Abstract
Aims
Regulatory T cells (Tregs) protect mice from angiotensin II (Ang-II)-induced abdominal aortic aneurysms (AAA). This study tested whether AAA patients are Treg-insufficient and the Treg molecular mechanisms that control AAA pathogenesis.
Methods and results
ELISA determined the Foxp3 concentration in blood cell lysates from 485 AAA patients and 204 age- and sex-matched controls. AAA patients exhibited lower blood cell Foxp3 expression than controls (P < 0.0001). Pearson's correlation test demonstrated a significant but negative correlation between Foxp3 and AAA annual expansion rate before (r = –0.147, P = 0.007) and after (r = –0.153, P = 0.006) adjustment for AAA risk factors. AAA in apolipoprotein E-deficient (Apoe–/–) mice that received different doses of Ang-II exhibited a negative correlation of lesion Foxp3+ Treg numbers with AAA size (r = –0.883, P < 0.0001). Adoptive transfer of Tregs from wild-type (WT) and IL10-deficient (Il10–/–) mice increased AAA lesion Treg content, but only WT mice Tregs reduced AAA size, AAA incidence, blood pressure, lesion macrophage and CD4+ and CD8+ T-cell accumulation, and angiogenesis with concurrent increase of lesion collagen content. Both AAA lesion immunostaining and plasma ELISA demonstrated that adoptive transfer of WT Tregs, but not Il10–/– Tregs, reduced the expression of MCP-1. In vitro cell culture and aortic ring assay demonstrated that only Tregs from WT mice, but not those from Il10–/– mice, reduced macrophage MCP-1 secretion, macrophage and vascular cell protease expression and activity, and aortic ring microvessel formation.
Conclusion
This study supports a protective role of Tregs in human and experimental AAA by releasing IL10 to suppress inflammatory cell chemotaxis, arterial wall remodelling, and angiogenesis.
Keywords: Abdominal aortic aneurysm, Regulatory T-cell, Interleukin-10, Inflammatory cell, Adoptive transfer
1. Introduction
Abdominal aortic aneurysm (AAA) is a chronic inflammatory aortic disease that occurs predominantly in men over 65 years old. Although its prevalence varies from 1.7 to 17.7% depending on the study and age population,1 most AAA are asymptomatic and can cause sudden death due to aortic rupture.2 The most serious concern of this aortic disease among the aged population is the reliance on surgical repair or endovascular aneurysm repair for treatment.3 Hence, it is important to understand the cellular and molecular mechanisms of AAA pathogenesis to aid the development of non-invasive therapies.
CD4+CD25+Foxp+ regulatory T cells (Tregs) account for 5–10% of the total peripheral CD4+ T cells in normal naïve mice and humans.4 These anti-inflammatory T cells prominently regulate autoimmunity, allergic reaction, inflammation, and response to infections.5 Tregs are often insufficient or functionally defective in patients with autoimmune diseases,6 as well as metabolic and cardiovascular diseases.7–9 Patients with acute coronary syndrome (ACS) exhibit reduced numbers and compromised functional properties of Tregs.10 An increase in Tregs in lymphoid tissues correlates with reduced atherosclerosis.8,9
Forkhead box P3 (Foxp3) is essential for Treg development and immunosuppression activity and is currently the most reliable molecular marker for Tregs.11 Foxp3 protein or mRNA levels have quantified Tregs in aortic tissues.12,13 At least three studies tested Foxp3 expression in human AAA lesions or in peripheral blood from 7 to 27 AAA patients.12–14 In four human AAA lesions, the expression (mRNA and protein levels) of Foxp3 was significantly reduced when compared with Foxp3 expression from an equal number of normal human aortas.13 Of note, there were comparable numbers of CD4+CD25+Foxp3+ Tregs in the pool of CD4+ T cells from the human AAA lesion intraluminal thrombus and peripheral blood, as determined by flow cytometry analysis in a study containing 27 AAA patients.14 Despite a limited number of AAA patients, this study suggested that Tregs in the peripheral may reflect those in AAA lesions and peripheral blood samples can be used directly to assess Treg changes among AAA patients. Indeed, peripheral blood preparations from AAA patients contained significantly lower numbers of CD4+CD25+Foxp3+ Tregs that expressed significantly lower Foxp3 (both mRNA and proteins) and exerted lower immunosuppressive activity against effector T cells than did peripheral Tregs from healthy controls.12 Although peripheral CD4+CD25+Foxp3+ flow cytometry analysis is a reliable method of quantifying human blood Treg numbers, it is impossible to apply this method to large population studies, which are often required to establish a meaningful correlation of these anti-inflammatory cells to the pathogenesis of AAAs.
A possible role of CD4+CD25+Foxp3+ Tregs in angiotensin II (Ang-II) infusion-induced AAA has been tested recently.13,15 In wild-type (WT) C57BL/6 mice, Treg depletion with an anti-CD25 antibody (250 µg intraperitoneal injection every 2 weeks) significantly increased AAA development. Deficiency of T cell co-stimulatory molecules CD28 and B7 increased AAA formation in these mice. In Cd28–/– mice, supplementation of WT Tregs significantly limited AAA development.15 In atherosclerosis-prone apolipoprotein E-deficient (Apoe–/–) mice, WT Treg repopulation also suppressed AAA development, although Treg depletion with the same anti-CD25 antibody (100 µg every 2 weeks) did not affect AAA formation, likely due to insufficient Treg depletion.13 Tregs mediate the immune response through the cell-to-cell contact and secretion of anti-inflammatory IL10 and TGF-β. Tregs use these cytokines to suppress activated T cell responses, thereby inhibiting atherosclerosis.8,9,16 Microvessels from hypertensive mice incubated with Treg-conditioned media have significantly improved endothelium-dependent relaxation responses through an IL10-depedent mechanism.17 These observations from independent experimental models suggest the role of Treg-derived IL10 in AAA. Non-selective systemic deficiency of IL10 in Il10–/– mice exacerbated AAA formation compared with C57BL/6 WT control mice. Treg depletion with anti-CD25 monoclonal antibody did not further enlarge AAA lesion size or increase mortality.15 These animal studies suggested the importance of Tregs in these experimental AAA models, but did not ask by exactly which molecule(s) that Tregs contributed AAA formation in C57BL/6 mice or Apoe–/– mice. Enhanced AAA in Il10–/– mice is possibly due to the absence of IL10 from any type of IL10-producing cells.
In this study, we used ELISA to test whether peripheral blood cell expression of Foxp3 from a prospective cohort of 485 AAA patients differs from that of 204 age- and sex-matched AAA-free controls, and if such Foxp3 levels correlate with AAA size and annual expansion rate. We then used Ang-II infusion-induced AAA in Apoe–/– mice and a Treg reconstitution method using cells from WT and Il10–/– mice to test whether Tregs produce IL10 to affect AAA formation.
2. Methods
2.1. Human subjects and Foxp3 ELISA
This study contained 485 AAA patients and 204 age- and sex-matched controls with their AAA sizes, AAA annual growth rates, and AAA lesion intraluminal thrombus (ILT) sizes (measured from ultrasound with built-in automated program)18 all available. Detailed study information has been reported previously.19 Blood samples were centrifuged at 3000 g for 12 min, plasma was removed, and a mixture containing both the red blood cell and the buffy coat (leucocytes) was stored at −80°C until analysis was performed. Written informed consent was obtained from all subjects before participation, and the study was approved by the Local Ethics Committee of the Central Region of Denmark, Denmark (RM20080028), the data protection authorities, and performed in accordance with the Helsinki Declaration. The use of non-coded human samples was also approved by the Partners Human Research Committee, Boston, MA, USA. Blood cell Foxp3 concentration was determined using the human Foxp3 ELISA Kit, according to the manufacturer (Antibodies-online Inc., Atlanta, GA, USA).
2.2. Mouse AAA production and lesion characterization
C57BL/6 WT, interleukin 10 (IL10)-deficient (Il10–/–, C57BL/6, N13), and apolipoprotein E-deficient (Apoe−/−, C57BL/6, N12) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). To induce AAA in 8-week-old male Apoe−/− mice, Apoe−/− mice were anaesthetized (200 mg/kg ketamine, 10 mg/kg xylazine, intraperitoneal) and infused with 500 or 1000 ng/kg/min Ang-II (Sigma-Aldrich, St Louis, MO) subcutaneously delivered by Alzet model 2004 osmotic minipumps (DURECT Corp., Cupertino, CA, USA) for 28 days while mice consumed a high-fat diet (C12108; Research Diets, Inc., New Brunswick, NJ, USA). Post-operative analgesia (buprenophine, 0.05 mg/kg/12 h, intraperitoneal) was administered every 12 h for 48 h. The body weight of the mice was recorded before and after Ang-II infusion. The systolic blood pressure and heart rate of mice were determined using the CODA non-invasive blood pressure system (Kent Scientific Co., Torrington, CT, USA). Mice were sacrificed with carbon dioxide narcosis, followed by cardiac puncture blood collection. Plasma monocyte chemoattractant protein-1 (MCP-1) levels were determined by ELISA, according to the manufacturer's instructions (Pepro Tech Inc., Rocky Hill, NJ, USA). Aortic diameters of mice were measured using a surgical microscope (Zeiss Stemi SV11) equipped with a micrometer eyepiece (14 mm/0.1, SG02.T0218c, Motic Instruments, Inc., Vancouver, British Columbia, Canada), which allowed for the reading of aortic diameters at any time during the surgical procedure or during tissue harvesting. Two independent investigators measured aortic diameters, with no significant interobserver or intraobserver variability. AAA incidence was defined by the increase of a suprarenal maximal aortic diameter >50% of the mean value from same-age mice that received saline alone (0.91 ± 0.04 mm, n = 10), according to previously reported methods.20 All animal procedures conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and were approved by the Harvard Medical School Standing Committee on Animals (protocol # 03759).
2.3. Mouse aortic tissue immunohistochemical analysis
Aorta segments for study were cut at the maximal suprarenal outer aortic diameter and embedded vertically with an optimal cutting temperature (OCT) compound, and at least 10–15 serial frozen sections covering the maximal dilated aorta were prepared for immunohistochemical analysis as described previously.21 For those with a similarly enlarged AAA diameter throughout the thoracic-abdominal aortas, we selected the segment at approximately the same distance from the renal artery as those of others with maximal AAA expansions. In cases with AAA lesions at multiple locations, we selected the largest lesion as close as possible to the same distance from the renal artery as those of others with maximal AAA expansions. Slides of each sample from identical levels were used for staining with each antibody. Serial cryostat cross-sections (6 μm) were used for immunostaining for Tregs (Foxp3, eBioscience, 1:100), macrophages (Mac-2, BD Biosciences, 1:1000), T cells (CD4, 1:90; BD Biosciences; and CD8, 1:300; Abcam, Cambridge, MA), MCP-1 (1:50, BD Biosciences), elastin (Verhoeff-van Gieson, Sigma-Aldrich), collagen (0.1% Sirius Red F3BA; Polysciences Inc., Warrington, PA, USA), and CD31 (angiogenesis marker, 1:1500; BD Biosciences). Elastin degradation and media smooth muscle cell (SMC) accumulation were graded according to the grading keys described previously.22 T cells, Foxp3-positive Tregs, and CD31-positive microvessels were counted blindly. Macrophage-, MCP-1-, and collagen-positive areas were determined using computer-assisted image analysis software (Image-Pro Plus; Media Cybernetics, Bethesda, MD, USA). Ang-II-induced AAA often show regions of disrupted media,23 and we calculated AAA lesion areas from regions with both intact and fragmented media.21
2.4. Treg culture and adoptive transfer
To perform Treg adoptive transfer in Apoe−/− mice, we purified splenic CD4+CD25+ Tregs from WT, Il10−/−, or CD45.1 transgenic mice according to the manufacturer (MiltenyiBiotec, Inc., Cambridge, MA, USA) after sacrificing mice by carbon dioxide narcosis. The resulting CD4+CD25+ Tregs were further purified with a cell sorter (The BD FACSAiraTMCell Sorter, BD Bioscience, San Jose, CA, USA). Treg purity was confirmed by both FACS analysis and anti-Foxp3 antibody-mediated immunostaining. Each 6-week-old male Apoe−/−recipient mouse received tail vein adoptive transfer of 5×106 donor Tregs. We started Ang-II infusion 2 weeks after the adoptive transfer for 28 days. Donor cells targeted to the aneurysmal wall were confirmed by immunostaining to detect CD45.1+ Tregs in AAA lesions from Apoe−/−recipient mice that received Tregs from CD45.1 transgenic mice (Supplementary material online, Figure S1).
2.5. Smooth muscle cell, endothelial cell, and peritoneal macrophage culture
Aortic SMCs24 and endothelial cells (ECs)25 were prepared from C57BL/6 mice as described and cultured on a 24-well plate to complete confluence. Peritoneal macrophages were isolated from C57Bl/6 mice. Mice were sacrificed by carbon dioxide narcosis according to our proved protocol (# 03759). Confluent SMCs, ECs, and peritoneal macrophages were co-cultured with purified Tregs (3×104/well) from WT or Il10–/– mice for 48 h. Cells were lysed in a lysis buffer containing 25 mM Tris–HCl (pH 7.6), 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% SDS. Cell lysate was used for immunoblot to detect collagenase matrix metalloproteinase (MMP)-13 expression with rabbit anti-mouse polyclonal antibody (1:500, Santa Cruz Biotechnologies, Santa Cruz, CA, USA). Immunoblot analysis with rabbit anti-mouse GAPDH polyclonal antibody (1:2000, Santa Cruz Biotechnologies) ensured equal protein loading (20 μg/lane). Cell lysate MMP activity was determined by a gelatin gel zymogram assay, as reported previously.25
2.6. Aortic ring assay
An aortic ring assay was used to test the role of Tregs in angiogenesis. In brief, a 96-well plate was coated with 50 μL of Matrigel (BD Biosciences, San Diego, CA, USA). Aortic rings of 1 mm in length from C57BL/6 mice were laid on top of the Matrigel and covered with 100 μL of Matrigel. After solidification, 150 μL of RPMI (10% fetal bovine serum and 10 ng/mL basic fibroblast growth factor bFGF, PeproTech, Inc.) with or without purified Tregs (1×104 cell/well) from WT and Il10–/– mice was added to each well. After 7 days of culture, the aortas were photographed, and the endothelial outgrowth was analysed using Image-Pro Plus software and presented as invasion area in (millmetre)2.
2.7. Statistical analysis
For human blood cell lysate Foxp3 concentration analysis, potential confounders were identified by univariate analyses of recorded baseline variables. Potential associations with a P-value below 0.05 were used in a multivariate linear regression analysis testing Foxp3 as an independent risk factor of AAA. The progression of AAA was calculated by individual linear regression analysis of AAA diameter over observation time allowing all observations to be included. Mouse and cell-culture data were presented as means ± SEM. The associations of Foxp3+ cell numbers were correlated to maximal aortic diameter by Spearman's correlation test. Because of relatively small sample sizes and often skewed data distribution, we selected the non-parametric Mann–Whitney U test for paired data sets and one-way ANOVA with post-hoc Bonferroni test was used for comparison among three or more groups to examine statistical significance for all data from cultured cells and experimental AAA. Fisher's exact test was used to compare the differences in AAA incidence and post-Ang-II mortality. P < 0.05 was considered statistically significant.
3. Results
3.1. Human blood cell Foxp3 level and correlation with AAA annual expansion rate
Here we screened over 50 000 men, among which 615 men were diagnosed with AAA, 495 men had blood samples taken at baseline, and 485 blood samples were finally analysed. The main reason for those which were not sampled was immediate referral for surgical repair due to symptoms or size. The mean age of all cases was 70.2 ± 3.0 years and 70.3 ± 2.5 years for 204 sex- and age-matched AAA-free controls. Human Foxp3 ELISA determined total blood cell Foxp3 protein levels from AAA patients (n = 485, 121.43 ± 46.63 ng/mL), which were normally distributed among cases (one-sample Kolmogorov–Smirnov Test, P = 0.550) and significantly lower than those from AAA-free donors (n = 204, 190.61 ± 39.02 ng/mL, P < 0.0001, Student's t-test) (Figure 1A). The mean patient follow-up time was 1.80 ± 0.57 years. Pearson's correlation test demonstrated that blood cell Foxp3 protein levels did not correlate with AAA size (r = 0.002, P = 0.958) or ILT size (r = −0.041, P = 0.378), but correlated significantly and negatively with AAA annual growth rate (r = −0.147, P = 0.007) (Table 1). Bivariate correlation analysis showed that AAA growth rate associated positively with smoking, but negatively associated with the use of low-dose aspirin, diabetes mellitus, and angina. In contrast, blood cell Foxp3 protein levels associated negatively with the use of low-dose aspirin (Table 2). Multivariate linear regression analysis using aneurysmal growth rate as the dependent variable showed that blood cell Foxp3 levels continued to associate independently and negatively with aneurysmal growth rate, as diabetes mellitus, while smoking and AAA size continued to associate positively with aneurysmal progression rate. The use of low-dose aspirin, angina, and relative size of ILT did not associate with aneurysmal growth rate (Table 3). Treg levels did not associate with age, previous acute myocardial infarction (AMI), hypertension, medication (use of ACE-inhibitors, calcium blockers, statins, warfarin, and NSAIDs), BMI, systolic or diastolic blood pressure (data not shown).
Figure 1.
(A) Human blood cell lysate Foxp3 levels in patients with and without AAA. Data are mean ± SD. Student's t-test. (B) Spearmen's correlation between AAA lesion Foxp3+ Treg numbers and aortic diameters from Apoe–/– mice received two different doses of Ang-II.
Table 1.
Associated continuous variables with Foxp3 or aneurysmal growth rate (correlation coefficient and significance)
| Variables | AAA size | Growth rate | Intraluminal thrombus | Foxp3 |
|---|---|---|---|---|
| Growth rate | r = 0.330 | r = 1.000 | r = 0.237 | r = –0.147 |
| P < 0.0001 | P < 0.0001 | P = 0.007 | ||
| Foxp3 | r = 0.002 | r = –0.147 | r = –0.041 | r = 1.000 |
| P = 0.958 | P = 0.007 | P = 0.378 |
Table 2.
Associated bivariate variables with Foxp3 or aneurysmal growth rate
| Variable | Smoking | Mean ± SD | P-value | Asprin | Mean ± SD | P-value |
|---|---|---|---|---|---|---|
| Foxp3 | 0 | 122.06 ± 47.51 | 0 | 125.59 ± 49.44 | ||
| 1 | 120.94 ± 45.41 | 0.796 | 1 | 117.38 ± 43.19 | 0.044 | |
| AAA size | 0 | 41.31 ± 12.51 | 0 | 40.71 ± 12.38 | ||
| 1 | 39.65 ± 10.58 | 0.074 | 1 | 40.53 ± 11.17 | 0.851 | |
| AAA growth rate | 0 | 3.57 ± 3.98 | 0 | 4.72 ± 4.26 | ||
| 1 | 4.97 ± 4.34 | 0.001 | 1 | 3.61 ± 4.08 | 0.007 | |
| Variable | Diabetes | Mean ± SD | P-value | Angina | Mean ± SD | P-value |
| Foxp3 | 0 | 121.74 ± 45.18 | 0 | 122.78 ± 45.62 | ||
| 1 | 121.19 ± 57.27 | 0.956 | 1 | 117.28 ± 53.73 | 0.418 | |
| AAA size | 0 | 40.60 ± 11.80 | 0 | 40.37 ± 11.36 | ||
| 1 | 40.69 ± 11.66 | 0.948 | 1 | 39.97 ± 11.34 | 0.752 | |
| AAA growth rate | 0 | 4.34 ± 4.23 | 0 | 4.43 ± 4.31 | ||
| 1 | 2.34 ± 3.31 | 0.002 | 1 | 3.15 ± 3.52 | 0.013 |
Table 3.
Multivariate linear regression analysis using aneurysmal growth rate as the dependent variable
| Variable | Unstandardized coefficienta |
P-value | 95% CI |
Pearson's correlation coefficient |
|||
|---|---|---|---|---|---|---|---|
| B | SEM | Lower | Upper | Unadjusted | Partialb | ||
| Foxp3 | −0.013 | 0.005 | 0.006 | −0.022 | −0.004 | −0.147 | −0.153 |
| Smoking | 1.499 | 0.449 | 0.001 | 0.615 | 2.383 | 0.203 | 0.182 |
| Diabetes | −1.977 | 0.799 | 0.014 | −3.550 | −0.404 | −0.163 | −0.136 |
| Aspirin | −0.540 | 0.462 | 0.244 | −1.450 | 0.370 | −0.125 | −0.065 |
| Angina | –1.115 | 0.647 | 0.086 | −2.387 | 0.158 | −0.084 | −0.095 |
| AAA size | 0.256 | 0.063 | 0.000 | 0.132 | 0.380 | 0.250 | 0.220 |
| ILT | 0.730 | 1.024 | 0.467 | −1.285 | 2.745 | 0.163 | 0.040 |
ILT, intraluminal thrombus size.
95% CI, 95% confidential interval.
aAverage change in the dependent variable associated with a 1-unit change, statistically controlling for the other independent variables. Both the coefficient (B) and standard error of measurement (SEM) are given.
bPearson's correlation coefficient adjusted for the other variables in first column.
3.2. Negative correlation of AAA lesion Treg numbers and AAA sizes in mice
Ang-II infusion-induced AAA has been used to test the role of Tregs in this aortic disease.13,15 Different doses of Ang-II may produce different sizes of AAA, which may allow us to correlate AAA size with lesion Treg content. We used both 500 and 1000 ng/kg/h of Ang-II to induce AAA in Apoe–/– mice. Abdominal aortic diameters correlated significantly and negatively with Foxp3+ Treg numbers in AAA lesions (r = −0.883, P < 0.0001) (Figure 1B). AAA lesion Foxp3+ Treg numbers from mice that received high-dose (1000 ng/kg/h) Ang-II exhibited significantly fewer Treg numbers than those from mice that received low-dose (500 ng/kg/h) Ang-II (Figure 2A and B). As expected, high-dose Ang-II produced larger AAA sizes and higher mortality rates, AAA incidence rates, and systolic blood pressure than low-dose Ang-II in Apoe–/– mice (Figure 2C and D). Although we did not include the Apoe–/– mice that received saline only, which we used to obtain the aortic diameters for non-AAA aortic size baseline, low-dose Ang-II (500 ng/kg/h) did not induce AAA from any of the 15 mice with their aortic diameters (0.78 ± 0.03 mm) similar to those of saline-treated mice (0.91 ± 0.04 mm, P = 0.07).
Figure 2.
AAA production and Treg adoptive transfer in Apoe–/– mice. (A) Foxp3 immunostaining to detect AAA lesion Tregs from Apoe–/– mice received 500 and 1000 ng/kg/h Ang-II and those received adoptive transfer of Tregs from WT and Il10–/– mice. Scale: 200 µm, inset scale: 50 µm. (B) Foxp3+ Tregs in AAA lesions from all four groups of mice. (C) Aortic diameters from four groups of mice. The number of mice in each group is indicated in the parenthesis. (D) Post-Ang-II infusion Apoe–/– mice survival rates, AAA incidence rates, and systolic blood pressures. The number of mice in each group is indicated in each bar.
3.3. Role of Treg-derived IL10 in AAA
A prior study used Il10–/– mice to hypothesize a role of IL10 in Ang-II-induced AAA in C57BL/6 mice,15 but did not test specifically whether Tregs controlled AAA using this anti-inflammatory cytokine. To test the direct role of Treg-derived IL10 in AAA, we performed adoptive transfer of Tregs (5×106 donor cells per recipient) from both WT and Il10–/– mice to Apoe–/– mice while recipient mice were given a high dose of Ang-II (1000 ng/kg/h). At harvest, 28 days after receiving Ang-II, we detected a significant increase of lesion Foxp3+ Tregs, regardless of the genotypes of donor Tregs (Figure 2A and B). Yet only Tregs from WT mice exhibited significantly reduced AAA lesion sizes, AAA incidence rates, and systolic blood pressure, although the reduction of mortality rates did not reach statistical significance. In contrast, Tregs from Il10–/– mice failed to significantly affect AAA development in Apoe–/– recipient mice among all tested variables (AAA size, mortality rate, AAA incidence, and blood pressure) (Figure 2C and D).
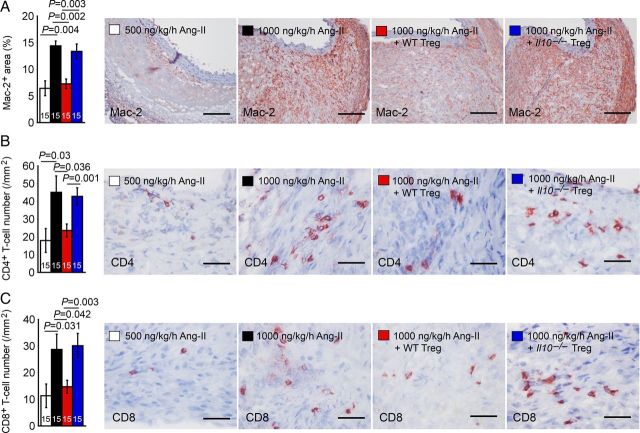
Inflammatory cell accumulation accompanies human and experimental AAA.26,27 Tregs may affect AAA lesion chemokine production and inflammatory cell accumulation.13,15 AAA lesions from mice receiving a high dose of Ang-II contained significantly more Mac-2+ macrophages and CD4+ and CD8+ T cells than those from mice receiving low-dose Ang-II (Figure 3A–C). As expected, reconstitution of WT Tregs brought elevated inflammatory cell accumulation in AAA lesions from high-dose Ang-II-treated mice to the level of that in mice that received low-dose Ang-II. In contrast, the same treatment of mice receiving a high dose of Ang-II with Tregs from Il10–/– mice did not affect the AAA lesion accumulation of these inflammatory cells (Figure 3A–C), suggesting the role of Tregs in suppressing AAA lesion accumulation of macrophages, T cells, and probably other untested inflammatory cells.
Figure 3.
AAA lesion Mac-2+ macrophages (A), CD4+ T cells (B), and CD8+ T cells (C) from Apoe–/– mice received 500 and 1000 ng/kg/h Ang-II and those received adoptive transfer of Tregs from WT and Il10–/– mice. The number of mice in each group is indicated in each bar. Representative figures are shown to the right. Scales: 200 µm (A) and 50 µm (B and C).
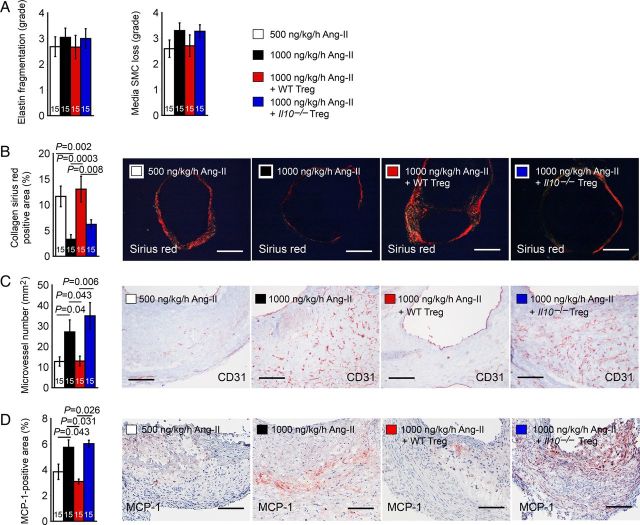
Arterial wall elastin degradation also characterizes human or experimental AAA. Increased elastin synthesis or the prevention of elastinolysis helps to stabilize AAA progression,28 even without interfering with inflammation (macrophage and T cell content), calcification, or high proteolytic activities.29 In Apoe–/– mice, high-dose Ang-II yielded higher degrees of arterial wall elastin fragmentation and SMC loss than low-dose Ang-II. Tregs from WT mice, but not from Il10–/– mice, also reduced arterial wall elastin fragmentation and SMC loss, but none of these differences reached statistical significance among the groups (Figure 4A). AAA growth and ultimate rupture also associate with impaired collagen homeostasis, although both type I and type III collagen have been increased30 or decreased31,32 in human AAA lesions. Picrosirius Red staining has been widely used to detect human and animal aortic aneurysm tissue collagen fibres,33–35 viewed with polarized light depends on the thickness of the fibres. As fibre thickness increases, the colour changes from green to orange. AAA lesion Picrosirius Red-positive collagen fibre contents were significantly lower in Apoe–/– mice that received high-dose Ang-II than those that received low-dose Ang-II. Tregs from WT mice, but not those from Il10–/– mice, significantly increased arterial collagen deposition (Figure 4B), suggesting the role of Treg-derived IL10 in regulating arterial wall matrix remodelling.
Figure 4.
AAA lesion media elastin fragmentation and SMC loss in grades (A), Sirius red-positive collagen area (B), CD31-positive microvessel numbers (C), and MCP-1-positive area (D) from Apoe–/– mice received 500 and 1000 ng/kg/h Ang-II and those received adoptive transfer of Tregs from WT and Il10–/– mice. The number of mice in each group is indicated in each bar. Representative figures for B–D are shown to the right. Scales: 500 µm (B) and 200 µm (C and D).
Microvessels localize in all layers of AAA lesions, and anti-angiogenic drugs limit AAA formation.36 High-dose Ang-II increased AAA lesion microvessel numbers in Apoe–/– mice. Tregs from WT mice, but not those from Il10–/– mice, significantly reduced AAA lesion microvessel numbers (Figure 4C), suggesting the role of Treg-derived IL10 in regulating angiogenesis during AAA development.
Adoptive transfer of Tregs from WT mice reduced AAA lesion accumulation of macrophages and CD4+ and CD8+ T cells (Figure 3A–C), increased AAA lesion collagen content (Figure 4B), and limited lesion angiogenesis (Figure 4C). Tregs from Il10–/– mice did not exert these actions, suggesting that Treg-derived IL10 controls inflammatory cell recruitment to AAA lesions, extracellular matrix macromolecule metabolism, and angiogenesis. MCP-1 immunostaining detected significantly higher MCP-1 expression in AAA lesions from high-dose Ang-II-treated Apoe–/– mice than in AAA lesions from low-dose Ang-II-treated mice. Tregs from WT mice, but not those from Il10–/– mice, significantly reduced the MCP-1 levels in AAA lesions from high-dose Ang-II-treated mice (Figure 4D).
3.4. Action of Treg-derived IL10 on chemokine production, MMP activity and expression, and angiogenesis
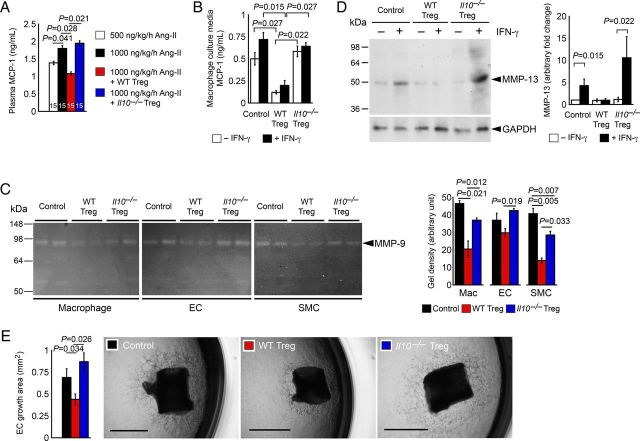
Tregs from WT mice, but not those from Il10–/– mice, reduced AAA lesion inflammatory cell (macrophages and CD4+ and CD8+ T cells) accumulation and chemokine MCP-1 expression, increased lesion collagen fibre content, and reduced lesion microvessel numbers from high-dose Ang-II-treated Apoe–/– mice (Figures 3A–C and 4B–D). These observations suggest a role of Treg-derived IL10 in inhibiting AAA lesion chemokine expression, collagenase expression, and neovascularization. Consistent to this hypothesis, we also found that increased plasma MCP-1 levels in high-dose Ang-II-treated Apoe–/– mice were reduced after mice received WT Tregs, but not Il10–/– Tregs (Figure 5A). To test a direct role of IL10 in regulating MCP-1 production, we co-cultured macrophages with Tregs from both WT and Il10–/– mice with and without interferon-γ (IFN-γ). Macrophage culture medium MCP-1 ELISA demonstrated that WT Tregs suppressed MCP-1 secretion from macrophages with or without IFN-γ. In contrast, Tregs from Il10–/– mice did not affect macrophage MCP-1 production (Figure 5B), supporting a hypothesis that Treg-derived IL10 controls lesion chemokine expression and lesion inflammatory cell (macrophages and CD4+ and CD8+ T cells) accumulation (Figures 3A–C and 4D).
Figure 5.
(A) Plasma MCP-1 levels from Apoe–/– mice received 500 and 1000 ng/kg/h Ang-II and those received adoptive transfer of Tregs from WT and Il10–/– mice. The number of mice in each group is indicated in each bar. (B) ELISA determined culture media MCP-1 levels from macrophages treated with and without IFN-γ and Tregs from WT and Il10–/– mice, n = 6. (C) MMP gelatin gel zymogram assay of mouse SMCs, ECs, and macrophages treated with and without Tregs from WT and Il10–/– mice. Representative gels are shown to the left, n = 6. (D) Immunoblot analysis of MMP-13 in peritoneal macrophages treated with and without IFN-γ and Tregs from WT and Il10–/– mice, n = 6. (E) Aortic ring assay. All rings were incubated in bFGF with and without Tregs from WT and Il10–/– mice. Data are from six 1-mm long aortic segments incubated with different Tregs from one preparation and data represent one of the three independent experiments. Representative data are shown to the right panels. Scale: 1 mm.
To investigate the mechanism by which WT Tregs increased AAA lesion collagen content, we incubated mouse aortic SMCs, aortic ECs, and thioglycolate-elicited peritoneal macrophages with or without Tregs from WT and Il10–/––/– mice, followed by detecting MMP activity using gelatin zymography. Tregs from WT mice suppressed MMP9 activity more potently than those from Il10–/– mice in inflammatory cells (macrophages) as well as vascular cells (SMCs and ECs) (Figure 5C), although our experiments did not allow the detection of MMP-2 activity. While type IV collagenase MMP-9 also exerts proteolytic activity against collagen types I and III,37,38 MMP-1, MMP-8, and MMP-13 are among the best known MMP collagenases. To detect collagenolytic MMP-13 expression in mouse peritoneal macrophages, we treated cells with and without IFN-γ and Tregs from WT and Il10–/– mice, followed by immunoblot analysis. IFN-γ-induced MMP-13 expression can be fully blocked by Tregs from WT mice, but not those from Il10–/– mice (Figure 5D).
Reduced numbers of microvessels in AAA lesions from Apoe–/– mice that received Tregs from WT mice, but not those from Il10–/– mice (Figure 4C), suggests that Treg-derived IL10 suppresses neovascularization in AAA lesions. We tested this hypothesis by culturing mouse aortic rings in Matrigel in the presence of bFGF with and without WT and Il10–/– Tregs. Tregs from WT mice, but not Il10–/––/– mice, significantly reduced microvessel growth from mouse aortic rings (Figure 5E). Reduced protease activity from ECs after incubating with WT Tregs (Figure 5C and D) may contribute in part to reduced angiogenesis.
4. Discussion
This study presented evidence for reduced expression of peripheral Foxp3 in 485 AAA patients compared with 204 age- and sex-matched AAA-free controls, and established a significant and negative association of this peripheral Treg marker with human AAA annual expansion rate. Instead of performing lesion protein extraction and immunoblot analysis,13 or performing peripheral blood flow cytometry analysis,12 blood-cell mixture freeze and thaw followed by ELISA analysis made large scale quantification of peripheral Foxp3 expression possible, although this method did not numerate Tregs as did the flow cytometry analysis. Significant and negative correlation between Foxp3 concentration and AAA annual growth rate from this large AAA patient population suggests the involvement of Tregs in human AAA progression. Uses of two different doses of Ang-II allowed for the determination of correlation between AAA lesion size and lesion Treg numbers from Apoe–/– mice. What differs from a prior study on systemic IL10-deficient mice,15 which did not specify the role of Treg-derived IL10 in AAA, is that this study used Tregs from Il10–/– mice and tested these cells in both in vitro and in vivo experimental models. Cell-to-cell interactions between Tregs and macrophages and between Tregs and vascular cells established a role of Treg-derived IL10 in regulating macrophage chemokine expression, promoting macrophage and vascular cell protease expression, and enhancing angiogenesis.
Besides releasing IL10, Tregs also produce immunosuppressive molecules TGF-β and IL35 and granzyme.39 By using these cytokines or proteases, or by direct cell-to-cell contact, Tregs may affect many other cell types pertinent to AAA development. For example, Tregs inhibit FcεR1-dependent degranulation of mast cells, which may be mediated by Treg surface OX40 and mast cell surface OX40L.40 Tregs also suppress dendritic cell maturation,39 promote alternatively activated M2 macrophage polarization,41 and induce neutrophil apoptosis,42 in addition to suppressing target T-cell responses. Mast cells, dendritic cells, macrophages, neutrophils, and T cells all contribute to AAA pathogenesis. Therefore, this study only tested few possible target cells of Tregs in AAA formation. Tregs likely have multiple functions in AAA, including more than just the aforementioned inflammatory cells and those vascular cells tested in this study.
A previous study in mice demonstrated that adoptive transfer of ex vivo-expanded polyclonal Tregs prevented xenograft rejection of pig pancreatic islets in mice.43 Similarly, adoptive transfer of Tregs can protect against ischaemic cardiac damage, reduce arterial blood pressure, and improve endothelium-dependent relaxation in coronary arterioles, and decrease inflammation (reduced inflammatory cytokine and macrophage infiltration into coronary arterioles) in Ang-II-induced arterial hypertension mice.17,44 In Cd28–/– and Apoe–/– mice,13,15 as in this study, adoptive transfer of WT Tregs reduced AAA development. These observations suggest the therapeutic potential of Tregs. Indeed, ex vivo-expanded Tregs have been injected into patients with graft-vs.-host disease (GVHD) to treat symptoms,45 or administered to children with recent-onset type-I diabetes to lower insulin requirements.46 Homologous Tregs prepared ex vivo from AAA patients might thus serve to treat AAA. IL2 together with TGF-β promote Treg differentiation. Administration of high-dose IL2 showed efficacy among individuals with metastatic renal cell carcinoma and melanoma.47,48 From recent clinical trials, IL2 administration also proved effective in patients with GVHD, hepatitis C-induced vasculitis, and type-I diabetes.49–51 Therefore, it is plausible that IL2 therapy may also be effective in stabilizing or regressing human AAA by promoting endogenous Treg differentiation and immunosuppression. This study illustrated a potential protective effect of Tregs in human AAA patients and in experimental AAA, and implicates IL10 in the mechanism of these anti-inflammatory CD4+ T cells in modulating AAA in this study and previous studies.13,15
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This study is supported by National Institutes of Health grants HL60942, HL81090, HL88547 (G.-P.S.), HL48743, and HL080472 (P.L.).
Acknowledgements
The authors thank Ms Eugenia Shvartz for her technical assistance and Ms Chelsea Swallom for her editorial assistance.
Conflict of interest: none declared.
References
- 1.Stather PW, Sidloff DA, Rhema IA, Choke E, Bown MJ, Sayers RD. A review of current reporting of abdominal aortic aneurysm mortality and prevalence in the literature. Eur J Vasc Endovasc Surg 2014;47:240–242. [DOI] [PubMed] [Google Scholar]
- 2.Tillman K, Lee OD, Whitty K. Abdominal aortic aneurysm: an often asymptomatic and fatal men's health issue. Am J Mens Health 2013;7:163–168. [DOI] [PubMed] [Google Scholar]
- 3.Cooper A. Endovascular repair of abdominal aortic aneurysm. Crit Care Nurse 2014;34:87–92. [DOI] [PubMed] [Google Scholar]
- 4.Duhen T, Duhen R, Lanzavecchia A, Sallusto F, Campbell DJ. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood 2012;119:4430–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 2008;133:775–787. [DOI] [PubMed] [Google Scholar]
- 6.Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev 2008;223:371–390. [DOI] [PubMed] [Google Scholar]
- 7.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 2009;15:930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med 2006;12:178–180. [DOI] [PubMed] [Google Scholar]
- 9.Mor A, Planer D, Luboshits G, Afek A, Metzger S, Chajek-Shaul T, Keren G, George J. Role of naturally occurring CD4+ CD25+ regulatory T cells in experimental atherosclerosis. Arterioscler Thromb Vasc Biol 2007;27:893–900. [DOI] [PubMed] [Google Scholar]
- 10.Mor A, Luboshits G, Planer D, Keren G, George J. Altered status of CD4(+)CD25(+) regulatory T cells in patients with acute coronary syndromes. Eur Heart J 2006;27:2530–2537. [DOI] [PubMed] [Google Scholar]
- 11.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003;299:1057–1061. [PubMed] [Google Scholar]
- 12.Yin M, Zhang J, Wang Y, Wang S, Böckler D, Duan Z, Xin S. Deficient CD4+CD25+ T regulatory cell function in patients with abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 2010;30:1825–1831. [DOI] [PubMed] [Google Scholar]
- 13.Meng X, Yang J, Zhang K, An G, Kong J, Jiang F, Zhang Y, Zhang C. Regulatory T Cells prevent angiotensin II-induced abdominal aortic aneurysm in apolipoprotein E knockout mice. Hypertension 2014;64:875–882. [DOI] [PubMed] [Google Scholar]
- 14.Sagan A, Mrowiecki W, Mikolajczyk TP, Urbanski K, Siedlinski M, Nosalski R, Korbut R, Guzik TJ. Local inflammation is associated with aortic thrombus formation in abdominal aortic aneurysms. Relationship to clinical risk factors . Thromb Haemost 2012;108:812–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ait-Oufella H, Wang Y, Herbin O, Bourcier S, Potteaux S, Joffre J, Loyer X, Ponnuswamy P, Esposito B, Dalloz M, Laurans L, Tedgui A, Mallat Z. Natural regulatory T cells limit angiotensin II-induced aneurysm formation and rupture in mice. Arterioscler Thromb Vasc Biol 2013;33:2374–2379. [DOI] [PubMed] [Google Scholar]
- 16.Mallat Z, Tedgui A. Cytokines as regulators of atherosclerosis in murine models. Curr Drug Targets 2007;8:1264–1272. [DOI] [PubMed] [Google Scholar]
- 17.Kassan M, Galan M, Partyka M, Trebak M, Matrougui K. Interleukin-10 released by CD4(+)CD25(+) natural regulatory T cells improves microvascular endothelial function through inhibition of NADPH oxidase activity in hypertensive mice. Arterioscler Thromb Vasc Biol 2011;31:2534–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behr-Rasmussen C, Grondal N, Bramsen MB, Thomsen MD, Lindholt JS. Mural thrombus and the progression of abdominal aortic aneurysms: a large population-based prospective cohort study. Eur J Vasc Endovasc Surg 2014;48:301–307. [DOI] [PubMed] [Google Scholar]
- 19.Lv BJ, Lindholt JS, Cheng X, Wang J, Shi GP. Plasma cathepsin S and cystatin C levels and risk of abdominal aortic aneurysm: a randomized population-based study. PLoS One 2012;7:e41813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang YX, Cassis LA, Daugherty A. Angiotensin II-induced aortic aneurysms. In: Xu Q, ed. Chichester, England: John Wiley & Sons; 2006. p125–135. [Google Scholar]
- 21.Schulte S, Sun J, Libby P, Macfarlane L, Sun C, Lopez-Ilasaca M, Shi GP, Sukhova GK. Cystatin C deficiency promotes inflammation in angiotensin II-induced abdominal aortic aneurisms in atherosclerotic mice. Am J Pathol 2010;177:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun J, Sukhova GK, Yang M, Wolters PJ, MacFarlane LA, Libby P, Sun C, Zhang Y, Liu J, Ennis TL, Knispel R, Xiong W, Thompson RW, Baxter BT, Shi GP. Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. J Clin Invest 2007;117:3359–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rateri DL, Howatt DA, Moorleghen JJ, Charnigo R, Cassis LA, Daugherty A. Prolonged infusion of angiotensin II in apoE(-/-) mice promotes macrophage recruitment with continued expansion of abdominal aortic aneurysm. Am J Pathol 2011;179:1542–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sukhova GK, Zhang Y, Pan JH, Wada Y, Yamamoto T, Naito M, Kodama T, Tsimikas S, Witztum JL, Lu ML, Sakara Y, Chin MT, Libby P, Shi GP. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J Clin Invest 2003;111:897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi GP, Sukhova GK, Kuzuya M, Ye Q, Du J, Zhang Y, Pan JH, Lu ML, Cheng XW, Iguchi A, Perrey S, Lee AM, Chapman HA, Libby P. Deficiency of the cysteine protease cathepsin S impairs microvessel growth. Circ Res 2003;92:493–500. [DOI] [PubMed] [Google Scholar]
- 26.Turner GH, Olzinski AR, Bernard RE, Aravindhan K, Boyle RJ, Newman MJ, Gardner SD, Willette RN, Gough PJ, Jucker BM. Assessment of macrophage infiltration in a murine model of abdominal aortic aneurysm. J Magn Reson Imaging 2009;30:455–460. [DOI] [PubMed] [Google Scholar]
- 27.Galle C, Schandené L, Stordeur P, Peignois Y, Ferreira J, Wautrecht JC, Dereume JP, Goldman M. Predominance of type 1 CD4+ T cells in human abdominal aortic aneurysm. Clin Exp Immunol 2005;142:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong J, Wang SM, Chen LH, Lin Y, Zhu YF, Ye CS. Elastic fibers reconstructed using adenovirus-mediated expression of tropoelastin and tested in the elastase model of abdominal aortic aneurysm in rats. J Vasc Surg 2008;48:965–973. [DOI] [PubMed] [Google Scholar]
- 29.Isenburg JC, Simionescu DT, Starcher BC, Vyavahare NR. Elastin stabilization for treatment of abdominal aortic aneurysms. Circulation 2007;115:1729–1737. [DOI] [PubMed] [Google Scholar]
- 30.Rizzo RJ, McCarthy WJ, Dixit SN, Lilly MP, Shively VP, Flinn WR, Yao JS. Collagen types and matrix protein content in human abdominal aortic aneurysms. J Vasc Surg 1989;10:365–373. [DOI] [PubMed] [Google Scholar]
- 31.Kuga T, Esato K, Zempo N, Fujioka K, Nakamura K. Detection of type III collagen fragments in specimens of abdominal aortic aneurysms. Surg Today 1998;28:385–390. [DOI] [PubMed] [Google Scholar]
- 32.Carmo M, Colombo L, Bruno A, Corsi FR, Roncoroni L, Cuttin MS, Radice F, Mussini E, Settembrini PG. Alteration of elastin, collagen and their cross-links in abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2002;23:543–549. [DOI] [PubMed] [Google Scholar]
- 33.Ruiz CE, Zhang HP, Butt AI, Whittaker P. Percutaneous treatment of abdominal aortic aneurysm in a swine model: understanding the behavior of aortic aneurysm closure through a serial histopathological analysis. Circulation 1997;96:2438–2448. [DOI] [PubMed] [Google Scholar]
- 34.Maegdefessel L, Azuma J, Toh R, Merk DR, Deng A, Chin JT, Raaz U, Schoelmerich AM, Raiesdana A, Leeper NJ, McConnell MV, Dalman RL, Spin JM, Tsao PS. Inhibition of microRNA-29b reduces murine abdominal aortic aneurysm development. J Clin Invest 2012;122:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maiellaro-Rafferty K, Weiss D, Joseph G, Wan W, Gleason RL, Taylor WR. Catalase overexpression in aortic smooth muscle prevents pathological mechanical changes underlying abdominal aortic aneurysm formation. Am J Physiol Heart Circ Physiol 2011;301:H355–H362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vijaynagar B, Bown MJ, Sayers RD, Choke E. Potential role for anti-angiogenic therapy in abdominal aortic aneurysms. Eur J Clin Invest 2013;43:758–765. [DOI] [PubMed] [Google Scholar]
- 37.Veidal SS, Vassiliadis E, Barascuk N, Zhang C, Segovia-Silvestre T, Klickstein L, Larsen MR, Qvist P, Christiansen C, Vainer B, Karsdal MA. Matrix metalloproteinase-9-mediated type III collagen degradation as a novel serological biochemical marker for liver fibrogenesis. Liver Int 2010;30:1293–1304. [DOI] [PubMed] [Google Scholar]
- 38.Bigg HF, Rowan AD, Barker MD, Cawston TE. Activity of matrix metalloproteinase-9 against native collagen types I and III. FEBS J 2007;274:1246–1255. [DOI] [PubMed] [Google Scholar]
- 39.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol 2008;8:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mekori YA, Hershko AY. T cell-mediated modulation of mast cell function: heterotypic adhesion-induced stimulatory or inhibitory effects. Front Immunol 2012;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci USA 2007;104:19446–19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewkowicz P, Lewkowicz N, Sasiak A, Tchórzewski H. Lipopolysaccharide-activated CD4+CD25+ T regulatory cells inhibit neutrophil function and promote their apoptosis and death. J Immunol 2006;177:7155–7163. [DOI] [PubMed] [Google Scholar]
- 43.Yi S, Ji M, Wu J, Ma X, Phillips P, Hawthorne WJ, O'Connell PJ. Adoptive transfer with in vitro expanded human regulatory T cells protects against porcine islet xenograft rejection via interleukin-10 in humanized mice. Diabetes 2012;61:1180–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kvakan H, Kleinewietfeld M, Qadri F, Park JK, Fischer R, Schwarz I, Rahn HP, Plehm R, Wellner M, Elitok S, Gratze P, Dechend R, Luft FC, Muller DN. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation 2009;119:2904–2912. [DOI] [PubMed] [Google Scholar]
- 45.Trzonkowski P, Bieniaszewska M, Juścińska J, Dobyszuk A, Krzystyniak A, Marek N, Myśliwska J, Hellmann A. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol 2009;133:22–26. [DOI] [PubMed] [Google Scholar]
- 46.Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Techmanska I, Juscinska J, Wujtewicz MA, Witkowski P, Mlynarski W, Balcerska A, Mysliwska J, Trzonkowski P. Administration of CD4+CD25highCD127- regulatory T cells preserves β-cell function in type 1 diabetes in children. Diabetes Care 2012;35:1817–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lotze MT, Chang AE, Seipp CA, Simpson C, Vetto JT, Rosenberg SA. High-dose recombinant interleukin 2 in the treatment of patients with disseminated cancer. Responses, treatment-related morbidity, and histologic findings. JAMA 1986;256:3117–3124. [PubMed] [Google Scholar]
- 48.Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, Matory YL, Skibber JM, Shiloni E, Vetto JT, Seipp CA, Simpson C, Reichert CM. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med 1985;313:1485–1492. [DOI] [PubMed] [Google Scholar]
- 49.Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP, III, Armand P, Cutler C, Ho VT, Treister NS, Bienfang DC, Prasad S, Tzachanis D, Joyce RM, Avigan DE, Antin JH, Ritz J, Soiffer RJ. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med 2011;365:2055–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V, Sene D, Cacoub P, Klatzmann D. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med 2011;365:2067–2077. [DOI] [PubMed] [Google Scholar]
- 51.Long SA, Rieck M, Sanda S, Bollyky JB, Samuels PL, Goland R, Ahmann A, Rabinovitch A, Aggarwal S, Phippard D, Turka LA, Ehlers MR, Bianchine PJ, Boyle KD, Adah SA, Bluestone JA, Buckner JH, Greenbaum CJ; Diabetes TrialNet and the Immune Tolerance Network. Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs β-cell function. Diabetes 2012;61:2340–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]