Abstract
Context: The identification of foods that can decrease the risk of cancer and type 2 diabetes may be helpful in reducing the burden of these diseases. Although nut consumption has been suggested to have a disease-preventive role, current evidence remains inconsistent.
Objective: The aim of this systematic review and meta-analysis was to clarify the association between nut consumption and risk of cancer or type 2 diabetes.
Data Sources: Six databases were searched for relevant studies from the time of database inception to August 2014. Reference lists of relevant review articles were hand searched, and authors were contacted when data were insufficient.
Study Selection: Eligible studies included epidemiological studies (case–control and cohort) or clinical trials that reported an association between nut consumption and the outcome of type 2 diabetes or specific cancers.
Data Extraction: Two investigators independently extracted descriptive, quality, and risk data from included studies.
Data Synthesis: Random-effects meta-analysis was used to pool relative risks from the included studies. The I2 statistic was used to assess heterogeneity. A total of 36 eligible observational studies, which included 30 708 patients, were identified. The studies had fair methodological quality, and length of follow-up ranged between 4.6 years and 30 years. Comparison of the highest category of nut consumption with the lowest category revealed significant associations between nut consumption and decreased risk of colorectal cancer (3 studies each with separate estimates for males and females, RR 0.76, 95% confidence interval [95%CI] 0.61–0.96), endometrial cancer (2 studies, RR 0.58, 95%CI 0.43–0.79), and pancreatic cancer (1 study, RR 0.68, 95%CI 0.48–0.96). No significant association was found with other cancers or type 2 diabetes. Overall, nut consumption was significantly associated with a reduced risk of cancer incidence (RR 0.85, 95%CI 0.76–0.95).
Conclusions: Nut consumption may play a role in reducing cancer risk. Additional studies are needed to more accurately assess the relationship between nut consumption and the prevention of individual types of cancer, given the scarcity of available data.
Keywords: cancer, meta-analysis, nuts, risk, type 2 diabetes.
INTRODUCTION
As the third leading cause of death worldwide,1 cancer represents a significant health burden. With the elderly population growing, the global burden of cancer is expected to rise by 50% by 2020.2 Cancer prevention is thus the optimal means of decreasing the cancer burden, and is particularly important given the severity of the disease. Type 2 diabetes is another highly prevalent disease that causes a significant public health burden in the United States as well as throughout the world.3–5 The age-standardized prevalence of adult diabetes in 2008 was nearly 10% worldwide, with the majority of cases being type 2 diabetes.6 The prevalence of this disease has continued to increase in developed as wells as developing countries for the last 3 decades.7,8 Finding an appropriate way to prevent type 2 diabetes is, thus, essential to reduce the health burden with which it is associated. One strategy for preventing cancer and type 2 diabetes is to promote the consumption of appropriate foods that can decrease the risk of disease occurrence.
Nuts are widely available around the world and contain many bioactive compounds, including fiber, vegetable protein, minerals, phytosterols, and phenolic compounds.9 Since the first report of an association between nut consumption and a lower risk of coronary heart disease in 1992,10 extensive research has been conducted to investigate the effects of nuts on health outcomes.9 Epidemiological studies suggest nuts have strong cardioprotective effects against nonfatal myocardial infarction, fatal coronary heart disease, and sudden cardiac death.10–13 Frequent nut consumption is also associated with a lower risk of developing gallstones.14,15 Additionally, consumption of nuts has not been shown to adversely affect body weight or energy balance.16
It has been hypothesized that nut consumption may reduce the risk of cancer and type 2 diabetes. Numerous mechanisms have been proposed, on the basis of basic research, to explain the potential roles of components of nuts in cancer prevention.17–19 For example, vitamin E and selenium in almonds and walnuts, as well as quercetin and resveratrol in pine nuts, are antioxidants.20,21 Vitamin E in almonds and hazelnuts can regulate cell differentiation and proliferation,17,22 and quercetin and resveratrol in almonds and pine nuts, as well as polyphenols in walnuts, can inhibit chemically induced carcinogenesis.20 Folic acid in almonds and pine nuts can reduce DNA damage,17,19,22 and resveratrol in pine nuts can regulate inflammatory response and immunological activity as well as induce phase 2 metabolic enzymes.18,20,23 Additionally, dietary fiber is supplied by almonds and walnuts and oleic acid is provided by hazelnuts; both of these components are recognized to be cancer protective.19,24,25 However, the current evidence from human studies is inconsistent. A 2006 review summarizing epidemiological studies that evaluated the association between nut consumption and cancer risk showed inconclusive results for the effects of nuts on the risk of various kinds of neoplasms, including colorectal, prostate, stomach, pancreatic, breast, and endometrial cancers.19 Similarly, 3 epidemiological studies estimating the relationship between consumption of nuts and type 2 diabetes risk suggested inconsistent conclusions.26 Two of them, the Nurses’ Health Study (n = 83 818, 14-y follow-up) and the Shanghai Women’s Health Study (n = 64 000, 4.6-year follow-up), suggested an inverse association, while the third, the Iowa Women’s Health Study (n = 1800, 11-y follow-up), did not detect such an association. Thus, the present systematic review and meta-analysis was conducted to comprehensively evaluate the association between nut consumption and risk of developing cancer and type 2 diabetes.
METHODS
The study protocol defined inclusion and exclusion criteria, search strategy, outcomes, and analysis methods. The PICOS (participants, interventions, comparisons, outcomes, and study design) criteria used to define the research question are presented in Table 1. The meta-analysis was performed in accordance with the MOOSE guidelines (see Appendix S1 in the Supporting Information for this article available online).27
Table 1.
PICOS criteria used to define the research question
| Parameter | Description |
|---|---|
| Participants | General population |
| Intervention/exposure | Consumption of nuts |
| Comparison | Individuals in highest category of nut consumption compared with individuals in lowest category of nut consumption |
| Outcomes | Cancer or type 2 diabetes |
| Study design | Cohort, case–control studies, and clinical trials |
Data sources and search strategies
A comprehensive search of 6 databases was conducted from each database’s earliest inception to December 2013, in any language, for any population. The databases included Medline In-Process & Other Non-Indexed Citations, MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, and Scopus. The search strategy was designed and conducted by an experienced librarian with input from the study’s principle investigator. Controlled vocabulary, supplemented with keywords, was used to search for studies evaluating the effect of nut consumption on the risk of cancer or type 2 diabetes. The precise search strategy is outlined in Appendix S2, available in the Supporting Information for this article online. References of related review articles were also reviewed to identify additional potential articles. The literature search was later updated to include articles published through August 29, 2014.
Study selection
Studies were eligible for inclusion if they were case–control studies, cohort studies, or clinical trials that evaluated the association between nut consumption and risk of developing cancer or type 2 diabetes. Studies were excluded if they used a cross-sectional study design. Studies were included regardless of publication status, sample size, length of follow-up, and language of publication. If multiple publications from the same study were identified, the study with the largest number of cases and most applicable information was included.
Data extraction and quality assessment
Two investigators (L.W. and J.Z.) independently carried out the abstract screening, full-text screening, data extraction, and quality assessment. Disagreements were resolved by consensus, with input from the senior investigator (M.H.M.). Data abstracted from each study included the following: authors’ names, year of publication, study region, study design, characteristics of study population (sample size, age, length of follow-up, measures and types of nut, along with consumption levels, and outcomes of interest). If multiple estimates of the association for the same outcome were reported, the estimate that adjusted for the most appropriate covariates was extracted. If no adjusted estimates were presented, the crude estimate was included. When an eligible study did not present enough data, the corresponding and first authors were contacted.
To assess study quality, the Newcastle-Ottawa Quality Assessment Scale28 was used; population and sample methods, exposure and outcome descriptions, and statistical matching/adjustments of the data were included in the assessment. The scale was used to assign a maximum of 9 points for each study.
Statistical methods
Relative risks (RRs) and the related 95% confidence intervals (95%CIs) were extracted from or calculated for each of the included studies. Due to the rarity of cancer in the general population, RR and odds ratio were deemed equivalent for the studies that focused on cancer. With regard to type 2 diabetes, RR was used because all of the available studies used it to estimate associations. The log-transformed RR, obtained using the DerSimonian and Laird random-effects method, was then pooled with the estimate of heterogeneity from the Mantel-Haenszel model.29 A subgroup analysis based on study design (either case–control study design or cohort study design) for each outcome was also conducted.
The I2 was used to assess heterogeneity across the included studies, where I2 > 50% suggests high heterogeneity.30 It was not possible to evaluate potential publication bias using visual inspection of symmetry of funnel plots and the Egger regression asymmetry test because of the small number of studies included and the high heterogeneity (I2 > 50%) in most analyses.31 All statistical analyses were conducted using STATA version 12.1 (StataCorp LP, College Station, TX, USA).
RESULTS
Literature search
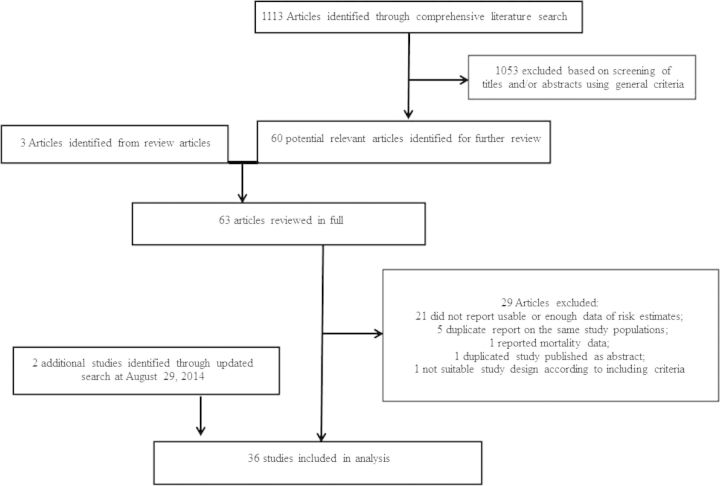
The detailed steps of the literature search are shown in Figure 1. A total of 36 studies met the inclusion criteria and were included in the review. The association of nut exposure was evaluated with type 2 diabetes (5 studies), breast cancer (4 studies), colorectal cancer (3 studies, including 2 of colon cancer), endometrial cancer (2 studies), leukemia (1 study), acute myeloid leukemia (2 studies), hepatocellular cancer (4 studies), ovarian cancer (3 studies), prostate cancer (5 studies), stomach cancer (2 studies), and 1 study each of gastric cancer, glioma, lymphoma, pancreatic cancer, and upper-aerodigestive tract cancer.
Figure 1.
Flow diagram of selection of eligible studies
Study characteristics
The detailed characteristics of the included studies are shown in Table 2.32–67 In total, 16 cohort studies and 20 case–control studies were evaluated. Overall, 10 studies were conducted in Europe, 15 in the Americas, 7 in Asia, 3 in Australia, and 1 in Africa. A total of 22 studies provided estimations of nut consumption with risk of disease, 6 provided estimations of peanut intake only, 4 provided estimations of nut and seed intake only, 2 provided estimations of pulse, nut, and seed intake only, 1 provided estimations of fruit/nut intake only, and 1 provided estimations of beans/lentil/nut/seed intake only. The studies enrolled 30 708 patients and had a median follow-up period of 10.15 years (range 4.6–30 y).
Table 2.
Characteristics of studies investigating nut consumption relative to the risk of cancer or type 2 diabetes
| Reference | Country/region (study type) | No. of cases/no. of overall subjects in cohort studies or no. of controls in case-control studies (age), duration of follow-up | Exposure categories (exposure/case assessment) | RR (95%CI) | Matched/adjusted factors | Outcome investigated |
|---|---|---|---|---|---|---|
| Cohort studies | ||||||
| Sonestedt et al. (2008)34 | Sweden (CS) | 544/15 773 | Tertiles of nut intake: no intake (Ref) | Season of data collection, diet interviewer, method version, age, total energy, weight, height, educational status, smoking habits, leisure-time physical activity, hours of household activities, alcohol consumption, age at menopause, parity, and current use of menopausal hormone therapy | Breast cancer | |
| (46–75 y), mean 10.3 y | 1 | 0.94 (0.72–1.22) | ||||
| 2 | 1.00 (0.77–1.29) | |||||
| 3 | 0.98 (0.75–1.27) | |||||
| (Trained interviewer/cancer registry) | ||||||
| Singh & Fraser (1998)33 | California, USA (CS) | 135/32 051 (≥25 y), 6 y | Nut consumption: never to <1 time/wk (Ref) | 0.67 (0.45-0.98) | Age at baseline, sex, BMI, physical activity, parental history of colon cancer, current smoking, past smoking, alcohol consumption, and aspirin use | Colon cancer |
| 1–4 times/wk | 0.68 (0.45-1.04) | |||||
| >4 times/wk | ||||||
| (Self-questionnaire/medical record confirmation and tumor registry) | ||||||
| Yeh et al. (2006)36 | Taiwan (CS) | 107/23 943 (30–65 y), 10 y | Peanut intake: 0–1 meal/wk (Ref) | Age, area of residence, cigarette smoking, BMI | Colorectal cancer | |
| ≥2 meals/wk | ||||||
| Men | 0.73 (0.44–1.21) | |||||
| Women | 0.42 (0.21–0.84) | |||||
| (Trained interviewer/cancer registry, confirmation by reviewing medical record and death certificate) | ||||||
| Thompson et al. (2010)35 | Iowa, USA (CS) | 415/35 159 (55–69 y), 19 y | Nut consumption: <1/mo (Ref) | Age, total energy intake | Lymphoma | |
| <1 time/wk | 1.24 (0.99–1.54) | |||||
| 1–4 times/wk | 0.99 (0.76–1.3) | |||||
| 5+ times/wk | 0.97 (0.52–1.81) | |||||
| (Self-questionnaire/cancer registry) | ||||||
| Hedelin et al. (2011)32 | Sweden (CS) | 163/47 140 (30–49 y), median 16 y | Nut intake: lowest category (Ref) | Age, use of oral ontraceptives, age at menarche, parity, hormone replacement therapy, and intakes of total energy, alcohol, saturated fat, meat, and fish | Ovarian cancer | |
| Highest category | 0.88 (0.56–1.38) | |||||
| (Self-questionnaire/cancer registry) | ||||||
| Bao et al. (2013)37 | USA, NHS (CS) | 466/75 680 (30–55 y), ∼30 y | Nut consumption frequency (28-g serving): never/almost never (Ref) | Age, height, physical activity, smoking, total energy intake, BMI, history of diabetes mellitus, alcohol consumption, multivitamin use, and intakes of red meat, fruits and vegetables, and vitamin D | Pancreatic Cancer | |
| 1–3 times/month | 0.90 (0.69–1.18) | |||||
| 1 time/wk | 0.71 (0.51–0.99) | |||||
| ≥2 times/wk | 0.68 (0.48–0.96) | |||||
| (Self-questionnaire/pathology reports or a secondary source) | ||||||
| Mills et al. (1989)39 | California, USA (CS) | 180/14 000 (25+ y), 6 y | Current nut intake: <1 time/wk (Ref) | Age, education, current intakes of meat, poultry, and fish; intakes of beans, legumes, or peas; intakes of citrus fruit and dried fruit; index of fruit, nuts, and tomatoes | Prostate Cancer | |
| 1–4 times/wk | 0.86 (0.59–1.24) | |||||
| ≥5 times/wk | 0.79 (0.51–1.22) | |||||
| (Self-questionnaire/medical record and tumor registry) | ||||||
| Pan et al. (2013)40 | USA, NHS–larger (CS) | 5 930/137 956 (35–77 y), 10 y | Nut consumption: rarely/never (Ref) | Age, BMI, race, family history of diabetes, smoking status, alcohol intake, physical activity, postmenopausal status and menopausal hormone use, multivitamin use, total energy intake, and other dietary variables, including intakes of whole grains, fruits, vegetables, fish, red meat, coffee, and sugar-sweetened beverages | Type 2 diabetes | |
| <1 time/wk | 0.99 (0.94–1.04) | |||||
| 1 time/wk | 1.03 (0.96–1.10) | |||||
| 2–4 times/wk | 0.99 (0.90–1.09) | |||||
| ≥5 times/wk | 1.01 (0.90–1.12) | |||||
| Total tree nuts: rarely/never (Ref) | ||||||
| <1 time/wk | 1.02 (0.97–1.07) | |||||
| 1 time/wk | 1.05 (0.96–1.15) | |||||
| ≥2 times/wk | 0.98 (0.87–1.10) | |||||
| Peanuts: rarely/never (Ref) | ||||||
| <1 time/wk | 1.02 (0.97–1.06) | |||||
| 1 time/wk | 1.07 (0.99–1.17) | |||||
| ≥2 times/wk | 1.04 (0.93–1.16) | |||||
| (Self-questionnaire/questionnaire checking and medical record reviewing) | ||||||
| Parker et al. (2003)41 | Iowa, USA (CS) | 1831/35 988 (NA), 12 y | Nut consumption: <1 time/mo (Ref) | Age, BMI, waist-to-hip ratio, physical activity, current smoking status, pack-years of smoking, alcohol consumption, total daily energy intake, educational attainment, current estrogen use, and intakes of fiber, polyunsaturated fat, saturated fat, monounsaturated fat, trans fatty acids, total fruit, total vegetables, whole grains, fish and seafood, and magnesium | Type 2 diabetes | |
| <1 times/wk | 0.98 (0.87–1.10) | |||||
| 1–4 times/wk | 1.06 (0.93–1.22) | |||||
| ≥5 times/wk | 1.51 (1.13–2.04) | |||||
| (Self-questionnaire/self-report in surveys) | ||||||
| Villegas et al. (2008)42 | Shanghai, China (CS) | 1608/64 227 (40–70 y), mean 4.6 y | Peanut consumption: Quintile 1 (Ref) | Age, energy intake, BMI, waist-to-hip ratio, smoking, alcohol consumption, vegetable intake, fiber intake, physical activity, income level, education level, occupation, and hypertension | Type 2 diabetes | |
| Quintile 2 | 0.80 (0.69–0.94) | |||||
| Quintile 3 | 0.95 (0.82–1.11) | |||||
| Quintile 4 | 0.79 (0.68–0.92) | |||||
| Quintile 5 | 0.80 (0.68–0.93) | |||||
| (Trained interviewer/self-report) | ||||||
| Kochar et al. (2010)38 | USA, PHS (CS) | 1828/20 224 (40.7–87.1 y), mean 19.2 y | Nut consumption: rarely/never (Ref) | Age, smoking (never, past, and current smokers), randomization arm, alcohol consumption, breakfast cereal consumption, dairy consumption (quintiles), red meat consumption (quintiles), physical activity, BMI, and history of hypertension | Type 2 diabetes | |
| <1 time/wk | 1.06 (0.93–1.20) | |||||
| 1 time/wk | 1.1 (0.95–1.26) | |||||
| 2–4 times/wk | 0.97 (0.82–1.14) | |||||
| 5–6 times/wk | 0.99 (0.76–1.3) | |||||
| ≥7 times/wk | 0.87 (0.61–1.24) | |||||
| (Self-questionnaire/self-report in questionnaires) | ||||||
| Thiebaut et al. (2009)46 | France (CS) | 1650/56 007 (40–65 y), mean 8 y | Quintile of 7.2% linoleic acid (from nut mixes): quintile I-0 (Ref) | Age, nonalcohol energy and ethanol intakes, smoking history, history of benign breast disease, history of breast cancer in first-degree relatives, age at menarche, parity, BMI, menopausal status, age at menopause, and use of menopausal hormone treatment | Breast cancer | |
| II | 0.92 (0.79–1.08) | |||||
| III | 1.01 (0.87–1.18) | |||||
| IV | 1.09 (0.94–1.27) | |||||
| V | 1.17 (1.01–1.37) | |||||
| (Self-questionnaire/self-report and/or pathology confirmation) | ||||||
| Jenab et al. (2004)43 | Europea (CS) | Males: | Nuts/seeds intake: category 1 – never (Ref) | Center, age, height, weight, intake of fruits (without nuts and seeds), intake of dietary fiber, physical activity, duration of smoking, gender, energy from alcohol, energy from fat, and energy from carbohydrates and proteins | Colorectal cancer | |
| 542/141 988; | Males: | |||||
| Females: | Category 2: <0.8 g/d | 1.10 (0.78–1.54) | ||||
| 787/336 052, | Category 3: 0.8–2.3 g/d | 1.03 (0.79–1.34) | ||||
| (35–70 y), | Category 4: 2.3–6.2 g/d | 1.13 (0.84–1.53) | ||||
| ∼10 y | Category 5: >6.2 g/d | 1.09 (0.81–1.49) | ||||
| Females: | ||||||
| Category 2: <0.8 g/d | 0.87 (0.64–1.15) | |||||
| Category 3: 0.8–2.3 g/d | 0.96 (0.77–1.20) | |||||
| Category 4: 2.3–6.2 g/d | 0.84 (0.67–1.07) | |||||
| Category 5: >6.2 g/d | 0.81 (0.63–1.04) | |||||
| Males: | Nuts/seeds intake: category 1 – never (Ref) | BMI, education, smoking, alcohol intake, physical activity, and total energy intake stratified by sex, center, and age at recruitment | Colon cancer | |||
| 327/141 988; | Males: | |||||
| Females: | Category 2: <0.8 g/d | 1.09 (0.70–1.69) | ||||
| 528/336 052, | Category 3: 0.8–2.3 g/d | 1.17 (0.84–1.63) | ||||
| (35–70 y), | Category 4: 2.3–6.2 g/d | 1.17 (0.80–1.73) | ||||
| ∼10 y | Category 5: >6.2 g/d | 1.01 (0.67–1.53) | ||||
| Females: | ||||||
| Category 2: <0.8 g/d | 1.01 (0.72–1.41) | |||||
| Category 3: 0.8–2.3 g/d | 1.01 (0.77–1.32) | |||||
| Category 4: 2.3–6.2 g/d | 0.92 (0.70–1.23) | |||||
| Category 5: >6.2 g/d | 0.69 (0.50–0.95) | |||||
| (Self-questionnaire/cancer registry and others) | ||||||
| Saberi Hosnijeh et al. (2014)45 | Europe (CS) | 773/477 325 (35–70 y), mean 11.34 y | Nuts/seeds intake: quartile 1 – never (Ref) | BMI, education, smoking, alcohol intake, physical activity, and total energy intake stratified by sex, center, and age at recruitment | Leukemia | |
| Quartile 2: 0.01–0.48 g/d | 1.01 (0.81–1.25) | |||||
| Quartile 3: 0.49–1.97 g/d | 0.99 (0.79–1.25) | |||||
| Quartile 4: 1.98–5.33 g/d | 1.04 (0.81–1.34) | |||||
| Quartile 5: 5.34–286.06 g/d | 1.08 (0.81–1.44) | |||||
| Nuts/seeds intake: quartile 1 – never (Ref) | BMI, education, smoking, alcohol intake, physical activity, and total energy intake stratified by sex, center, and age at recruitment | Acute myeloid leukemia | ||||
| Quartile 2: 0.01–0.7 g/d | 0.69 (0.43–1.13) | |||||
| Quartile 3: 0.8–4 g/d | 0.94 (0.60–1.47) | |||||
| Quartile 4: 4.1–286.1 g/d | 1.27 (0.79–2.04) | |||||
| (Self-questionnaire/cancer registry and others) | ||||||
| Nettleton et al. (2008)44 | USA (CS) | 413/5 011 (45–84 y), ∼5 y | Nuts/seeds intake: per 1-unit change (servings/d) | 0.94 (0.84–1.06) | Energy intake, study center, age, sex, race/ethnicity, education, active leisure-time physical activity, inactive leisure-time physical activity, current smoking status, smoking pack-years, current weekly supplement use, and intakes of whole grains, vegetables, low-fat dairy, coffee, soda, red meat, processed meat, high-fat dairy, white potatoes | Type 2 diabetes |
| (Self-questionnaire/self-report, exam, or medication use) | ||||||
| Farvid et al. (2014)66 USA, CS | 2830/88 803 (24–43 y), 20 y | Nuts intake: Category 1 – no intake (Ref) | Age, race, family history of breast cancer in mother or sisters, history of benign breast disease, smoking, height, BMI, age at menarche, parity and age at first birth, oral contraceptive use, alcohol intake, and energy intake | Breast cancer | ||
| Category 2 (median 0.07 serving/d) | 1.03 (0.92–1.15) | |||||
| Category 3 (median 0.14 serving/d) | 0.94 (0.83–1.06) | |||||
| Category 4 (median 0.21 serving/d) | 0.96 (0.85–1.09) | |||||
| Category 5-highest (median 0.57 serving/d) | 0.94 (0.83–1.05) | |||||
| (Self-questionnaire/self-report followed by review of medical records and pathology reports) | ||||||
| Case–control studies | ||||||
| Takayama et al. (2013)48 | Japan (HC-CS) | 161/380 (mean 54 y), NA | Peanut intake frequency: no intake (Ref) | BMI, diabetes history, and hypertension history | Endometrial endometrioid cancer | |
| 1–3 times/mo | 1.22 (0.76-1.95) | |||||
| ≥1–2 times/wk | 0.48 (0.27–0.86) | |||||
| Nut intake: ≤0.53 g/1000 kcal (Ref) | ||||||
| 0.54–1.16 g/1000 kcal | 0.93 (0.53–1.54) | |||||
| 1.17–2.25 g/1000 kcal | 1.33 (0.77–2.31 | |||||
| ≥2.26 g/1000 kcal | 0.46 (0.25–0.86) | |||||
| (Self-questionnaire and telephone/clinic histological confirmation) | ||||||
| Wang et al. (2012)49 | China (PC-CS) | 257/514 (30–79 y), NA | Nut intake: Tertile 1 (Ref) | Sex, age, area, education, smoking, alcohol consumption, family history, total vegetable intake, total fruit intake, pickled food intake, soy products intake, total energy intake, meat intake, and Helicobacter pylori | Gastric cancer | |
| Tertile 2 | 0.9 (0.2–2.7) | |||||
| Tertile 3 | 0.9 (0.3–3.3) | |||||
| (Trained interviewer/hospital pathological confirmation) | ||||||
| Giles et al. (1994)47 | Australia (PC-CS) | Males: 243/243 | Nut intake: no intake (Ref) | Age, sex, alcohol consumption, tobacco use | Glioma | |
| Females: 166/166 (20–70 y), NA | High intake | |||||
| Males | 1.36 (0.76–2.35) | |||||
| Females | 0.92 (0.49–1.75) | |||||
| (Self-questionnaire/cancer registry) | ||||||
| Chen et al. (1991)50 | Taiwan (HC-CS) | 200/200 (NA), NA | Peanut consumption: <1 meal/wk (Ref) | Age, sex, ethnic group, residential area | Liver cancer | |
| ≥1 meal/wk | 1.44 (0.94–2.21) | |||||
| (Trained interviewer/clinic pathological and/or cytological confirmation) | ||||||
| Zhang et al. (1998)57 | China (HC-CS) | 152/115 (mean 52 y), NA | Peanut consumption (involving aflatoxin intake): no intake (Ref) | Sex, age, individual history of liver disease, family history of liver disease, history of drinking alcohol, corn consumption, psychological stress, HBV infection | Liver cancer | |
| Intake | 13.75 (3.69–51.16) | |||||
| (Trained interviewer/clinic diagnosis) | ||||||
| Yu et al. (2002)56 | China (HC-CS) | 248/248 (25–79 y), NA | Peanut intake: <3 times/wk (Ref) | Sex, age, residence, HBV infection | Liver cancer | |
| ≥3 times/wk | 0.66 (0.32–1.36) | |||||
| (Trained interviewer/clinic confirmation) | ||||||
| Soliman et al. (2010)54 | Egypt (HC-CS) | 150/150 (NA), NA | Peanut consumption: no intake (Ref) | Age, sex, viral infection | Liver cancer | |
| 1–2 times/y | 0.64 (0.16–2.57) | |||||
| >2 times/y | 0.59 (0.13–2.61) | |||||
| (Trained interviewer/cancer registry) | ||||||
| Pan et al. (2004)52 | Canada (PC-CS) | 442/2135 (mean 55 y), NA | Nut products intake (servings/wk): | Age, sex, 10-y age group, province of residence, education, alcohol consumption, cigarette pack-years, BMI, total caloric intake, recreational physical activity, number of live births, menstruation years, and menopause status | Ovarian cancer | |
| First quartile (Ref) | ||||||
| Second quartile | 1.22 (0.89–1.67) | |||||
| Third quartile | 1.04 (0.75–1.45) | |||||
| Fourth quartile | 1.13 (0.82–1.55) | |||||
| (Self-questionnaire and phone follow-up/cancer registry) | ||||||
| Raimondi et al. (2010)53 | Canada (HC-CS) | 197/197 (35–84 y), NA | Nut consumption: 0 g/d (Ref) | Age, place of residence, family history of prostate cancer, total energy intake | Prostate cancer | |
| 0.1–1.2 g/d | 0.91 (0.47–1.76) | |||||
| 1.3–3.0 g/d | 1.10 (0.52–2.29) | |||||
| >3.0 g/d | 0.43 (0.22–0.85) | |||||
| (Trained interviewer/clinic pathological confirmation) | ||||||
| Trichopoulos et al. (1985)55 | Greece (HC-CS) | 110/100 (NA), NA | Nut consumption frequency: 0 (Ref) | None | Stomach cancer | |
| 2 times/mo | 0.64 (0.30–1.39) | |||||
| 4 times/mo | 0.45 (0.20–0.99) | |||||
| 10 times/mo | 1.96 (0.75–5.17) | |||||
| 30 times/mo | 1.88 (1.22–2.89) | |||||
| (Trained interviewer/clinic histological confirmation) | ||||||
| Hoshiyama & Sasaba (1992)51 | Japan (PC-CS) | 294/294 (NA), NA | Nut intake: never (Ref) | Sex, age, area, smoking status, intakes of salty foods, rice, miso soup, boiled fish, pickled vegetables, seaweed, soybean products, fruits, green–yellow vegetables, raw vegetables | Stomach cancer | |
| ≤2 times/mo | 0.7 (0.4–1.3) | |||||
| ≥3 times/mo | 0.6 (0.3–1.0) | |||||
| (Trained interviewer/clinic histological confirmation) | ||||||
| Jackson et al. (2013)58 | Jamaica (HC-CS) | 243/273 (40–80 y), NA | Nut intake: Tertile 1 (Ref) | Age, family history of prostate cancer, education, BMI, smoking, physical activity, and total energy intake | Prostate cancer | |
| Tertile 2 | 1.31 (0.79, 2.18) | |||||
| Tertile 3 | 0.81 (0.46–1.42) | |||||
| (Trained interviewer/clinic histological confirmation) | ||||||
| Moller et al. (2013)59 | Sweden (PC-CS) | 1482/1108 (35–79 y), NA | Nut intake: low intake: <75th centile intake among controls (Ref) | Age, region, education, smoking, BMI, energy intake, physical activity, diabetes, and family history of PC | Prostate cancer | |
| High intake: >75th centile intake among controls | 1.03 (0.84–1.25) | |||||
| (Self-questionnaire/cancer registry) | ||||||
| Petridou et al. (2002)60 | Greece (HC-CS) | 84/84 (NA), NA | Pulses, nuts, and seeds intake: | Age, education, BMI, pregnancy, and total energy intake | Endometrial cancer | |
| First quartile (Ref) | ||||||
| Every quartile increase | 0.63 (0.44–0.88) | |||||
| (Self-questionnaire/clinic histologic confirmation) | ||||||
| Yamamura et al. (2013)61 | Texas, USA (HC-CS) | Males: 171/186; Females: 152/194 (18–80 y), NA | Nuts/seeds intake: | Age, gender, place of residence, race, total energy intake, education, smoking, obesity, and exposure to solvents Age, education, obesity, exposure to solvents, alcohol consumption, dark-green vegetable intake, orange vegetable intake | Acute myeloid leukemia | |
| First quartile (<0.10) (Ref) | ||||||
| Males: | ||||||
| Second quartile (0.10–0.27) | 1.14 (0.48–2.69) | |||||
| Third quartile (0.27–0.54) | 1.32 (0.57–3.06) | |||||
| Fourth quartile (>0.54) | 0.49 (0.20–1.20) | |||||
| Females: | ||||||
| Second quartile (0.10–0.29) | 0.38 (0.19–0.78) | |||||
| Third quartile (0.29–0.51) | 0.17 (0.08–0.39) | |||||
| Fourth quartile (>0.51) | 0.26 (0.11–0.60) | |||||
| (Self-questionnaire/clinic registry) | ||||||
| Ibiebele et al. (2012)62 | Australia (PC-CS) | 1366/1414 (18–79 y), NA | Omega-6 fatty acid (g) from nuts: | Age, education, BMI, smoking status, oral contraceptive use, parity, menopausal status, hormonal replacement therapy , total fat intake, and total energy intake | Ovarian cancer | |
| 0.13 (0.0–0.29) (Ref) | ||||||
| 0.45 (0.29–0.68) | 0.83 (0.67–1.03) | |||||
| 1.48 (0.73–2.59) | 0.88 (0.71–1.09) | |||||
| 3.35 (2.59–25.9) | 0.72 (0.57–0.92) | |||||
| (Self-questionnaire/cancer registry and clinic ascertainment) | ||||||
| Jain et al. (1999)63 | Canada (HC-CS) | 617/636 (NA), NA | Beans, lentils, nuts, and seeds intake: | Log total energy, vasectomy, age, ever smoked, marital status, BMI, education, ever used multivitamin supplements in 1 y before diagnosis interview, area of study, and log-converted amounts for grains, fruit, vegetables, total plants, total carotenoids, folic acid, dietary fiber, conjugated linoleic acid, vitamin E, vitamin C, retinol, total fat, and linoleic acid | Prostate cancer | |
| Category 1 (Ref) | ||||||
| Category 2 | 0.79 (0.61–1.04) | |||||
| Category 3 | 0.72 (0.55–0.95) | |||||
| Category 4 | 0.69 (0.53–0.91) | |||||
| (Trained interviewer/cancer registry or clinic histological confirmation) | ||||||
| Samoli et al. (2010)67 | Greece (HC-CS) | 239/194 (mean 61 y), NA | Fruits and nuts intake: < median (Ref) | Energy intake, alcohol consumption, and intakes of vegetables, legumes, dairy products, cereals, fish, meat and meat products, and monounsaturated to saturated lipidsAge | Upper-aerodigestive tract cancer | |
| ≥ median | 0.84 (0.52, 1.35) | |||||
| (Trained interviewer/hospital pathological confirmation) | ||||||
| Kune et al. (1987)64 | Australia (HC-CS) | Males: | Pulses, nuts, and seeds intake: quintile 1 (Ref) | Colorectal cancer | ||
| 388/398 | For males: | |||||
| Females: 327/329, (NA), NA | Quintile 2 | 1.10 (0.76–1.60) | ||||
| Quintile 3 | 1.03 (0.70–1.51) | |||||
| Quintile 4 | 0.83 (0.56–1.22) | |||||
| For females: | ||||||
| Quintile 2 | 1.05 (0.69–1.60) | |||||
| Quintile 3 | 0.54 (0.35–0.83) | |||||
| Quintile 4 | 0.55 (0.35–0.84) | |||||
| (Trained interviewer/NA) | ||||||
| Liu et al. (2014)65 | Canada (PC-CS) | 2865/3299 (25–74 y), NA | Nut intake: <1 time/mo (Ref) | Age, family history of breast cancer in mother and sisters, age at menarche, parity, age at first birth, education, ethnicity, oral contraceptive use, adult BMI, breastfeeding, menopausal status, hormone replacement therapy, and alcohol consumption 2 y before study enrollment | Breast cancer | |
| 1–3 times/mo | 0.86 (0.71–1.04) | |||||
| 1–6 times/wk | 0.86 (0.72–1.04) | |||||
| ≥1 times/d | 0.76 (0.61–0.95) | |||||
Abbreviations: BMI, body mass index; CI, confidence interval; CS, cohort study; HBV, hepatitis B virus; HC-CS, hospital-based case–control study; NA, not available; NHS, Nurses' Health Study; PC-CS, population-based case–control study; PC, prostate cancer; PHS, Physicians' Health Study; Ref, reference; RR, relative risk.
The detailed quality ratings for each study are listed in Tables 3 and 4. Overall, the studies had fair methodological quality. All cohort studies and all but 2 case–control studies reported the estimations after adjusting for covariates. Exposure (nut consumption) was ascertained through interview in 41.7% of the studies and through self-completed questionnaire in the remaining studies.
Table 3.
Quality assessment of reviewed case–control studies
| Reference | Cases defined with independent validation | Representativeness of the cases | Controls selected from community | Inclusion of statement that controls had no history of outcome | Cases and controls matched and/or adjusted by factors | Exposure ascertained by blinded structured interview | Same method of ascertainment used for cases and controls | Same response rate for both groups |
|---|---|---|---|---|---|---|---|---|
| Kune et al. (1987)64 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| Takayama et al. (2013)48 | 1 | 1 | 0 | 1 | 2 | 0 | 1 | 0 |
| Wang et al. (2012)49 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 |
| Giles et al. (1994)47 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 0 |
| Chen et al. (1991)50 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 |
| Zhang et al. (1998)57 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 0 |
| Yu et al. (2002)56 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 |
| Soliman et al. (2010)54 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 0 |
| Pan et al.52 (2004) | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 |
| Raimondi et al.53 (2010) | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 0 |
| Trichopoulos et al. (1985)55 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 |
| Hoshiyama & Sasaba (1992)51 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 0 |
| Jackson et al. (2013)58 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 0 |
| Moller et al. (2013)59 | 1 | 1 | 1 | 0 | 2 | 0 | 1 | 1 |
| Petridou et al. (2002)60 | 1 | 1 | 0 | 1 | 2 | 0 | 1 | 0 |
| Yamamura et al. (2013)61 | 1 | 1 | 1 | 0 | 2 | 0 | 1 | 0 |
| Ibiebele et al. (2012)62 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 |
| Jain et al. (1999)63 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 0 |
| Samoli et al. (2010)67 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 |
| Liu et al. (2014)65 | 1 | 1 | 1 | 0 | 2 | 0 | 1 | 1 |
aScoring: 1 means the study adequately fulfilled a quality criterion (2 for case–control, fully matched and adjusted), 0 means it did not. Quality scale does not imply that items are of equally relevant importance.
Table 4.
Quality assessment of reviewed cohort studies
| Reference | Exposed cohort represented average in community | Nonexposed cohort selected from same community | Exposure ascertained through records or structured interviews | Outcome demonstrated to be not present at study start | Exposed and nonexposed subjects matched and/or adjusted by factors | Outcome ascertained via independent blind assessment or record linkage | Follow-up long enough for outcome to occur | Loss to follow-up <20% |
|---|---|---|---|---|---|---|---|---|
| Sonestedt et al. (2008)34 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| Singh & Fraser (1998)33 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 |
| Yeh et al. (2006)36 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 |
| Thompson et al. (2010)35 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 |
| Hedelin et al. (2011)32 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 1 |
| Bao et al. (2013)37 | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 1 |
| Mills et al. (1989)39 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 1 |
| Pan et al. (2013)40 | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 1 |
| Parker et al. (2003)41 | 1 | 1 | 0 | 1 | 2 | 0 | 1 | 1 |
| Villegas et al. (2008)42 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 |
| Kochar et al. (2010)38 | 0 | 1 | 0 | 1 | 2 | 0 | 1 | 0 |
| Thiebaut et al. (2009)46 | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 1 |
| Jenab et al. (2004)43 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 |
| Saberi Hosnijeh et al. (2014)45 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 |
| Nettleton et al. (2008)44 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 |
| Farvid et al. (2014)66 | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 1 |
aScoring: 2 means the subjects were fully matched and/or the analysis was fully adjusted. 1 means the study adequately fulfilled a quality criterion, 0 means it did not. Quality scale does not imply that items are of equally relevant importance.
Meta-analysis
The combined effect sizes are shown in Table 5. The estimations were based on the comparison of the highest category of nut consumption with the lowest category. Cancers for which only 1 study was available (gastric cancer, glioma, lymphoma, pancreatic cancer, and upper-aerodigestive tract cancer) were not meta-analyzed individually but were included in the overall meta-analysis for cancer. Significant associations were found between nut consumption and decreased risk of developing colorectal cancer (3 studies, each with separate estimates for males and females [RR 0.76; 95%CI 0.61–0.96; I2 51.3%], 2 of which examined colon cancer with 1 including separate estimates for males and females [RR 0.77; 95%CI 0.60–0.98; I2 18.0%]), endometrial cancer (2 studies [RR 0.58; 95%CI 0.43–0.79; I2 0%]), and pancreatic cancer (1 study [RR 0.68; 95%CI 0.48–0.96; I2 not available]). There was no statistically significant association between consumption of nuts and risk of developing upper-aerodigestive tract cancer (1 study [RR 0.84; 95%CI 0.52–1.35; I2 not available]), acute myeloid leukemia (2 studies, including 1 with separate estimates for males and females [RR 0.57; 95%CI 0.21–1.57; I2 82.7%]), breast cancer (4 studies [RR 0.96; 95%CI 0.81–1.14; I2 72.0%]), gastric cancer (1 study [RR 0.90; 95%CI 0.30–3.30; I2 not available]), glioma (1 study with separate estimates for males and females [RR 1.15; 95%CI 0.75–1.75; I2 0%]), hepatocellular carcinoma (4 studies [RR 1.57; 95%CI 0.56–4.40; I2 82.3%]), leukemia (1 study [RR 1.08; 95%CI 0.81–1.44; I2 not available]), lymphoma (1 study [RR 0.97; 95%CI 0.52–1.81; I2 not available]), ovarian cancer (3 studies [RR 0.88; 95%CI 0.66–1.19; I2 59.5%]), prostate cancer (5 studies [RR 0.78; 95%CI 0.60–1.01; I2 59.7%]), stomach cancer (2 studies [RR 1.08; 95%CI 0.35–3.32; I2 89.1%]), or type 2 diabetes (5 studies [RR 0.98; 95%CI 0.84–1.14; I2 74.2%]).
Table 5.
Summary risk estimates for the association between nut consumption and risk of cancer or type 2 diabetes
| Disease | RR (95%CI) | P value | I2 (%) | Heterogeneity P value |
|---|---|---|---|---|
| Overall cancer (31 studies) | 0.85 (0.76–0.95) | 0.003 | 66.5 | <0.001 |
| Upper-aerodigestive tract cancer (1 study) | 0.84 (0.52–1.35) | 0.474 | N/A | N/A |
| Acute myeloid leukemia (2 studies) | 0.57 (0.21–1.57) | 0.280 | 82.7 | 0.003 |
| Breast cancer (4 studies) | 0.96 (0.81–1.14) | 0.640 | 72.0 | 0.013 |
| Colon cancer (2 studies) | 0.77 (0.60–0.98) | 0.031 | 18.0 | 0.295 |
| Colorectal cancer (3 studies) | 0.76 (0.61–0.96) | 0.021 | 51.3 | 0.068 |
| Endometrial cancer (2 studies) | 0.58 (0.43–0.79) | <0.001 | 0.0 | 0.384 |
| Gastric cancer (1 study) | 0.90 (0.27–2.99) | 0.863 | N/A | N/A |
| Glioma (1 study) | 1.15 (0.75–1.75) | 0.530 | 0.0 | 0.368 |
| Hepatocellular carcinoma (4 studies) | 1.57 (0.56–4.40) | 0.387 | 82.3 | 0.001 |
| Leukemia (1 study) | 1.08 (0.81–1.44) | 0.600 | N/A | N/A |
| Lymphoma (1 study) | 0.97 (0.52–1.81) | 0.924 | N/A | N/A |
| Ovarian cancer (3 studies) | 0.88 (0.66–1.19) | 0.407 | 59.5 | 0.084 |
| Pancreatic cancer (1 study) | 0.71 (0.51–0.99) | 0.043 | N/A | N/A |
| Prostate cancer (5 studies) | 0.78 (0.60–1.01) | 0.059 | 59.7 | 0.042 |
| Stomach cancer (2 studies) | 1.08 (0.35–3.32) | 0.888 | 89.1 | 0.003 |
| Type 2 diabetes (5 studies) | 0.98 (0.84–1.14) | 0.774 | 74.2 | 0.004 |
Abbreviations: NA, not applicable; RR, relative risk.
Overall, nut consumption was significantly associated with a reduced risk of developing cancer (RR 0.85, 95%CI 0.76–0.95; I2 66.5%). No significant association with risk of type 2 diabetes was detected (RR 0.98; 95%CI 0.84–1.14; I2 74.2%). In subgroup analysis (Table 6), no significant difference between the prospective cohort studies and the case–control studies was found for colorectal cancer, ovarian cancer, prostate cancer, or overall cancer; however, for acute myeloid leukemia, the case-control study showed a significantly lower RR (RR 0.35, 95%CI 0.19–0.65, P = 0.001) than the prospective cohort study (RR 1.27, 95%CI: 0.79–2.04, P = 0.32). Similarly, for breast cancer, the case–control study showed a significantly lower RR than did the cohort studies (RR 0.76 [95%CI 0.61–0.95] vs RR 1.03 [95%CI 0.88–1.20]).
Table 6.
Subgroup analyses for the association between nut consumption and risk of cancers or type 2 diabetes
| Disease | RR (95%CI) | P value | I2 (%) | P value for difference |
|---|---|---|---|---|
| Overall cancer | ||||
| Cohort (11 studies) | 0.91 (0.81–1.02) | 0.095 | 49.0 | 0.33 |
| Case–control (20 studies) | 0.82 (0.69–0.98) | 0.028 | 71.0 | |
| Upper-aerodigestive cancer | ||||
| Cohort | ||||
| Case–control (1 study) | 0.840 (0.521–1.353) | 0.474 | N/A | |
| Acute myeloid leukemia | ||||
| Cohort (1 study) | 1.270 (0.790–2.041) | 0.323 | N/A | 0.001 |
| Case–control (1 study) | 0.351 (0.189–0.652) | 0.001 | 1.3 | |
| Breast cancer | ||||
| Cohort (3 studies) | 1.03 (0.88–1.20) | 0.735 | 60.4 | 0.03 |
| Case–control (1 study) | 0.76 (0.61–0.95) | 0.015 | N/A | |
| Colon cancer | ||||
| Cohort (3 studies) | 0.767 (0.602–0.976) | 0.031 | 18.0 | |
| Case–control | ||||
| Colorectal cancer | ||||
| Cohort (2 studies) | 0.798 (0.591–1.077) | 0.140 | 57.3 | 0.55 |
| Case–control (1 study) | 0.684 (0.458–1.023) | 0.065 | 47.2 | |
| Endometrial cancer | ||||
| Cohort | ||||
| Case–control (2 studies) | 0.584 (0.432–0.791) | 0.000 | 0.0 | |
| Gastric cancer | ||||
| Cohort | ||||
| Case–control (1 study) | 0.900 (0.271–2.985) | 0.863 | N/A | |
| Glioma | ||||
| Cohort | ||||
| Case–control (1 study) | 1.145 (0.751–1.747) | 0.530 | 0.0 | |
| Hepatocellular carcinoma | ||||
| Cohort | ||||
| Case–control (4 studies) | 1.574 (0.563–4.401) | 0.387 | 82.3 | |
| Leukemia | ||||
| Cohort (1 study) | 1.080 (0.810–1.440) | 0.600 | N/A | |
| Case–control | ||||
| Lymphoma | ||||
| Cohort (1 study) | 0.970 (0.520–1.810) | 0.924 | N/A | |
| Case–control | ||||
| Ovarian cancer | ||||
| Cohort (1 study) | 0.880 (0.561–1.381) | 0.578 | N/A | 0.97 |
| Case–control (2 studies) | 0.891 (0.573–1.384) | 0.607 | 79.7 | |
| Pancreatic cancer | ||||
| Cohort (study) | 0.710 (0.510–0.989) | 0.043 | N/A | |
| Case–control | ||||
| Prostate cancer | ||||
| Cohort (1 study) | 0.790 (0.511–1.222) | 0.289 | N/A | 0.90 |
| Case–control (4 studies) | 0.762 (0.549–1.059) | 0.105 | 69.4 | |
| Stomach cancer | ||||
| Cohort | ||||
| Case–control (2 studies) | 1.084 (0.354–3.316) | 0.888 | 89.1 | |
| Type 2 diabetes | ||||
| Cohort (5 studies) | 0.978 (0.841–1.138) | 0.774 | 74.2 | |
| Case–control | ||||
Abbreviations: NA, not applicable; RR, relative risk.
DISCUSSION
The present comprehensive systematic review and meta-analysis assessed the associations between nut consumption and risk of developing cancer and type 2 diabetes.
After summarizing all of the available evidence, nut intake was found to be associated with a decreased risk of developing colorectal cancer, endometrial cancer, and pancreatic cancer. There was no significant association with upper-aerodigestive tract cancer, breast cancer, gastric cancer, glioma, hepatocellular carcinoma, leukemia (including acute myeloid leukemia), lymphoma, ovarian cancer, prostate cancer, stomach cancer, or type 2 diabetes. As far as can be determined, this is the first systematic review and meta-analysis to summarize the available evidence for determining the associations between nut intake and cancer. During the course of this review, several studies investigating a similar association in type 2 diabetes were published.68–70 The finding of a null association with type 2 diabetes in the present review is largely consistent with the findings of those studies.
The present systematic review and meta-analysis has several strengths. First, the search strategy is exhaustive and reproducible. Second, two reviewers independently performed selection, review, and extraction of data, thus decreasing potential biases and errors.
Several limitations affect inferences from this systematic review. It was not possible to test for publication bias, which is likely to exist when evidence consists of observational studies that do not require trial registration. It is plausible that studies with negative findings were conducted but not published. In addition, since the included studies were observational it is possible that patients who consumed more nuts were healthier or had other characteristics that reduced their risk of disease, and these factors could not be fully adjusted for in the analysis. In some of the cohort studies, investigators did not update the nut consumption information during the follow-up period, which could potentially cause measurement error to further affect the associations. In case–control studies, recall bias may result in deviations of estimates from actual nut consumption. Other common challenges encountered in nutrition research, such as the accuracy of dietary records and the effect of cointerventions (other nutrients consumed with nuts), also apply to this study.
Nuts contain nutrients that are widely thought to be beneficial for human health. As stated above, numerous mechanisms have been proposed to explain the potential effect of nuts on the risk of cancer. More investigations on the role of nuts in each of the cancers examined in the present review (colorectal, endometrial, and pancreatic) are warranted.
Although the results of several studies suggested nuts may play a protective role in type 2 diabetes, a significant association between nut consumption and risk of type 2 diabetes was not found in this review. Interestingly, one randomized clinical trial published in 2008 found that patients assigned to a Mediterranean diet that included nuts had a lower risk of developing type 2 diabetes when compared with the control group assigned to a low-fat diet (hazard ratio 0.48; 95%CI 0.24–0.96).71 This study was not included in the present analysis, however, because it was a study of the Mediterranean diet including nuts, rather than a study of nuts alone.
It should also be acknowledged that there was significant heterogeneity among the studies of type 2 diabetes in this analysis. Research has demonstrated that nuts are associated with beneficial glycemic responses in healthy individuals. For example, almonds are shown to reduce the glycemic impact of carbohydrate foods72; pistachio nuts can attenuate the relative glycemic response when taken with a carbohydrate meal73; and nuts were demonstrated to have a dose-dependent effect on the glycemic response.74 Nuts have also been shown to contain a high proportion of unsaturated fatty acids,9 and nut consumption is inversely associated with circulating inflammatory cytokines and positively associated with plasma adiponectin.75 Since all of these factors are linked to diabetes,76,77 the exact relationship between nut consumption and type 2 diabetes, as well as between nut consumption and glycemic control, warrants further exploration. Additional well controlled, well designed studies are needed to clarify this question.
In this review, the role of nut intake in reducing the risk of developing cancer and type 2 diabetes was estimated. Several other studies have evaluated the role of nut consumption in attenuating mortality due to specific diseases.78,79 A large cohort study using data from the Nurses' Health Study and the Health Professionals Follow-up Study found that nut consumption could decrease total mortality in a dose–response manner. Moreover, nut consumption was associated with decreased mortality from cancer and heart disease, but not from type 2 diabetes. A combined evaluation of the relationships between 1) intake of nuts and disease incidence and 2) intake of nuts and mortality may provide a more comprehensive picture of the benefits of nut consumption in decreasing the burden of diseases.
In addition to the plausible benefits of nut consumption on the risk of some cancers, other benefits have been suggested. An inverse association between nut consumption and the risk of coronary heart disease was demonstrated in 5 large prospective cohort studies.80 The 2013 American College of Cardiology/American Heart Association Guideline on Lifestyle Management to Reduce Cardiovascular Risk recommends that a heart-healthy eating pattern, when based on a diet containing 2000 calories per day, should include 4–5 servings of nuts, legumes, and seeds per week.81 Nevertheless, while nuts have health benefits, it is important to remember they are a calorie-dense food. Nuts can contain 160–200 calories per ounce. Therefore, weight gain is a concern. The recommendation from the American Heart Association (5 servings per week, with an average recommended serving size of 28 g) is consistent with the highest category of intake in most of the studies summarized in this systematic review. This level of intake is associated with a net increase of 800–1000 calories per week. Weight gain may not occur, however, if nuts are incorporated into a healthy diet in which they are substituted for other foods, as opposed to being added to an existing diet. Indeed, diets enriched with nuts did not increase body weight, body mass index, or waist circumference.82 Several mechanisms have been proposed to explain a less pronounced effect of nut intake on weight, including increased satiety with nut consumption and a possible decrease in desire to consume carbohydrates.83
In addition, the caloric and fat contents of nuts vary across the different types of nuts, and consumers can make choices to ensure the greatest personal benefit. The US Food and Drug Administration reviewed 11 interventional and observational human studies and approved a qualified health claim about nut consumption and heart disease and recognized that walnuts were the more commonly studied nut type.84 Nuts with the lowest saturated fatty acid content (as a percentage of total fats) are pecans, walnuts, hazelnuts, almonds, pine nuts, and pistachios. Conversely, peanuts, macadamia nuts, cashews, and Brazil nuts have the highest saturated fatty acid content.83 In conclusion, a practical recommendation for individuals interested in making better food choices to reduce the risk of cancer and heart disease is to consume nuts 4–5 times per week, to aim for a serving size of 1–1.5 ounces, to use nuts as a substitution for other foods high in saturated fat and carbohydrates and to choose healthier nut options.
CONCLUSION
Based on an analysis of evidence from 36 cohort and case–control studies, nut consumption was inversely associated with risk of colorectal cancer, endometrial cancer, and pancreatic cancer, but not other types of cancer or type 2 diabetes. Overall, nut intake was associated with a decreased risk of cancer. Given the scarcity of currently available data, however, evidence from additional studies is required to more precisely determine the relationship between nut consumption and risk of individual cancer types.
Acknowledgments
The authors extend thanks to the Mayo Clinic library staff and several authors of related studies for obtaining manuscripts and providing information needed to complete this study. Gratitude is also expressed to Dr Maria Jackson of the University of the West Indies, Dr Elisabeth Möller of the Karolinska Institute, Dr Fatemeh Saberi Hosnijeh of Utrecht University, and Dr Jennifer Nettleton, who returned original data on request.
Funding. L. Wu was partially supported by UL1 TR000135 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health.
Declaration of interest. The authors have no relevant interests to declare.
Supporting Information
The following Supporting Information is available through the online version of the article at the publisher’s website.
Appendix S1. Completed MOOSE checklist
Appendix S1. Detailed search strategy
References
- 1.Ma X, Yu H. Global burden of cancer. Yale J Biol Med. 2006;79:85–94. [PMC free article] [PubMed] [Google Scholar]
- 2.Popat K, McQueen K, Feeley TW. The global burden of cancer. Best Pract Res Clin Anaesthesiol. 2013;27:399–408. [DOI] [PubMed] [Google Scholar]
- 3.Boyle JP, Thompson TJ, Gregg EW, et al. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29 doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vinicor F. The public health burden of diabetes and the reality of limits. Diabetes Care. 1998;21(Suppl 3):C15–C18. [DOI] [PubMed] [Google Scholar]
- 5.Lam DW, LeRoith D. The worldwide diabetes epidemic. Curr Opin Endocrinol Diabetes Obes. 2012;19:93–96. [DOI] [PubMed] [Google Scholar]
- 6.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. [DOI] [PubMed] [Google Scholar]
- 7.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. [DOI] [PubMed] [Google Scholar]
- 8.van Dieren S, Beulens JW, van der Schouw YT, et al. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil. 2010;17(Suppl 1):S3–S8. [DOI] [PubMed] [Google Scholar]
- 9.Ros E. Health benefits of nut consumption. Nutrients. 2010;2:652–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser GE, Sabate J, Beeson WL, et al. A possible protective effect of nut consumption on risk of coronary heart disease. The Adventist Health Study. Arch Intern Med. 1992;152:1416–1424. [PubMed] [Google Scholar]
- 11.Kushi LH, Folsom AR, Prineas RJ, et al. Dietary antioxidant vitamins and death from coronary heart disease in postmenopausal women. N Engl J Med. 1996;334:1156–1162. [DOI] [PubMed] [Google Scholar]
- 12.Hu FB, Stampfer MJ, Manson JE, et al. Frequent nut consumption and risk of coronary heart disease in women: prospective cohort study. BMJ. 1998;317:1341–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albert CM, Gaziano JM, Willett WC, et al. Nut consumption and decreased risk of sudden cardiac death in the Physicians' Health Study. Arch Intern Med. 2002;162:1382–1387. [DOI] [PubMed] [Google Scholar]
- 14.Tsai CJ, Leitzmann MF, Hu FB, et al. Frequent nut consumption and decreased risk of cholecystectomy in women. Am J Clin Nutr. 2004;80:76–81. [DOI] [PubMed] [Google Scholar]
- 15.Tsai CJ, Leitzmann MF, Hu FB, et al. A prospective cohort study of nut consumption and the risk of gallstone disease in men. Am J Epidemiol. 2004;160:961–968. [DOI] [PubMed] [Google Scholar]
- 16.Rajaram S, Sabate J. Nuts, body weight and insulin resistance. Br J Nutr. 2006;96(Suppl 2):S79–S86. [DOI] [PubMed] [Google Scholar]
- 17.Greenwald P, Clifford CK, Milner JA. Diet and cancer prevention. Eur J Cancer. 2001;37:948–965. [DOI] [PubMed] [Google Scholar]
- 18.Kris-Etherton PM, Hecker KD, Bonanome A, et al. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002;113(Suppl 9B):71S–88S. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez CA, Salas-Salvado J. The potential of nuts in the prevention of cancer. Br J Nutr. 2006;96(Suppl 2):S87–S94. [DOI] [PubMed] [Google Scholar]
- 20.Yang CS, Landau JM, Huang MT, et al. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001;21:381–406. [DOI] [PubMed] [Google Scholar]
- 21.Kannamkumarath SS, Wrobel K, Vonderheide A, et al. HPLC-ICP-MS determination of selenium distribution and speciation in different types of nut. Anal Bioanal Chem. 2002;373:454–460. [DOI] [PubMed] [Google Scholar]
- 22.Glade MJ. Food, nutrition, and the prevention of cancer: a global perspective. American Institute for Cancer Research/World Cancer Research Fund, American Institute for Cancer Research, 1997. Nutrition. 1999;15:523–526. [DOI] [PubMed] [Google Scholar]
- 23.Stoner GD, Mukhtar H. Polyphenols as cancer chemopreventive agents. J Cell Biochem Suppl. 1995;22:169–180. [DOI] [PubMed] [Google Scholar]
- 24.Martin-Moreno JM. The role of olive oil in lowering cancer risk: is this real gold or simply pinchbeck? J Epidemiol Community Health . 2000;54:726–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bingham SA, Day NE, Luben R, et al. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet. 2003;361:1496–1501. [DOI] [PubMed] [Google Scholar]
- 26.Sabate J, Ang Y. Nuts and health outcomes: new epidemiologic evidence. Am J Clin Nutr. 2009;89:1643S–1648S. [DOI] [PubMed] [Google Scholar]
- 27.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 28.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute website. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed January 15, 2014. [Google Scholar]
- 29.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ. 2007;176:1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hedelin M, Lof M, Andersson TML, et al. Dietary phytoestrogens and the risk of ovarian cancer in the Women's Lifestyle and Health Cohort Study. Cancer Epidemiol Biomarkers Prev. 2011;20:308–317. [DOI] [PubMed] [Google Scholar]
- 33.Singh PN, Fraser GE. Dietary risk factors for colon cancer in a low-risk population. Am J Epidemiol. 1998;148:761–774. [DOI] [PubMed] [Google Scholar]
- 34.Sonestedt E, Borgquist S, Ericson U, et al. Plant foods and oestrogen receptor alpha- and beta-defined breast cancer: observations from the Malmo Diet and Cancer cohort. Carcinogenesis. 2008;29:2203–2209. [DOI] [PubMed] [Google Scholar]
- 35.Thompson CA, Habermann TM, Wang AH, et al. Antioxidant intake from fruits, vegetables and other sources and risk of non-Hodgkin's lymphoma: the Iowa Women's Health Study. Int J Cancer. 2010;126:992–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeh C-C, You S-L, Chen C-J, et al. Peanut consumption and reduced risk of colorectal cancer in women: a prospective study in Taiwan. World J Gastroenterol. 2006;12:222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bao Y, Hu FB, Giovannucci EL, et al. Nut consumption and risk of pancreatic cancer in women. Br J Cancer. 2013;109:2911–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kochar J, Gaziano JM, Djousse L. Nut consumption and risk of type II diabetes in the Physicians' Health Study. Eur J Clin Nutr. 2010;64:75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mills PK, Beeson WL, Phillips RL, et al. Cohort study of diet, lifestyle, and prostate cancer in Adventist men. Cancer. 1989;64:598–604. [DOI] [PubMed] [Google Scholar]
- 40.Pan A, Sun Q, Manson JE, et al. Walnut consumption is associated with lower risk of type 2 diabetes in women. J Nutr. 2013;143:512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parker ED, Harnack LJ, Folsom AR. Nut consumption and risk of type 2 diabetes. JAMA. 2003;290:38–39; author reply 39–40. [DOI] [PubMed] [Google Scholar]
- 42.Villegas R, Gao Y-T, Yang G, et al. Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women's Health Study. Am J Clin Nutr. 2008;87:162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jenab M, Ferrari P, Slimani N, et al. Association of nut and seed intake with colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2004;13:1595–1603. [PubMed] [Google Scholar]
- 44.Nettleton JA, Steffen LM, Ni H, et al. Dietary patterns and risk of incident type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care. 2008;31:1777–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saberi Hosnijeh F, Peeters P, Romieu I, et al. Dietary intakes and risk of lymphoid and myeloid leukemia in the European Prospective Investigation into Cancer and Nutrition (EPIC). Nutr Cancer. 2014;66:14–28. [DOI] [PubMed] [Google Scholar]
- 46.Thiebaut ACM, Chajes V, Gerber M, et al. Dietary intakes of omega-6 and omega-3 polyunsaturated fatty acids and the risk of breast cancer. Int J Cancer. 2009;124:924–931. [DOI] [PubMed] [Google Scholar]
- 47.Giles GG, McNeil JJ, Donnan G, et al. Dietary factors and the risk of glioma in adults: results of a case-control study in Melbourne, Australia. Int J Cancer. 1994;59:357–362. [DOI] [PubMed] [Google Scholar]
- 48.Takayama S, Monma Y, Tsubota-Utsugi M, et al. Food intake and the risk of endometrial endometrioid adenocarcinoma in Japanese women. Nutr Cancer. 2013;65:954–960. [DOI] [PubMed] [Google Scholar]
- 49.Wang X-Q, Yan H, Terry PD, et al. Interaction between dietary factors and Helicobacter pylori infection in noncardia gastric cancer: a population-based case-control study in China. J Am Coll Nutr. 2012;31:375–384. [DOI] [PubMed] [Google Scholar]
- 50.Chen CJ, Liang KY, Chang AS, et al. Effects of hepatitis B virus, alcohol drinking, cigarette smoking and familial tendency on hepatocellular carcinoma. Hepatology. 1991;13:398–406. [PubMed] [Google Scholar]
- 51.Hoshiyama Y, Sasaba T. A case-control study of stomach cancer and its relation to diet, cigarettes, and alcohol consumption in Saitama Prefecture, Japan. Cancer Causes Control. 1992;3:441–448. [DOI] [PubMed] [Google Scholar]
- 52.Pan SY, Ugnat A-M, Mao Y, et al. A case-control study of diet and the risk of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1521–1527. [PubMed] [Google Scholar]
- 53.Raimondi S, Mabrouk JB, Shatenstein B, et al. Diet and prostate cancer risk with specific focus on dairy products and dietary calcium: a case-control study. Prostate. 2010;70:1054–1065. [DOI] [PubMed] [Google Scholar]
- 54.Soliman AS, Hung CW, Tsodikov A, et al. Epidemiologic risk factors of hepatocellular carcinoma in a rural region of Egypt. Hepatol Int. 2010;4:681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trichopoulos D, Ouranos G, Day NE, et al. Diet and cancer of the stomach: a case-control study in Greece. Int J Cancer. 1985;36:291–297. [PubMed] [Google Scholar]
- 56.Yu S-Z, Huang X-E, Koide T, et al. Hepatitis B and C viruses infection, lifestyle and genetic polymorphisms as risk factors for hepatocellular carcinoma in Haimen, China. Jpn J Cancer Res. 2002;93:1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang JY, Wang X, Han SG, et al. A case-control study of risk factors for hepatocellular carcinoma in Henan, China. Am J Trop Med Hyg. 1998;59:947–951. [DOI] [PubMed] [Google Scholar]
- 58.Jackson M, Tulloch-Reid M, Walker S, et al. Dietary patterns as predictors of prostate cancer in Jamaican men. Nutrition and Cancer. 2013;65:367–374. [DOI] [PubMed] [Google Scholar]
- 59.Moller E, Galeone C, Andersson TML, et al. Mediterranean Diet Score and prostate cancer risk in a Swedish population-based case-control study. J Nutr Sci. 2013;2 doi: 10.1017/jns.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petridou E, Kedikoglou S, Koukoulomatis P, et al. Diet in relation to endometrial cancer risk: a case-control study in Greece. Nutr Cancer. 2002;44:16–22. [DOI] [PubMed] [Google Scholar]
- 61.Yamamura Y, Oum R, Gbito KYE, et al. Dietary intake of vegetables, fruits, and meats/beans as potential risk factors of acute myeloid leukemia: a Texas case-control study. Nutr Cancer. 2013;65:1132–1140. [DOI] [PubMed] [Google Scholar]
- 62.Ibiebele TI, Nagle CM, Bain CJ, et al. Intake of omega-3 and omega-6 fatty acids and risk of ovarian cancer. Cancer Causes Control. 2012;23:1775–1783. [DOI] [PubMed] [Google Scholar]
- 63.Jain MG, Hislop GT, Howe GR, et al. Plant foods, antioxidants, and prostate cancer risk: findings from case-control studies in Canada. Nutr Cancer. 1999;34:173–184. [DOI] [PubMed] [Google Scholar]
- 64.Kune S, Kune GA, Watson LF. Case-control study of dietary etiological factors: the Melbourne Colorectal Cancer Study. Nutr Cancer. 1987;9:21–42. [DOI] [PubMed] [Google Scholar]
- 65.Liu Y, Colditz GA, Cotterchio M, et al. Adolescent dietary fiber, vegetable fat, vegetable protein, and nut intakes and breast cancer risk. Breast Cancer Res Treat. 2014;145:461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Farvid MS, Cho E, Chen WY, et al. Dietary protein sources in early adulthood and breast cancer incidence: prospective cohort study. BMJ. 2014;348:g3437 doi: 10.1136/bmj.g3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Samoli E, Lagiou A, Nikolopoulos E, et al. Mediterranean diet and upper aerodigestive tract cancer: the Greek segment of the Alcohol-Related Cancers and Genetic Susceptibility in Europe study. Br J Nutr. 2010;104:1369–1374. [DOI] [PubMed] [Google Scholar]
- 68.Guo K, Zhou Z, Jiang Y, et al. Meta-analysis of prospective studies on the effects of nut consumption on hypertension and type 2 diabetes mellitus. J Diabetes. 2015;7:202–212. [DOI] [PubMed] [Google Scholar]
- 69.Luo C, Zhang Y, Ding Y, et al. Nut consumption and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a systematic review and meta-analysis. Am J Clin Nutr. 2014;100:256–269. [DOI] [PubMed] [Google Scholar]
- 70.Zhou D, Yu H, He F, et al. Nut consumption in relation to cardiovascular disease risk and type 2 diabetes: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2014;100:270–277. [DOI] [PubMed] [Google Scholar]
- 71.Salas-Salvado J, Bullo M, Babio N, et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care. 2011;34:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Josse AR, Kendall CW, Augustin LS, et al. Almonds and postprandial glycemia – a dose-response study. Metabol: Clin Exp. 2007;56:400–404. [DOI] [PubMed] [Google Scholar]
- 73.Kendall CW, Josse AR, Esfahani A, et al. The impact of pistachio intake alone or in combination with high-carbohydrate foods on post-prandial glycemia. Eur J Clin Nutr. 2011;65:696–702. [DOI] [PubMed] [Google Scholar]
- 74.Kendall CW, Esfahani A, Josse AR, et al. The glycemic effect of nut-enriched meals in healthy and diabetic subjects. Nutr Metab Cardiovasc Dis. 2011;21(Suppl 1):S34–S39. [DOI] [PubMed] [Google Scholar]
- 75.Ros E. Nuts and novel biomarkers of cardiovascular disease. Am J Clin Nutr. 2009;89:1649S–1656S. [DOI] [PubMed] [Google Scholar]
- 76.Pradhan AD, Ridker PM. Do atherosclerosis and type 2 diabetes share a common inflammatory basis? Eur Heart J . 2002;23:831–834. [DOI] [PubMed] [Google Scholar]
- 77.Wu JH, Micha R, Imamura F, et al. Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Br J Nutr. 2012;107(Suppl 2):S214–S227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guasch-Ferre M, Bullo M, Martinez-Gonzalez MA, et al. Frequency of nut consumption and mortality risk in the PREDIMED nutrition intervention trial. BMC Med. 2013;11:164 doi:10.1186/1741-7015-11-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bao Y, Han J, Hu FB, et al. Association of nut consumption with total and cause-specific mortality. N Engl J Med. 2013;369:2001–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hu FB, Stampfer MJ. Nut consumption and risk of coronary heart disease: a review of epidemiologic evidence. Curr Atheroscler Rep. 1999;1:204–209. [DOI] [PubMed] [Google Scholar]
- 81.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S76–S99. [DOI] [PubMed] [Google Scholar]
- 82.Flores-Mateo G, Rojas-Rueda D, Basora J, et al. Nut intake and adiposity: meta-analysis of clinical trials. Am J Clin Nutr. 2013;97:1346–1355. [DOI] [PubMed] [Google Scholar]
- 83.Vadivel V, Kunyanga CN, Biesalski HK. Health benefits of nut consumption with special reference to body weight control. Nutrition. 2012;28:1089–1097. [DOI] [PubMed] [Google Scholar]
- 84.Tarantino LM. Qualified Health Claims: Letter of Enforcement Discretion – Walnuts and Coronary Heart Disease (Docket no. 02P-0292). http://www.fda.gov/Food/IngredientsPackagingLabeling/LabelingNutrition/ucm072910.htm. Published March 9, 2004. [Google Scholar]