Abstract
Background
The Institute of Medicine has identified the comparative effectiveness of renal replacement therapies as a kidney-related topic among the top 100 national priorities. Given the importance of ensuring internal and external validity, the goal of this study was to identify potential sources of bias in observational studies that compare outcomes with different dialysis modalities.
Methods
This observational cohort study used data from the electronic medical records of all patients that started maintenance dialysis in the calendar years 2007–2011 and underwent treatment for at least 60 days in any of the 2217 facilities operated by DaVita Inc. Each patient was assigned one of six dialysis modalities for each 91-day period from the date of first dialysis (thrice weekly in-center hemodialysis (HD), peritoneal dialysis (PD), less-frequent HD, home HD, frequent HD and nocturnal in-center HD).
Results
Of the 162 644 patients, 18% underwent treatment with a modality other than HD for at least one 91-day period. Except for PD, patients started treatment with alternative modalities after variable lengths of treatment with HD; the time until a change in modality was shortest for less-frequent HD (median time = 6 months) and longest for frequent HD (median time = 15 months). Between 30 and 78% of patients transferred to another dialysis facility prior to change in modality. Finally, there were significant differences in baseline and time-varying clinical characteristics associated with dialysis modality.
Conclusions
This analysis identified numerous potential sources of bias in studies of the comparative effectiveness of dialysis modalities.
Keywords: bias, comparative effectiveness research, end-stage renal disease, hemodialysis, peritoneal dialysis
INTRODUCTION
Over the past 30 years, the risks of hospitalization and death for patients undergoing maintenance dialysis in the USA have declined significantly; yet, challenges remain [1]. The median life expectancy of patients starting renal replacement therapy in the USA is only approximately 3 years, and patients spend an average of 12 days in the hospital annually [1, 2]. The overwhelming majority of patients are treated with thrice-weekly in-center hemodialysis (TWICHD) and most of the rest perform peritoneal dialysis (PD) at home. However, an increasingly larger number of patients are being treated with modified hemodialysis (HD) regimens that include significantly longer treatment times (nocturnal), or different frequency (two to six times/week), or alternative platforms (e.g. NxStage System One) [3–5]. Most of these alternative regimens differ significantly from the ones tested in clinical trials conducted by the Frequent Hemodialysis Network [6, 7].
Treatment with these alternative dialysis modalities significantly alters and/or increases the burden of treatment on patients. It is critically important to perform a rigorous assessment of the true nature of the benefit, if any, of these alternative modalities on patient-centered outcomes. Underscoring the importance of this issue, the Institute of Medicine identified comparing the effectiveness of renal replacement therapies as the only kidney-disease-related topic among the top 100 initial national priorities for comparative effectiveness research [8]. Given the challenges in randomly assigning patients to modalities with disparate effects on lifestyles, observational studies have remained the mainstay of such comparative effectiveness research. In order to generate valid estimates of effects of different modalities, it is important to identify and account for all potential sources of bias in such comparisons. Most studies, however, thus far have considered only differences in patient characteristics at the time of start of maintenance dialysis [3, 5, 9–12]. This study was undertaken to test the hypothesis that there are significant differences not only in baseline, but also time-varying patient- and facility-level characteristics among individuals treated with six distinct maintenance dialysis modalities.
METHODS
Study population and data source
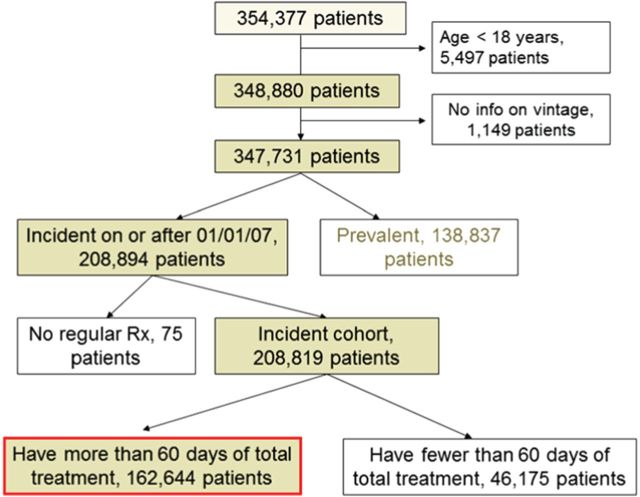
The study cohort comprised all patients who started maintenance dialysis in calendar years 2007–2011 and received treatment in one of the facilities operated by DaVita Inc. Patients <18 years of age at baseline, or who did not receive treatment for at least 60 days were excluded. Our study population is comprised of 162 644 individuals (Figure 1). All data were obtained from electronic records at DaVita.
FIGURE 1:
Consort diagram describing the creation of the study cohort.
Dialysis modality, access type and dialysis facility assignments
The entire follow-up period for each patient was divided into successive 91-day periods from the date of first dialysis of that patient; follow-up was available for up to 20 such periods. Each patient was assigned one of six different dialysis modalities for each 91-day period—TWICHD, PD, less-frequent in-center HD (less-frequent HD; ≤two times/week with identical pattern of days of the week for treatment), home HD, frequent in-center HD (frequent HD; ≥three times/week) and nocturnal in-center HD (NICHD). Each patient was considered to be treated with a given modality if she/he was treated with that particular modality for at least 60 consecutive days. The modality assigned for any given 91-day period was the therapy with which the patient was treated for ≥45 days of the period. The dialysis access with which the patient was treated for more than 45 days was assigned as the access for the period. Each patient was also assigned a dialysis facility where the patient received care for ≥45 days in the period.
Hemodynamic, other dialysis-related and laboratory parameters were summarized for each 91-day period as arithmetic means. Similarly, summary values of each parenteral medication were computed for each period.
Statistical analysis
Patients who were treated for at least one 91-day period with PD, less-frequent HD, home HD, frequent HD or NICHD were categorized as ‘ever-PD’, ‘ever-less-frequent HD’, ‘ever home HD’, ‘ever frequent HD’ and ‘ever NICHD’, respectively. Patients who were treated only with TWICHD during follow-up were grouped as ‘only TWICHD’. Descriptive statistics were calculated for patients in each of the six categories. At baseline, data for spKt/V were missing for 8% of subjects, pre-dialysis body weight for 6%, and hemoglobin, hematocrit, transferrin saturation, ferritin, albumin, calcium, phosphorous, parathyroid hormone and alkaline phosphatase for 1–2%. Multiple imputation was used for missing data for all regression analyses. Standard graphical diagnostics for linear regression was performed to assess the fit of the multiple imputation models. In addition, differences in summary statistics between the complete case data and missing values and the imputed data were also checked.
The characteristics of patients treated with each of the five alternative modalities were compared with those treated only with TWICHD. In order to build parsimonious descriptive models, multivariate backward stepwise logistic regression models were fitted to assess the strength of association between candidate covariates and the assignment of ever being treated with an alternative modality. A threshold of P < 0.05 was set for inclusion and removal from the model. Age, gender and race were kept in the models, regardless of statistical significance.
To compare characteristics that might be related to transferring from TWICHD to an alternative modality, a nested case–control design was used that matched subjects on treatment history and the calendar year of start of maintenance dialysis. Specifically, patients that transferred from TWICHD into PD, less-frequent HD, Home HD, frequent HD or NICHD (cases) were 1:1 matched with patients who continued treatment with TWICHD up to the quarter of transfer (controls), and the year of start of maintenance dialysis. Descriptive statistics were calculated for relevant covariates for patients who transferred and their associated matched controls. For these patients, in the 91 days prior to the transfer, 10–15% of the data were missing for pre-dialysis body weight, hemoglobin, hematocrit, transferrin saturation, ferritin, albumin, calcium, phosphorus, parathyroid hormone, alkaline phosphatase and spKt/V, which were imputed using multiple imputation. Five different multivariate backward stepwise conditional logistic regression models were fitted using the same approach as for analyzing predictors of ‘ever’ being treated with each of the alternative dialysis modalities. Each regression included the appropriate matched pairs of cases and controls.
All statistical analyses were performed with Stata 13.0 for Windows (Stata Corp, College Station, TX, USA) and R version 3.0.0 for Windows (R Foundation for statistical computing, Vienna, Austria).
RESULTS
Utilization of alternative dialysis modalities from the time of initiation of dialysis
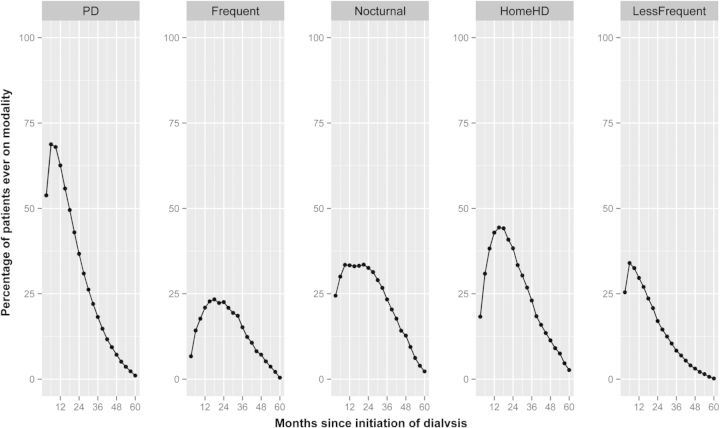
Of the 162 644 incident patients over the 5-year period, 18% underwent treatment with a dialysis modality other than TWICHD for at least one 91-day period: PD, 11%; less-frequent HD, 3%; home HD, 2%; frequent HD, 1% and nocturnal HD, 1% (Table 1). While most patients that were ever treated with PD utilized the therapy as the initial dialysis modality, the vast majority of patients treated with alternative HD modalities started treatment with the alternative modality after variable lengths of initial treatment with TWICHD (Table 1). The median time from the initiation of dialysis to start of treatment with the specific modality was shortest for less-frequent HD (6 months), followed by nocturnal and home HD (9 months each), and longest for frequent HD (15 months). The accrual of patients into each of the alternative dialysis modalities from the time of initiation of dialysis is illustrated in Figure 2.
Table 1.
Accrual of patients into dialysis modalities other than thrice-weekly in-center hemodialysis
| Peritoneal dialysis n (%) | Less-frequent in-center hemodialysis n (%) | Home hemodialysis n (%) | Frequent in-center hemodialysis n (%) | Nocturnal in-center hemodialysis n (%) | |
|---|---|---|---|---|---|
| Patients who started maintenance dialysis with this modality | 9835 (54) | 1173 (26) | 485 (18) | 126 (7) | 355 (24) |
| Patients who entered the cohort ≥91 days after first dialysis while being treated with this modality | 1667 (9) | 96 (2) | 398 (15) | 18 (1) | 112 (8) |
| Patients who transferred after continuous treatment with thrice-weekly in-center hemodialysis | 6461 (35) | 3292 (71) | 1513 (57) | 1653 (87) | 827 (57) |
| Patients treated with thrice-weekly in-center hemodialysis initially but information on dialysis modality in the 91-days period preceding transfer was unavailable | 128 (1) | 0 (0) | 62 (2) | 1 (0) | 45 (3) |
| Patients who transferred to this modality after treatment with modality other than thrice-weekly in-center hemodialysis | 186 (1) | 51 (1) | 195 (8) | 89 (5) | 113 (8) |
| All | 18 277 (100) | 4612 (100) | 2653 (100) | 1887 (100) | 1452 (100) |
FIGURE 2:
Summary illustration of utilization of five different dialysis modalities by patients in the cohort relative to the time of initiation of maintenance dialysis. The entire follow-up period for any given patient was divided into 91-day intervals from the day of first dialysis. Each data point in each panel represents the proportion of all patients ever treated with the dialysis modality (viz., peritoneal dialysis for up to 5-years of follow-up) who were undergoing treatment with that particular dialysis modality at that point of time. For example, of the 2653 patients treated with home hemodialysis over the 5-year study period, 25% were being treated with the modality 24 months from the date of first dialysis treatment.
Baseline and time-varying predictors of treatment with alternative dialysis modalities
Of the 18 277 patients ever treated with PD, 16 612 (91%) entered the cohort within the first 91 days of start of dialysis; PD was the initial dialysis modality for 59% (Supplementary data, Table S1). Compared with individuals treated only with TWICHD, patients ever treated with PD were younger, more likely to be white or to have had insurance other than Medicare or Medicaid, and treated in a region other than the Northeast (Table 2). They were also more likely to have diabetes, dyslipidemia or atherosclerotic heart disease at baseline. However, they were less likely to have congestive heart failure or be hospitalized in the first quarter after initiating dialysis. They had higher baseline serum albumin, lower body weight and serum ferritin, and required lower cumulative iron dose in the first 91-day period (Table 2 and Supplementary data, Table S1). After a median treatment of 5 months with TWICHD, 6461 patients transferred to PD; 36% transferred to another dialysis facility at the same time as the change in modality. In the 91-day period immediately preceding the transfer, patients who transferred to PD were more likely to be hospitalized, to have lower serum ferritin and higher cumulative iron dose compared with matched controls (Table 3 and Supplementary data, Table S1).
Table 2.
Predictors from the first 91-day period of start of first dialysis of treatment with dialysis modalities other than thrice-weekly in-center hemodialysis at any time during follow-up (adjusted odds ratio with 95% confidence interval) with each group compared only to patients who were treated only with thrice-weekly in-center hemodialysis during the entire period of follow-up (n = 113 129)
| Peritoneal dialysis | Less-frequent hemodialysis | Home hemodialysis | Frequent in-center hemodialysis | Nocturnal in-center hemodialysis | |
|---|---|---|---|---|---|
| Baseline variables | |||||
| Age, per 5 years | 0.86 (0.85, 0.87) | 1.04 (1.03, 1.06) | 0.82 (0.80, 0.84) | 0.92 (0.90, 0.94) | 0.83 (0.81, 0.85) |
| Race (reference, white) | |||||
| Blacks | 0.43 (0.40, 0.46) | 0.82 (0.69, 0.99) | 0.41 (0.35, 0.47) | NS | NS |
| Hispanics | 0.68 (0.59, 0.79) | 0.56 (0.50, 0.63) | 0.60 (0.42, 0.86) | 0.58 (0.51, 0.67) | 0.80 (0.68, 0.94) |
| Asian | 0.68 (0.59, 0.79) | 0.82 (0.69, 0.99) | 0.60 (0.42, 0.86) | NS | NS |
| Other | 0.54 (0.47, 0.63) | 0.82 (0.67, 1.00) | 0.39 (0.27, 0.58) | 0.66 (0.50, 0.86) | 0.37 (0.22, 0.63) |
| Gender (reference, females) | 0.87 (0.82, 0.92) | 1.17 (1.08, 1.26) | 1.16 (1.02, 1.31) | NS | NS |
| Primary health insurance (reference, Medicare) | |||||
| Medicaid | 0.64 (0.57, 0.72) | NS | 0.60 (0.44, 0.81) | NS | NS |
| Other Insurance | 1.17 (1.11, 1.25) | NS | 1.61 (1.43, 1.82) | NS | 1.43 (1.23, 1.66) |
| Cause of ESRD (reference, diabetes) | |||||
| Hypertension | 1.14 (1.05, 1.23) | 1.23 (1.11, 1.36) | 0.58 (0.49, 0.69) | NS | NS |
| Glomerular disease | 1.63 (1.49, 1.77) | 1.55 (1.35, 1.77) | 1.72 (1.43, 2.06) | NS | NS |
| Other | 1.17 (1.08, 1.27) | 1.37 (1.22, 1.54) | 1.55 (1.31, 1.84) | NS | NS |
| H/O previous transplant | 1.27 (1.07, 1.50) | NS | 2.14 (1.64, 2.79) | NS | 1.69 (1.11, 2.59) |
| Comorbidities | |||||
| Diabetes | 1.82 (1.71, 1.94) | 1.12 (1.02, 1.22) | 1.34 (1.17, 1.55) | 1.16 (1.02, 1.31) | 1.33 (1.13, 1.57) |
| Hypertension | 1.13 (1.06, 1.21) | NS | 3.32 (2.90, 3.81) | NS | 1.78 (1.54, 2.06) |
| Congestive heart failure | 0.60 (0.57, 0.64) | NS | 1.60 (1.42, 1.79) | 61.65 (42.90, 88.58) | NS |
| Atherosclerotic heart disease | 1.58 (1.48, 1.68) | 1.18 (1.07, 1.30) | 1.79 (1.57, 2.05) | NS | NS |
| Other cardiovascular disease | NS | NS | NS | NS | 1.70 (1.43, 2.02) |
| Dyslipidemia | 2.26 (2.17, 2.45) | NS | 1.71 (1.52, 1.93) | NS | NS |
| Time-varying variables | |||||
| Hospitalized in the first 91-days | 0.84 (0.80, 0.89) | 0.73 (0.67, 0.80) | 0.72 (0.64, 0.82) | 1.26 (1.13, 1.41) | NS |
| Body weight, >100 kg | 0.89 (0.83, 0.95) | NS | 1.57 (1.37, 1.78) | 1.86 (1.65, 2.09) | 1.39 (1.20, 1.62) |
| Vascular access type (reference: AV Fistula) | |||||
| Central venous catheter | 1.11 (1.00, 1.23) | 0.55 (0.48, 0.63) | NS | 0.68 (0.57, 0.82) | |
| AV graft | NS | 0.65 (0.47, 0.91) | NS | NS | |
| Unknown | NS | 0.76 (0.59, 0.97) | NS | NS | |
| Treatment variables (if first modality, in-center hemodialysis) | |||||
| Length of each hemodialysis session, minutes (per 30 min) | 1.06 (1.02, 1.09) | 0.52 (0.50, 0.55) | 0.92 (0.86, 1.00) | NS | 2.89 (2.70, 3.09) |
| Pre-dialysis systolic blood pressure (per 10 mm Hg) | NS | NS | NS | NS | NS |
| Pre-dialysis diastolic blood pressure (per 10 mm Hg) | 1.21 (1.19, 1.25) | 1.07 (1.03, 1.12) | 1.09 (1.03, 1.16) | NS | 1.14 (1.07, 1.22) |
| Maximum change in blood pressure per treatment (per 5 mm Hg) | 0.96 (0.94, 0.97) | NS | 0.94 (0.91, 0.97) | NS | NS |
| Weight change during treatment, kg | NS | NS | NS | NS | NS |
| Week-day interdialytic weight gain, per 1% | 0.98 (0.97, 0.99) | 0.87 (0.85, 0.89) | 0.94 (0.91, 0.98) | 1.01 (1.00, 1.02) | NS |
| Weekend interdialytic weight gain, per 1% | NS | NS | NS | NS | NS |
| Lab variables | |||||
| Hemoglobin, g/dL (per 1 g/dL | 1.04 (1.02, 1.07) | NS | NS | NS | 0.93 (0.87, 0.99) |
| Iron saturation, (per 1%) | NS | NS | NS | NS | NS |
| Serum ferritin (per 1 ng/mL) | NS | NS | NS | NS | NS |
| Serum albumin, (per 1 g/dL) | 1.46 (1.38, 1.56) | 1.59 (1.44, 1.74) | 1.64 (1.44, 1.88) | NS | 1.38 (1.17, 1.62) |
| spKt/V (per 0.1 units) | 0.99 (0.98, 1.00) | 1.12 (1.11, 1.13) | NS | 0.95 (0.94, 0.97) | NS |
| Serum calcium (per 1 mg/dL) | NS | NS | NS | NS | NS |
| Serum phosphorus (per 1 mg/dL) | NS | 0.81 (0.78, 0.84) | NS | NS | NS |
| Parathyroid hormone (per 100 pg/mL) | 1.01 (1.01, 1.02) | 0.97 (0.95, 0.98) | 0.98 (0.96, 1.00) | NS | NS |
| Alkaline phosphatase (per 1 IU/L) | NS | NS | NS | NS | NS |
| Hemoglobin A1c, (per 1%) | NS | NS | NS | NS | NS |
| Parenteral medications | |||||
| Cumulative Iron Dose per month (per 100 mg) | (1.00, 1.00) | (1.00, 1.00) | NS | 1.0 (1.00, 1.00) | NS |
| Median Epo Dose (per 1000 units) | NS | NS | NS | NS | NS |
| Geographic location (reference: northeast) | |||||
| Midwest | 1.40 (1.27, 1.55) | NS | 1.41 (1.17, 1.71) | NS | 2.25 (1.74, 2.93) |
| West | 1.48 (1.35, 1.61) | 1.21 (1.06, 1.37) | NS | 0.69 (0.56, 0.85) | NS |
| South | 1.43 (1.29, 1.57) | 1.44 (1.25, 1.65) | NS | 3.19 (2.64, 3.85) | 1.90 (1.44, 2.51) |
| Year of Incidence (reference: 2007) | |||||
| 2008 | 1.16 (1.07, 1.25) | NS | NS | NS | NS |
| 2009 | 1.25 (1.15, 1.35) | NS | NS | 0.86 (0.75, 0.99) | 0.75 (0.62, 0.92) |
| 2010 | 1.40 (1.30, 1.52) | 0.78 (0.69, 0.88) | NS | 0.55 (0.46, 0.65) | 0.53 (0.42, 0.66) |
| 2011 | 1.18 (1.08, 1.29) | 0.39 (0.33, 0.45) | 0.62 (0.50, 0.77) | 0.23 (0.18, 0.31) | 0.28 (0.20, 0.39) |
NS, not significant.
P < 0.05.
Table 3.
Predictors of transfer from thrice-weekly in-center hemodialysis to alternative modality, with time-varying variables derived from the 91-day period immediately preceding the transfer, compared with a cohort treated only with thrice-weekly in-center hemodialysis, matched for the length of time after date of first dialysis to the time of transfer (data are presented as adjusted odds ratio with 95% confidence interval)
| Peritoneal dialysis (n = 6461) | Less-frequent hemodialysis (n = 3292) | Home hemodialysis (n = 1513) | Frequent in-center hemodialysis (n = 1653) | Nocturnal in-center hemodialysis (n = 827) | |
|---|---|---|---|---|---|
| Baseline variables | |||||
| Age, per 5 years | 0.88 (0.86, 0.91) | 1.07 (1.03, 1.11) | 0.73 (0.67, 0.78) | 0.90 (0.85, 0.95) | 0.75 (0.69, 0.81) |
| Race (reference, white) | |||||
| Blacks | 0.37 (0.32, 0.42) | 0.55 (0.35, 0.86) | 0.29 (0.19, 0.44) | NS | NS |
| Hispanics | 0.46 (0.39, 0.54) | 0.56 (0.44, 0.72) | 0.15 (0.08, 0.28) | 0.54 (0.36, 0.80) | NS |
| Asian | 0.55 (0.41, 0.74) | 0.75 (0.57, 0.97) | 0.26 (0.09, 0.71) | 0.47 (0.30, 0.74) | NS |
| Other | 0.54 (0.41, 0.71) | NS | 0.18 (0.07, 0.50) | 0.23 (0.10, 0.49) | NS |
| Gender (reference, females) | 0.83 (0.74, 0.92) | 1.63 (1.34, 1.98) | 1.67 (1.16, 2.40) | NS | NS |
| Primary health insurance (reference, Medicare) | |||||
| Medicaid | NS | NS | NS | NS | NS |
| Other Insurance | 1.15 (1.04, 1.28) | NS | 1.50 (1.04, 2.17) | NS | NS |
| Cause of ESRD (reference, diabetes) | |||||
| Hypertension | NS | NS | NS | NS | NS |
| Glomerular Disease | 2.06 (1.71, 2.48) | 1.84 (1.33, 2.55) | NS | NS | NS |
| Other | 1.32 (1.12, 1.55) | 1.42 (1.09, 1.86) | NS | NS | NS |
| H/O previous transplant | NS | NS | NS | NS | NS |
| Comorbidities | |||||
| Diabetes | 1.82 (1.60, 2.07) | NS | 1.96 (1.27, 3.03) | NS | NS |
| Hypertension | 1.14 (1.01, 1.30) | NS | 3.36 (2.25, 5.02) | NS | NS |
| Congestive heart failure | 0.56 (0.50, 0.62) | NS | NS | 48.01 (23.88, 96.49) | 1.57 (1.00, 2.45) |
| Atherosclerotic heart disease | 1.62 (1.42, 1.86) | NS | 1.92 (1.24, 2.98) | NS | 1.83 (1.09, 3.06) |
| Other cardiovascular disease | NS | NS | 2.03 (1.39, 2.98) | NS | NS |
| Dyslipidemia | 2.27 (2.02, 2.52) | NS | NS | NS | NS |
| Time-varying variables | |||||
| Hospitalized in the 91-day period prior to transfer | 1.13 (1.01, 1.28) | 0.69 (0.56, 0.85) | 0.58 (0.38, 0.88) | 2.13 (1.55, 2.93) | NS |
| Body weight, >100 kg | NS | NS | 1.90 (1.23, 2.93) | 2.37 (1.68, 3.33) | 1.69 (1.05, 2.70) |
| Vascular Access Type (Reference: AV Fistula) | |||||
| Central Venous Catheter | 1.40 (1.13, 1.73) | 0.31 (0.21, 0.47) | 0.65 (0.47, 0.91) | 0.48 (0.30, 0.77) | |
| AV Graft | NS | 0.50 (0.27, 0.94) | NS | NS | |
| Unknown | NS | NS | 0.36 (0.15, 0.85) | NS | |
| Treatment Variables (in-center hemodialysis in the preceding 91 days) | |||||
| Length of each hemodialysis session, minutes (per 30 min) | NS | 0.39 (0.35, 0.44) | 0.57 (0.46, 0.70) | NS | 2.94 (2.35, 3.69) |
| Pre-dialysis systolic blood pressure (per 10 mm Hg) | NS | NS | NS | NS | NS |
| Pre-dialysis diastolic blood pressure (per 10 mm Hg) | 1.31 (1.24, 1.39) | 1.19 (1.08, 1.32) | NS | NS | NS |
| Maximum change in blood pressure per treatment (per 5 mm Hg) | 0.95 (0.93, 0.97) | NS | NS | NS | NS |
| Weight change during treatment, kg | NS | NS | NS | NS | NS |
| Week-day interdialytic weight gain, per 1% | 0.96 (0.94, 0.98) | 0.87 (0.83, 0.92) | NS | 1.21 (1.10, 1.33) | NS |
| Weekend interdialytic weight gain, per 1% | NS | NS | NS | NS | NS |
| Lab Variables | |||||
| Hemoglobin, g/dL (per 1 g/dL | 1.07 (1.02, 1.13) | NS | NS | 0.81 (0.70, 0.95) | NS |
| Iron Saturation, (per 1%) | NS | NS | NS | NS | NS |
| Serum Ferritin (per 1 ng/mL) | NS | NS | NS | NS | NS |
| Serum albumin, (per 1 g/dL) | 1.41 (1.26, 1.62) | NS | 1.97 (1.26, 3.09) | NS | NS |
| spKt/V (per 0.1 units) | 0.98 (0.96, 0.99) | 1.15 (1.12, 1.18) | NS | 0.95 (0.91, 0.99) | NS |
| Serum calcium (per 1 mg/dL) | NS | NS | NS | NS | NS |
| Serum phosphorus (per 1 mg/dL) | NS | 0.77 (0.71, 0.84) | 0.81 (0.70, 0.94) | NS | NS |
| Parathyroid hormone (per 100 pg/mL) | 1.03 (1.01, 1.05) | NS | NS | NS | NS |
| Alkaline phosphatase (per 1 IU/L) | NS | NS | NS | NS | NS |
| Hemoglobin A1c, (per 1%) | NS | NS | NS | NS | NS |
| Parenteral Medications | |||||
| Cumulative Iron Dose per month (per 100 mg) | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) | 1.00 (0.99, 1.00) | NS | NS |
| Median Epo Dose (per 1000 units) | 1.00 (0.99, 1.00) | NS | NS | NS | NS |
| Geographic Location (Reference: Northeast) | |||||
| Midwest | 1.28 (1.06, 1.54) | NS | 2.15 (1.21, 3.84) | NS | 2.56 (1.23, 5.33) |
| West | 1.32 (1.11, 1.57) | NS | 1.67 (1.00, 2.81) | NS | NS |
| South | 1.27 (1.06, 1.54) | NS | NS | 5.09 (2.92, 8.89) | 2.78 (1.27, 6.06) |
NS, not significant.
P < 0.05.
Of the 4612 patients ever treated with Less-Frequent HD, 4517 (98%) entered the cohort within the first 91 days of start of dialysis; less-frequent HD was the initial dialysis modality for 28% (Supplementary data, Table S2). Compared with individuals treated only with TWICHD, less-frequent HD patients were older and more commonly white, male and treated in the South. They were less likely to have had diabetes as their cause of end-stage renal disease, and had lower body weight (Table 2 and Supplementary data, Table S2). In the first 91-day period from the initiation of dialysis, they had significantly lower adjusted odds of being hospitalized, and had higher serum albumin. After a median treatment of 9 months with TWICHD, 3292 patients transferred to less-frequent HD; 2% transferred to another dialysis facility at the same time as the change in modality (Supplementary data, Table S2). In the 91-day period immediately preceding the transfer, patients who transferred to less-frequent HD had significantly shorter HD treatment time compared with matched controls (Table 3 and Supplementary data, Table S2).
Of the 2653 patients ever treated with home HD, 2255 (85%) entered the cohort within the first 91 days of start of dialysis; home HD was the initial dialysis modality for 25% (Supplementary data, Table S3). On an average, the patients received 3.7 treatments per week for 165 min per session. Compared with individuals treated only with TWICHD, home HD patients were younger, more likely to be white, male and had insurance other than Medicare or Medicaid (Table 3 and Supplementary data, Table S3). They were more likely to have had a prior kidney transplant, diabetes, hypertension, congestive heart failure, atherosclerotic heart disease, dyslipidemia or a body weight >100 kg. In the first 91-day period from the initiation of dialysis, they were more likely to have been dialyzed with an arteriovenous fistula, had higher serum albumin and lower serum ferritin and cumulative iron dose. After a median treatment of 11 months with TWICHD, 1513 patients transferred to home HD; 78% transferred to another dialysis facility at the same time as the change in modality (Supplementary data, Table S3). In the 91-day period immediately preceding the transfer, patients who transferred to home HD were less likely to be hospitalized, had higher serum albumin and received lower cumulative dose of parenteral iron compared with matched controls who continued treatment with TWICHD (Table 3 and Supplementary data, Table S3).
Of the 1887 patients ever treated with frequent HD, 1879 (99.5%) entered the cohort within the first 91 days of start of dialysis; frequent HD was the initial dialysis modality for 8% (Supplementary data, Table S4). Compared with individuals treated only with TWICHD, frequent HD patients were younger, more likely to be white and male. They were significantly more likely to have diabetes or dyslipidemia and had a higher body weight (Table 2 and Supplementary data, Table S4). The overwhelming majority of frequent HD patients have a history of congestive heart failure (Table 2). In the first 91-day period from the initiation of dialysis, they had significantly lower serum ferritin levels. After a median treatment of 15 months with TWICHD, 1653 patients transferred to frequent HD; 2% transferred to another dialysis facility at the same time as the change in modality (Supplementary data, Table S4). In the 91-day period immediately preceding the transfer, patients who transferred to frequent HD were more likely to have been hospitalized and received higher cumulative dose of parenteral iron compared with controls (Table 3 and Supplementary data, Table S4).
Of the 1452 patients ever treated with NICHD, 1340 (92%) entered the cohort within the first 91 days of start of dialysis; NICHD was the initial dialysis modality for 29% (Supplementary data, Table S5). Compared with individuals who were treated only with TWICHD, NICHD patients were younger, more likely to be male, Hispanic or Asian, and had insurance other than Medicare or Medicaid (Table 2 and Supplementary data, Table S5). They were more likely to have a history of kidney transplant, diabetes, hypertension, congestive heart failure, atherosclerotic heart disease or dyslipidemia, and had higher body weight. In the first 91-day period from the initiation of dialysis, they were more likely to have been dialyzed with an arteriovenous fistula and had lower serum ferritin and phosphorous levels. After a median treatment of 14 months with TWICHD, 827 patients transferred to NICHD; 30% of these individuals transferred to another dialysis facility at the same time as the change in dialysis modality (Supplementary data, Table S5). In the 91-day period immediately preceding the transfer, patients who transferred to NICHD were more likely to be hospitalized, compared with controls (Table 3 and Supplementary data, Table S5).
Dialysis modalities and facility
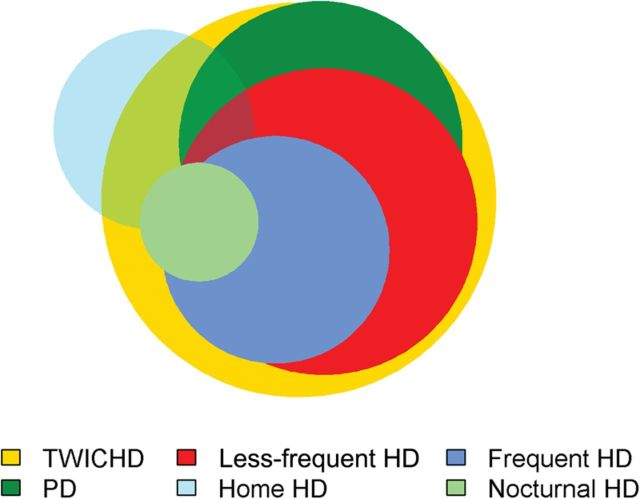
Over the 5-year period, the study cohort received care in 2217 facilities in 45 states. Patients received care for TWICHD, PD, less-frequent HD, home HD, frequent HD and NICHD in 2020, 1042, 1219, 520, 671 and 183 facilities, respectively (Figure 3). Of the 520 facilities where patients received care for home HD, 30% provided care only to patients with that modality. Of the 1042 facilities where patients received care for PD, only 4% provided care exclusively to patients with that modality.
FIGURE 3:
Overlap of availability of different dialysis modalities in the 2217 facilities in 45 states where patients received care. Each circle represents the facilities that offered treatment with any of the six different dialysis modalities, with the size of each circle proportional to the number of facilities. Of the 2217 facilities in 45 states where patients received care, patients received care for TWICHD, PD, less-frequent HD, home HD, frequent HD and NICHD in 2020, 1042, 1219, 520, 671 and 183 facilities, respectively.
DISCUSSION
Maintenance dialysis is a life-saving therapy for patients with end-stage renal disease but it imposes a significant burden of treatment. Because the nature of this burden varies by dialysis modality, it is critically important to generate robust data for the comparative effectiveness of various modalities for diverse group of individuals and a broad range of outcomes to allow patients to make informed choices. Given the challenges in conducting randomized controlled clinical trials of modalities with disparate effects on patients' lifestyle, observational studies are the mainstay of comparative effectiveness research in this field. Our examination of data from a large dialysis provider illustrates at least four sources of potential confounding or bias: time course of accrual into and treatment with various dialysis modalities, patient characteristics at the time of start of maintenance dialysis, change in health status over time and the facilities where care is delivered. Many of these factors have not been routinely considered in studies to date and importantly, vary by dialysis modality.
The overwhelming majority of patients in the USA are treated with TWICHD; however, a much larger proportion is treated with alternative dialysis modalities than is reflected by point-prevalent counts. Except for PD, most of the patients started treatment with alternative dialysis modalities after varying periods of TWICHD. Failure to consider this staggered start may lead to biased estimates of the comparative effectiveness of dialysis modalities because the first few months around the time of initiation of maintenance dialysis is a high-risk period for adverse outcomes [13]. Under-representation of alternative dialysis modalities in this high-risk period, as shown in this study, may create a survival bias against TWICHD [14]. Even after the first few months, the risk for death for patients undergoing maintenance dialysis is quite high. Hence, the longer the interval from the time of dialysis initiation to the transfer to an alternative dialysis modality, the greater is the risk for survivor bias. As an illustration of the same concept, several studies have demonstrated that PD patients who transfer to the therapy after a period of treatment with TWICHD have poorer outcomes compared with those who start maintenance dialysis with PD [15, 16]. Whether the same pattern of risk applies to patients treated with home HD or NICHD or other modalities is presently not known. These considerations highlight the importance of minimizing the potential bias deriving from the staggered start of dialysis modalities.
There were also differences in the demographic and clinical characteristics at the time of start of maintenance dialysis between patients treated with various dialysis modalities. The bias from differences in age or race is easy to understand and simple to account for in survival analyses. However, the potential bias from other measured characteristics may not be readily evident. For example, the body weight of patients treated with PD was lower, and the weight of home HD patients higher than individuals treated exclusively with TWICHD. While a higher body weight is associated with a lower risk of death among patients undergoing TWICHD, the implications of differences in body weight in comparing various dialysis modalities are far less clear [17, 18]. Moreover, there are significant differences in the burden of co-existing diseases between patients treated with different dialysis modalities. For example, while patients treated with PD were more likely to have a history of atherosclerotic heart disease but less likely to have congestive heart failure. The patients treated with home HD had a higher prevalence of both these conditions, and virtually every patient treated with frequent HD had a history of congestive heart failure. Most studies comparing the outcomes of patients treated with different dialysis modalities have considered differences in baseline demographic and clinical characteristics to some extent, either by including them as covariates or with the use of propensity scores [2–5].
In contrast to differences in health status of patients at the time of start of maintenance dialysis, most studies have not considered differences in these parameters over time. This is particularly important when comparing different HD modalities, because few patients start maintenance dialysis with the modality to which their outcomes are being attributed (Table 1). In these cases, using data on clinical characteristics at the time of start of maintenance dialysis is often far removed from and hence, less important to the outcomes being attributed to the modality. The time-varying parameters include risk factors such as dialysis access, surrogate measures of health such as hospitalizations or serum albumin or ferritin or change in body weight, residual kidney function, exposure to medications such as iron, erythropoiesis-stimulating drugs or vitamin D receptor activators, and experience with TWICHD (such as inter-dialytic weight gain or hemodynamic tolerability) or results of other laboratory parameters. These factors likely have effects on clinical outcomes and may also affect the decision of switching dialysis modality. Our study illustrates some of these differences in time-varying parameters, shows how they vary by dialysis modality and hence underscores the importance of accounting for potential bias arising from these differences.
Finally, facility-level differences are another important potential source of bias as illustrated by our study. There are significant geographic differences in the utilization of various dialysis modalities (Tables 2 and 3). Facility-level differences in both practice patterns and outcomes of patients undergoing maintenance dialysis are well described [15, 19–21]. Furthermore, the availability of different dialysis modalities varied considerably across facilities. Thus, 30–78% of patients that transferred from TWICHD to home dialysis modalities or NICHD also changed the dialysis facility where they received care. There are several potential reasons why facility-level differences may introduce bias. These include differences in practice patterns, staff experience and demographic, case-mix, and socioeconomic characteristics of patients treated in the facility. It is also likely that goals and preferences of healthcare providers in different facilities may vary and are an important but unmeasured source of bias. Hence, facility-level covariates might be important in examining heterogeneity in comparative effects of different modalities for subgroups of patients.
The results of our study should be interpreted in light of some potential limitations. First, the data were derived from facilities operated by a single dialysis provider. However, this constitutes almost one-third of all patients undergoing maintenance dialysis in the country. Moreover, studies both from within and outside the USA suggest similar sources of bias when comparing dialysis modalities [2, 3, 5, 22, 23]. Second, data were available only from the time the patients received care in facilities operated by a single provider, but not after they switched to facilities operated by other dialysis providers. Using data from the United States Renal Data System might have partially overcome this limitation, but the information on many of these dialysis modalities (such as NICHD or frequent or less-frequent HD) and the granularity of data that are used in the present study are not available from the national registry. Third, data on residual kidney function at the time of initiation of dialysis that are not available may have influenced the selection of dialysis modality, which is an important but often unmeasured source of bias.
In conclusion, our analysis illustrates several potential important sources of bias at both patient and facility levels that would need to be considered to validly study the comparative effectiveness of dialysis modalities, including both patient- and facility-level characteristics. The potential sources of bias vary by dialysis modality and it is imperative to consider and account for these in comparative effectiveness research studies for valid identification and estimation of the benefits and risks with any given dialysis modality.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
R.M. has received honoraria from Baxter Healthcare Inc. S.B. and A.N. are employees of DaVita Inc.
FUNDING
The work in this manuscript has been performed with the support of grant R01DK95668 and R21AG047306 (M.M., K.K.Z., and R.M.).
Supplementary Material
REFERENCES
- 1.U.S. Renal Data System. USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2008 2013
- 2.Mehrotra R, Chiu YW, Kalantar-Zadeh K, et al. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med 2011; 171: 110–118 [DOI] [PubMed] [Google Scholar]
- 3.Lacson E, Jr, Xu J, Suri RS, et al. Survival with three-times weekly in-center nocturnal versus conventional hemodialysis. J Am Soc Nephrol 2012; 23: 687–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suri RS, Lindsay RM, Bieber BA, et al. A multinational cohort study of in-center daily hemodialysis and patient survival. Kidney Int 2013; 83: 300–307 [DOI] [PubMed] [Google Scholar]
- 5.Weinhandl ED, Liu J, Gilbertson DT, et al. Survival in daily home hemodialysis and matched thrice-weekly in-center hemodialysis patients. J Am Soc Nephrol 2012; 23: 895–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chertow GM, Levin NW, Beck GJ, et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med 2010; 363: 2287–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rocco MV, Lockridge RS, Jr, Beck GJ, et al. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney Int 2011; 80: 1080–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Committee on Comparative Effectiveness Research Prioritization: Initial National Priorities for Comparative Effectiveness Research, in Medicine Io (ed). Washington, DC: National Academic Press, 2009 [Google Scholar]
- 9.Lacson E, Jr, Wang W, Lester K, et al. Outcomes associated with in-center nocturnal hemodialysis from a large multicenter program. Clin J Am Soc Nephrol 2010; 5: 220–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nesrallah GE, Lindsay RM, Cuerden MS, et al. Intensive hemodialysis associates with improved survival compared with conventional hemodialysis. J Am Soc Nephrol 2012; 23: 696–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansen KL, Zhang R, Huang Y, et al. Survival and hospitalization among patients using nocturnal and short daily compared to conventional hemodialysis: a USRDS study. Kidney Int 2009; 76: 984–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pauly RP, Gill JS, Rose CL, et al. Survival among nocturnal home haemodialysis patients compared to kidney transplant recipients. Nephrol Dial Transplant 2009; 24: 2915–2919 [DOI] [PubMed] [Google Scholar]
- 13.Chan KE, Maddux FW, Tolkoff-Rubin N, et al. Early outcomes among those initiating chronic dialysis in the United States. Clin J Am Soc Nephrol 2011; 6: 2642–2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quinn RR, Hux JE, Oliver MJ, et al. Selection bias explains apparent differential mortality between dialysis modalities. J Am Soc Nephrol 2011; 22: 1534–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehrotra R, Story K, Guest S, et al. Neighborhood location, rurality, geography, and outcomes of peritoneal dialysis patients in the United States. Perit Dial Int 2012; 32: 322–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nessim SJ, Bargman JM, Jassal SV, et al. The impact of transfer from hemodialysis on peritoneal dialysis technique survival. Perit Dial Int 2015; 35: 297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khawar O, Kalantar-Zadeh K, Lo WK, et al. Is the declining use of long-term peritoneal dialysis justified by outcome data? Clin J Am Soc Nephrol 2007; 2: 1317–1328 [DOI] [PubMed] [Google Scholar]
- 18.Lievense H, Kalantar-Zadeh K, Lukowsky LR, et al. Relationship of body size and initial dialysis modality on subsequent transplantation, mortality and weight gain of ESRD patients. Nephrol Dial Transplant 2012; 27: 3631–3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez RA, Sen S, Mehta K, et al. Geography matters: relationships among urban residential segregation, dialysis facilities, and patient outcomes. Ann Intern Med 2007; 146: 493–501 [DOI] [PubMed] [Google Scholar]
- 20.Pisoni RL, Arrington CJ, Albert JM, et al. Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: an instrumental variable analysis. Am J Kidney Dis 2009; 53: 475–491 [DOI] [PubMed] [Google Scholar]
- 21.Mehrotra R, Chiu YW, Kalantar-Zadeh K, et al. The outcomes of continuous ambulatory and automated peritoneal dialysis are similar. Kidney Int 2009; 76: 97–107 [DOI] [PubMed] [Google Scholar]
- 22.Yeates K, Zhu N, Vonesh E, et al. Hemodialysis and peritoneal dialysis are associated with similar outcomes for end-stage renal disease treatment in Canada. Nephrol Dial Transplant 2012; 27: 3568–3575 [DOI] [PubMed] [Google Scholar]
- 23.Marshall MR, Hawley CM, Kerr PG, et al. Home hemodialysis and mortality risk in Australian and New Zealand populations. Am J Kidney Dis 2011; 58: 782–793 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.