In this first-in-human phase I trial of adenoviral-delivered E. coli purine nucleoside phosphorylase and fludarabine, a dose-dependent tumor response was observed. The regimen was safe and no dose-limiting toxicities were encountered in this study of 12 patients with solid tumors. Further studies are warranted.
Keywords: purine nucleoside phosphorylase, fludarabine, gene therapy, human clinical trial
Abstract
Background
The use of Escherichia coli purine nucleoside phosphorylase (PNP) to activate fludarabine has demonstrated safety and antitumor activity during preclinical analysis and has been approved for clinical investigation.
Patients and methods
A first-in-human phase I clinical trial (NCT 01310179; IND 14271) was initiated to evaluate safety and efficacy of an intratumoral injection of adenoviral vector expressing E. coli PNP in combination with intravenous fludarabine for the treatment of solid tumors. The study was designed with escalating doses of fludarabine in the first three cohorts (15, 45, and 75 mg/m2) and escalating virus in the fourth (1011–1012 viral particles, VP).
Results
All 12 study subjects completed therapy without dose-limiting toxicity. Tumor size change from baseline to final measurement demonstrated a dose-dependent response, with 5 of 6 patients in cohorts 3 and 4 achieving significant tumor regression compared with 0 responsive subjects in cohorts 1 and 2. The overall adverse event rate was not dose-dependent. Most common adverse events included pain at the viral injection site (92%), drainage/itching/burning (50%), fatigue (50%), and fever/chills/influenza-like symptoms (42%). Analysis of serum confirmed the lack of systemic exposure to fluoroadenine. Antibody response to adenovirus was detected in two patients, suggesting that neutralizing immune response is not a barrier to efficacy.
Conclusions
This first-in-human clinical trial found that localized generation of fluoroadenine within tumor tissues using E. coli PNP and fludarabine is safe and effective. The pronounced effect on tumor volume after a single treatment cycle suggests that phase II studies are warranted.
ClinicalTrials.gov Identifier
introduction
Most conventional chemotherapeutic agents derive antitumor specificity from the ability to kill dividing (as opposed to non-dividing) cancer cells. Many therapy-resistant solid tumors are refractory precisely because they have a low-growth fraction, which includes the regenerative or tumor ‘stem cell’ compartment.
Enzyme–prodrug therapy has the potential to treat this compartment. The enzyme Escherichia coli purine nucleoside phosphorylase (PNP) converts relative nontoxic purine nucleosides to very potent adenine analogs, an activity that human PNP lacks. Fludarabine monophosphate is a clinically approved chemotherapeutic that is converted by E. coli PNP into fluoroadenine, which is phosphorylated into its ATP analog (F-ATP) and is incorporated into RNA, a process which disrupts RNA and protein synthesis [1]. In this way, PNP and fludarabine confer cytotoxicity to cells of any proliferative stage, circumventing limitations in targeting DNA. Furthermore, fluoroadenine has the unique ability to extend its cytotoxic effect to neighboring cells (i.e. bystander killing) [2–5].
We and others have previously demonstrated robust antitumor activity following the intratumoral injection of E. coli PNP followed by systemic administration of fludarabine in preclinical models [6–11]. Based on these studies, a dose-escalation, phase I trial was conducted. We hypothesized that direct tumor infiltration of non-replicative adenovirus engineered to encode E. coli PNP (Ad/PNP) followed by intravenous administration of fludarabine would be safe and effective in the treatment of solid tumors (Figure 1).
Figure 1.
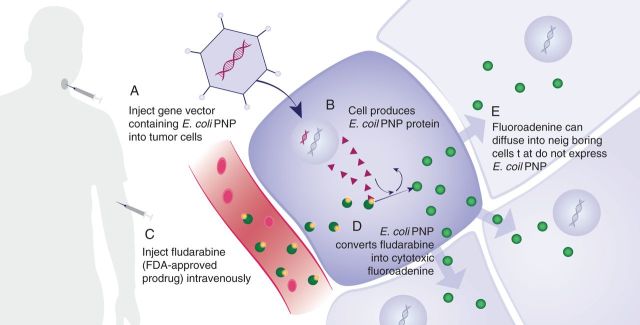
Mechanism of E. coli purine nucleoside phosphorylase prodrug activation using fludarabine.
methods
study design
We conducted an open-label, two-center dose-escalating phase 1 trial evaluating the safety and antitumor activity of a non-replicative (E1- and E3-deleted adenovirus) engineered to encode E. coli PNP (Ad/PNP) in combination with systemic fludarabine phosphate (referred to here as fludarabine) administered to patients with solid tumors who failed or exhausted all other standard or approved therapies. We investigated three dose levels of intravenous fludarabine and two dose levels of intratumoral Ad/PNP. A 3 + 3 dose-escalation format for fludarabine along with an additional dose-escalation arm for PNP was designed and institutional review board (IRB) approval was obtained at both study sites. The trial was registered with Clinicaltrials.gov as NCT01310179. Patient recruitment was from February 2011 to April 2014.
inclusion and exclusion criteria
Subjects were recruited at the University of Alabama at Birmingham (n = 11) and Vanderbilt University (n = 1). Each study site tumor board reviewed screening data to determine eligibility. Inclusion criteria included: biopsy-confirmed diagnosis of solid tumor, failure or exhaustion of all standard or approved treatment options that would provide substantive palliation; at least one measurable primary or metastatic tumor (>5 × 5 mm based on physical examination) accessible for direct intratumoral injection; age ≥19 years; life expectancy >12 weeks; Karnofsky performance status of at least 60%; and acceptable serum laboratory results.
Exclusion criteria included: diagnosis of leukemia; prior receipt of any gene therapy product or oncolytic viral therapy; current allopurinol treatment; radiation or chemotherapy treatment <4 weeks before initiating Ad/PNP; significant baseline neuropathy (>grade 2 based on CTCAE v4.0); treatment with systemic antibiotics for infection within a week before Ad/PNP; FEV1 <40% of predicted; or immunosuppressive medications.
study drug and dosing
Ad/PNP was manufactured by the biological division of Sigma-Aldrich, SAFC Pharma (Carlsbad, CA) and supplied as a liquid formulation in vials containing 2 × 1011 viral particles (VP)/100 μl in 20 mM Tris (pH 8.0), 25 mM NaCl, and 2.5% glycerol and stored at less than −60°C. Dilutions of Ad/PNP in sterile normal saline USP were prepared immediately before use. Commercially available fludarabine phosphate (Fludara®) was utilized as the prodrug.
In cohorts 1–3, Ad/PNP dose was held constant at 3 × 1011 VP, whereas total fludarabine dose was increased from 15 mg/m2 (cohort 1) to 45 mg/m2 (cohort 2) and then to 75 mg/m2 (cohort 3). Cohort 4 increased dosing of intratumoral Ad/PNP to 2.73 × 1012 VP while maintaining the highest fludarabine dose (75 mg/m2). For each subject, one target tumor was chosen for intratumoral injection while non-target tumors, if present, were only observed. Ad/PNP was administered in three intratumoral inoculations (0.5 ml each) over the first 2 days (two injections separated by 4 h on day 1 and a single injection the next day) followed by intravenous infusions of fludarabine (on days 3, 4, and 5) for each patient.
safety monitoring and other laboratory measurements
Patients were assessed by physical examination, vital signs, performance status, and chemistry and hematology panels daily on days 1–5, with subsequent clinic visits on days 8, 10, 14, 21, 28, and 56. Adverse events were monitored and graded according to the NCI Common Terminology Criteria for Adverse Events (CTCAE v4.0). Attribution of adverse events to the study drug was carried out by the investigator according to National Cancer Institute Adverse Event Reporting Requirements [12] and was assessed for each component of treatment. Serum samples were obtained before and 15–30 min following each infusion of fludarabine. Serum samples were obtained to detect anti-viral antibodies. In cohorts 3 and 4, a tumor biopsy was obtained on day 3 using a 3-mm punchand. E. coli PNP activity was evaluated enzymatically as previously described [13].
treatment outcomes
Tumor size was assessed at all visits up to day 56. Tumor length and width (cm2) were obtained clinically using calipers or ruler and assessed by two clinicians. Standardized color photos were obtained for all patients except for patient 11. Primary efficacy end point was tumor size change from baseline (tumor dimensions on day 3 of treatment) versus the final measurement using the following percentage cutoffs: complete response was defined as disappearance of the target lesion; partial response as ≥30% decrease in size. Progressive disease was reported for tumors with a ≥20% increase in size, and stable disease as the absence of either tumor response or progressive disease. Non-target lesions were tumors identified on radiographs or examinations that were not treated with vector.
statistical analysis
Statistical analysis was carried out using SPSS Statistics for Windows version 22.0 (IBM Corp., Released 2013, Armonk, NY) and R v3.0.3 [14]. We calculated descriptive statistics for tumor volume at baseline by cohort versus linear regression to examine whether percent change from baseline to last follow-up varied by treatment cohort, with or without adjustment for baseline tumor dimensions.
results
patients' characteristics
Twelve patients who presented with recurrent disease following multi-modality treatment and had exhausted all other curative therapies were enrolled in the study (Table 1). All subjects completed the full course of therapy. Mean age of enrolled individuals was 61.5 years. Head and neck squamous cell carcinoma was the most common pathologic diagnosis (n = 8), followed by adenoid cystic carcinoma (n = 2) and melanoma (n = 2). Target lesions were located in the neck (n = 6), face (2), scalp (1), oral cavity (1), oropharynx (1), and lower extremity (1). Target lesion volumes at baseline averaged 5.9 ± 5.4 cm2 (median 4.7, range 1.5–20). Seven patients presented with non-target lesions amenable to concurrent serial evaluation.
Table 1.
Patient characteristics
| No. | Age/sex | Ethnicity | Perf. status (%) | Tumor site | Histology | Recurrence | Prior chemo | Prior XRT | Prior surgery | Non-target lesions | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort 1 (3 × 1011 VP + 15 mg/m2) | 1 | 54/F | Caucasian | 80 | Neck | SCC—mod diff. | Regional | Y | Y | Y | N |
| 2 | 55/M | Caucasian | 90 | Neck | Adenoid cystic ca. | Regional | Y | Y | Y | Y | |
| 3 | 59/M | Caucasian | 80 | Neck | SCC | Regional | Y | Y | Y | N | |
| Cohort 2 (3 × 1011 VP + 45 mg/m2) | 4 | 65/M | Caucasian | 70 | Cheek | SCC | Local | Y | Y | Y | Y |
| 5 | 60/M | Caucasian | 90 | Hard palate | Adenoid cystic ca. | Regional | Y | Y | Y | Y | |
| 6 | 65/M | Caucasian | 100 | Tonsil | SCC | Local | Y | Y | Y | N | |
| Cohort 3 (3 × 1011 VP + 75 mg/m2) | 7 | 61/F | African-American | 80 | Neck | SCC—poor diff. | Local | Y | Y | Y | N |
| 8 | 53/M | Caucasian | 90 | Neck | SCC—poor diff. | Local | Y | Y | Y | Y | |
| 9 | 56/M | Caucasian | 70 | Neck | SCC | Local | Y | N | Y | Y | |
| Cohort 4 (2.7 × 1012 VP + 75 mg/m2) | 10 | 64/F | Caucasian | 70 | Lower lip | SCC—mod diff. | Local | Y | Y | Y | N |
| 11 | 82/M | Caucasian | 70 | Scalp | Melanoma | Distant | Y | Y | Y | Y | |
| 12 | 64/M | Caucasian | 100 | Lower ext. | Nevoid melanoma | Local | N | Y | Y | Y |
Patients enrolled in this study are listed by cohort, age, sex, ethnicity, performance status, cancer type, site, treatment history, and presence of non-target lesions. Doses of fludarabine are listed by body surface area (mg/m2).
VP, viral particles; SCC, squamous cell carcinoma; Ca., carcinoma; Perf. status, Performance status; mod diff., moderately differentiated; poor diff., poorly differentiated.
drug delivery
Each patient received three direct intratumoral Ad/PNP injections over a 2-day period, followed by systemic administration of fludarabine for 3 consecutive days. Serum fluoroadenine levels were found below detection level (1 ng/ml). Serum adenovirus antibodies were obtained on days 1 and 28. Patient 3 in cohort 1 and patient 10 in cohort 3 demonstrated measurable antibody levels to the vector at day 28, while all other samples were below detection threshold. Antibody levels did not appear to correlate with response to treatment. Attempts to monitor E. coli PNP enzymatic activity were frustrated by difficulty preparing soluble extract from small tissue samples. One sample indicated a level of 208 nmol/mg h, but very little activity was detected in other samples. One unit of PNP activity was defined as the amount of tumor extract necessary to cleave 1 nmol of 6-methylpurine-2′-deoxyriboxide per mg protein in a 1-h period.
safety
Patients tolerated the therapy well without discontinuation or delay in treatment. Although there were adverse events attributable to study drug (Figure 2), dose-limiting toxicities were not observed. Moreover, no association between escalating dose and the number or grade of adverse events was noted. There were two grade 3 events attributed to therapy. One subject experienced a decreased lymphocyte count, which resolved spontaneously and was considered possibly related to fludarabine. The second treatment related grade 3 event involved pain at the injection site following Ad/PNP administration. Grade 1 and 2 toxicities did not increase with escalating doses of Ad/PNP or fludarabine.
| All Patients N =12 n (%) [n related] |
Cohort 1 N =3 n (%) [n related] |
Cohort 2 N =3 n (%) [n related] |
Cohort 3 N =3 n (%) [n related] |
Cohort 4 N =3 n (%) [n related] |
|
|---|---|---|---|---|---|
| Any AE | 12 (100) [12] | 3 (100) [3] | 3 (100) [3] | 3 (100) [3] | 3 (100) [3] |
| Patients with Serious AEs (Grade 3–5) | 5 (42) [0] | 2 (66) [0] | 1 (33) [0] | 1 (33) [0] | 1 (33) [0] |
| Dehydration | 1 (8) [0] | 1 (33) [0] | |||
| Pericardial Effusion | 1 (8) [0] | 1 (33) [0] | |||
| Cardiac Tamponade | 1 (8) [0] | 1 (33) [0] | |||
| Pain | 1 (8) [0] | 1 (33) [0] | |||
| Nausea/Vomiting | 1 (8) [0] | 1 (33) [0] | |||
| Bacteremia | 1 (8) [0] | 1 (33) [0] | |||
| Partial Seizure | 1 (8) [0] | 1 (33) [0] | |||
| Lower Ext. Weakness | 1 (8) [0] | 1 (33) [0] | |||
| Chronic Wound Infection | 1 (8) [0] | 1 (33) [0] | |||
| AEs in 3 or more (≥25%) patients (All Grades) | |||||
| Injection site symptoms | 12 (100) [10] | 3 (100) [3] | 3 (100) [1] | 3 (100) [3] | 3 (100) [3] |
| Fatigue | 8 (66) [6] | 2 (66) [2] | 2 (66) [1] | 2 (66) [1] | 2 (66) [2] |
| Pain (excluding injection site) | 8 (66) [4] | 2 (33) [2] | 2 (66) [0] | 3 (100) [2] | 1 (33) [0] |
| Nausea/Vomiting/Diarrhea | 6 (50) [4] | 2 (66) [2] | 1 (33) [0] | 3 (100) [2] | |
| Flu-Like Symptoms/Chills | 5 (42) [5] | 2 (66) [2] | 1 (33) [1] | 2 (66) [2] | |
| Facial Edema/Pitting Edema | 5 (42) [3] | 1 (33) [1] | 1 (33) [1] | 2 (66) [1] | 1 (33) [0] |
| Dizziness | 5 (42) [2] | 3 (100) [2] | 1 (33) [0] | 1 (33) [0] | |
| Cough/Dyspnea/Wheeze/Sore Throat | 5 (42) [2] | 2 (66) [1] | 2 (66) [0] | 1 (33) [1] | |
| Headache | 4 (33) [3] | 2 (66) [2] | 1 (33) [0] | 1 (33) [1] | |
| Decreased Lymphocytes | 4 (33) [2] | 2 (66) [1] | 1 (33) [1] | 1 (33) [0] | |
| Decreased Hemoglobin | 4 (33) [2] | 2 (66) [2] | 1 (33) [0] | 1 (33) [0] | |
| Rash | 4 (33) [0] | 1 (33) [0] | 3 (100) [0] | ||
| Pruritis | 3 (25) [2] | 2 (66) [2] | 1 (33) [0] | ||
| Decreased Appetite | 3 (25) [2] | 1 (33) [1] | 2 (66) [1] | ||
| Neck/Extremity Stiffness | 3 (25) [3] | 2 (66) [2] | 1 (33) [1] | ||
| Thrush | 3 (25) [0] | 1 (33) [0] | 1 (33) [0] | 1 (33) [0] | |
| Hypertension (or worsening) | 3 (25) [0] | ||||
| Noteworthy AEs | |||||
| Increased AST/ALT | 1 (8) [0] | 1 (33) [0] |
Figure 2.
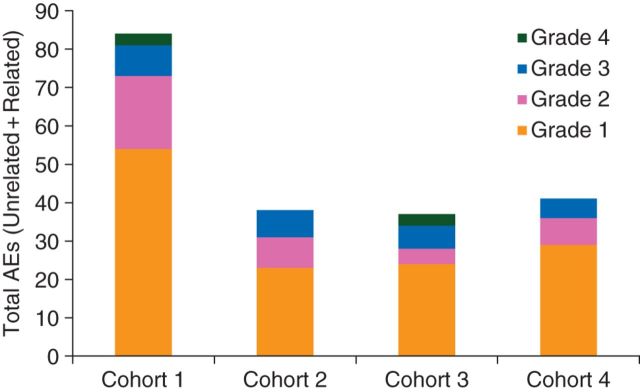
Adverse events (AEs) by grade, relatedness, and cohort. Adverse events were tabulated on a per-patient basis (top table). All adverse events, including repeat events within patients, were graphed (bottom histogram). ALT, alanine transaminase; AST, aspartate transaminase.
Additional adverse events attributed to treatment were grade 1 (82%) and grade 2 (15%), and included pain at the injection site (83%), drainage/itching/burning at the injection site (50%), fatigue (50%), and fever/chills/influenza-like symptomatology (42%). Hematologic events possibly related to study drug included leukopenia in two patients (16.6%) and anemia in two others (16.6%). One patient exhibited a grade 1 alanine transaminase (ALT) transient increase 1.05 times outside the normal range. This was not deemed clinically significant.
response to therapy
Tumor response using tumor volume percent cutoffs and mean tumor size change from baseline to last measurement for each cohort are illustrated in Figure 3. Indication of therapeutic benefit was achieved for each sequential cohort, with a linear response to dose escalation of fludarabine. A significantly higher proportion of responders occurred at the highest dose of fludarabine (5 of 6 patients in cohorts 3 and 4) compared with lower doses (0 of 6 in cohorts 1 and 2). Although Ad/PNP was increased 10-fold from cohort 3 to 4, similar antitumor effect in the two groups suggests that expression of E. coli PNP is saturated at the lower dose. A single cycle of fludarabine has no known activity against these tumor types and this was evident when comparing target lesions with non-target lesions. Moreover, dose dependence of tumor response indicates specificity of combination of Ad/PNP and fludarabine, rather than either component individually.
| Baseline to Last Size | Cohort 1 | Cohort 2 | Cohort 3 | Cohort 4 |
|---|---|---|---|---|
| Progression (>+20%) | 2 | – | – | – |
| Stable (+20 to −30%) | 1 | 3 | – | 1 |
| Partial Response (>−30%) | – | – | 3 | 2 |
Figure 3.
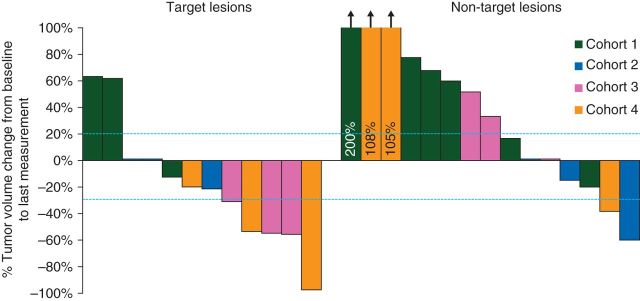
Treatment outcome and percent change in tumor volume by cohort. Treatment outcome was defined with tumor volume percentage cutoffs (marked with dashed lines) where progression is a percentage change in tumor volume of ≥20% increase, partial response is defined as a change of ≥30% decrease, and stable a change between −30% and +20%. Measurements were obtained at baseline before study drug injection and each patient's last measurement for the study. The response rate in cohorts 3 and 4 was significantly higher than the rate in cohorts 1 and 2.
Five of the six patients in cohort 3 and 4 demonstrated a partial response (Figure 3), and relative tumor volume measurements demonstrated a reduction for these subjects. Two patients experienced complete tumor regression of otherwise refractory, multiply treated masses (Figure 4). In these subjects (8 and 11), complete regression was achieved by days 14–21 and was present for 1 week before regrowth, which suggests that regression might be more sustainable with repeated administration. Subjects with partial or complete tumor regression exhibited healing at the treatment site without evidence of ulceration or non-target tissue necrosis (Figure 5).
Figure 4.
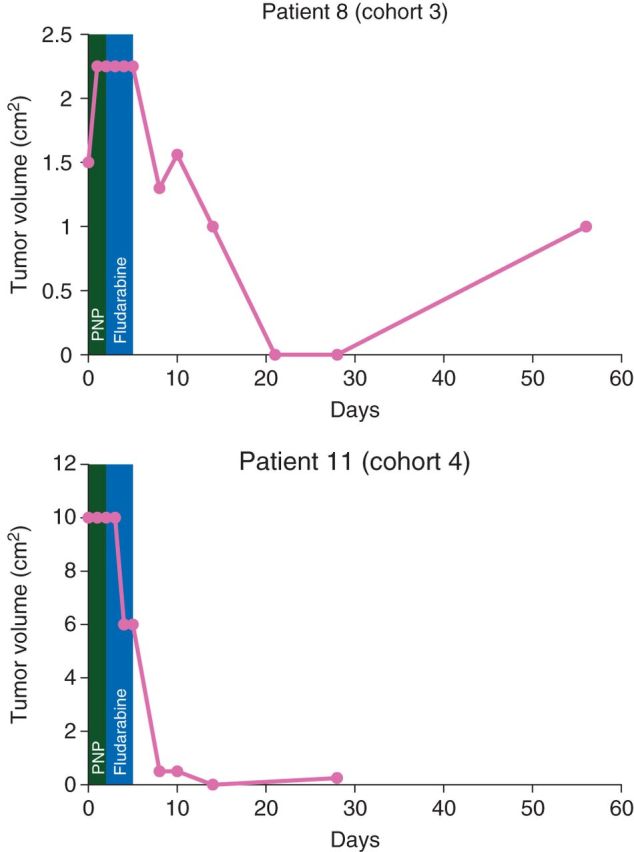
Serial tumor volume measurements for patients with complete tumor regression. Complete tumor volume measurements are plotted for two patients with complete tumor regression. A green box identifies the timeframe for the viral injection of purine nucleoside phosphorylase (PNP) and a blue box identifies the timeframe for fludarabine injection. Patient 11 was lost to follow-up for day 56.
Figure 5.
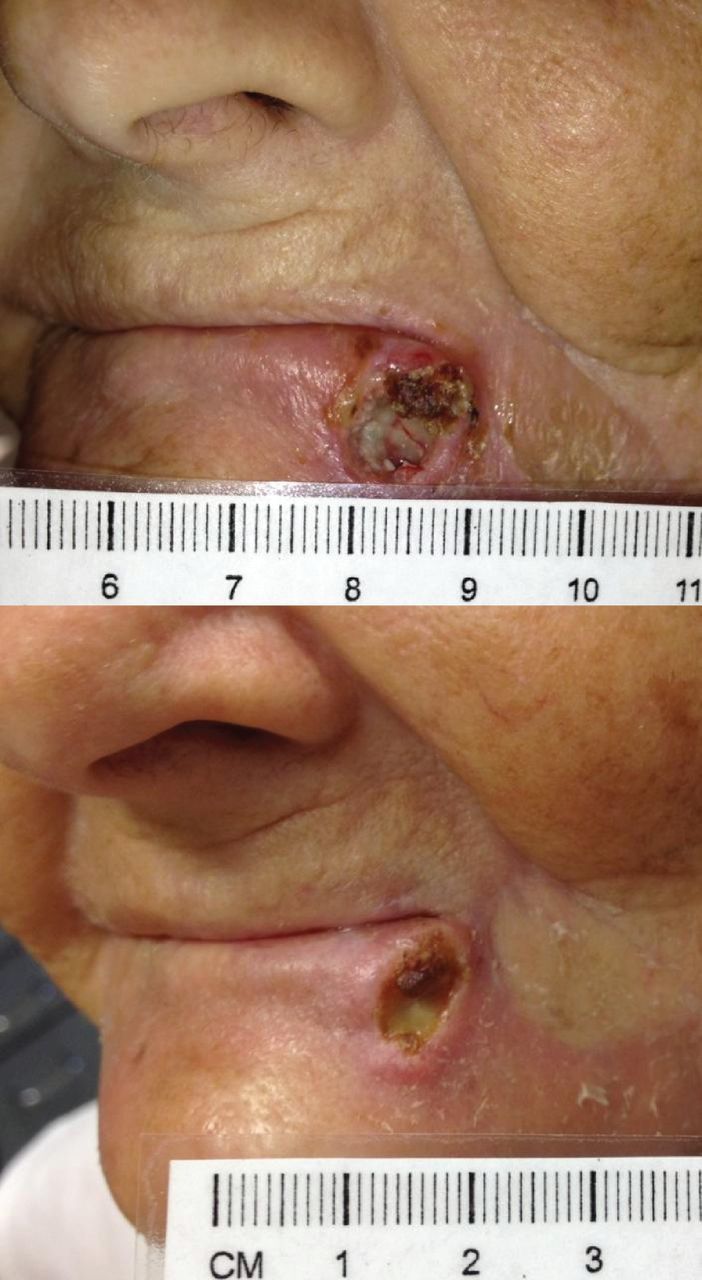
Tumor response in a cohort 4 patient. Sample images from a cohort 4 patient demonstrates serial changes from baseline (top) to day 14 (bottom).
discussion
We present the first-in-human study evaluating viral delivery of E. coli PNP followed by systemic fludarabine. Our findings demonstrate safety and efficacy in human subjects, corroborating results seen in preclinical studies. Because non-target lesions were stable or progressed, our results confirm that fludarabine has negligible efficacy against solid tumors, and antitumor activity is confined to lesions that received intratumoral injection.
No dose-limiting events or dose-related increases in toxicity were identified in this study. The lack of toxicity is consistent with the local nature of injectable treatments and extends preclinical studies, demonstrating that co-administration of E. coli PNP/fludarabine is safe [4, 5, 15]. The absence of detectable serum fluoroadenine indicates that release of the compound into systemic circulation occurs at levels below the toxic range. This absence of toxicity and evidence of dose-related tumor response in this early phase trial suggest that therapeutic efficacy might be improved with repeated treatment cycles, increasing volume of the vector, and increasing the fludarabine dose (the maximum dose of fludarabine used in our study was 60% of a standard weekly dose for the treatment of chronic leukemia). Although one may expect increased doses to confer additional toxicity associated with fludarabine, this risk must be weighed against potential gains in efficacy (Figure 3).
Several published studies have compared PNP with first-generation suicide gene strategies such as activation of ganciclovir by herpes simplex virus thymidine kinase (HSV-TK) or 5-fluorocytosine by E. coli cytosine deaminase (CD), and several advantages have been established for E. coli PNP [16–20]. In contrast to HSV-TK or CD, E. coli PNP confers pronounced and reproducible in vivo bystander activity in preclinical studies using human tumor xenografts. The strategy is capable of eliminating at least 95% of tumor cells that do not express the enzyme. To our knowledge, no other prodrug activation strategy has shown bystander killing of this magnitude [21, 22]. It should also be noted that fluoroadenine is highly potent in its ability to kill cancer cells (∼1000 times more active on a molar basis than 5-fluorouracil, the compound generated by CD [1]), indicating only a small amount of fluoroadenine is required to reach surrounding tumor cells (that do not express E. coli PNP) to be effective. Finally, inhibition of RNA and protein synthesis via fluoroadenine kills both proliferating and nonproliferating tumor cells, and may improve outcomes when used in conjunction with radiation therapy [15].
A key limitation of the treatment strategy described by this report is a requirement for intratumoral injection using multiple passes with a narrow gauge needle to ensure optimal distribution of virus within tumor tissue. Although there are techniques to improve drug diffusion after direct intratumoral injection [23, 24], Ad/PNP is limited at present to neoplasms that are amenable to local inoculation. Nevertheless, head and neck, cutaneous, breast, and many gynecologic malignancies may still benefit. Furthermore, there has been significant improvement in delivery strategies for the PNP gene which include replication-deficient retrovirus, measles vectors, vaccinia, and herpes simplex virus, among others.
In summary, the present report describes clinical validation and shows safety and clinical responses in refractory tumors using E. coli PNP/fludarabine. More aggressive treatment strategies are warranted in next phase trials and could be used to treat refractory, needle accessible solid tumors with curative intent, for tumors palliation, or as a neoadjuvant (preoperative) means to ‘de-bulk’ needle accessible tumors (such as breast or prostate) before surgical resection.
conclusion
Successful regression of tumors without significant toxicity was demonstrated in this first-in-human study of an effective prodrug activation strategy using E. coli PNP. These results warrant future clinical trials with multiple cycles until the tumor mass has been eliminated or sufficiently reduced in size, so that it could be more safely and completely excised with limited morbidity.
funding
This work was supported by PNP Therapeutics, Inc. and a grant from the NIH T32CA091078.
disclosure
EJS, JH, FRH, and WBP have significant equity interests in PNP Therapeutics, Inc., which has licensed the patents concerning use of E. coli PNP for the treatment of cancer. EJS is also a faculty member at the University of Alabama Birmingham. WP is an employee of Southern Research Institute in Birmingham, AL. All remaining authors have declared no conflicts of interest.
references
- 1.Parker WB, Allan PW, Shaddix SC, et al. Metabolism and metabolic actions of 6-methylpurine and 2-fluoroadenine in human cells. Biochem Pharmacol 1998; 55: 1673–1681. [DOI] [PubMed] [Google Scholar]
- 2.Hughes BW, Wells AH, Bebok Z, et al. Bystander killing of melanoma cells using the human tyrosinase promoter to express the Escherichia coli purine nucleoside phosphorylase gene. Cancer Res 1995; 55: 3339–3345. [PubMed] [Google Scholar]
- 3.Hughes BW, King SA, Allan PW, et al. Cell to cell contact is not required for bystander cell killing by Escherichia coli purine nucleoside phosphorylase. J Biol Chem 1998; 273: 2322–2328. [DOI] [PubMed] [Google Scholar]
- 4.Hong JS, Waud WR, Levasseur DN, et al. Excellent in vivo bystander activity of fludarabine phosphate against human glioma xenografts that express the Escherichia coli purine nucleoside phosphorylase gene. Cancer Res 2004; 64: 6610–6615. [DOI] [PubMed] [Google Scholar]
- 5.Parker WB, Allan PW, Waud WR, et al. Effect of expression of adenine phosphoribosyltransferase on the in vivo anti-tumor activity of prodrugs activated by E. coli purine nucleoside phosphorylase. Cancer Gene Ther 2011; 18: 390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kikuchi E, Menendez S, Ozu C, et al. Delivery of replication-competent retrovirus expressing Escherichia coli purine nucleoside phosphorylase increases the metabolism of the prodrug, fludarabine phosphate and suppresses the growth of bladder tumor xenografts. Cancer Gene Ther 2007; 14: 279–286. [DOI] [PubMed] [Google Scholar]
- 7.Ungerechts G, Springfeld C, Frenzke ME, et al. Lymphoma chemovirotherapy: CD20-targeted and convertase-armed measles virus can synergize with fludarabine. Cancer Res 2007; 67: 10939–10947. [DOI] [PubMed] [Google Scholar]
- 8.Deharvengt S, Wack S, Uhring M, et al. Suicide gene/prodrug therapy for pancreatic adenocarcinoma by E. coli purine nucleoside phosphorylase and 6-methylpurine 2′-deoxyriboside. Pancreas 2004; 28: E54–E64. [DOI] [PubMed] [Google Scholar]
- 9.Krohne TU, Shankara S, Geissler M, et al. Mechanisms of cell death induced by suicide genes encoding purine nucleoside phosphorylase and thymidine kinase in human hepatocellular carcinoma cells in vitro. Hepatology 2001; 34: 511–518. [DOI] [PubMed] [Google Scholar]
- 10.Mohr L, Shankara S, Yoon SK, et al. Gene therapy of hepatocellular carcinoma in vitro and in vivo in nude mice by adenoviral transfer of the Escherichia coli purine nucleoside phosphorylase gene. Hepatology 2000; 31: 606–614. [DOI] [PubMed] [Google Scholar]
- 11.Ungerechts G, Springfeld C, Frenzke ME, et al. An immunocompetent murine model for oncolysis with an armed and targeted measles virus. Mol Ther 2007; 15: 1991–1997. [DOI] [PubMed] [Google Scholar]
- 12.National Cancer Institute NCI Guidelines for Investigators. Adverse Event Reporting Requirements for DCTD (CTEP and CIP) and DCP INDs and IDEs. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/aeguidelines.pdf (3 March 2015, date last accessed). [Google Scholar]
- 13.Gadi VK, Alexander SD, Kudlow JE, et al. In vivo sensitization of ovarian tumors to chemotherapy by expression of E. coli purine nucleoside phosphorylase in a small fraction of cells. Gene Ther 2000; 7: 1738–1743. [DOI] [PubMed] [Google Scholar]
- 14.R Core Team. R: A language and environment for statistical computing. http://www.R-project.org (20 January 2015, date last accessed). [Google Scholar]
- 15.Sorscher EJ, Hong JS, Allan PW, et al. In vivo antitumor activity of intratumoral fludarabine phosphate in refractory tumors expressing E. coli purine nucleoside phosphorylase. Cancer Chemother Pharmacol 2012; 70: 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nestler U, Heinkelein M, Lucke M, et al. Foamy virus vectors for suicide gene therapy. Gene Ther 1997; 4: 1270–1277. [DOI] [PubMed] [Google Scholar]
- 17.Lockett LJ, Molloy PL, Russell PJ, Both GW. Relative efficiency of tumor cell killing in vitro by two enzyme-prodrug systems delivered by identical adenovirus vectors. Clin Cancer Res 1997; 3: 2075–2080. [PubMed] [Google Scholar]
- 18.Xie X, Guo J, Kong Y, et al. Targeted expression of Escherichia coli purine nucleoside phosphorylase and Fludara(R) for prostate cancer therapy. J Gene Med 2011; 13: 680–691. [DOI] [PubMed] [Google Scholar]
- 19.Martiniello-Wilks R, Garcia-Aragon J, Daja MM, et al. In vivo gene therapy for prostate cancer: preclinical evaluation of two different enzyme-directed prodrug therapy systems delivered by identical adenovirus vectors. Hum Gene Ther 1998; 9: 1617–1626. [DOI] [PubMed] [Google Scholar]
- 20.Puhlmann M, Gnant M, Brown CK, et al. Thymidine kinase-deleted vaccinia virus expressing purine nucleoside phosphorylase as a vector for tumor-directed gene therapy. Hum Gene Ther 1999; 10: 649–657. [DOI] [PubMed] [Google Scholar]
- 21.Yao SY, Ng AM, Cass CE, et al. Nucleobase transport by human equilibrative nucleoside transporter 1 (hENT1). J Biol Chem 2011; 286: 32552–32562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Visser F, King KM, et al. The role of nucleoside transporters in cancer chemotherapy with nucleoside drugs. Cancer Metastasis Rev 2007; 26: 85–110. [DOI] [PubMed] [Google Scholar]
- 23.Currier MA, Adams LC, Mahller YY, Cripe TP. Widespread intratumoral virus distribution with fractionated injection enables local control of large human rhabdomyosarcoma xenografts by oncolytic herpes simplex viruses. Cancer Gene Ther 2005; 12: 407–416. [DOI] [PubMed] [Google Scholar]
- 24.Bazan-Peregrino M, Carlisle RC, Purdie L, Seymour LW. Factors influencing retention of adenovirus within tumours following direct intratumoural injection. Gene Ther 2008; 15: 688–694. [DOI] [PubMed] [Google Scholar]


