Abstract
The functions of blood cells extend well beyond the immune functions of leucocytes or the respiratory and hemostatic functions of erythrocytes and platelets. Seen as a whole, the bloodstream is in charge of nurturing and protecting all organs by carrying a mixture of cell populations in transit from one organ to another. To optimize these functions, evolution has provided blood and the vascular system that carries it with various mechanisms that ensure the appropriate influx and egress of cells into and from the circulation where and when needed. How this homeostatic control of blood is achieved has been the object of study for over a century, and although the major mechanisms that govern it are now fairly well understood, several new concepts and mediators have recently emerged that emphasize the dynamism of this liquid tissue. Here we review old and new concepts that relate to the maintenance and regulation of leucocyte homeostasis in blood and briefly discuss the mechanisms for platelets and red blood cells.
Keywords: Leucocyte homeostasis, Leucocyte mobilization, Leucocyte recruitment, Cholesterol metabolism
1. Introduction
Making and maintaining blood requires a symphony of finely tuned microenvironments and mechanisms that regulate self-renewal, differentiation, migration, and removal of its ageing elements. The generative arm is orchestrated in the bone marrow where distinct niches have been shown to maintain haematopoietic stem cells (HSCs) and regulate progenitor commitment and differentiation into mature blood cells, whereas the recycling arm is shared among the marrow, spleen, and liver. Although the response of the bone marrow and extramedullary organs to acute blood loss or severe infection can be spectacular—and mechanistically insightful—the precise regulation of haematopoiesis during homeostasis illustrates the exquisite level with which haematopoietic organs must operate; billions of blood cells are generated every hour, whereas the same number must be effortlessly removed and recycled to maintain steady-state blood. We review herein the differential mechanisms mediating the release, migration, and removal of major blood cell subsets to paint a picture in which common pathways will emerge.
2. Mechanisms governing cellular mobilization into the blood stream
The most critical parameter for blood cell homeostasis in the circulation is the regulation of cellular numbers. The production of haematopoietic cells and their release into blood needs to be tightly balanced to match the removal rate from blood into tissues. Thus, levels of circulating leucocytes, platelets, and red blood cells are regulated by input and output mechanisms, whose combined net effects are reflected in how many cells are present in the circulation at any given time. For leucocytes, homeostasis does not necessarily mean a constant number of cells in the circulation at all times but rather an oscillation with peak counts occurring during the behavioural rest phase of the organism.1 Thus, the appropriate number of circulating blood cells reflects the current need for specific cell populations at specific times.
In humans and mice, circulating leucocytes account for ∼3–10 × 106 cells per millilitre of blood. This relatively small pool lies in stark contrast to the huge cellular reservoirs of haematopoietic cells that reside within bone marrow, thymus, spleen, and lymph nodes from where cells can be readily released into blood in a process termed mobilization or egress. Thus, while circulating white blood cell counts are an important rheostat of altered homeostasis, disease states, and behavioural activity of the organism, a small difference in the amount of cells being released from or recruited to organs can have a dramatic effect on numbers, phenotypes, as well as cellular composition and may not necessarily reflect what is happening within individual organs. This also illustrates that circulating leucocytes represent cells in transition, in the process of migrating from one organ to another. Only few leucocyte subtypes such as neutrophils and monocyte subsets do not normally leave the circulation (or rather the intravascular compartment, as detailed below) during their lifetime under steady-state conditions. Since leucocytes do not undergo apoptosis in blood itself, the key input and output mechanisms of blood counts are mobilization and recruitment, respectively, which we will discuss below focusing on the individual cell populations.
Cellular egress into blood and the migration of cells into tissues are the result of extensive communication among organs. A local inflammatory reaction in the skin for example provides the cue for the bone marrow to enhance granulopoiesis and release mature neutrophils into the blood. In contrast, hypoxia or bleeding provides the necessary signals for the release of erythrocytes or platelets, respectively. The key communication system that relays this information between tissues is the vasculature itself, acting as transport vessels for numerous endocrine signals, as well as the autonomic nervous system which comprise opposing sympathetic and parasympathetic branches. Thus, local release of hormones or cytokines in peripheral tissues reaches the bone marrow via the bloodstream, whereas neural circuits provide signals outside the blood. The sympathetic nervous system (SNS) for example drives mobilization of cells from the bone marrow via local adrenergic output from nerve varicosities in that tissue.2 The parasympathetic nervous system (PNS), in turn, can provide long-distance anti-inflammatory reflexes in response to pathogen or injury as has been elegantly demonstrated in the spleen.3 Together, extensive signalling pathways between organs are responsible for keeping homeostasis in blood cell counts.
The bone marrow is the most important haematopoietic organ in the adult from which all blood cells are derived. Recent research indicates that different niches exist within the bone marrow that are responsible for the generation of specific haematopoietic cell subsets.4 While the niche for HSCs appears to be localized in close proximity to sinusoids and arterioles,5 the generation of B cells has been suggested to occur close to bone lining osteoblasts.6 Due to incomplete understanding of different niches for different haematopoietic lineages, the mechanisms of how distinct niches are targeted to induce specificity in the egress of haematopoietic subsets into blood are not well understood. It is evident, however, that some stimuli are more specific for the mobilization of certain haematopoietic lineages than others. This is an emerging concept in haematology and will not be further discussed here.
2.1. Haematopoietic stem and progenitor cells
Haematopoietic stem and progenitor cells (HSPCs) can circulate throughout the body after release from the bone marrow and percolate via blood and lymph to reach target organs.7 The physiological role of spontaneous HSPC mobilization is not entirely understood and may occur to replenish emptied niches and perform immunosurveillance of peripheral organs.7,8 In the context of inflammation, mobilized HSPCs can feed the spleen for further differentiation into myeloid cells that can then be deployed into the affected tissues.9 The possibility to mobilize haematopoietic cells at will has had impact in the clinic where HSPC mobilization has provided enormous benefits for bone marrow transplantation procedures. G-CSF is a cytokine that induces the bone marrow to produce granulocytes and HSPCs. In addition, it stimulates the release of these cells into the bloodstream, which is why it is currently the most commonly used mobilizing agent for HSPCs with its peak effect occurring 5–7 days after initial administration.10 This drug also mobilizes committed myeloid, megakaryocytic, as well as erythroid progenitors, indicating similar egress mechanisms for different myeloid lineages. For G-CSF-induced mobilization of HSPCs to occur, expression of the G-CSF-receptor (G-CSF-R) is required on haematopoietic cells. The precise nature of this haematopoietic cell had long been a matter of debate as G-CSF-R is found on a variety of cell types in the bone marrow, including neutrophils, monocytes as well as a broad range of progenitor cell types, which could explain some of the common egress pathways of these cells. Recent data now indicate the essential involvement of bone marrow macrophages in G-CSF-induced mobilization of HSPCs.11,12 Expression of G-CSF-R exclusively on CD68+ mononuclear phagocytes as well as depletion of CD169+ bone marrow macrophages has revealed the importance of these cells in the mobilization after G-CSF. Interestingly, the effect of G-CSF antagonizes macrophage function in the bone marrow HSPC niche as depletion of monocytes/macrophages results in the mobilization of HSPCs. This indicates a critical role for this cell type in the retention of stem and progenitor cells in the bone marrow.
G-CSF acts in part by inhibiting the ability of macrophages to support the expression of the HSPC retention factors CXCL12, VCAM-1, Kitl, and Angpt1 in Nestin+ niche cells. CXCL12 (also known as SDF-1) is the most important chemoattractant expressed in the bone marrow parenchyma, which is recognized by the chemokine receptor CXCR4, expressed throughout haematopoietic lineages from immature HSCs to mature myeloid and lymphoid cells. The CXCL12-CXCR4 chemokine axis therefore plays a predominant role in the retention/mobilization of most haematopoietic cells from the bone marrow (Figure 1). This interaction can be disrupted by using the CXCR4 antagonist AMD3100 (also known or plerixafor), which induces the mobilization of HSPCs and neutrophils13 by interfering with CXCR4-induced chemoattraction and adhesion within the bone marrow or other organs, as well as by preventing their emigration from the circulation.14 HSPCs can also be mobilized from the bone marrow during infection. Systemic infection of mice with Escherichia coli resulted in the deployment of HSPCs to the spleen and associated extramedullary haematopoiesis, which was dependent on expression of toll-like receptor (TLR) 4 and nucleotide-binding oligomerization domain-containing protein 1 (NOD1) on radio-resistant cells.15
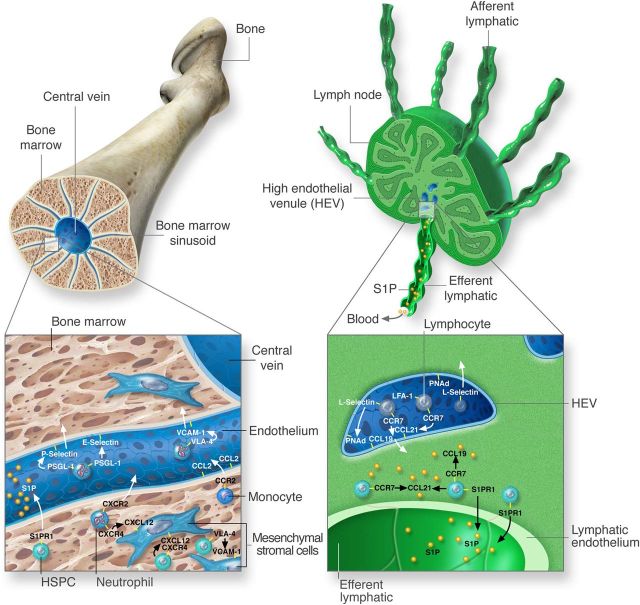
Figure 1.
Key pathways in the mobilization and recruitment of leucocytes. The key recruitment and mobilization pathways involved in the trafficking of leucocyte populations are exemplified for the bone marrow and lymph node. In the bone marrow (left), leucocytes are recruited from sinusoids via interactions with P- and E-selectin expressed on the endothelium and leucocyte glycoproteins such as PGSL-1. By rolling on the endothelium, leucocytes become activated via CXCR4-CXCL12 interactions and up-regulate the integrin VLA-4, which binds to vascular expressed VCAM-1, to migrate into the parenchyma. Within the bone marrow parenchyma, cells adhere via VLA-4 and CXCR4 with stromal cells expressing VCAM-1 and CXCL12, respectively. The function of CXCR2 can counteract the attractive forces of CXCR4 to induce mobilization in neutrophils. For monocytes, CCR2 detects CCL2 on sinusoidal endothelial cells for mobilization. An egress signal for the mobilization of HSPCs is S1P, which acts via the receptor S1PR1. Within lymph nodes (right) lymphocytes are recruited from blood due to interactions with molecules expressed on HEV. Key factors in this process are the chemokine receptor CCR7, which recognizes the chemokines CCL19 and CCL21. In addition, L-selectin as well as the integrin LFA-1 binds to peripheral node addressins (PNAd) and immunoglobulin superfamily members expressed on HEVs. For their egress, lymphocytes up-regulate S1PR1 and down-modulate the retention factor CCR7. S1PR1 detects higher concentration of S1P in efferent lymph and induces the immigration of cells into lymph and subsequently back into blood.
Vascular cell adhesion molecule (VCAM)-1 contributes to anchoring HSPCs to bone marrow stromal cells by engaging with the integrin very late antigen (VLA)-4 (α4β1; CD49d/CD29) expressed on haematopoietic cells. Consequently, interfering with this axis causes mobilization of HSPCs as shown by blockade of VCAM-1 or VLA-4 with antibodies16,17 (Figure 1). In addition, genetic ablation of VLA-4 or VCAM-1 results in the constitutive mobilization of HSPCs in the bone marrow, which is enhanced after G-CSF.10 Both the chemokine and adhesion molecule retention pathways are highly coordinated as engagement of CXCL12 present on the bone marrow vasculature by HSPCs results in a high affinity conformation of the integrin VLA-4 within the marrow space leading to increased retention of transfused HSPCs in the bone marrow.18 In contrast to the retention factors CXCL12 and VCAM-1 whose down-modulation induces the mobilization of HSPCs, the phospholipid sphingosine 1 phosphate (S1P) is an HSPC egress factor. S1P is an 18-carbon amino alcohol with an unsaturated hydrocarbon chain that is phosphorylated by sphingosine kinase. S1PR1, the main receptor for S1P, is expressed on HSPCs and is involved in HSPC mobilization in steady state. It also enhances mobilization in combination with plerixafor administration.19,20
While macrophages provide key retention factors for HSPCs, the SNS down-regulates the same factors to induce HSPC mobilization. Pharmacological inhibition or genetic ablation of catecholamine neurotransmission blunts bone marrow mobilization while agonism of β2 or β3 adrenergic receptors induces mobilization.2 Recent evidence points to a non-continuous mobilization of HSPCs from bone marrow into blood and to a rhythmic recruitment pattern back to the marrow (see below), both appearing to be responsible for the rhythmic oscillations of blood leucocyte counts.21 Together, macrophages as local haematopoietic cells and the SNS as an organism-wide neural circuit exert opposite functions in the mobilization of HSPCs from the bone marrow11 and are key players for regulating their homeostatic numbers in blood.
2.2. Neutrophils
Neutrophils are the most abundant circulating leucocytes in humans and represent a significant white blood cell fraction in mice. Mobilization of mature neutrophils from the bone marrow occurs at a dramatic rate of ∼1011 cells per day (107 in mice, respectively).22–26 However, this rate is further increased in an inflammatory setting as the bone marrow reserve of neutrophils can be mobilized in a matter of hours.22 This is in stark contrast to the mobilization of HSPCs, which occurs robustly after few days. Mature neutrophils that are about to be released reside next to bone marrow sinusoids from where they pass through the sinusoidal endothelium to reach the blood stream. In addition to the general mode of leucocyte migration into peripheral organs where leucocytes mostly migrate at the junction between endothelial cells, newly formed leucocytes can migrate into sinusoids transcellularly, by passing through the endothelial cell body independently of its junctions.27,28 As mentioned above, G-CSF also induces the mobilization of neutrophils from the bone marrow. This cytokine is also critical for proliferation and differentiation of myeloid cells. In contrast to G-CSF, CXCR4 inhibition via plerixafor may augment the frequency of circulating neutrophils through their release from the marginated pool present in the lung, while simultaneously preventing neutrophil return to the bone marrow.14 An interesting paradigm of how elimination of leucocytes in tissues communicates with their mobilization into blood to maintain homeostasis in blood is the regulation of granulopoiesis in the bone marrow by peripheral tissue-resident phagocytes and γδT cells. Dying neutrophils are taken up in tissues by phagocytes (in equal parts by the spleen, liver, and bone marrow25), which curbs the release of IL-23 (Figure 2). IL-23 induces IL-17 production by T cells, which in turn increases G-CSF levels and activates granulopoiesis.29 Therefore, phagocytosis of neutrophils in peripheral tissues may provide negative feedback for their mobilization from the bone marrow to fine tune neutrophil homeostasis in blood.
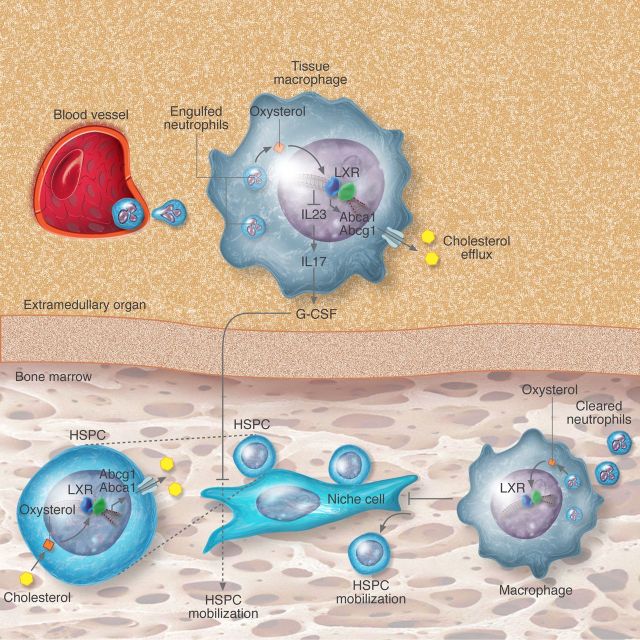
Figure 2.
Cholesterol metabolism and control of leucocyte homeostasis. Modified forms of cholesterol within haematopoietic cells (macrophages or haematopoietic progenitors; HSPC) function as agonists for LXR. Activation of these transcription factors then causes increased transcription of ABC transporters required for cholesterol efflux from the cells and trans-repression of inflammatory cytokines. When LXR or ABC transporters are absent, accumulation of cholesterol in the cells leads to elevated cytokine production (e.g. IL-23) or enhanced signalling through membrane-associated receptors (e.g. IL-3/GM-CSF-R or c-MPL) that promotes leucocyte mobilization, HSPC expansion or thrombocytosis, respectively. Regulation of these processes can occur in extramedullary sites (macrophages in tissues) or in the bone marrow (HSPC or megakaryocytic precursors).
Similar to HSPCs, neutrophils are retained in the bone marrow mainly through CXCL12-CXCR4 and VCAM-1-VLA-4 axes. In contrast, however, to the relatively slow process of mobilization with G-CSF, neutrophils are rapidly mobilized into blood in inflammatory scenarios. This is reflected in the rapid increase of blood neutrophil numbers after peripheral stimulation with the proinflammatory mediators leukotriene B4, C5a, and IL-8. In addition, the chemokines CXCL1 and CXCL2 induce rapid and selective mobilization of neutrophils.30 However, under conditions of hypercholesterolaemia CXCL1 can also induce the mobilization of inflammatory monocyte subsets (see below) from bone marrow and spleen.31 Consistent with these observations, studies from the Link and Rankin laboratories have established that CXCR2 and CXCR4 provide negative feedback for the homeostatic release of neutrophils into the circulation. In this model, CXCR4 signalling antagonizes that of CXCR2 to promote retention in the bone marrow, while predominant CXCR2 signalling induces rapid and efficient mobilization.32–34 Importantly, this mechanism also operates in the steady state as shown by the cell-autonomous retention of CXCR2-deficient neutrophils in bone marrow chimeras.32 These studies have illustrated that different mobilization mechanisms can operate during steady state and inflammatory conditions for neutrophils.
2.3. Monocytes
Monocytic lineages consist of macrophages as well as Ly6Chi inflammatory or classical and Ly6Clo non-inflammatory or non-classical monocytes. Macrophages are by definition cells that reside in tissues and are critical mediators for the mobilization of HSPCs as discussed above. They also represent important regulators of the erythroid niche in the bone marrow as erythroblast islands, formed by erythrocyte precursors surrounding a central macrophage, are depleted upon removal of CD169 macrophages, thereby reducing bone marrow erythroblasts.35 In their role as phagocytes in peripheral tissues they provide critical long-distance feedback for the egress of cell types from the bone marrow (discussed below). Ly6Chi monocytes express the chemokine receptor CCR2 on their surface and are thus able to respond to inflammatory signals consisting of the chemokine CCL2 (also known as MCP-1). This axis is critical for their deployment from the marrow as CCR2-deficient mice exhibit blood monocytopaenia and an accumulation of Ly6Chi monocytes in the bone marrow.36 Together with CXCR4, this chemokine receptor demonstrates the central role of chemokines in the spontaneous mobilization of myeloid cells. Interestingly, the response to CCL2 is not due to systemic levels of CCL2 in the blood stream. Instead, a local decoration of bone marrow sinusoids with the chemokine is necessary, into which inflammatory monocytes then enter (Figure 1). This mechanism is induced by low levels of TLR ligands such as TLR4, the receptor for the bacterial cell wall component LPS, which act on mesenchymal stromal cells to release CCL2.37
In addition to these models of pathogen-associated molecular pattern (PAMP)-induced mobilization, sterile acute myocardial infarction can induce the release of Ly6Chi monocytes from the bone marrow.38 This is dependent on mature B cells, which selectively release the chemokine CCL7 that in turn induces monocyte mobilization.39 An additional reservoir for monocytes is the spleen, which is highly sensitive to inflammation and can rapidly mobilize these cells. The current paradigm suggests that the bone marrow is the primary organ to maintain monocyte numbers in the circulation in steady state, whereas the spleen contributes to their release under inflammatory conditions.40 In a manner analogous to neutrophils in the bone marrow, the splenic reservoir of monocytes is clustered in cords within the subcapsular red pulp. Ischaemic myocardial injury increases the motility of monocytes within this compartment and induces their mobilization via an angiotensin II-mediated mechanism. Splenic monocytes express the angiotensin type-1 receptor on their surface and are thus specifically able to react to this signal.41 Interestingly, myocardial infarction additionally induces the egress of HSPCs from the bone marrow via SNS signalling pathways. HSPCs then seed the spleen, yielding a sustained boost in monocyte production.9 These data underline the intricate relationships between various leucocyte subsets in different tissues and the extensive means of communication between them.
2.4. Lymphocytes
In a manner similar to myeloid cells, down-modulation of CXCR4 and VLA-4 is thought to play the major role in the egress of B lymphocytes from the bone marrow. However, interfering with either the CXCR4-CXCL12 or the VCAM-1-VLA-4 axis induces the release of immature B cell progenitors to a greater extent than mature B cells, indicating mechanistic differences in the mobilization at different stages of maturation.42 For the retention of immature B cells in the bone marrow, the VCAM-1-VLA-4 axis seems to play a more dominant role than CXCR4. Instead, the Gαi-coupled cannabinoid receptor 2 is required for the retention of these cells in sinusoids.43 In keeping with the prominent role of this signalling axis in general B cell egress into the bloodstream, maturation along the B cell lineage is accompanied by diminished function of CXCR4 which may be important for the relocalization of maturing B cells within the bone marrow or mobilization into blood.44
In contrast to the other haematopoietic lineages, the central organ for the generation of mature T lymphocytes and their release into blood is the thymus. T-cell progenitors leave the bone marrow to seed the thymus, where they undergo differentiation and are being released into the circulation as mature single-positive CD4+ or CD8+ T cells. A critical axis involved in the egress of lymphocytes from organs into blood is S1P and its receptors S1PR1-5.45 S1P levels exhibit a strong concentration gradient between blood and tissues, which is essential for egress of lymphocytes from tissues, being highest in blood (∼1 μM) and lowest in organs (∼1 nM). Fully mature T and B cells express the S1P-receptor S1PR1, which is the main S1P receptor for lymphocyte egress as cells deficient in this molecule fail to leave the thymus and lymph nodes resulting in severe lymphopenia. S1P also promotes the egress of lymphocytes from splenic white to red pulp and back into the circulation.46
The egress mechanisms of lymphocytes have been investigated in most detail in the lymph node due to better accessibility for in vivo imaging techniques. In contrast to the bone marrow, thymus or spleen, egress of cells into blood from lymph nodes is not direct but occurs via the lymph. For most of the body (except the right arm) lymph drains into the thoracic (or left lymphatic) duct, which at the level of the subclavicular bone merges with blood vessels allowing cells to reach the blood circulation. Therefore, egress from lymph nodes into blood is not immediate but occurs with a delay. In addition, this means that cells must migrate across lymphatic endothelial cells to reach the blood. S1P provides the egress signal via S1PR1 for lymphocytes in the lymph node, whereas chemokine receptors such as CCR7 provide retention signals and are critical for their recruitment (discussed below) (Figure 1). T cell activation (e.g. in response to infection) delays lymphocyte egress and provides the necessary time for specific T-cell clones to find their cognate antigen and proliferate. This is due to a combination of enhanced S1P levels in tissues, which desensitizes S1P receptors, and the up-regulation of activation markers such as CD69, which counteracts S1PR1 signalling.45 In the clinic, functional antagonism of S1PRs is induced by treating multiple sclerosis patients with FTY720 (also known as fingolimod), a drug that inhibits lymphocytes from leaving lymph nodes, from entering the circulation and infiltrating the CNS.47 Recent data indicate the importance of the SNS in the retention of lymphocytes within lymph nodes: Adrenergic signals via the β2-adrenergic receptor enhance retention-promoting signals through CCR7 and CXCR4, and consequently inhibit lymphocyte egress from lymph nodes.48 These data demonstrate the central importance of G-protein-coupled receptor signalling for lymphocyte homeostasis in blood.
2.5. Platelets and erythrocytes
Platelets and erythrocytes play important roles in the regulation of blood leucocyte homeostasis due to interactions with adherent leucocytes, which can promote vascular injury.49,50 Several millions of platelets are produced every hour by bone marrow megakaryocytes. Megakaryocytes are localized in the perivascular environment of bone marrow sinusoids where they form long, dynamic pseudopods that reach across endothelial cells into the blood stream.51 CXCL12 is an important chemokine for the positioning of megakaryocytes next to bone marrow sinusoids.52 In addition, thrombopoietin (THPO), which is produced in the liver and kidneys induces their production and differentiation and is thus the most critical hormone for thrombopoiesis. S1P is also a critical cue during thrombopoiesis as it is needed for the shedding of platelets into blood. Mice lacking S1PR1 exhibit severe thrombocytopenia due to defects in shedding and formation of pro-platelets.53 The S1P gradient between blood and bone marrow parenchyma is important to drive polarized pro-platelet extension of megakaryocytes into the sinusoidal lumen via S1PR1 expression on megakaryocytes. Mature megakaryocytes form intravascular pro-platelet extensions that grow from the megakaryocyte body at a rate of 10 µm/min under shear. The immunosuppressive drug FTY720 leads to shedding of intravascular pro-platelet extensions into the blood stream, paralleled by an acute but transient increase in circulating platelets. This suggests that FTY720 acts as an agonist for megakaryocytic S1PR1 and has the potential to rapidly mobilize pro-platelets into the blood, most likely by supporting fragmentation of intravascular pro-platelets.
Erythropoietin (EPO) is the principal hormone promoting erythropoiesis. Similar to THPO, it is produced in the liver and kidneys. While the liver is the major producing organ during development, the kidneys are the main producers of EPO during adulthood. EPO levels in the circulation are relatively low in steady state. However, during periods of hypoxic stress, the levels can increase up to a 1000-fold. The fates of erythrocytes and leucocytes are highly intertwined as demonstrated by diseases of altered red blood cell production, such as is the case in the red blood cell disorders sickle cell disease, β-thalassaemia, and polycythemia vera. In these conditions, blood leucocyte counts and functions are changed, which contributes to clinical manifestations.54
3. Recruitment and clearance of cells from the circulation
Together with the mechanisms regulating the influx of leucocytes, erythrocytes, and platelets into the bloodstream, those that control their emigration back into the tissues are equally essential to confer homeostasis in blood cell numbers. The efflux of blood cells into haematopoietic, lymphoid, or other tissues serves three major purposes: definitive elimination, replenishment, and recirculation of cells. The first accounts for the necessity to eliminate cells that have accumulated structural or other alterations during their transit in blood and are no longer functional (e.g. erythrocytes, platelets, or neutrophils). Replenishment of leucocytes is needed in the case of leucocyte subtypes that do not self-renew in tissues. Finally, recirculation allows various leucocyte subsets to survey tissues in search of danger signals and/or in search of appropriate environments to carry out their functions, as is the case of lymphocytes and haematopoietic precursors.
The basic mechanisms that guide leucocyte extravasation into tissues were largely defined over two decades ago and summarized by Butcher and Springer as a series of sequential and distinct molecular interactions between the leucocyte and the endothelial cells that coat the vessels.55,56 These interactions are initiated by pairs of lectin-type receptors and their glycoconjugate ligands present on leucocytes and endothelial cells (Figure 1): L-selectin on leucocytes and P- or E-selectins on endothelial cells recognize sialyl-Lewis x, a tetrasaccharide structure presented by specific glycoproteins including PSGL-1, CD44, and GlyCAM-1. That selectins display very high affinity but low avidity for their ligands57 is particularly relevant for the initial capture or tethering of cells under the conditions of high shear stress present in most blood vessels. This relevant role in early leucocyte egress from blood is illustrated by alterations in spleen size or leucocyte counts in mice lacking one or more of the selectins or other adhesion receptors.29,58,59 Subsequent steps in the extravasation process include the activation of β1 and β2 integrins induced by various chemokines or upon selectin binding, firm engagement of immunoglobulin-superfamily ligands present on the endothelial surface, leucocyte crawling, and finally transmigration between or across endothelial cells into the tissue (reviewed in more detail in Ref. 60 and 61). Although this sequence of steps has been defined almost exclusively during inflammation, a context in which many of the relevant chemokines and receptors are strongly up-regulated, it is likely that similar mechanisms mediate leucocyte egress under steady-state conditions as described below. For example, the bone marrow, skin, and lymph node microvasculatures display constitutive expression of many of the molecules required for extravasation and leucocytes are therefore continuously found rolling and arrested on these vessels.62–65 A subset of patrolling monocytes has also been shown to adhere to and crawl on resting vascular beds in the absence of infectious or inflammatory stimuli.66
Below, we discuss basic mechanisms of recruitment and clearance of the different blood cells into their preferred target organs, and when relevant, we describe how this process varies during steady-state or inflammatory conditions.
3.1. HSPCs
As indicated above, levels of haematopoietic precursors in the circulation oscillate according to circadian cycles. While the mechanisms underlying HSPC mobilization (spontaneous or induced by various agents) have been thoroughly studied because of its therapeutic potential for transplantation procedures, those regulating their migration to extramedulary tissues remain largely unexplored. In contrast, interest in understanding the cues that guide their migration back to the bone marrow has been fostered by the potential benefit of improving engraftment when the number of donating progenitors are limiting, as is the case when cord blood units are used for transplantation or with poorly mobilizing patients.67 For this reason, there is thorough knowledge of the chemotactic and adhesive pathways regulating their homing to the bone marrow. Predominant chemotactic and adhesive cues in mice and humans are those mediated by CXCR4 and VLA-4 expressed on HSPCs,18 which recognize CXCL12 and VCAM-1, respectively. These molecules are complemented by endothelial selectins,68 which are constitutively expressed in the bone marrow microvasculature.18,21,69 Evidence that the CXCR4/CXCL12 interaction regulates the directionality of HSPC migration (blood to bone marrow, or bone marrow to blood) comes from studies showing that the expression of CXCR4 on HSPC is regulated by the circadian clock and is up-regulated at those times when the progenitors naturally adhere to bone marrow microvessels and migrate to this organ,69,70 which coincides with elevated expression of its cognate ligand in the marrow.21,71 These studies highlighted a coordinated action of the migrating cells and the target organ for efficient clearance of HSPCs from the circulation. The question remains as to the physiological cues that underlie the specific increase of the receptor and ligand that promote the natural reductions of HSPCs in the circulation. A major regulatory pathway in the bone marrow appears to be catecholamines delivered by local nerves and signalling through the β3-adrenergic receptor, as demonstrated by the finding that pharmacological stimulation enhances expression of relevant adhesive pathways by the medullary microvasculature and promotes HSPC homing.69 An additional mechanism controlling the steady-state reductions of HSPC from blood is the elevation in niche ‘activity’ within the bone marrow induced by tissue-resident macrophages,11,72 whose function is in turn regulated in a time-dependent manner by the presence of senescent neutrophils cleared from blood.71
Although the bone marrow is the major organ for HSPC release and recruitment, haematopoietic precursors can be also found in multiple organs in the steady state, including the spleen, liver, and fat tissues.7 The contribution of these organs in regulating HSPC numbers in the circulation is unclear, however, and deserves further study. As discussed above, HSPCs with long-term reconstituting capacity are also found in lymph. Experiments in parabiotic models concluded that they are derived from the circulation and reach the lymphatics through extramedullary tissues rather than entering through the lymph nodes.7 These studies revealed that, similarly to lymphocytes, the recirculation of HSPCs from tissues into lymph depends on S1PR1, while the return of progenitor cells from blood back into the bone marrow occurs through the canonical CXCR4/CXCL12 pathway described above.
3.2. Neutrophils
As in the case of HSPCs, neutrophils are most efficiently cleared from the circulation at specific times of the day. This natural clearance of neutrophils from the blood is believed to result in degradation within bone marrow, liver, and spleen,23 organs that are specialized in the turnover of blood cells. Other organs are also possibly involved in the clearance of neutrophils, but this has not been explored in detail. A peculiarity of neutrophils, compared with other blood populations, is their short lifetime in the circulation (∼12 h),73 and a high reactivity against danger signals that allows them to rapidly respond to pathogens, but is in turn an underlying factor of vascular inflammation.74 Both features are likely to have driven an evolutionary selection for efficient mechanisms for neutrophil disposal. Interestingly, the elimination of neutrophils from blood is preceded by specific molecular and morphological alterations collectively defined as ‘ageing,’71 including increases in adhesion receptors such as CD11b, CD49d, and CXCR4 that facilitate their migration into peripheral tissues and the bone marrow.34,69,71 As indicated for HSPCs, these studies suggested a coordinated program between neutrophils (cell intrinsic) and the surrounding tissues (environmental) that promote their homeostatic elimination from blood. While the migration to the marrow clearly involves CXCR4 signalling,34,71 the mechanisms guiding elimination in other tissues are less clear. β2 integrins together with endothelial selectins are likely adhesive mediators of the emigration of neutrophils from blood in the absence of inflammation, as evidenced by the marked neutrophilia displayed by mice mutant in these pathways.29 In contrast, the chemotactic gradients that promote the extramedullary clearance of neutrophil have not been defined. It is possible, however, that CXCR2—a prominent chemokine receptor on these cells—plays an active role in the process, although most data related to the functions of CXCR2 have been obtained in the context of inflammation,75 and different receptors may be specialized for migration into specific organs.76
Another peculiarity of neutrophils is the dynamic fluctuations of their number in blood, which may not necessarily involve extravasation and require instead firm adhesion on the vessel wall, resulting in their removal from the circulation. This process, referred to as margination, is thought to occur in organs endowed with a rich network of small capillaries such as the lung and the liver.77 Recent data have demonstrated that the reverse process, or de-margination, from pulmonary vessels actually accounts for the rapid mobilization of neutrophils into blood in response to CXCR4 antagonists but, interestingly, not in response to G-CSF.14 This suggests that the CXCR4/CXCL12 pathway is important in the control of neutrophil numbers even in extramedullary tissues.
Although neutrophils are believed to be eliminated and die in the tissues that they infiltrate during active inflammation, evidence obtained in zebrafish and mice suggest that a fraction can return back to the vasculature and into the circulation.78,79 Although in mice this represented a small proportion of cells that could be recruited to secondary inflammatory sites,79 the contribution of this phenomenon to balancing neutrophil numbers and/or promoting distant inflammation remains to be determined.
3.3. Monocytes
As previously indicated, two subsets of monocytes are found in the circulation.80 The less abundant, non-inflammatory subset can be found crawling on vascular beads even in the absence of injury.66,81 This patrolling behaviour allows encounter with damaged cells or pathogen that results in the uptake of the foreign body and restoration of endothelial cell and vascular homeostasis.82 Mechanisms regulating the removal of these cells from the circulation are poorly defined. In contrast, the mechanisms regulating the natural egress of Ly6Chi CX3CR1lo inflammatory monocytes from the circulation have been recently identified using elegant experimental approaches. Expression of CCR2, one of the major chemokine receptors in inflammatory monocytes, is repressed by the concerted action of Bmal1, a component of the circadian clock, and the polycomb repressor complex.81 When Bmal1 levels are low, or in mice with myeloid-specific deficiency in this gene, CCR2 expression is constitutively high and causes an elevated migration of these cells into tissues. The relevance of these findings in the context of inflammation was illustrated by the enhanced response of the myeloid-specific Bmal1-deficient mice to bacterial infection and their susceptibility to metabolic disease.81 These studies revealed that at least some of the molecular pathways underlying homeostatic clearance and inflammatory responses in specific leucocyte subsets are similar, and that mechanisms have evolved to quantitatively modulate these pathways to ensure blunted or enhanced migration as needed. Interestingly, a recent study reported that these inflammatory monocytes actively recirculate between blood, tissues, and lymphoid organs,83 suggesting that the levels of monocytes in circulation could be dynamically regulated by their differential dwell times in various compartments.
3.4. Lymphocytes
The basic mechanisms governing lymphocyte recirculation were elucidated almost 50 years ago through the pioneering work of Gowans and colleagues.84 The mechanisms of natural lymphocyte recirculation from the blood into the different tissues markedly differ from that of myeloid cells and are driven by the need of the various lymphocyte subsets to search for rare antigens that will initiate their activation and immunological response.
Guided mostly by intravital imaging of the vessels that feed lymph nodes85 and supported by in vitro assays using flow chambers,86 the processes by which lymphocytes leave the bloodstream are now well understood. Egress of lymphocytes from blood typically occurs by engagement of dedicated ligands on the surface of high endothelial venules (HEV) on secondary lymphoid organs (SLO), which comprise a specialized endothelium that constitutively expresses sulfated Lexis × glycoproteins that are recognized by L-selectin. Peyer's Patches additionally express MadCAM-1, which is recognized by the α4β7 integrin.87,88 Interactions mediated by these ligands initiate a rolling-like motion that facilitates secondary interactions between subset-specific chemokine receptors (mainly CCR7, the receptor for the chemokines CCL19 and CCL21; but also CXCR4 on B cells) and its cognate ligands presented on the surface of HEV which trigger arrest mediated by LFA-1 (αLβ2; CD11a/CD18), and subsequent transendothelial migration (Figure 1).87 As discussed earlier, if naïve lymphocytes do not encounter their cognate ligand in a specific SLO, they will gain access to efferent lymphatic vessel and return to the circulation through the thoracic duct to restart a new cycle. This unique recirculatory migration pattern conditions their numbers in blood, but it is unclear how different checkpoints in each tissue may regulate their numbers in the circulation in the steady state or under conditions of infection or inflammation. The use of agonists for S1PR1 that efficiently impair the function of the receptor has demonstrated the essential dependence of homeostatic lymphocyte trafficking on the S1P-S1PR1 axis.89
Once activated, T and B cells gain new migratory properties that allow their migration to specific tissues. Interestingly, this specificity depends on the SLO where they were primed and the mediators produced by tissue-specific dendritic cells. For example, activation in gut-associated lymphoid structures promotes tropism for the gut by inducing expression of CCR9 and α4β7. In contrast, activation in other SLOs creates a skin-homing profile mediated by the induction of P- and E-selectin ligands and CCR4.90–92
3.5. Platelets and erythrocytes
Platelets circulate in the bloodstream for relatively short times (∼10 days in humans, 5 days in mice) before they are eliminated in the spleen and liver.93 It has been argued that this prompt elimination could be explained, because the high reactivity of platelets in adverse conditions could generate fatal disseminated thrombosis. For example, cold induces rapid clearance of platelets, probably to prevent thrombotic events when the organism's temperature decreases.94 This notion has been better studied during bacterial infections. In this context, platelet clearance caused by bacterial neuraminidase exposes de-sialylated structures that are recognized by the Ashwell receptor in the liver, and clearance in this setting has been clearly shown to protect the organism from coagulopathy.95 Intriguingly, other forms of platelet activation, such as thrombin stimulation, do not alter their lifetime in circulation.96 Massive platelet depletion can cause serious bleeding complications as seen in certain pathologies such as ITP or thombotic thrombocytopenic purpura (TTP). Studies in patients with ITP, or in patients transfused with platelets stored in cold, have shown that opsonizing anti-platelet antibodies or surface glycan modifications mediate their premature recognition and engulfment by liver or splenic macrophages.94,97 In certain models of inflammation, platelet numbers moderately but rapidly decrease as they accumulate in specific intravascular compartments or are captured by activated neutrophils.49,98,99 Recent studies have highlighted the expression and function of TLRs (most prominently TLR3, TLR4, and TLR7) as sensors of pathogens or injury that can initiate platelet activation and clearance from the circulation, either by phagocytosis or through deposition in vascular beds, and that can further promote an efficient innate immune response against the microbe.100–102 In contrast to these well-characterized processes of clearance associated to pathological conditions, the mechanisms that mediate the spontaneous elimination of aged platelets from blood are not completely understood. There is however evidence that platelet ageing is accompanied by appearance of phosphatidylserine moieties on the cell surface or loss of mitochondrial inner potential, which can mark the cells for natural elimination.103 Beyond their haemostatic functions, platelets can also participate in regulating leucocyte migration, as demonstrated by their capacity to enhance lymphocyte rolling on SLO104 and to stimulate intravascular crawling and migration of neutrophils during inflammation.105
Because erythrocytes are produced at a remarkable rate of 1010 per hour and do not actively extravasate, a critical aspect in regulating homeostatic number of erythrocytes is their efficient removal from the circulation when their functional capacity declines. The lifespan of erythrocytes (∼120 days in humans; 60 days in rodents) is uniform within a given species. This is determined by the removal of ‘aged’ erythrocytes from the circulation by macrophages present in filter organs,106 which occurs at a calculated rate of 5 million cells per second in humans. Macrophages in the red pulp of the spleen are responsible for the bulk of erythrocyte clearance,107 and although the recognition of aged erythrocytes involves specific receptor-ligand pairs, the unique structure of splenic sinuses also facilitates their clearance on the basis of their physical properties.108 The prevailing theory explaining how erythrocytes are marked for destruction states that molecular changes that accumulate on circulating cells drive their specific recognition and engulfment by macrophages.106 The critical alterations that appear over time include changes in the anti-oxidative capacity of the cells, leading to the appearance of oxidized proteins (most prominently haemoglobin),109 clustering of the protein Band3 and exposure of phosphatidylserine, all of which can be recognized and opsonized by naturally occurring antibodies and complement proteins, or bridging molecules106,110 that are finally recognized by specific receptors on filtering macrophages in the spleen or liver. Additionally, loss of functional CD47, a ‘don't-eat-me’ signal expressed by multiple cell types, appears to promote the engulfment of aged erythrocytes.106,107
Finally, alterations in the structural properties of major erythrocyte proteins, haematological stress, and chronic inflammation normally result in accelerated removal of erythrocytes from the circulation, as illustrated by the dramatic reduction in the lifespan of erythrocytes in models of sickle cell disease or β-thalassaemia.111 Interestingly, inflammatory signals originating by chronic activation of the vascular beds in the context of sickle cell disease (caused by a mutation present only in erythrocytes) result in marked increases in leucocyte numbers and activation.112 These studies illustrate the multiple levels of interactions that exist among circulating cells that result in mutual regulation of their levels in the circulation.
4. Regulation of leucocyte homeostasis by cholesterol metabolism
A prominent and clinically relevant example of how leucocyte numbers are regulated in blood, and may contribute to inflammatory disease, comes from the study of atherosclerosis. This chronic disease of the vascular wall of medium to large arteries is a primary cause of myocardial infarction and stroke.113 The formation and growth of the atherosclerotic plaque tightly correlate with hypercholesterolaemia in patients, and a formal link between both parameters was discovered by studies showing that lipid-lowering drugs significantly reduce major ischaemic events,114 and that human populations or animals with naturally low levels of cholesterol in the form of low-density lipoproteins are protected from cardiovascular disease.115,116 Similarly, circulating leucocyte counts show a predictive correlation with cardiovascular events,117 and in animal models neutrophil and monocyte counts correlate with lesion burden.118,119 Lipid-lowering drugs that reduce cholesterol levels also result in reduced leucocyte counts.120 These studies in the context of atherosclerosis suggested a possible connection between cholesterol handling and the homeostasis of circulating leucocytes.
Intracellular cholesterol homeostasis is orchestrated by liver X receptors (LXR) (Figure 2). The two members of this family of nuclear receptors, LXRα and LXRβ, are endogenously activated by modified forms of cholesterol and control the expression of genes important for cholesterol uptake, transport, and efflux.121 Among these genes, the ATP-binding cassette (ABC) transporters Abca1 and Abcg1 mediate cholesterol efflux.122 Studies in mice deficient in Abca1 and Abcg1 showed that they display elevated blood neutrophil counts123 and marked expansion and proliferation of HSPCs.124 Molecular analyses furthermore demonstrated that the haematopoietic expansion was caused by increased Erk-mediated signalling and expression of the common β subunit of the IL-3/GM-CSF-receptors, and that downstream up-regulation of the PU.1 transcription factors introduced a differentiation bias towards myeloid lineages.124 While these effects were intrinsic to HSPCs, both ABC transporters are also important for cholesterol handling in differentiated myeloid leucocytes. Studies in mice with macrophage- and dendritic cell-specific deficiency in Abca1 and Abcg1 demonstrated that cholesterol efflux in these cells was important to maintain homeostatic production of IL-23. Systemic elevation of this cytokine resulted in activation of the IL-17/G-CSF axis in these mice which in turn inhibited stromal niche cells in the bone marrow, causing local reductions in CXCL12 that promoted the release of HSPCs into blood.125 Interestingly, a different ABC transporter specifically expressed in megakaryocytic progenitors, Abcg4, was shown to control cholesterol efflux in these cells and to regulate thrombopoiesis.126 Deficiency in the receptor caused abnormal activation of the Lyn kinase and c-CBL, leading to abnormal recycling of c-MPL, the receptor for THPO, and resulting in elevated production of platelets. Interestingly, treatment with a recombinant form of high-density lipoprotein to stimulate cholesterol efflux normalized platelet counts in the blood of these mice.126
LXR receptors, on the other hand, have also been associated with the homeostatic control of at least two other leucocyte populations, neutrophils and HSPCs. Mice deficient in both LXR receptors display increased neutrophil counts in blood, spleen, and liver, which were caused in part by impaired clearance of neutrophils from the circulation.127 These studies revealed that engulfment of cleared neutrophils in extramedullary tissues activates LXR receptors on phagocytes and curbs the expression of IL-23, and the downstream IL-17 and G-CSF effector cytokines, resulting in blunted production and release of neutrophil into blood.29,127 Phagocytosis of cleared neutrophils also occurs in the bone marrow,23,128 where haematopoietic niches are located. However, the consequences of clearance in this tissue were shown to differ from those described above. Marrow-resident macrophages efficiently engulf cleared neutrophils, resulting in activation of LXR and up-regulation of Abca1 gene expression. Activation of this pathway resulted in reductions in the number of stromal cells producing CXCL12 and concomitant release of HSPC into the circulation.71 Because LXR receptors in both cases are likely activated by recycled cholesterol molecules derived from the engulfed cells,99 these studies highlight the dependence of circulating leucocyte homeostasis on cholesterol metabolism. Whether similar mechanisms operate for the regulation of other leucocyte populations in blood is presently unknown. More broadly, these studies illustrate the various mechanisms by which cholesterol handling and appropriate disposal regulate the homeostatic production and release of blood cells, and identify therapeutic opportunities to reverse alterations in blood composition that may be associated with atherosclerosis and other haematological diseases.
4.1. Concluding remarks
As a tissue of easy access, blood has been for a long time—and continues to be—a preferred window for physicians to interrogate the body's condition. We have summarized here studies illustrating that alterations in blood cell composition are in fact a reflection of intricate pathways that aim to preserve homeostasis; leucocytes enter and egress the circulation for a purpose that often meets a demand-supply equilibrium, either because of an environmental stress (e.g. injury or infection) or because aged elements need to be replaced by new functional ones. Even in the absence of environmental stress, various mechanisms are at play to ensure that the optimal number of cells is present in the circulation. For many leucocyte subsets, this does not mean constant numbers, as the blood displays like other tissues circadian variations that are believed to anticipate normal changes in the environment. At the same time, blood composition is also conditioned by socioenvironmental stressors that are part of modern lifestyle. Repeated stress causes alterations in the bone marrow (e.g. increased myelopoiesis), which may be relayed from the blood into peripheral tissues to ultimately promote disease.129 Thus, beyond its use in every-day clinical practice, understanding the mechanisms that maintain blood homeostasis may reveal new secrets about the function and dysfunction of any tissue of the body.
Acknowledgements
C.S. is supported by an Emmy-Noether-grant from the German Research Foundation (DFG; SCHE 1645/2-1). P.S.F. is supported by grants from the US National Institutes of Health (NIH; R01 grants DK056638, HL069438, HL116340), the Leukemia and Lymphoma Society, and the New York State Department of Health (NYSTEM Program). A.H. is supported by SAF2012-31142 from Ministerio de Economia y Competitividad (MINECO), S2010/BMD-2314 from Comunidad de Madrid. The CNIC is supported by the MINECO and the Pro-CNIC Foundation.
Conflict of interest: none declared.
References
- 1.Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol 2013;13:190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 2006;124:407–421. [DOI] [PubMed] [Google Scholar]
- 3.Andersson U, Tracey KJ. Reflex principles of immunological homeostasis. Annu Rev Immunol 2012;30:313–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature 2014;505:327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, Mar JC, Bergman A, Frenette PS. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 2013;502:637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu J, Garrett R, Jung Y, Zhang Y, Kim N, Wang J, Joe GJ, Hexner E, Choi Y, Taichman RS, Emerson SG. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood 2007;109:3706–3712. [DOI] [PubMed] [Google Scholar]
- 7.Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, von Andrian UH. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell 2007;131:994–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science 2001;294:1933–1936. [DOI] [PubMed] [Google Scholar]
- 9.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M. Myocardial infarction accelerates atherosclerosis. Nature 2012;487:325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenbaum AM, Link DC. Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia 2011;25:211–217. [DOI] [PubMed] [Google Scholar]
- 11.Chow A, Lucas D, Hidalgo A, Mendez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, Tanaka M, Merad M, Frenette PS. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med 2011;208:261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christopher MJ, Rao M, Liu F, Woloszynek JR, Link DC. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J Exp Med 2011;208:251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB, Calandra G, Bridger G, Dale DC, Srour EF. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med 2005;201:1307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devi S, Wang Y, Chew WK, Lima R, A-González N, Mattar CN, Chong SZ, Schlitzer A, Bakocevic N, Chew S, Keeble JL, Goh CC, Li JL, Evrard M, Malleret B, Larbi A, Renia L, Haniffa M, Tan SM, Chan JK, Balabanian K, Nagasawa T, Bachelerie F, Hidalgo A, Ginhoux F, Kubes P, Ng LG. Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. J Exp Med 2013;210:2321–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burberry A, Zeng MY, Ding L, Wicks I, Inohara N, Morrison SJ, Nunez G. Infection mobilizes hematopoietic stem cells through cooperative NOD-like receptor and Toll-like receptor signaling. Cell Host Microbe 2014;15:779–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Craddock CF, Nakamoto B, Andrews RG, Priestley GV, Papayannopoulou T. Antibodies to VLA4 integrin mobilize long-term repopulating cells and augment cytokine-induced mobilization in primates and mice. Blood 1997;90:4779–4788. [PubMed] [Google Scholar]
- 17.Papayannopoulou T, Craddock C, Nakamoto B, Priestley GV, Wolf NS. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc Natl Acad Sci USA 1995;92:9647–9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peled A, Grabovsky V, Habler L, Sandbank J, Arenzana-Seisdedos F, Petit I, Ben-Hur H, Lapidot T, Alon R. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34(+) cells on vascular endothelium under shear flow. J Clin Invest 1999;104:1199–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golan K, Vagima Y, Ludin A, Itkin T, Cohen-Gur S, Kalinkovich A, Kollet O, Kim C, Schajnovitz A, Ovadya Y, Lapid K, Shivtiel S, Morris AJ, Ratajczak MZ, Lapidot T. S1P promotes murine progenitor cell egress and mobilization via S1P1-mediated ROS signaling and SDF-1 release. Blood 2012;119:2478–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juarez JG, Harun N, Thien M, Welschinger R, Baraz R, Pena AD, Pitson SM, Rettig M, DiPersio JF, Bradstock KF, Bendall LJ. Sphingosine-1-phosphate facilitates trafficking of hematopoietic stem cells and their mobilization by CXCR4 antagonists in mice. Blood 2012;119:707–716. [DOI] [PubMed] [Google Scholar]
- 21.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature 2008;452:442–447. [DOI] [PubMed] [Google Scholar]
- 22.Furze RC, Rankin SM. Neutrophil mobilization and clearance in the bone marrow. Immunology 2008;125:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furze RC, Rankin SM. The role of the bone marrow in neutrophil clearance under homeostatic conditions in the mouse. FASEB J 2008;22:3111–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pitchford SC, Furze RC, Jones CP, Wengner AM, Rankin SM. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell 2009;4:62–72. [DOI] [PubMed] [Google Scholar]
- 25.Rankin SM. The bone marrow: a site of neutrophil clearance. J Leukoc Biol 2010;88:241–251. [DOI] [PubMed] [Google Scholar]
- 26.Wengner AM, Pitchford SC, Furze RC, Rankin SM. The coordinated action of G-CSF and ELR + CXC chemokines in neutrophil mobilization during acute inflammation. Blood 2008;111:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burdon PC, Martin C, Rankin SM. Migration across the sinusoidal endothelium regulates neutrophil mobilization in response to ELR + CXC chemokines. Br J Haematol 2008;142:100–108. [DOI] [PubMed] [Google Scholar]
- 28.De Bruyn PP, Michelson S, Thomas TB. The migration of blood cells of the bone marrow through the sinusoidal wall. J Morphol 1971;133:417–437. [DOI] [PubMed] [Google Scholar]
- 29.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 2005;22:285–294. [DOI] [PubMed] [Google Scholar]
- 30.Hosking MP, Liu L, Ransohoff RM, Lane TE. A protective role for ELR+ chemokines during acute viral encephalomyelitis. PLoS Pathogens 2009;5:e1000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soehnlein O, Drechsler M, Doring Y, Lievens D, Hartwig H, Kemmerich K, Ortega-Gomez A, Mandl M, Vijayan S, Projahn D, Garlichs CD, Koenen RR, Hristov M, Lutgens E, Zernecke A, Weber C. Distinct functions of chemokine receptor axes in the atherogenic mobilization and recruitment of classical monocytes. EMBO Mol Med 2013;5:471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest 2010;120:2423–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eash KJ, Means JM, White DW, Link DC. CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood 2009;113:4711–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity 2003;19:583–593. [DOI] [PubMed] [Google Scholar]
- 35.Chow A, Huggins M, Ahmed J, Hashimoto D, Lucas D, Kunisaki Y, Pinho S, Leboeuf M, Noizat C, van Rooijen N, Tanaka M, Zhao ZJ, Bergman A, Merad M, Frenette PS. CD169(+) macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat Med 2013;19:429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol 2006;7:311–317. [DOI] [PubMed] [Google Scholar]
- 37.Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity 2011;34:590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ingersoll MA, Platt AM, Potteaux S, Randolph GJ. Monocyte trafficking in acute and chronic inflammation. Trends Immunol 2011;32:470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zouggari Y, Ait-Oufella H, Bonnin P, Simon T, Sage AP, Guerin C, Vilar J, Caligiuri G, Tsiantoulas D, Laurans L, Dumeau E, Kotti S, Bruneval P, Charo IF, Binder CJ, Danchin N, Tedgui A, Tedder TF, Silvestre JS, Mallat Z. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med 2013;19:1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bronte V, Pittet MJ. The spleen in local and systemic regulation of immunity. Immunity 2013;39:806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science (New York, NY) 2009;325:612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park SY, Wolfram P, Canty K, Harley B, Nombela-Arrieta C, Pivarnik G, Manis J, Beggs HE, Silberstein LE. Focal adhesion kinase regulates the localization and retention of pro-B cells in bone marrow microenvironments. J Immunol 2013;190:1094–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pereira JP, An J, Xu Y, Huang Y, Cyster JG. Cannabinoid receptor 2 mediates the retention of immature B cells in bone marrow sinusoids. Nat Immunol 2009;10:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Honczarenko M, Douglas RS, Mathias C, Lee B, Ratajczak MZ, Silberstein LE. SDF-1 responsiveness does not correlate with CXCR4 expression levels of developing human bone marrow B cells. Blood 1999;94:2990–2998. [PubMed] [Google Scholar]
- 45.Cyster JG, Schwab SR. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol 2012;30:69–94. [DOI] [PubMed] [Google Scholar]
- 46.Arnon TI, Cyster JG. Blood, sphingosine-1-phosphate and lymphocyte migration dynamics in the spleen. Curr Top Microbiol Immunol 2014;378:107–128. [DOI] [PubMed] [Google Scholar]
- 47.Massberg S, von Andrian UH. Fingolimod and sphingosine-1-phosphate--modifiers of lymphocyte migration. N Engl J Med 2006;355:1088–1091. [DOI] [PubMed] [Google Scholar]
- 48.Nakai A, Hayano Y, Furuta F, Noda M, Suzuki K. Control of lymphocyte egress from lymph nodes through β2-adrenergic receptors. J Exp Med 2014;211:2583–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hidalgo A, Chang J, Jang JE, Peired AJ, Chiang EY, Frenette PS. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat Med 2009;15:384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scheiermann C, Kunisaki Y, Jang JE, Frenette PS. Neutrophil microdomains: linking heterocellular interactions with vascular injury. Curr Opin Hematol 2010;17:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Junt T, Schulze H, Chen Z, Massberg S, Goerge T, Krueger A, Wagner DD, Graf T, Italiano JE, Jr., Shivdasani RA, von Andrian UH. Dynamic visualization of thrombopoiesis within bone marrow. Science 2007;317:1767–1770. [DOI] [PubMed] [Google Scholar]
- 52.Niswander LM, Fegan KH, Kingsley PD, McGrath KE, Palis J. SDF-1 dynamically mediates megakaryocyte niche occupancy and thrombopoiesis at steady state and following radiation injury. Blood 2014;124:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L, Orban M, Lorenz M, Barocke V, Braun D, Urtz N, Schulz C, von Bruhl ML, Tirniceriu A, Gaertner F, Proia RL, Graf T, Bolz SS, Montanez E, Prinz M, Muller A, von Baumgarten L, Billich A, Sixt M, Fassler R, von Andrian UH, Junt T, Massberg S. A novel role of sphingosine 1-phosphate receptor S1pr1 in mouse thrombopoiesis. J Exp Med 2012;209:2165–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turhan A, Weiss LA, Mohandas N, Coller BS, Frenette PS. Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm. Proc Natl Acad Sci USA 2002;99:3047–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell 1991;67:1033–1036. [DOI] [PubMed] [Google Scholar]
- 56.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 1994;76:301–314. [DOI] [PubMed] [Google Scholar]
- 57.McEver RP, Zhu C. Rolling cell adhesion. Annu Rev Cell Dev Biol 2010;26:363–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arbones ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon DJ, Tedder TF. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity 1994;1:247–260. [DOI] [PubMed] [Google Scholar]
- 59.Frenette PS, Mayadas TN, Rayburn H, Hynes RO, Wagner DD. Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell 1996;84:563–574. [DOI] [PubMed] [Google Scholar]
- 60.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 2007;7:678–689. [DOI] [PubMed] [Google Scholar]
- 61.Zarbock A, Ley K, McEver RP, Hidalgo A. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood 2011;118:6743–6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chiang EY, Hidalgo A, Chang J, Frenette PS. Imaging receptor microdomains on leukocyte subsets in live mice. Nat Methods 2007;4:219–222. [DOI] [PubMed] [Google Scholar]
- 63.Mazo IB, Quackenbush EJ, Lowe JB, von Andrian UH. Total body irradiation causes profound changes in endothelial traffic molecules for hematopoietic progenitor cell recruitment to bone marrow. Blood 2002;99:4182–4191. [DOI] [PubMed] [Google Scholar]
- 64.Nacher M, Blazquez AB, Shao B, Matesanz A, Prophete C, Berin MC, Frenette PS, Hidalgo A. Physiological contribution of CD44 as a ligand for E-Selectin during inflammatory T-cell recruitment. Am J Pathol 2011;178:2437–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schweitzer KM, Drager AM, van der Valk P, Thijsen SF, Zevenbergen A, Theijsmeijer AP, van der Schoot CE, Langenhuijsen MM. Constitutive expression of E-selectin and vascular cell adhesion molecule-1 on endothelial cells of hematopoietic tissues. Am J Pathol 1996;148:165–175. [PMC free article] [PubMed] [Google Scholar]
- 66.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 2007;317:666–670. [DOI] [PubMed] [Google Scholar]
- 67.Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE, Stevens C, Barker JN, Gale RP, Lazarus HM, Marks DI, van Rood JJ, Scaradavou A, Horowitz MM. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med 2004;351:2265–2275. [DOI] [PubMed] [Google Scholar]
- 68.Mazo IB, Gutierrez-Ramos JC, Frenette PS, Hynes RO, Wagner DD, von Andrian UH. Hematopoietic progenitor cell rolling in bone marrow microvessels: parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. J Exp Med 1998;188:465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scheiermann C, Kunisaki Y, Lucas D, Chow A, Jang JE, Zhang D, Hashimoto D, Merad M, Frenette PS. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity 2012;37:290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lucas D, Battista M, Shi PA, Isola L, Frenette PS. Mobilized hematopoietic stem cell yield depends on species-specific circadian timing. Cell Stem Cell 2008;3:364–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Casanova-Acebes M, Pitaval C, Weiss LA, Nombela-Arrieta C, Chevre R, A-González N, Kunisaki Y, Zhang D, van Rooijen N, Silberstein LE, Weber C, Nagasawa T, Frenette PS, Castrillo A, Hidalgo A. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 2013;153:1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, Poulton IJ, van Rooijen N, Alexander KA, Raggatt LJ, Levesque JP. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood 2010;116:4815–4828. [DOI] [PubMed] [Google Scholar]
- 73.Tak T, Tesselaar K, Pillay J, Borghans JA, Koenderman L. What's your age again? Determination of human neutrophil half-lives revisited. J Leukoc Biol 2013;94:595–601. [DOI] [PubMed] [Google Scholar]
- 74.Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nat Med 2011;17:1381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 2010;330:362–366. [DOI] [PubMed] [Google Scholar]
- 76.Rossaint J, Zarbock A. Tissue-specific neutrophil recruitment into the lung, liver, and kidney. J Innate Immun 2013;5:348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hogg JC. Neutrophil kinetics and lung injury. Physiol Rev 1987;67:1249–1295. [DOI] [PubMed] [Google Scholar]
- 78.Mathias JR, Perrin BJ, Liu TX, Kanki J, Look AT, Huttenlocher A. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J Leukoc Biol 2006;80:1281–1288. [DOI] [PubMed] [Google Scholar]
- 79.Woodfin A, Voisin MB, Beyrau M, Colom B, Caille D, Diapouli FM, Nash GB, Chavakis T, Albelda SM, Rainger GE, Meda P, Imhof BA, Nourshargh S. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol 2011;12:761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003;19:71–82. [DOI] [PubMed] [Google Scholar]
- 81.Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science 2013;341:1483–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, Hedrick CC, Cook HT, Diebold S, Geissmann F. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell 2013;153:362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, van Rooijen N, Grainger JR, Belkaid Y, Ma'ayan A, Riches DW, Yokoyama WM, Ginhoux F, Henson PM, Randolph GJ. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity 2013;39:599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gowans JL, Steer HW. The function and pathways of lymphocyte recirculation. Ciba Found Symp 1980;71:113–126. [DOI] [PubMed] [Google Scholar]
- 85.Halin C, Mora JR, Sumen C, von Andrian UH. In vivo imaging of lymphocyte trafficking. Annu Rev Cell Dev Biol 2005;21:581–603. [DOI] [PubMed] [Google Scholar]
- 86.Alon R, Chen S, Puri KD, Finger EB, Springer TA. The kinetics of L-selectin tethers and the mechanics of selectin-mediated rolling. J Cell Biol 1997;138:1169–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bargatze RF, Jutila MA, Butcher EC. Distinct roles of L-selectin and integrins alpha 4 beta 7 and LFA-1 in lymphocyte homing to Peyer's patch-HEV in situ: the multistep model confirmed and refined. Immunity 1995;3:99–108. [DOI] [PubMed] [Google Scholar]
- 88.Berg EL, Robinson MK, Warnock RA, Butcher EC. The human peripheral lymph node vascular addressin is a ligand for LECAM-1, the peripheral lymph node homing receptor. J Cell Biol 1991;114:343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rosen H, Sanna G, Alfonso C. Egress: a receptor-regulated step in lymphocyte trafficking. Immunol Rev 2003;195:160–177. [DOI] [PubMed] [Google Scholar]
- 90.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity 2004;21:527–538. [DOI] [PubMed] [Google Scholar]
- 91.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature 2003;424:88–93. [DOI] [PubMed] [Google Scholar]
- 92.Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J Exp Med 2005;201:303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dormehl IC, Kilian JG, Maree M, Jacobs L. Investigation by scintigraphic methods of platelet kinetics under normal and septic shock conditions in the experimental baboon model. Am J Physiol Imaging 1990;5:75–79. [PubMed] [Google Scholar]
- 94.Hoffmeister KM, Felbinger TW, Falet H, Denis CV, Bergmeier W, Mayadas TN, von Andrian UH, Wagner DD, Stossel TP, Hartwig JH. The clearance mechanism of chilled blood platelets. Cell 2003;112:87–97. [DOI] [PubMed] [Google Scholar]
- 95.Grewal PK, Uchiyama S, Ditto D, Varki N, Le DT, Nizet V, Marth JD. The Ashwell receptor mitigates the lethal coagulopathy of sepsis. Nat Med 2008;14:648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Berger G, Hartwell DW, Wagner DD. P-Selectin and platelet clearance. Blood 1998;92:4446–4452. [PubMed] [Google Scholar]
- 97.Stasi R, Evangelista ML, Stipa E, Buccisano F, Venditti A, Amadori S. Idiopathic thrombocytopenic purpura: current concepts in pathophysiology and management. Thromb Haemost 2008;99:4–13. [DOI] [PubMed] [Google Scholar]
- 98.Looney MR, Nguyen JX, Hu Y, Van Ziffle JA, Lowell CA, Matthay MA. Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. J Clin Invest 2009;119:3450–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maugeri N, Rovere-Querini P, Evangelista V, Covino C, Capobianco A, Bertilaccio MT, Piccoli A, Totani L, Cianflone D, Maseri A, Manfredi AA. Neutrophils phagocytose activated platelets in vivo: a phosphatidylserine, P-selectin, and {beta}2 integrin-dependent cell clearance program. Blood 2009;113:5254–5265. [DOI] [PubMed] [Google Scholar]
- 100.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 2007;13:463–469. [DOI] [PubMed] [Google Scholar]
- 101.D'Atri LP, Etulain J, Rivadeneyra L, Lapponi MJ, Centurion M, Cheng K, Yin H, Schattner M. Expression and functionality of Toll-like receptor 3 in the megakaryocytic lineage. J Thromb Haemost 2015, [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Koupenova M, Vitseva O, MacKay CR, Beaulieu LM, Benjamin EJ, Mick E, Kurt-Jones EA, Ravid K, Freedman JE. Platelet-TLR7 mediates host survival and platelet count during viral infection in the absence of platelet-dependent thrombosis. Blood 2014;124:791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rand ML, Wang H, Bang KW, Packham MA, Freedman J. Rapid clearance of procoagulant platelet-derived microparticles from the circulation of rabbits. J Thromb Haemost 2006;4:1621–1623. [DOI] [PubMed] [Google Scholar]
- 104.Diacovo TG, Puri KD, Warnock RA, Springer TA, von Andrian UH. Platelet-mediated lymphocyte delivery to high endothelial venules. Science 1996;273:252–255. [DOI] [PubMed] [Google Scholar]
- 105.Sreeramkumar V, Adrover JM, Ballesteros I, Cuartero MI, Rossaint J, Bilbao I, Nacher M, Pitaval C, Radovanovic I, Fukui Y, McEver RP, Filippi MD, Lizasoain I, Ruiz-Cabello J, Zarbock A, Moro MA, Hidalgo A. Neutrophils scan for activated platelets to initiate inflammation. Science 2014;346:1234–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.de Back DZ, Kostova EB, van Kraaij M, van den Berg TK, van Bruggen R. Of macrophages and red blood cells; a complex love story. Front Physiol 2014;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science 2000;288:2051–2054. [DOI] [PubMed] [Google Scholar]
- 108.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol 2005;5:606–616. [DOI] [PubMed] [Google Scholar]
- 109.Rifkind JM, Nagababu E. Hemoglobin redox reactions and red blood cell aging. Antioxid Redox Signal 2013;18:2274–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schluter K, Drenckhahn D. Co-clustering of denatured hemoglobin with band 3: its role in binding of autoantibodies against band 3 to abnormal and aged erythrocytes. Proc Natl Acad Sci USA 1986;83:6137–6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kean LS, Brown LE, Nichols JW, Mohandas N, Archer DR, Hsu LL. Comparison of mechanisms of anemia in mice with sickle cell disease and beta-thalassemia: peripheral destruction, ineffective erythropoiesis, and phospholipid scramblase-mediated phosphatidylserine exposure. Exp Hematol 2002;30:394–402. [DOI] [PubMed] [Google Scholar]
- 112.Manwani D, Frenette PS. Vaso-occlusion in sickle cell disease: pathophysiology and novel targeted therapies. Blood 2013;122:3892–3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med 2011;17:1410–1422. [DOI] [PubMed] [Google Scholar]
- 114.Nissen SE, Tuzcu EM, Schoenhagen P, Crowe T, Sasiela WJ, Tsai J, Orazem J, Magorien RD, O'Shaughnessy C, Ganz P; Reversal of Atherosclerosis with Aggressive Lipid Lowering I. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med 2005;352:29–38. [DOI] [PubMed] [Google Scholar]
- 115.Egusa G, Yamane K. Lifestyle, serum lipids and coronary artery disease: comparison of Japan with the United States. J Atheroscler Thromb 2004;11:304–312. [DOI] [PubMed] [Google Scholar]
- 116.Mills GL, Taylaur CE. The distribution and composition of serum lipoproteins in eighteen animals. Comp Biochem Physiol 1971;40:489–501. [DOI] [PubMed] [Google Scholar]
- 117.Friedman GD, Klatsky AL, Siegelaub AB. The leukocyte count as a predictor of myocardial infarction. N Engl J Med 1974;290:1275–1278. [DOI] [PubMed] [Google Scholar]
- 118.Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation 2010;122:1837–1845. [DOI] [PubMed] [Google Scholar]
- 119.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest 2007;117:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tani S, Nagao K, Anazawa T, Kawamata H, Furuya S, Takahashi H, Iida K, Matsumoto M, Washio T, Kumabe N, Hirayama A. Association of leukocyte subtype counts with coronary atherosclerotic regression following pravastatin treatment. Am J Cardiol 2009;104:464–469. [DOI] [PubMed] [Google Scholar]
- 121.A-Gonzalez N, Castrillo A. Liver X receptors as regulators of macrophage inflammatory and metabolic pathways. Biochim Biophys Acta 2011;1812:982–994. [DOI] [PubMed] [Google Scholar]
- 122.Westerterp M, Bochem AE, Yvan-Charvet L, Murphy AJ, Wang N, Tall AR. ATP-binding cassette transporters, atherosclerosis, and inflammation. Circ Res 2014;114:157–170. [DOI] [PubMed] [Google Scholar]
- 123.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation 2008;118:1837–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 2010;328:1689–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Westerterp M, Gourion-Arsiquaud S, Murphy AJ, Shih A, Cremers S, Levine RL, Tall AR, Yvan-Charvet L. Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell Stem Cell 2012;11:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Murphy AJ, Bijl N, Yvan-Charvet L, Welch CB, Bhagwat N, Reheman A, Wang Y, Shaw JA, Levine RL, Ni H, Tall AR, Wang N. Cholesterol efflux in megakaryocyte progenitors suppresses platelet production and thrombocytosis. Nat Med 2013;19:586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hong C, Kidani Y, A-González N, Phung T, Ito A, Rong X, Ericson K, Mikkola H, Beaven SW, Miller LS, Shao WH, Cohen PL, Castrillo A, Tontonoz P, Bensinger SJ. Coordinate regulation of neutrophil homeostasis by liver X receptors in mice. J Clin Invest 2012;122:337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Suratt BT, Young SK, Lieber J, Nick JA, Henson PM, Worthen GS. Neutrophil maturation and activation determine anatomic site of clearance from circulation. Am J Physiol Lung Cell Mol Physiol 2001;281:L913–L921. [DOI] [PubMed] [Google Scholar]
- 129.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, Lin CP, Vinegoni C, Libby P, Swirski FK, Weissleder R, Nahrendorf M. Chronic variable stress activates hematopoietic stem cells. Nat Med 2014;20:754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]