hedges g = 1.56, n = 20, P < .001; 95% CI = 1.35, 1.84).
DISCUSSION
The most important result of this analysis is that vibration type may influence blood-flow response. Peripheral blood flow increases during side-alternating vibration but not during vertical vibration. Thus, vibration type may be key in the use of therapeutic WBV to influence blood flow. Given that no commercially available device can switch between vertical and side-alternating vibration modes, this factor may be important for athletic trainers when deciding which type of device to purchase. Very few researchers have directly compared side-alternating vibration with vertical vibration using commercially available devices to examine changes in peripheral blood flow.2 In a study directly comparing vertical and side-alternating WBV, Gojanovic and Henchoz2 found that a 20-minute exercise protocol of side-alternating WBV increased heart rate compared with vertical WBV and no-WBV conditions. This supports our finding that side-alternating WBV may cause a different or greater response than vertical vibrations. No investigators have identified how side-alternating vibration elicits different physiologic responses than vertical vibration. Future studies in which researchers directly compare vertical vibration with side-alternating vibration are needed to more clearly understand how vibration type influences peripheral blood flow and muscle oxygenation and to understand the physiologic pathway by which each type of vibration may elicit physiologic changes.
Vibration frequencies of 50, 30, 25, 20, 15, 10, and 5 Hz resulted in mean effects of therapeutic WBV on peripheral blood flow, with the Hedges g ranging from 0.943 (50 Hz) to 4.172 (10 Hz). These findings agree with the meta-regression analysis, suggesting that a mean effect of therapeutic WBV on peripheral blood flow would be greater at lower frequencies than at higher frequencies. Meta-analysis cannot examine the mechanisms by which the observed changes occurred. Yet by objectively measuring the reported effects of WBV, meta-analysis may uncover the effect of an acute bout of WBV. The results from both the individual subanalyses and the meta-regression demonstrate that vibration frequency influences the amount of change produced in peripheral blood flow. Our meta-analyses indicate that lower frequencies (5–25 Hz) produce a greater observed effect than higher frequencies (30–50 Hz) of WBV. This evidence suggests that lower frequencies should be used to increase peripheral blood flow.
One possible explanation is that the increased blood flow may be influenced by the rate of muscle contraction. Lower frequencies may provide increased time between contractions, allowing for greater perfusion. Higher frequencies, on the other hand, may not allow for this perfusion, resulting in lower blood flow during WBV application. This is simply a theory based on our knowledge; investigators have not comprehensively examined the effects of vibration frequency on peripheral blood flow in skeletal muscle or vascular tissues (eg, arteries). Lythgo et al23 examined the effects of vibration frequency and amplitude of blood velocity in the femoral artery and found that lower frequencies (10–30 Hz) increased blood velocity more than did higher vibration frequencies (20–30 Hz) (33% versus 27%).
Another important WBV variable is the position of the participants and whether the position is loaded or unloaded with body weight. Our supplemental subanalyses showed that loading influenced peripheral blood-flow responses at higher frequencies and support our meta-regression data, suggesting that lower frequencies resulted in a greater peripheral blood-flow response than did higher frequencies. These supplemental results must be interpreted carefully because all of the segmental vibration data were from 1 source study and 1 test location (ie, forearm) and may not hold true for different WBV settings or application sites.
Measures taken at large vascular structures (eg, femoral artery) revealed that peripheral blood flow increased after WBV (Hedges g = 2.64) compared with pre-WBV control measures. Cutaneous blood flow (ie, skin blood flow) also showed an increase in peripheral blood flow after WBV (Hedges g = 1.48) compared with pre-WBV control measures. However, compared with control measures, peripheral blood flow measured in skeletal muscle was not altered after WBV. The data suggest that vascular tissues and cutaneous blood flow demonstrated larger responses to therapeutic WBV than did skeletal muscle. All measures of peripheral blood flow were recorded superficially from the skin, but the measurement location of the vascular tissue changed. For example, measures taken from skeletal muscles examined the capillary changes within the muscle. If the blood flow through the large vessels was altered the most, this may hold promise for using therapeutic WBV as a nonpharmacologic treatment for diseases of the peripheral vasculature. Comparing human WBV models with animal models and exercise-hyperemia models of blood perfusion, we see mechanisms by which WBV could elicit changes in peripheral blood flow. Recent WBV findings in a mouse model of hind-limb ischemia support our findings of increases in peripheral blood flow in the vascular tissue through vasodilation, stimulated by the endothelial nitric oxide synthase mechanism.28 Yet these researchers did not examine mice without hind-limb ischemia, and the mechanism by which nitric oxide creates vasodilation may be altered. Further, examination of the exercise-hyperemia literature suggested that a large number of potential vasodilatory agents, including potassium,29 adenosine,30 and nitric oxide,31 may be released with muscle contractions. Contraction-induced hyperemia has been shown to occur after only 1 muscle contraction.32 Whereas exercise-hyperemia studies support increased blood flow with muscle contractions, the frequency at which the contractions take place may not be comparable with the frequencies that occur during a bout of WBV. Clearly, more work is needed in this area, specifically to examine WBV. Many mechanisms (metabolic, humeral, and neuronal factors) potentially play a role in the increased blood flow observed after WBV exposure. Additional work is needed to determine the mechanism by which blood flow increases during therapeutic WBV exposure.
Vibration frequency was revealed to selectively influence muscle-oxygenation levels. A vibration frequency of 30 Hz decreased muscle-oxygenation levels (Hedges g = −0.53) compared with control levels. This finding suggests that the physiologic oxygen demand is greater than the oxygen supply during WBV at 30 Hz in the skeletal muscles tested. Compared with higher vibration frequencies, 30 Hz may preferentially activate concentric and eccentric muscle- contraction cycles. Preferential stimulation of skeletal muscle by WBV has been supported in recent work by investigators33 who found that vibration frequencies of 25 to 35 Hz preferentially activated the lateral gastrocnemius muscle as measured with electromyography. In contrast, we found that a WBV frequency of 25 Hz greatly increased muscle-oxygenation levels (Hedges g = 4.55) compared with baseline values, indicating that the oxygen supply far exceeded demand during WBV exposure. These results are difficult to explain given the large range of scores (Hedges g range, −0.53 to 4.55) after only a 5-Hz frequency change. A closer investigation of the data showed that the 25-Hz data consisted of only 2 data points and were from the same study with a small sample (N = 10); therefore, these data should be interpreted with caution. Also, the 30-Hz treatments used vertical vibration, whereas the 25-Hz treatments used side-alternating vibration. Vertical vibration and side-alternating vibration may affect oxygen demand and delivery differently. All data points included in the muscle-oxygenation meta-analysis were obtained with vertical vibration except for the data point at 25 Hz, which was obtained with side-alternating vibration. Further examination of the role of vibration type on muscle oxygenation is not possible at this point given the limited data available. The ability of the oxygen supply to meet oxygen demand under WBV conditions could be attributed to 2 hypotheses: (1) therapeutic WBV does not elicit a metabolic demand in the muscle greater than what is required during rest or (2) therapeutic WBV does elicit a greater metabolic demand in muscle than at rest, but this demand is met with increased peripheral blood flow. The second theory is supported by research suggesting that WBV elicits reflexive muscle contractions.34 The muscle-oxygenation data indicate that this increased demand is matched by increased supply during therapeutic WBV. Additionally, our results from the meta-analysis of peripheral blood flow support the hypothesis that WBV elicits increased metabolic demand, which is adequately met. Peripheral blood flow increases overall during and immediately after an acute bout of therapeutic WBV.
Additional research is warranted to determine if WBV is safe and effective in populations with injury, illness, or disease. Authors of all of the reports we examined studied young, healthy participants. Populations with injury, illness, or disease may produce very different results. Investigators have examined therapeutic WBV in populations with osteoporosis35,36 and obesity,37,38 but few39 have examined the effect of WBV on blood-flow and oxygenation measures in special populations. Research needs to be conducted on the dose-response of WBV after repeated treatments and the training effects of WBV on measures of blood flow and muscle oxygenation. Common reporting standards are also needed. Recent recommendations (eg, common terminology, proper intervention descriptions) by the International Society of Musculoskeletal and Neuronal Interactions for reporting WBV intervention studies have begun to address this concern40; however, not all researchers have implemented these reporting recommendations. Comparison among studies is difficult without common reporting standards.
Our study had several limitations that must be considered when interpreting our results. Care must be taken in generalizing these results, as we assessed studies with large variations in measurement tools and vibration settings. A risk of bias always exists at both the study and outcome level. Although we attempted to retrieve as much of the WBV literature as possible, we may not have completely retrieved the data we identified for inclusion, including articles that were not published in English and articles published in journals that were not indexed in the search engines. Finally, the meta-analysis technique is designed to uncover the true effect of a treatment by quantitatively examining the effects of individual data points. This aids in our understanding of the effects of WBV on blood flow and muscle oxygenation but does not uncover the mechanism by which the observed effects are occurring. Mechanistic studies are needed to understand the pathway by which WBV influences blood flow and muscle oxygenation.
CONCLUSIONS
Analysis of the literature suggested that an acute bout of therapeutic WBV increases peripheral blood flow but does not alter muscle-oxygenation levels. Vibration type and frequency influence the effects of peripheral blood flow and muscle oxygenation during an acute bout of WBV.
ACKNOWLEDGMENTS
A portion of the data, using a different analysis, was presented as an abstract/poster presentation at the 60th Annual Meeting of the American College of Sports Medicine, May 29–June 1, 2013, Indianapolis, IN.
REFERENCES
- 1.Cloak R, Nevill AM, Clarke F, Day S, Wyon MA. Vibration training improves balance in unstable ankles. Int J Sports Med. 2010;31(12):894–900. doi: 10.1055/s-0030-1265151. [DOI] [PubMed] [Google Scholar]
- 2.Gojanovic B, Henchoz Y. Whole-body vibration training: metabolic cost of synchronous, side-alternating or no vibrations. J Sports Sci. 2012;30(13):1397–1403. doi: 10.1080/02640414.2012.710756. [DOI] [PubMed] [Google Scholar]
- 3.Fort A, Romero D, Bagur C, Guerra M. Effects of whole-body vibration training on explosive strength and postural control in young female athletes. J Strength Cond Res. 2012;26(4):926–936. doi: 10.1519/JSC.0b013e31822e02a5. [DOI] [PubMed] [Google Scholar]
- 4.Pellegrini MJ, Lythgo ND, Morgan DL, Galea MP. Voluntary activation of the ankle plantar flexors following whole-body vibration. Eur J Appl Physiol. 2010;108(5):927–934. doi: 10.1007/s00421-009-1304-2. [DOI] [PubMed] [Google Scholar]
- 5.Poston B, Holcomb WR, Guadagnoli MA, Linn LL. The acute effects of mechanical vibration on power output in the bench press. J Strength Cond Res. 2007;21(1):199–203. doi: 10.1519/00124278-200702000-00036. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs PL, Burns P. Acute enhancement of lower-extremity dynamic strength and flexibility with whole-body vibration. J Strength Cond Res. 2009;23(1):51–57. doi: 10.1519/JSC.0b013e3181839f19. [DOI] [PubMed] [Google Scholar]
- 7.Marshall LC, Wyon MA. The effect of whole-body vibration on jump height and active range of movement in female dancers. J Strength Cond Res. 2012;26(3):789–793. doi: 10.1519/JSC.0b013e31822a5ce8. [DOI] [PubMed] [Google Scholar]
- 8.Cardinale M, Ferrari M, Quaresima V. Gastrocnemius medialis and vastus lateralis oxygenation during whole-body vibration exercise. Med Sci Sports Exerc. 2007;39(4):694–700. doi: 10.1249/mss.0b013e31803084d8. [DOI] [PubMed] [Google Scholar]
- 9.Games KE, Sefton JM. Whole-body vibration influences lower extremity circulatory and neurological function. Scand J Med Sci Sports. 2013;23(4):516–523. doi: 10.1111/j.1600-0838.2011.01419.x. [DOI] [PubMed] [Google Scholar]
- 10.Lohman EB, III, Sackiriyas KS, Bains GS, et al. A comparison of whole body vibration and moist heat on lower extremity skin temperature and skin blood flow in healthy older individuals. Med Sci Monit. 2012;18(7):CR415–CR424. doi: 10.12659/MSM.883209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rittweger J, Beller G, Felsenberg D. Acute physiological effects of exhaustive whole-body vibration exercise in man. Clin Physiol. 2000;20(2):134–142. doi: 10.1046/j.1365-2281.2000.00238.x. [DOI] [PubMed] [Google Scholar]
- 12.Yamada E, Kusaka T, Miyamoto K, et al. Vastus lateralis oxygenation and blood volume measured by near-infrared spectroscopy during whole body vibration. Clin Physiol Funct Imaging. 2005;25(4):203–208. doi: 10.1111/j.1475-097X.2005.00614.x. [DOI] [PubMed] [Google Scholar]
- 13.Brooks GA, Fahey TD, Baldwin KM. Exercise Physiology: Human Bioenergetics and Its Applications. 4th ed. Boston, MA: McGraw-Hill;; 2005. [Google Scholar]
- 14.Prentice WE. Using therapeutic modalities to affect the healing process. In: Prentice WE, editor. Therapuetic Modalities: For Sports Medicine and Athletic Training. Boston, MA: McGraw-Hill;; 2009. pp. 17–32. In. ed. [Google Scholar]
- 15.Merrick MA. Secondary injury after musculoskeletal trauma: a review and update. J Athl Train. 2002;37(2):209–217. [PMC free article] [PubMed] [Google Scholar]
- 16.Calvisi V, Angelozzi M, Franco A, et al. Influence of whole-body vibration static exercise on quadriceps oxygenation. Adv Exp Med Biol. 2006;578:137–141. doi: 10.1007/0-387-29540-2_22. [DOI] [PubMed] [Google Scholar]
- 17.Coza A, Nigg BM, Dunn JF. Effects of vibrations on gastrocnemius medialis tissue oxygenation. Med Sci Sports Exerc. 2011;43(3):509–515. doi: 10.1249/MSS.0b013e3181f2589f. [DOI] [PubMed] [Google Scholar]
- 18.Rittweger J, Moss AD, Colier W, Stewart C, Degens H. Muscle tissue oxygenation and VEGF in VO-matched vibration and squatting exercise. Clin Physiol Funct Imaging. 2010;30(4):269–278. doi: 10.1111/j.1475-097X.2010.00937.x. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari M, Mottola L, Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol. 2004;29(4):463–487. doi: 10.1139/h04-031. [DOI] [PubMed] [Google Scholar]
- 20.Maloney-Hinds C, Petrofsky JS, Zimmerman G. The effect of 30 Hz vs. 50 Hz passive vibration and duration of vibration on skin blood flow in the arm. Med Sci Monit. 2008;14(3):CR112–CR116. [PubMed] [Google Scholar]
- 21.Hazell TJ, Thomas GW, Deguire JR, Lemon PW. Vertical whole-body vibration does not increase cardiovascular stress to static semi-squat exercise. Eur J Appl Physiol. 2008;104(5):903–908. doi: 10.1007/s00421-008-0847-y. [DOI] [PubMed] [Google Scholar]
- 22.Kerschan-Schindl K, Grampp S, Henk C, et al. Whole-body vibration exercise leads to alterations in muscle blood volume. Clin Physiol. 2001;21(3):377–382. doi: 10.1046/j.1365-2281.2001.00335.x. [DOI] [PubMed] [Google Scholar]
- 23.Lythgo N, Eser P, de Groot P, Galea M. Whole-body vibration dosage alters leg blood flow. Clin Physiol Funct Imaging. 2009;29(1):53–59. doi: 10.1111/j.1475-097X.2008.00834.x. [DOI] [PubMed] [Google Scholar]
- 24.Laser Doppler Theory. Perimed Web site. http://www.perimed-instruments.com/support/theory/laser-doppler. Accessed January 10, 2014. [Google Scholar]
- 25.Jayanthy AK, Sujatha N, Ramasubba-Reddy M. Measuring blood flow: techniques and applications. Int J Res Rev Appl Sci. 2011;6(2):203–216. [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG. The PG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. Chichester, UK: John Wiley & Sons;; 2009. [Google Scholar]
- 28.Rokutanda T, Izumiya Y, Miura M, et al. Passive exercise using whole-body periodic acceleration enhances blood supply to ischemic hindlimb. Arterioscler Thromb Vasc Biol. 2011;31(12):2872–2880. doi: 10.1161/ATVBAHA.111.229773. [DOI] [PubMed] [Google Scholar]
- 29.Lott ME, Hogeman CS, Vickery L, Kunselman AR, Sinoway LI, MacLean DA. Effects of dynamic exercise on mean blood velocity and muscle interstitial metabolite responses in humans. Am J Physiol Heart Circ Physiol. 2001;281(4):H1734–H1741. doi: 10.1152/ajpheart.2001.281.4.H1734. [DOI] [PubMed] [Google Scholar]
- 30.Hellsten Y, Maclean D, Radegran G, Saltin B, Bangsbo J. Adenosine concentrations in the interstitium of resting and contracting human skeletal muscle. Circulation. 1998;98(1):6–8. doi: 10.1161/01.cir.98.1.6. [DOI] [PubMed] [Google Scholar]
- 31.Tidball JG, Lavergne E, Lau KS, Spencer MJ, Stull JT, Wehling M. Mechanical loading regulates NOS expression and activity in developing and adult skeletal muscle. Am J Physiol. 1998;275((1, pt 1)):C260–C266. doi: 10.1152/ajpcell.1998.275.1.C260. [DOI] [PubMed] [Google Scholar]
- 32.Shoemaker JK, Tschakovsky ME, Hughson RL. Vasodilation contributes to the rapid hyperemia with rhythmic contractions in humans. Can J Physiol Pharmacol. 1998;76(4):418–427. doi: 10.1139/cjpp-76-4-418. [DOI] [PubMed] [Google Scholar]
- 33.Di Giminiani R, Masedu F, Tihanyi J, Scrimaglio R, Valenti M. The interaction between body position and vibration frequency on acute response to whole body vibration. J Electromyogr Kinesiol. 2013;23(1):245–251. doi: 10.1016/j.jelekin.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 34.Pollock RD, Woledge RC, Martin FC, Newham DJ. Effects of whole body vibration on motor unit recruitment and threshold. J Appl Physiol. 2012;112(3):388–395. doi: 10.1152/japplphysiol.01223.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwamoto J, Sato Y, Takeda T, Matsumoto H. Whole body vibration exercise improves body balance and walking velocity in postmenopausal osteoporotic women treated with alendronate: Galileo and Alendronate Intervention Trail (GAIT) J Musculoskelet Neuronal Interact. 2012;12(3):136–143. [PubMed] [Google Scholar]
- 36.Slatkovska L, Alibhai SM, Beyene J, Hu H, Demaras A, Cheung AM. Effect of 12 months of whole-body vibration therapy on bone density and structure in postmenopausal women: a randomized trial. Ann Intern Med. 2011;155(10):668–679. doi: 10.7326/0003-4819-155-10-201111150-00005. W205. [DOI] [PubMed] [Google Scholar]
- 37.Giunta M, Cardinale M, Agosti F, et al. Growth hormone-releasing effects of whole body vibration alone or combined with squatting plus external load in severely obese female subjects. Obes Facts. 2012;5(4):567–574. doi: 10.1159/000342066. [DOI] [PubMed] [Google Scholar]
- 38.Vissers D, Verrijken A, Mertens I, et al. Effect of long-term whole body vibration training on visceral adipose tissue: a preliminary report. Obes Facts. 2010;3(2):93–100. doi: 10.1159/000301785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Figueroa A, Gil R, Wong A, et al. Whole-body vibration training reduces arterial stiffness, blood pressure and sympathovagal balance in young overweight/obese women. Hypertens Res. 2012;35(6):667–672. doi: 10.1038/hr.2012.15. [DOI] [PubMed] [Google Scholar]
- 40.Rauch F, Sievanen H, Boonen S, et al. Reporting whole-body vibration intervention studies: recommendations of the International Society of Musculoskeletal and Neuronal Interactions. J Musculoskelet Neuronal Interact. 2010;10(3):193–198. [PubMed] [Google Scholar]


