Systemic drug reactions occurred more frequently with weekly rifapentine plus isoniazid and were mostly flu-like: white race, female sex, lower body mass index, and older age increased risk. Severe reactions were associated with concomitant medication and white race.
Keywords: treatment of latent M. tuberculosis infection, flu-like syndrome, adverse drug reaction, rifapentine, isoniazid
Abstract
Background. Weekly rifapentine plus isoniazid for 3 months (3HP) is as effective as daily isoniazid for 9 months (9H) for latent tuberculosis infection in high-risk persons, but there have been reports of possible flu-like syndrome.
Methods. We identified clinically significant systemic drug reactions (SDR) and evaluated risk factors in patients who did not complete treatment in the PREVENT Tuberculosis study.
Results. Among 7552 persons who received ≥1 dose of study drug, 153 had a SDR: 138/3893 (3.5%) with 3HP vs 15/3659 (0.4%) with 9H (P < .001). In the 3HP arm, 87 (63%) had flu-like syndrome and 23 (17%) had cutaneous reactions; 13/3893 (0.3%) had severe reactions (6 were hypotensive) and 6 reported syncope. Symptoms occurred after a median of 3 doses, and 4 hours after the dose; median time to resolution was 24 hours. There were no deaths. In multivariate logistic regression analysis, factors independently associated with SDR included receipt of 3HP (adjusted odds ratio [aOR] 9.4; 95% confidence interval [CI], 5.5, 16.2), white non-Hispanic race/ethnicity (aOR 3.3; 95% CI, 2.3, 4.7), female sex (aOR 2.0; 95% CI, 1.4, 2.9), age ≥35 years (aOR 2.0; 95% CI, 1.4, 2.9), and lower body mass index (body mass index [BMI]; P = .009). In a separate multivariate analysis among persons who received 3HP, severe SDR were associated with white non-Hispanic race/ethnicity (aOR 5.4; 95% CI, 1.8, 16.3), and receipt of concomitant non-study medications (aOR 5.9; 95% CI, 1.3, 27.1).
Conclusions. SDR were more common with 3HP, and mostly flu-like. Persons of white race, female sex, older age, and lower BMI were at increased risk. Severe reactions were rare and associated with 3HP, concomitant medication, and white race. The underlying mechanism is unclear.
Clinical Trials Registration. NCT00023452.
Once-weekly rifapentine plus isoniazid for 3 months (3HP) is effective against latent Mycobacterium tuberculosis infection [1–3] and is an alternative to 9 months of daily isoniazid (9H) [4]. The PREVENT Tuberculosis study was a randomized open-label trial of once-weekly directly-observed 3HP vs daily self-administered 9H [1]. Early in the trial there were reports of possible drug hypersensitivity or flu-like syndrome. This had not been reported with rifapentine plus isoniazid for the treatment of active tuberculosis [5, 6]. A “flu-like syndrome” characterized by fever, chills, fatigue, malaise, headache, myalgia, and arthralgia has been reported with intermittent, high doses of rifampin [7–14]. Among persons receiving once-weekly rifampin as part of their anti-tuberculosis regimen, a flu-like syndrome developed among 35%–57% of persons who received 1200–1800 mg of rifampin, 22%–31% of those who received 900 mg, and 10% of those who received 600 mg rifampin once-weekly [11]. In contrast, among persons treated with twice-weekly rifampin plus isoniazid, flu syndrome was reported in 8% of those receiving 900 mg and 4% of those receiving 600 mg rifampin [11].
The rifampin flu-like syndrome usually develops after 3–6 months of treatment [11, 15]. It may be less common in patients who initially have lower, daily dosing [5, 12, 16, 17]. Patients who develop the syndrome on high-dose intermittent regimens often subsequently tolerate lower daily dosing [15]. This is different from the severe immunologically-mediated reaction to other drugs, which typically occurs within 2 months, is less dependent on dose, and often intensifies with continued and subsequent exposure following a reaction [18, 19]. Symptoms with rifampin appear 1–2 hours after drug administration and last up to 8 hours [9, 11]. These symptoms are more common in women than men, and the incidence increases with age [20–22].
There have also been case reports of a flu syndrome with isoniazid [23–29]. Manifestations include pruritic rash, appetite loss, myalgia, arthralgia, fatigue, weight loss, malaise, headache, fever, red eyes, leukocytosis, and hypotension.
We sought to evaluate reactions with systemic manifestations in all participants in the PREVENT Tuberculosis clinical trial, to ascertain their severity, and to identify risk factors for these reactions.
METHODS
Study Population
The PREVENT Tuberculosis study was a prospective, open-label, randomized trial of 3 months of once-weekly rifapentine 900 mg (graduated dosing for persons ≤50 kg) plus isoniazid 15–25 mg/kg (rounded up to nearest 50 mg; 900 mg maximum) given under direct observation (3HP), compared to 9 months of daily self-administered isoniazid 5–15 mg/kg (rounded up to nearest 50 mg; 300 mg maximum) (9H). Persons ≥12 years of age with latent M. tuberculosis infection were enrolled between June 2001 and February 2008. Participants from one PREVENT Tuberculosis study site were excluded from this analysis due to discrepancies regarding receipt of study drug and directly-observed therapy. The PREVENT Tuberculosis study and a dedicated substudy were approved by the institutional review boards of the Centers for Disease Control and Prevention (CDC) and all study sites. Written informed consent was obtained from all study participants.
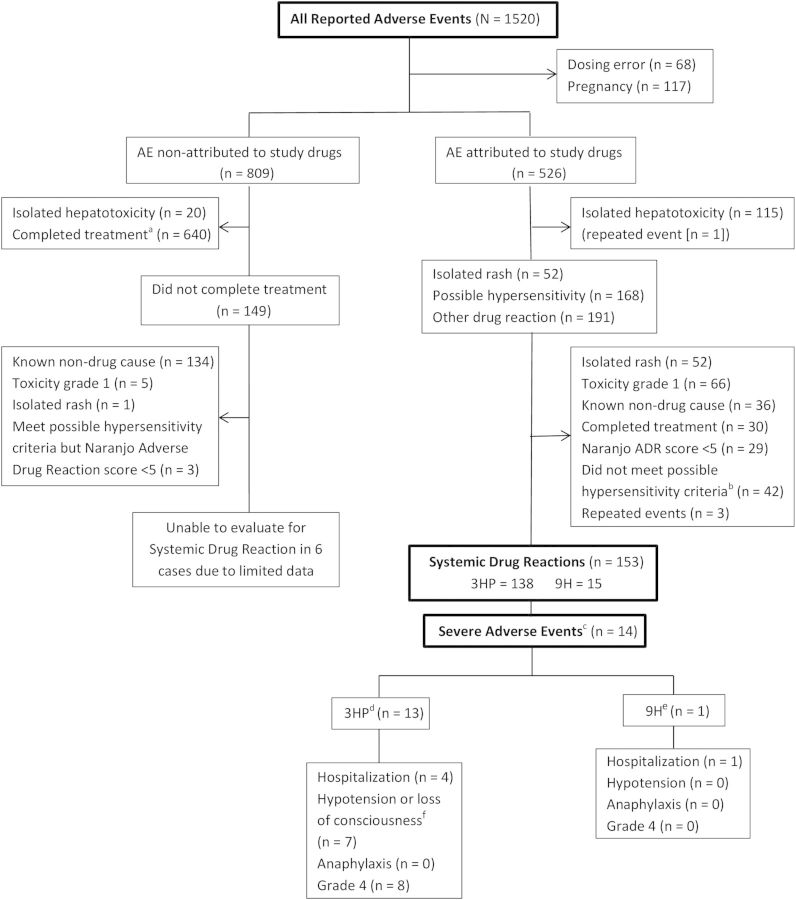
Information about adverse events (AE) was collected monthly during treatment and until 30 days after last study dose, graded by the Cancer Therapy Evaluation Program common toxicity criteria [30]. All AE from patients who received ≥1 dose of study drug were included in this analysis. Details have been published previously [1]. All AE, excluding pregnancies and dosing errors, were evaluated. The events were classified as attributed to study drugs (definitely, probably, or possibly) or not-attributed to study drugs (unlikely, non-related, or unclassifiable), as reported by the local site investigator (Supplementary Table 1). To focus on clinically significant systemic reactions, isolated hepatotoxicity, isolated rash, AE with known non-drug cause, AE of grade 1 severity [30], and events in participants able to complete treatment (11 doses in the 3HP arm or 240 doses in the 9H arm), were excluded. The Naranjo adverse drug reaction probability scale [31] was used to objectively evaluate AE regardless of regimen. The scale was modified to increase specificity for possible hypersensitivity events (Supplementary Table 2). AE with low score (≤5) were excluded. In addition, after the first AE reports of possible hypersensitivity had been received and no clear definition was available, the PREVENT Tuberculosis sub-study protocol team established two broad criteria for such a drug reaction; (1) hypotension (systolic blood pressure <90 mm Hg), urticaria (hives), angioedema, acute bronchospasm, or conjunctivitis (red eyes); and (2) >4 of the following symptoms occurring concurrently (>1 of which had to be grade 2 or higher): weakness, fatigue, nausea, vomiting, headache, fever, aches, sweats, dizziness, shortness of breath, flushing, or chills. AE that met either of the above criteria were termed systemic drug reactions (SDR).
Severe AE were defined as those resulting in hospitalization, hypotension (systolic blood pressure <90 mm Hg) or loss of consciousness, anaphylaxis, or grade 4 toxicity [30]. Anaphylaxis was defined as at least one major dermatological reaction and at least 1 cardiovascular and/or respiratory criterion [32].
SDR were classified in one of five hierarchical and mutually exclusive categories: cutaneous, flu-like, gastrointestinal, respiratory, or not defined (see Table 2 for definitions) [13, 15]. Concomitant medications received before or up to 7 days after the SDR (the latter to ensure capture of medications received prior to the event) were recorded and coded according to the WHO Drug B2 Herbal / DDE drug dictionary (June 2010 version). Vitamins, calcium carbonate, mineral supplements, and antioxidants were considered nonstudy concomitant medications and included in the analysis. Medications given to treat AE were not considered concomitant medications. An in-depth study record review was performed (by R. M., E. P., G. S., N. F. A., M. E. V.) for all AE attributed to study drugs. Drug rechallenge was allowed at the discretion of the site study investigators (See guidelines for re-challenge in the Supplemental Material, including Figure 1). Drug restart was defined as receipt of the full protocol-recommended regimen after the event.
Table 2.
Characterization of the 153 Systemic Drug Reactions According to Syndrome
| 3HP (n = 138) | 9H (n = 15) | |
|---|---|---|
| Cutaneousa | 23 (17%) | 9 (60%) |
| Severe | 3 | 1 |
| Nonsevere | 20 | 8 |
| Flu-likeb | 87 (63%) | 2 (13%) |
| Severe | 6 | 0 |
| Nonsevere | 81 | 2 |
| Gastrointestinalc | 7 (5%) | 1 (7%) |
| Severe | 2 | 0 |
| Nonsevere | 5 | 1 |
| Respiratoryd | 5 (4%) | 0 (0%) |
| Severe | 1 | 0 |
| Nonsevere | 4 | 0 |
| Not definede | 16 (12%) | 3 (20%) |
| Severe | 1 | 0 |
| Nonsevere | 15 | 3 |
The syndromes were compiled from definitions in the literature and the study protocol.
The syndromes were hierarchically ordered (as presented below) and mutually exclusive.
a Angioedema, urticaria, rash and itching, or anaphylaxis (defined as cutaneous plus circulatory or respiratory reaction) that occurred within 24 hours of the study dose.
b Presence of (fever or chills) and (weakness, fatigue or muscle pain) and (aches, syncope, heart rate >100, palpitations, flushing, dizziness, conjunctivitis (red eyes), or sweats). This includes 6 syncope events.
c Presence of vomiting (with or without nausea) or abdominal pain.
d Bronchospasm, cough, shortness of breath, or chest pain.
e Did not meet any of the above definitions.
Figure 1.
Adverse events (AE) evaluated in the PREVENT Tuberculosis study. aCompletion of treatment definition: 11/12 doses in the 3HP or 240/270 in the 9H arm; bCriteria for possible hypersensitivity: (1) hypotension (systolic blood pressure <90 mm Hg), urticaria, angioedema (defined as swelling around the lips or eyes), acute bronchospasm, or conjunctivitis (red eyes); or (2) ≥4 of these symptoms concurrently, ≥1 of which was grade 2 or higher: weakness, fatigue, nausea, vomiting, headache, fever, aches, sweats, dizziness, shortness of breath, flushing, or chills; cSevere Adverse Event: hospitalization, hypotension (systolic blood pressure <90 mmHg) or loss of consciousness, anaphylaxis, or grade 4 toxicity. These categories were not mutually exclusive; d3HP: 3 months of once-weekly rifapentine plus isoniazid given under direct observation; e9H: 9 months of daily isoniazid given self-administered; fOne report was received after the 6 cases of hypotension reported in the parent PREVENT Tuberculosis study. Abbreviation: ADR, adverse drug reaction.
Statistical Analysis
Categorical variables were compared using the Pearson's chi-squared test. Continuous variables were compared using the 2-sample Kolmogorov—Smirnov (asymptotic) nonparametric test [33]. Univariate and multivariate logistic regression analyses were performed; clinically important variables (HIV infection and smoking) and all variables with P < .05 in the univariate analysis were included in the multivariate model. Interaction terms between study arms and all factors were also included in the multivariate model; this analytical strategy reveals whether arm-specific associations are statistically different between arms; this comparison is not possible in stratified analysis [34]. To control for variations among enrolling sites, we constructed a generalized linear mixed model where each site was treated as a random effect [35]. In addition, an analysis of residuals was used to analyze age as a continuous variable; no pattern was identified, so median age of the study population was used in the model. Body mass index (BMI) was evaluated as a continuous variable but grouped according to standard categories [36]. A cluster analysis clustered participants with similar signs and symptoms (Supplemental Material) [37]. A nonparametric test compared the time of SDR onset after the last dose ingested, and the time to resolution. Statistical analyses were conducted using SAS version 9.3; the cluster analysis was performed using R.
RESULTS
Among the 7552 (3HP = 3893 and 9H = 3659) study participants who received ≥1 dose of study drug, there were 1520 AE reported. After excluding pregnancies (n = 117) and dosing errors (n = 68), 809 were reported as nonattributed and 526 attributed to study drugs. No SDR were identified in the nonattributed group, although 6 events could not be evaluated due to limited data. Of 526 AE attributed to study drugs, 153 SDR occurred in 153 individual participants (2.0% of all 7552 participants): 138/3893 (3.5%) in the 3HP arm vs 15/3659 (0.4%) in the 9H (P < .001) (Figure 1 and Table 1). There were 14 severe SDR, of which 13 occurred in the 3HP arm (13/3893 = 0.3%).
Table 1.
Adverse Events Attributed to Study Drug Among PREVENT Tuberculosis Participants—by Study Arm
| 3HP | 9H | |
|---|---|---|
| Received ≥1 dose study drug | 3893 | 3659 |
| AE attributable to study drug | 327 (8.4%) | 199 (5.4%) |
| Hepatotoxicity | 17 | 97 |
| Rash only | 31 | 21 |
| Toxicity grade 1 | 39 | 27 |
| Other known reason for AE | 31 | 5 |
| Completed treatment | 27 | 3 |
| Naranjo score <5, Possible or Doubtfula | 21 | 8 |
| Did not meet possible HS criteria 1) or 2) | 20 | 22 |
| Repeated events | 3 | 1 |
| Systemic drug reactions | 138 (3.5%) | 15 (0.4%) |
| Met possible HS criterion 1b | 63 (1.6%) | 9 (0.2%) |
| Met only HS criterion 2c | 75 (1.9%) | 6 (0.2%) |
| Clinically severe events (n = 14)d | 13 | 1 |
| Hospitalization | 4e | 1 |
| Hypotension or loss of consciousness | 7 | 0 |
| Anaphylaxis | 0 | 0 |
| Grade 4 toxicity | 8f | 0 |
There were 6 cases of hypotension and 6 reported syncopal events (2 syncopal episodes were severe events); these 12 episodes did not overlap. One case of syncope had loss of consciousness.
Abbreviations: AE, adverse event; HS, hypersensitivity.
a Of those who remained, AE among participants receiving 3HP: Highly probable (22), Probable (134); AE among participants receiving 9H: Highly probable (0), Probable (35).
b Includes 1 case of syncope.
c Includes 5 cases of syncope.
d The categories are not mutually exclusive.
e Includes 0 cases of syncope.
f Includes 1 case of syncope.
Among the 138 events in the 3HP arm, 23 (17%) were cutaneous and 87 (63%) were flu-like syndrome. In contrast, among the 15 events in the 9H arm, 9 (60%) were cutaneous and 2 (13%) were flu-like (Table 2). Of the 138 participants in the 3HP arm who developed an SDR, 73 were re-challenged with study drug (Supplementary Table 3). Of the 51 participants initially rechallenged with rifapentine, 36 (71%) tolerated it. Of the 20 participants initially rechallenged with isoniazid, 3 (15%) tolerated it. Only 2/73 (3%) tolerated both isoniazid and rifapentine on re-challenge, and none completed study treatment. In addition, none of the 153 study participants who developed an SDR completed study treatment, per study criteria. Data on whether an alternative regimen was completed were not available.
Persons developing an SDR were more likely to receive 3HP, were older, more likely to be female, of white non-Hispanic race/ethnicity, and to receive concomitant nonstudy medication (Supplementary Table 4). Among persons developing an SDR, the event occurred after a median of 3 once-weekly doses in the 3HP arm and 16 daily doses in the 9H arm (Table 3). In the 3HP arm, the median time from drug ingestion to symptom onset was 4 hours, and the median time from symptom onset to resolution was 24 hours (Table 3). There was no difference in time to symptom resolution among those who did vs did not develop fever (data not shown).
Table 3.
Time to Symptom Onset and Resolution of Systemic Drug Reactions
| Event | 3HP (N = 138) | 9H (N = 15) | P Valuea |
|---|---|---|---|
| Median doses received prior to event onset (IQR) | 3 (2.0–5.0) (n = 138) | 17 (9–57.0) (n = 15) | NAb |
| Median time from drug ingestion to event onset (hours; IQR) | 4 (1.0–8.0) (n = 135) | 1.5 (1.0–13.5) (n = 8) | .60 |
| Median time to symptom resolution (hours; IQR) | 24 (12–48) (n = 132) | 24 (2–48) (n = 11) | .89 |
| Median time to symptom resolution—nonsevere events (n = 139) (hours; IQR) | 24 (12–62) (n = 119) | 36 (12–48) (n = 10) | … |
| Median time to symptom resolution—severe events (n = 14) (hours; IQR) | 21 (6–24) (n = 13) | 2 (n = 1) | … |
Abbreviation: IQR, interquartile range.
a Two sample Kolmogorov–Smirnov (Asymptotic) test, a nonparametric test due to the small sample size and not normally distributed.
b NA: Not applicable since the comparison is between regimens with different dosing intervals.
Signs and symptoms of the SDR are in Supplementary Table 5. In the 3HP arm, fatigue, headache, nausea, weakness, chills, and myalgia were most common. All of the signs and symptoms were associated in the cluster analysis, distinct from a cluster that included rash and itching. Fever (>100°F) was present in approximately half.
There were 3 persons younger than 18 (age range 14–17): 2 received 3HP and 1 received 9H; none developed a severe event and all recovered in <24 hours.
The univariate and multivariate logistic regression analysis of risk factors for SDR are in Tables 4 and 5. In the multivariate analysis, factors independently associated with these reactions were receipt of 3HP, white race, female sex, age ≥35 years, and lower BMI. To control for variations according to study site, a generalized linear mixed model was performed; results were similar (data not shown). In the multivariate analysis, an interaction between regimen and sex was noted; the risk of SDR according to regimen and sex is in Table 6. After adjusting for the variables in the multivariate model, among those receiving 9H, the risk of SDR was 15-fold higher in women than men. Among those receiving 3HP, the risk of SDR was approximately twice as high in women as men. In those persons at highest risk of SDR (white non-Hispanic women >35 years old who received 3HP), the probability of developing SDR was 12.3% (95% CI, 8.1%, 24.4%) (Supplementary Table 6).
Table 4.
Univariate Logistic Regression Analysis of Risk Factors for Systemic Drug Reactions
| OR | 95% CI | P Value | |
|---|---|---|---|
| 3HP (n = 3893) vs 9H (n = 3659) | 8.9 | 5.2, 15.2 | <.001 |
| White-non-Hispanic race | 3.4 | 2.4, 4.8 | <.001 |
| Female sex | 2.1 | 1.5, 2.9 | <.001 |
| Age ≥35 y (mediana) | 2.0 | 1.4, 2.8 | <.001 |
| Body mass index | .03 | ||
| 18.5–24.9 (normal) | reference | ||
| <18.5 (underweight) | 0.8 | .3, 1.8 | .55 |
| 25–29.9 (overweight) | 0.5 | .4, .8 | .004 |
| ≥30 (obese) | 0.9 | .6, 1.3 | .58 |
| Any concomitant nonstudy drug | 1.6 | 1.1, 2.2 | .006 |
| HIV infection | 0.3 | .04, 2.3 | .25 |
| Smoking | 1.0 | .7, 1.5 | .99 |
There were 153 systemic drug reactions among 7552 study participants.
There was no interaction between age and receipt of concomitant nonstudy drugs.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio.
a Of the 7552 study participants.
Table 5.
Multivariate Logistic Regression of Risk Factors for Systemic Drug Reactions
| Adjusted OR | 95% CI | P Value | |
|---|---|---|---|
| 3HP vs 9H | 9.4 | 5.5, 16.2 | <.001 |
| White-non-Hispanic race | 3.3 | 2.3, 4.7 | <.001 |
| Female sex | 2.0 | 1.4, 2.9 | <.001 |
| Age ≥35 y (mediana) | 2.0 | 1.4, 2.9 | <.001 |
| Body mass index (BMI) | .009 | ||
| 18.5–24.9 (normal) | reference | ||
| <18.5 (underweight) | 0.9 | .4, 2.2 | .88 |
| 25–29.9 (overweight) | 0.5 | .3, .7 | .001 |
| ≥30 (obese) | 0.7 | .4, 1.0 | .05 |
| Any concomitant non-study drug | 1.2 | .8, 1.7 | .33 |
There were 153 systemic drug reactions among 7552 persons.
The model also included HIV infection and smoking because of their potential role in systemic drug reactions, but these variables were not statistically significant. We tested for the following possible individual interactions: a) regimen with race, sex, age, concomitant medications, BMI, HIV, smoking; b) concomitant medications with race, sex, and age. The only interaction was between regimen and sex, therefore the results (Table 6) are presented for each category of regimen and sex.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio.
a Of the 7552 study participants.
Table 6.
Final Multivariate Logistic Regression Model of Risk Factors for Systemic Drug Reactions, Evaluating Regimen by Sex
| Adjusted OR | 95% CI | P Value | |
|---|---|---|---|
| Regimen – sex | <.001 | ||
| 9H male (reference) | … | ||
| 9H female | 15.1 | 2.0, 115.5 | .009 |
| 3HP male | 53.4 | 7.4, 386.3 | <.001 |
| 3HP female | 94.4 | 13.1, 680.6 | <.001 |
| White-non-Hispanic | 3.3 | 2.3, 4.7 | <.001 |
| Age ≥35 y (mediana) | 2.0 | 1.4, 2.9 | <.001 |
| Body mass index | .01 | ||
| 18.5–24.9 (normal) | reference | ||
| <18.5 (underweight) | 0.9 | .4, 2.3 | .9 |
| 25–29.9 (overweight) | 0.5 | .3, .7 | .001 |
| ≥30 (obese) | 0.7 | .5, 1.0 | .05 |
There were 153 systemic drug reactions among 7552 persons.
This model included HIV infection, use of any concomitant medication, and smoking, but these variables were not statistically significant.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio.
a Of the 7552 study participants.
Severe SDR were uncommon. Among the 13 persons who received 3HP and developed a severe SDR, the median number of doses received before event onset was 5. Six persons developed hypotension, and 2 others reported syncope. The lowest reported systolic blood pressure was 70 mmHg (in one participant), though none required vasopressor support and the median time to recovery was 24 hours. Overall, 6 persons were reported to develop syncope, of whom 2 had severe SDR; none of them had documented hypotension and all of them recovered in <24 hours. Only one of the 6 episodes of syncope had loss of consciousness.
There were no deaths or permanent sequelae from SDR; there were no instances of Stevens-Johnson syndrome, toxic epidermal necrolysis, or drug reaction with eosinophilia and systemic symptoms, and no episodes of acute renal failure, bleeding due to thrombocytopenia, or hemolytic anemia. There was 1 episode each of thrombocytopenia (without evidence of bleeding), anemia (not hemolytic), and neutropenia (see Supplemental Material for details). There were no reports of renal failure.
In a logistic regression analysis of predictors of clinically severe SDR among persons who received 3HP, white non-Hispanic race/ethnicity and concomitant nonstudy medication were the only statistically significant variables (Table 7). Of the 14 persons who developed clinically severe SDR, 9 (64%) received ≥2 concomitant medications. Of the 28 concomitant medications received, 14 (50%) were for the alimentary tract/metabolism and 8 (29%) were for cardiovascular indications. There was no predominant medication or medication class associated with severe SDR. In those persons at highest risk of severe SDR (white non-Hispanic persons who received 3HP plus concomitant medications), the probability of developing SDR was 1.8% (95% CI, .9%, 4%) (Supplementary Table 6).
Table 7.
Univariate and Multivariate Logistic Regression Analysis of Predictors of Clinically Severe Systemic Drug Reactions (n = 13) Among 3893 Study Participants Who Received ≥1 Dose of the 3HP Regimen
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | Adjusted OR | 95% CI | P Value | |
| White non-Hispanic | 7.4 | 2.5, 22.0 | <.001 | 5. | 1.8, 16.3 | .003 |
| Female sex | 1.4 | .5, 4.3 | .51 | … | … | … |
| Age ≥35 y (mediana) | 2.1 | .6, 6.7 | .23 | … | … | … |
| Any nonstudy concomitant medicationb | 7.8 | 1.7, 35.4 | .008 | 5.9 | 1.3, 27.1 | .02 |
| BMI (per 1 unit increase) | 1.0 | .9, 1.1 | .69 | |||
| Smoking | 0.2 | .03, 1.7 | .15 | … | … | … |
Of those concomitant medications reported by persons who developed a severe event, 52% were classified as for the alimentary tract/metabolism (including vitamins) and 28% were classified as cardiovascular.
Prior to performing these analyses among persons who received 3HP, the following interactions were evaluated among the full dataset of 7552 participants, but none were statistically significant: (1) regimen with race, sex, age, concomitant medication, BMI; (2) concomitant medication with race, sex, age; (3) BMI with sex.
Abbreviations: BMI, body mass index; CI, confidence interval; OR, odds ratio.
a Of the 7552 study participants.
b This includes concomitant medications given before or up to 7 days after the onset date. Medications to treat adverse events were excluded.
DISCUSSION
In the PREVENT Tuberculosis study SDR were uncommon but occurred significantly more frequently among persons who received 3HP than 9H. Among persons in the 3HP arm, the most common presentation was flu-like syndrome, which accounted for 63% of the SDR episodes. These reactions were associated with female sex, white non-Hispanic race/ethnicity, age ≥35 years old, and lower BMI. Female sex, white race, and increased age have been reported as risk factors for flu-like syndrome among persons who received intermittent rifampin—usually at high doses and given together with isoniazid or ethambutol [11, 12, 15, 20, 38]. In 2 recent studies of 3HP for treatment of latent M. tuberculosis infection, there were no reports of flu-like syndrome, but both studies were smaller than PREVENT Tuberculosis and were conducted in Brazil and South Africa; very few white women of older age were enrolled [2, 3]. The inconsistency in reproducibility of symptoms upon single and multiple drug rechallenge, female sex, the association with lower BMI, and the successful completion of treatment in many patients without morbidity despite the previous adverse drug reactions, suggest that the SDR and flu-like symptoms may not be immunologically mediated. This SDR differs from flu-like syndrome associated with immunologically mediated reactions such as with abacavir, where there is significant intensification with continued dosing and severe re-challenge morbidity [19]. However, the possibility of an immune-mediated reaction cannot be ruled out.
Previous reports of the rifampin flu-like syndrome noted a possible association with rifampin antibodies [39, 40]. However, the presence of antibodies in persons who have received and tolerated antibiotics is relatively common, and of uncertain importance.
Isoniazid can rarely cause a classic delayed immunologically mediated drug hypersensitivity reaction. Although it can be associated with fever and flu-like symptoms, it can have features distinct from the SDR described in this study, such as skin eruptions, lymphadenopathy, hepatitis, and eosinophilia [23, 24, 27–29, 41]. Isoniazid can also interact with foods rich in monoamines, particularly tyramine [25, 42, 43].
Given the similarity of published reports of flu-like syndrome associated with rifampin and the reactions seen in this study, and given the 9-fold greater frequency of such reactions in the 3HP arm, one might expect rifapentine to more likely cause these symptoms than isoniazid. However, rifapentine was better tolerated than isoniazid on rechallenge (Supplementary Table 3). In a recent multicenter randomized clinical trial of intermittent continuation-phase therapy after 2 months of daily therapy, participants (64% of whom were black males) received 900 mg rifapentine twice-weekly or 1200 mg rifapentine once-weekly, both in combination with moxifloxacin, pyrazinamide, and ethambutol (not isoniazid). There were no reports of possible hypersensitivity or flu-like syndrome [44] (Amina Jindani, unpublished data), but it is possible that the lack of flu-like syndrome was due to the regimens or the population studied.
There were 14 patients with clinically severe reactions: 13/3893 (0.3%) in the 3HP arm and 1/3659 (0.03%) in the 9H arm. Among the 13 episodes in the 3HP arm, 4 patients were hospitalized and 6 developed hypotension. However, most recovered within 24 hours without sequelae. Basic data (onset date, seriousness, attribution) on all AE in the PREVENT Tuberculosis study were collected systematically and prospectively; however, additional data (eg, symptoms on rechallenge, vital signs) were retrospectively and not uniformly collected for severe SDR. This could have contributed the lack of reports of hypotension among those with reported syncope. Decisions regarding rechallenge in participants with SDR were made by the site investigator. About half of participants with an SDR, including the severe events, were not rechallenged. Therefore, it is difficult to ascertain whether isoniazid, rifapentine, or their combination was the cause of SDR. We were also unable to determine whether there were early clinical predictors of subsequent SDR, including severe reactions, since symptoms of adverse drug reactions were ascertained monthly, and the median time to onset in the 3HP arm was before the first monthly visit (week 3). Whether particular symptoms predict subsequent SDR, and discontinuation of treatment in persons with such symptoms decreases SDR risk merits further investigation. It would also appear that SDR would not be mitigated by directly observed therapy, because the median time to symptom onset was 4 hours, and the healthcare worker would likely not be present at that time.
Clinically severe SDR were associated with receipt of 3HP, concomitant nonstudy medications, and white non-Hispanic race/ethnicity. These associations merit further investigation. Although half of the patients received concomitant medications, we found no association with receipt of a particular medication or medication class.
There were several limitations of this study. First, it was a post hoc analysis of data collected in an open-label clinical trial. It is difficult to know if the open label design led to under- or over-estimation of endpoints. Additional data on severe SDR were not systematically collected on all study participants specifically related to possible flu-like syndrome. Second, there was no case definition for this syndrome at the start of the study. However, a case definition was developed during the trial, after the first reports of flu-like syndrome were identified. Third, drug levels that correlate with drug exposure were not obtained in this study. Fourth, the data instruments for surveillance of AE in the clinical trial were not well-suited to investigate potentially novel clinical syndromes.
In conclusion, SDR in the PREVENT Tuberculosis study occurred primarily among persons who received 3HP, were mostly flu-like, did not meet objective drug hypersensitivity criteria, and had features that differ from severe immunologically mediated drug reactions. Most SDR were mild and resolved within 24 hours. Attribution to rifapentine or isoniazid separately or together is not possible with the available data, though the syndromes observed were similar to those reported previously with rifampin. Persons of white race, female sex, increased age, and lower BMI were at increased risk. Severe reactions were rare and appeared to be associated with the receipt of concomitant medication and white race. As 3HP is introduced into clinical practice, including populations that differ from our study population, clinical monitoring and continued vigilance for SDR are warranted.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. PREVENT Tuberculosis substudy on systemic drug reactions protocol team members: Timothy R. Sterling, MD (Chair), N. Franklin Adkinson, Jr, MD, Andrey Borisov, MD, MPH, Jacques Grosset, MD, Judith Hackman, RN, Robert G. Hamilton, PhD, M. Elsa Villarino, MD, MPH, (Centers for Disease Control and Prevention [CDC] Project Officer), Stephen Weis, DO.
PREVENT Tuberculosis protocol team members: Timothy R. Sterling, MD (Chair), M. Elsa Villarino, MD, MPH, (CDC Project Officer), Andrey S. Borisov, MD, MPH, Nong Shang, PhD, Fred Gordin, MD, Awal Khan, PhD, Judith Hackman, RN, Carol Dukes Hamilton, MD, Dick Menzies, MD, MSc, Amy Kerrigan, RN, MSN, C. Robert Horsburgh, Jr, MD, Richard E. Chaisson, MD, George McSherry, MD, Bert Arevalo, BS, Andrew Vernon, MD, MHS (CDC) provided important guidance in final analysis and manuscript preparation. David G. Kleinbaum, PhD (Emory University) provided guidance on some statistical analyses. Charles Heilig, PhD (CDC) provided statistical consultation. Kimberly Fryer, Isabelle Sanchez, and Suet K. Lam performed medical record reviews. Nigel Scott, MS (CDC) assisted with data management. The study investigators and coordinators gratefully acknowledge all study participants.
Disclaimer. The findings and conclusions of this article are those of the authors and do not necessarily represent the views of the CDC. Sanofi did not participate in design of the study; in the collection, analysis, or interpretation of data; in the writing of this manuscript; or in the decision to submit this manuscript for publication.
Financial support. This work was supported by the CDC. Sanofi donated the rifapentine used in this study, and donated $2.5 million to the CDC Foundation to supplement available US federal funding for rifapentine research. Details on the uses of these funds are available. Ruth N. Moro is employed by the CDC Foundation.
Potential conflicts of interest. T. R. S. one-day consultation for Sanofi for presentation of PREVENT Tuberculosis study data to the US Food and Drug Administration in 2012. Data safety monitoring board for a clinical trial sponsored by Otsuka. R. N. M. employed by CDC Foundation, which receives funds for rifapentine research from Sanofi. G. S. consultant to Genzyme (Sanofi) for drug reactions related to the lysosomal storage diseases and alemtuzumab. N. F. A. consultant and data safety monitoring board member for Genzyme (Sanofi). All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: for the Tuberculosis Trials Consortium, Timothy R. Sterling, N. Franklin Adkinson, Andrey Borisov, Jacques Grosset, Judith Hackman, Robert G. Hamilton, M. Elsa Villarino, Stephen Weis, Timothy R. Sterling, M. Elsa Villarino, Andrey S. Borisov, Nong Shang, Fred Gordin, Awal Khan, Judith Hackman, Carol Dukes Hamilton, Dick Menzies, Amy Kerrigan, C. Robert Horsburgh, Richard E. Chaisson, George McSherry, Bert Arevalo, Andrew Vernon, David G. Kleinbaum, Charles Heilig, Kimberly Fryer, Isabelle Sanchez, Suet K. Lam, and Nigel Scott
References
- 1.Sterling TR, Villarino ME, Borisov AS, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med 2011; 365:2155–66. [DOI] [PubMed] [Google Scholar]
- 2.Schechter M, Zajdenverg R, Falco G, et al. Weekly rifapentine/isoniazid or daily rifampin/pyrazinamide for latent tuberculosis in household contacts. Am J Respir Crit Care Med 2006; 173:922–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinson NA, Barnes GL, Moulton LH, et al. New regimens to prevent tuberculosis in adults with HIV infection. N Engl J Med 2011; 365:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep 2011; 60:1650–3. [PubMed] [Google Scholar]
- 5.Bock NN, Sterling TR, Hamilton CD, et al. A prospective, randomized, double-blind study of the tolerability of rifapentine 600, 900, and 1,200 mg plus isoniazid in the continuation phase of tuberculosis treatment. Am J Respir Crit Care Med 2002; 165:1526–30. [DOI] [PubMed] [Google Scholar]
- 6.Benator D, Bhattacharya M, Bozeman L, et al. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomized clinical trial. Lancet 2002; 360:528–34. [DOI] [PubMed] [Google Scholar]
- 7.Girling DJ. Adverse reactions to rifampicin in antituberculosis regimens. J Antimicrob Chemother 1977; 3:115–32. [DOI] [PubMed] [Google Scholar]
- 8.Naafs B, Matemera BO. A possible ‘flu’ syndrome on once-monthly rifampicin. Lepr Rev 1986; 57:271–2. [PubMed] [Google Scholar]
- 9.Martinez E, Collazos J, Mayo J. Hypersensitivity reactions to rifampin. Medicine 1999; 78:361–9. [DOI] [PubMed] [Google Scholar]
- 10.Dhar S, Kaur I, Sharma VK, Kumar B. “Flu” syndrome due to rifampin; experience with four cases. Int J Lepr Other Mycobact Dis 1995; 63:92–4. [PubMed] [Google Scholar]
- 11.Grosset J, Leventis S. Adverse effects of rifampin. Clin Infect Dis 1983; 5:S440–6. [DOI] [PubMed] [Google Scholar]
- 12.Riska NV, Mattson K. Systemic reactions to intermittent rifampicin. Bull Int Union Tuberc 1974; 49(suppl 1):280–5. [PubMed] [Google Scholar]
- 13.Riska N, Mattson K. Adverse reactions during rifampicin treatment. Scand J Respir Dis 1972; 53:87–96. [PubMed] [Google Scholar]
- 14.Salafia A, Candida. Rifampicin induced flu-syndrome and toxic psychosis. Indian J Lepr 1992; 64:537–9. [PubMed] [Google Scholar]
- 15.Aquinas M, Allan WG, Horsfall PA, et al. Adverse reactions to daily and intermittent rifampicin regimens for pulmonary tuberculosis in Hong Kong. Br Med J 1972; 1:765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poole G, Stradling P, Worlledge S. Potentially serious side-effects of high-dose twice-weekly rifampicin. Postgrad Med J 1971; 47:727–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dutt AK, Moers D, Stead WW. Undesirable side effects of isoniazid and rifampin in largely twice-weekly short-course chemotherapy for tuberculosis. Am Rev Respir Dis 1983; 128:419–24. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro M, Ward KM, Stern JJ. A near-fatal hypersensitivity reaction to abacavir: case report and literature review. AIDS Read 2001; 11:222–6. [PubMed] [Google Scholar]
- 19.Rive CM, Bourke J, Phillips EJ. Testing for drug hypersensitivity syndromes. Clin Biochem Rev 2013; 34:15–38. [PMC free article] [PubMed] [Google Scholar]
- 20.Hong Kong Tuberculosis Treatment Services/British Medical Research Council. The influence of age and sex on the incidence of the ‘flu’ syndrome and rifampicin-dependent antibodies in patients on intermittent rifampicin for tuberculosis. Tubercle 1975; 56:173–8. [DOI] [PubMed] [Google Scholar]
- 21.Eule H, Werner E, Winsel K, Iwainsky H. Intermittent chemotherapy of pulmonary tuberculosis using rifampicin and isoniazid for primary treatment: the influence of various factors on the frequency of side-effects. Tubercle 1974; 55:81–9. [PubMed] [Google Scholar]
- 22.A comparative study of daily followed by twice or once weekly regimens of ethambutol and rifampicin in retreatment of patients with pulmonary tuberculosis. The results at 1 year. A cooperative tuberculosis chemotherapy study in Poland. Tubercle 1975; 56:1–26. [DOI] [PubMed] [Google Scholar]
- 23.Motion S, Humphries MJ, Gabriel SM. Severe “flu”-like symptoms due to isoniazid--a report of three cases. Tubercle 1989; 70:57–60. [DOI] [PubMed] [Google Scholar]
- 24.Rubira N, Baltasar MA, Marti E. Hypersensitivity syndrome from isoniazid. Allergy 1999; 54:1011–2. [DOI] [PubMed] [Google Scholar]
- 25.Smith CK, Durack DT. Isoniazid and reaction to cheese. Ann Intern Med 1978; 88:520–1. [DOI] [PubMed] [Google Scholar]
- 26.Pandit S, Choudhury S, Das A, Datta S, Das SK. Isoniazid-induced flu-like syndrome: a rare side effect. Lung India 2013; 30:61–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabrail NY. Severe febrile reaction to isoniazid. Chest 1987; 91:620–1. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs NF, Jr, Thompson SE., III Spiking fever from isoniazid simulating a septic process. JAMA 1977; 238:1759–60. [PubMed] [Google Scholar]
- 29.Crook MJ. Isoniazid-induced anaphylaxis. J Clin Pharmacol 2003; 43:545–6. [PubMed] [Google Scholar]
- 30.Cancer Therapy Evaluation Program. The revised common toxicity criteria: version 2.0. Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf#search=“revisedcommontoxicitycriteria” Accessed 5 May 2015.
- 31.Naranjo CA, Busto V, Sellers E, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30:239–45. [DOI] [PubMed] [Google Scholar]
- 32.Ruggeberg JU, Gold MS, Bayas JM, et al. Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2007; 25:5675–84. [DOI] [PubMed] [Google Scholar]
- 33.Shao J. Kolmogorov-Smirov test. Mathematical Statistics. 2nd ed New York: Springer-Verlag, 2003:446. [Google Scholar]
- 34.Rothman KJ, Greenland S, Lash TL. Stratification. Modern Epidemiology. 3rd ed Philadelphia: Lippincott, Williams & Wilkins, 2008:179. [Google Scholar]
- 35.McCulloch CE, Searle SR. GLLM with random effects. Generalized, Linear, and Mixed Models. New York: John Wiley & Sons, Inc, 2001:222. [Google Scholar]
- 36.Centers for Disease Control and Prevention. Body Mass Index. 2014.
- 37.Kaufman L, Rousseeuw PJ. Finding groups in data: an introduction to cluster analysis, 2005.
- 38.Patki AH, Jadhav VH, Mehta JM. ‘Flu’ syndrome on once monthly rifampicin. Indian J Lepr 1988; 60:84–6. [PubMed] [Google Scholar]
- 39.Hong Kong Tuberculosis Treatment Services/British Medical Research Council. A controlled clinical trial of small daily doses of rifampicin in the prevention of adverse reactions to the drug in a once-weekly regimen of chemotherapy in Hong Kong: second report--the results at 12 months. Tubercle 1974; 55:193–210. [DOI] [PubMed] [Google Scholar]
- 40.Worlledge S. The detection of rifampicin-dependent antibodies. Scand J Resp Dis 1973; 84(suppl):60–3. [PubMed] [Google Scholar]
- 41.Haber E, Osborne RK. Icterus and febrile reactions in response to isonicotinic acid hydrazine; report of two cases and review of the literature. N Engl J Med 1959; 260:417–20. [DOI] [PubMed] [Google Scholar]
- 42.Kaneko T, Ishigatsubo Y. Isoniazid and food interactions: fish, cheese, and wine. Intern Med 2005; 44:1120–1. [DOI] [PubMed] [Google Scholar]
- 43.Miki M, Ishikawa T, Okayama H. An outbreak of histamine poisoning after ingestion of the ground saury paste in eight patients taking isoniazid in tuberculous ward. Intern Med 2005; 44:1133–6. [DOI] [PubMed] [Google Scholar]
- 44.Jindani A, Harrison TS, Nunn AJ, et al. High-dose rifapentine with moxifloxacin for pulmonary tuberculosis. N Engl J Med 2014; 371:1599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.