Abstract
Aims
Previously we demonstrated that both hypoxia inducible factor-1 (HIF-1) and bone morphogenetic protein-4 (BMP4) up-regulate transient receptor potential canonical (TRPC) 1 and TRPC6, resulting in increased basal intracellular Ca2+ concentration ([Ca2+]i) in pulmonary arterial smooth muscle cells (PASMCs), driving development of chronic hypoxia (CH)-induced pulmonary hypertension (CHPH). This study aims to determine whether HIF-1 regulates BMP4, and whether BMP4 mediates TRPC and basal [Ca2+]i increases in hypoxic PASMCs.
Methods and results
The level of BMP4 mature protein was increased for ∼183% in distal pulmonary arterial smooth muscle (PA) from CH (10% O2 for 21 days; CH) exposed rats, and 143% in PASMCs cultured under prolonged hypoxia (4% O2 for 60 h). In rat PASMCs, HIF-1α overexpression up-regulated, whereas HIF-1α knockdown under hypoxia decreased BMP4 expression; site-mutation identified two functional HIF-1-binding sites in Bmp4 gene promoter; noggin or BMP4 siRNA treatment blocked hypoxia-induced increases of TRPC1 and TRPC6 expression and basal [Ca2+]i. Likewise, in mice, exposure to CH increased BMP4 expression in distal PA for ∼80%, which was absent in HIF-1α heterozygous mutant mice. Comparing with wild-type littermates, BMP4 heterozygous mutant mice exposed to CH displayed lower BMP4 and TRPC levels in PA, decreased basal [Ca2+]i in PASMCs, and attenuated CHPH. In human PASMCs, HIF-1α knockdown attenuated hypoxia-induced BMP4 expression and knockdown of either HIF-1α or BMP4 abolished hypoxia-induced TRPC expression and basal [Ca2+]i.
Conclusions
BMP4 acts downstream of HIF-1 and mediates hypoxia-induced up-regulation of TRPC, leading to increased basal [Ca2+]i in PASMCs, promoting CHPH pathogenesis.
Keywords: HIF-1, BMP4, TRPC, Basal [Ca2+]i, PASMCs
1. Introduction
Pulmonary hypertension (PH) is a group of progressive diseases characterized by increased pulmonary arterial pressure and pulmonary vascular remodelling, leading to right heart failure and death in the absence of suitable therapy. PH is frequently a complication of chronic obstructive pulmonary disease (COPD), in which global alveolar hypoxia is an important trigger for hypoxic pulmonary vasoconstriction, endothelial dysfunction, and medial wall smooth muscle hypertrophy in the pulmonary vasculature, contributing to development of PH.1–3 Experimental animals with chronic hypoxia (CH)-induced PH (CHPH) manifest pulmonary vascular changes similar to those in PH patients associated with COPD, particularly with chronic bronchitis.4–6
Increased intracellular calcium concentration ([Ca2+]i) is a critical signal facilitating both contraction and growth of pulmonary arterial smooth muscle cells (PASMCs).7,8 Elevation of [Ca2+]i may occur via Ca2+ release from sarcoplasmic reticulum stores, enhanced Ca2+ influx through L-type voltage-dependent Ca2+ channels (VDCCs), receptor-operated Ca2+ channels, store-operated Ca2+ channels (SOCCs), and/or reduced Ca2+ efflux via Na+/Ca2+ exchanger and plasma membrane Ca2+ ATPase.9 PASMCs from rats or mice exhibit elevated [Ca2+]i upon development of CHPH or after in vitro exposure to prolonged hypoxia.7 We previously demonstrated that CH induced increased basal [Ca2+]i in PASMCs by up-regulating the expression of SOCCs components, thereby enhancing store-operated calcium entry (SOCE).8,10 Prior to the recent identification of ORAI members, SOCCs had been primarily thought to be composed of transient receptor potential canonical (TRPC) proteins.11–13 Out of the three most abundantly expressed TRPC family members (TRPC1, 4, and 6) in distal pulmonary artery smooth muscle (PA) and PASMCs,14 CH selectively up-regulates TRPC1 and TRPC6.8 By using siRNA knockdown, both TRPC1 and TRPC6 contribute to hypoxia-elevated SOCE and basal [Ca2+]i in PASMCs.15 Moreover, deletion of TRPC1 or TRPC6 suppressed vascular remodelling and CHPH development in mice.16
Hypoxia inducible factor-1 (HIF-1) is a key transcription factor that regulates the expression of hundreds of genes in the adaptive response to hypoxia.17 HIF-1 is a heterodimeric protein that consists of HIF-1α and HIF-1β subunits. HIF-1α is found at low level under normoxia due to rapid proteasomal degradation. However, under hypoxia, HIF-1α protein is stabilized, followed by accumulation in the nucleus, where HIF-1α dimerizes with ubiquitously expressed HIF-1β and binds to its cognate binding site 5′-(A/G)CGTG-3′, within hypoxia response elements (HREs), resulting in the transactivation of target genes.18 Previous studies utilizing adenoviral vector encoding a constitutively active form of HIF-1α (AdCA5) and HIF-1α knockout mice indicated HIF-1α plays a critical role in modulating TRPC expression and Ca2+ homeostasis in PASMCs.8,19 Hypoxia increased TRPC expression, SOCE and basal [Ca2+]i in PA and PASMCs from HIF-1α+/+ mice, whereas these responses were diminished in HIF-1α+/− littermates.8
Bone morphogenetic proteins (BMPs) are a subgroup of multifunctional ligands that belong to the transforming growth factor beta (TGF-β) superfamily and regulate various cell functions, including proliferation, differentiation, and apoptosis. Accumulating evidence suggests that dysregulated BMP signalling is involved in the pathogenesis of PH.20 BMP4, a member of BMPs, was found being increased during CH in mouse lung and promoted PA remodelling during the pathogenesis of CHPH.21,22 We previously demonstrated that BMP4 treatment increased TRPC expression, leading to enhanced SOCE and elevated basal [Ca2+]i in distal PASMCs,23 indicating BMP4 is an important regulator of intracellular Ca2+ homeostasis. In the present study, we investigated whether BMP4 acts downstream of HIF-1 and mediates the hypoxia-induced expression of TRPC1 and TRPC6 and elevation of basal [Ca2+]i in PASMCs during the pathogenesis of CHPH.
2. Methods
2.1. COPD patients and control subjects
Twenty-nine lung cancer patients who underwent lobectomy were identified and studied with the approval by the Ethic Committee of The First Affiliated Hospital of Guangzhou Medical University. The human study was performed in conformity with the declaration of Helsinki. These 29 patients were divided into three groups with normal lung function (n = 9), COPD (n = 10), and COPD plus PH (n = 10). The detailed recruitment criteria were described in Supplementary material online.
2.2. Animal protocols and hypoxic exposure
All animal procedures were based on the National Institutes of Health guidelines for use of live animals and approved by the Institutional Animal Care and Use Committee (IACUC) of Guangzhou Medical University and Johns Hopkins University. All surgery was performed under anaesthesia with sodium pentobarbital (65 mg/kg, i.p.), and all efforts were made to minimize animal suffering. Mice or rats were put under 10% O2 for 21 days to create CHPH models.
2.3. Haemodynamic measurements and assessment of pulmonary vascular remodelling
Right ventricular pressure and right ventricular hypertrophy (RVH) were measured as we described previously.15,24
2.4. Isolation of distal PA and culture of PASMCs
Isolation of distal (fourth generation or more) intrapulmonary artery smooth muscle and also culture of mouse, rat, and human PASMCs were performed as we described previously.15,24
2.5. Overexpression of HIF-1α in rat distal PASMCs
Infection of AdCA5, an adenoviral vector encoding a constitutively active form of HIF-1α, and control vector AdLacZ that encodes β-galactosidase were performed as we described previously.8,25
2.6. Cloning of the 5′-flanking region of rat Bmp4 gene
A 3-kb 5′-UTR sequence from rat Bmp4 gene was amplified and cloned.
2.7. Site-directed deletion
Bmp4 promoter mutants with HRE deletions were created by site-directed deletion.
2.8. Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assays were carried out with ChIP-IT™ Express kit (Active Motif).
The detailed procedures for the above and all other experiments in this study were described in the Supplementary material online.
2.9. Statistical analysis
Data are expressed as mean ± SEM, and n is the number of experiments, which equals the number of animals providing lung, PA, or cells. Fura-2 fluorescence was measured in 20–30 cells per experiment. Statistical analyses were performed using analysis of variance (ANOVA). If significant F ratios were obtained with the ANOVA, pairwise comparison of means was performed using Student's t-test. When necessary, a Bonferroni correction was performed to correct for multiple comparisons. Otherwise, differences were considered significant when P < 0.05.
3. Results
3.1. CH induces BMP4 expression in rat distal PA and PASMCs
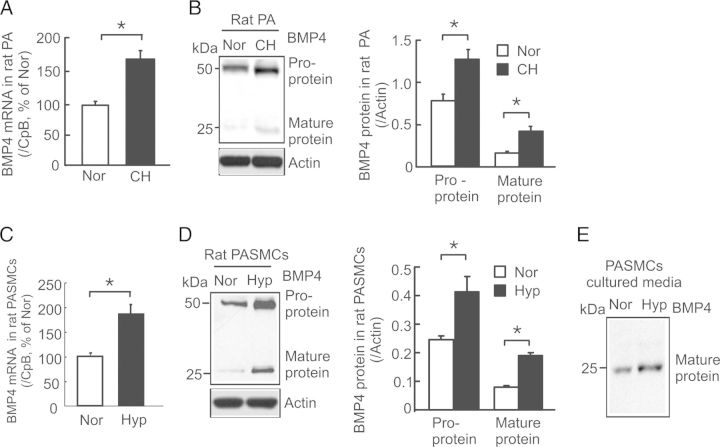
In rats exposed to CH (10% O2 for 21 days), the mean levels of BMP4 mRNA, pro- and mature proteins in distal PA were increased by 72% (Figure 1A), 62% (Figure 1B), and 183% (Figure 1B), respectively. In cultured PASMCs exposed to prolonged hypoxia (4% O2 for 60 h), the mean levels of BMP4 mRNA, pro- and mature proteins were increased by 89% (Figure 1C), 82% (Figure 1D), and 143% (Figure 1D), respectively, and the level of secreted mature BMP4 protein (Figure 1E) was also remarkably increased compared with normoxic control cells. These results indicate hypoxia directly up-regulates BMP4 production in PASMCs in a cell-autonomous manner.
Figure 1.
Effects of hypoxia on BMP4 mRNA and protein expression in rat distal PA and PASMCs. BMP4 mRNA was determined by real-time quantitative PCR and normalized to cyclophilin B (CpB). BMP4 protein was detected by western blotting and normalized to actin. (A and B) Levels of BMP4 mRNA (A; n = 7 per group) and protein (B; n = 6 per group) expression in distal PA from normoxic (Nor) or chronically hypoxic (CH; 10% O2 for 21 days) rats. (C and D) Levels of BMP4 mRNA (C; n = 4 per group) and protein (D; n = 4 per group) expression in rat distal PASMCs exposed to normoxia or prolonged hypoxia (Hyp; 4% O2 for 60). (E) Representative western blots of secreted BMP4 mature protein in cultured media of PASMCs exposed to Nor or Hyp (n = 4 per group). Bar values are mean ± SEM. *P < 0.05.
3.2. HIF-1α up-regulates BMP4 expression in rat distal PASMCs
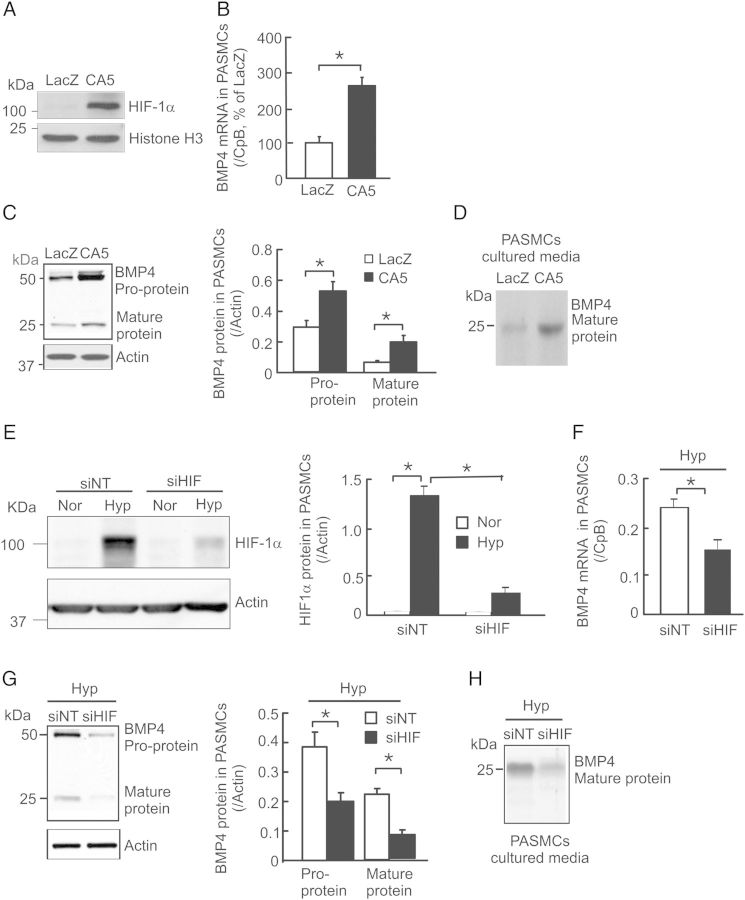
To determine whether the hypoxia-induced BMP4 up-regulation in PASMCs is mediated by HIF-1α, we utilized both gain- and loss-of-function approaches. Initially, we overexpressed HIF-1α in rat PASMCs by infection with AdCA5. As shown, the level of HIF-1α protein in nuclear extracts from AdCA5-infected cells (Figure 2A) was greatly increased when compared with AdLacZ-infected control cells. Consequently, HIF-1α overexpression caused BMP4 mRNA increase by 165% (Figure 2B), pro-protein by 83% and mature protein by 233% (Figure 2C), and resulted in a remarkable increase in BMP4 secretion (Figure 2D) in AdCA5 treated cells comparing with control. Secondly, we determined whether knockdown of endogenous HIF-1α alters hypoxia-induced BMP4 expression in PASMCs. As seen in Figure 2E, HIF-1α protein in siNT-treated normoxic PASMCs was barely detectable; yet, it was markedly up-regulated after exposure of cells to prolonged hypoxia (4% O2 for 60 h). Treatment with HIF-1α siRNA (siHIF) reduced hypoxia-induced HIF-1α protein expression by ∼85% (Figure 2E). Under hypoxic conditions, knockdown of HIF-1α by siHIF treatment led to significantly decreased levels of BMP4 mRNA (Figure 2F), intracellular pro- (Figure 2G) and mature BMP4 proteins (Figure 2G), and secreted mature protein (Figure 2H) when compared with siNT-treated cells. Notably, comparing with non-treatment controls, treatments with siNT did not alter the levels of HIF-1α nuclear accumulation (Supplementary material online, Figure S1A and B), BMP4 mRNA (Supplementary material online, Figure S1C), and pro- and mature protein expression (Supplementary material online, Figure S1D and E), and secreted BMP4 protein (Supplementary material online, Figure S1F) in rat PASMCs under both normoxic and prolonged hypoxic conditions. In conclusion, these results suggest that HIF-1α mediates hypoxia-induced up-regulation of BMP4 expression in rat PASMCs.
Figure 2.
Effects of HIF-1α on BMP4 mRNA and protein expression in rat PASMCs. BMP4 mRNA was determined by real-time quantitative PCR and normalized to cyclophilin B (CpB). HIF-1α and BMP4 proteins were detected by western blotting and normalized to histone H3 or actin. (A) Representative western blots of HIF-1α and histone H3 protein in the nuclear extract from cells transfected with AdCA5 (CA5) or AdLacZ (LacZ). (B–D) BMP4 mRNA (B; n = 3 for each group), pro- and mature proteins (C; n = 3 for each group), as well as secretion of BMP4 protein (D; n = 3 for each group) in LacZ or CA5 transfected cells. (E) Levels of HIF-1α protein (n = 3 for each group) in PASMCs exposed to normoxia (Nor) or prolonged hypoxia (Hyp; 4% O2 for 60) with treatment of non-targeting control siRNA (siNT) or HIF-1α targeting siRNA (siHIF). (F and G) Levels of BMP4 mRNA (F; n = 4 per group), and protein (G; n = 3 for each group) in siNT or siBMP4 treated rat PASMCs under prolonged hypoxic condition (Hyp). (H) Representative western blots of secreted BMP4 mature protein in the cultured media of PASMCs treated with siNT or siHIF under Hyp (n = 3 per group). Bar values are mean ± SEM. *P < 0.05.
3.3. Identification of HREs in rat Bmp4 gene
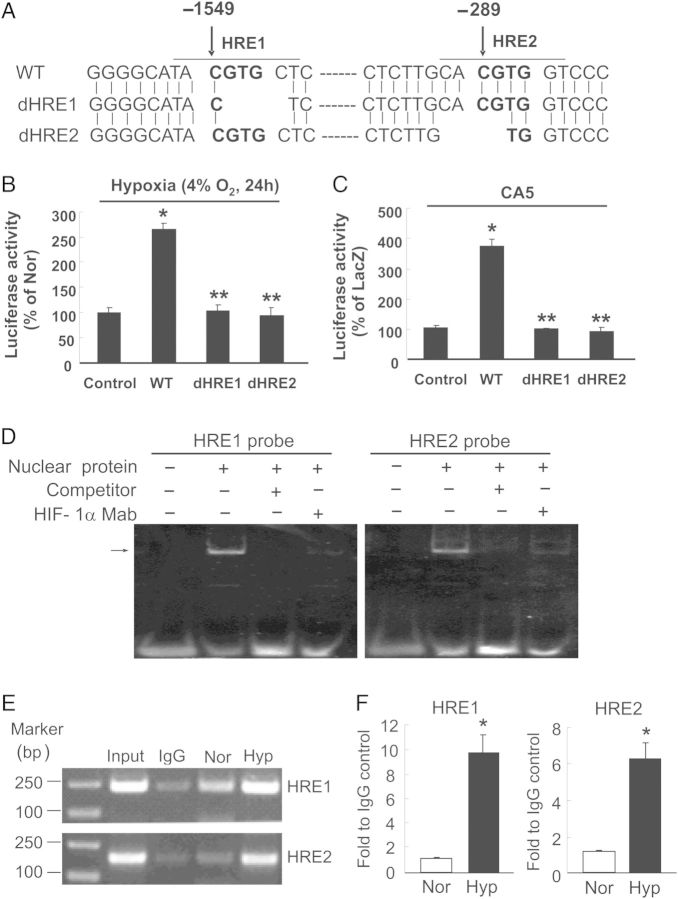
To determine the mechanisms of HIF-1α regulation on BMP4 expression, we examined whether HIF-1α could bind to Bmp4 promoter and regulate the transcription of BMP4 in rat PASMCs. As illustrated in Figure 3A, two putative HIF-1-binding sites were identified in the 5′-flanking region of Bmp4 gene between −1551 to −1544 (HRE1) and −291 to −284 (HRE2) relative to the transcription start site. We next constructed the promoter–reporter plasmids by inserting in upstream of the firefly luciferase reporter a 3-kb fragment of the 5′-flanking region of Bmp4 containing either the wild-type (WT) or a 4-bp deletion of HRE1 or HRE2. We found that augmentation of HIF-1α protein levels, either by exposure of rat PASMCs to 4% O2 for 24 h (Figure 3B) or by infection with AdCA5 (Figure 3C), led to greater activation of the WT Bmp4 promoter as indicated by significant increases of luciferase activity compared with a control vector lacking the Bmp4 promoter. Deletion of either HRE1 or HRE2 blunted these responses (Figure 3B and C). Moreover, EMSA demonstrated binding of HIF-1α to HRE1 and HRE2 probes, which was absent when excess unlabelled oligonucleotide with the same sequence as the HRE1 or HRE2 probe was included as competitor (Figure 3D). Complex formation was also blocked by prior incubation of PASMC nuclear proteins with mAb against HIF-1α (Figure 3D). In addition, ChIP assays showed that rat PASMCs exposed to prolonged hypoxia exhibited approximately seven- and four-fold more binding of HIF-1α to HRE1 and HRE2, respectively, when compared with normoxic cells (Figure 3E and F). These results indicated that both HRE1 and HRE2 in the rat Bmp4 promoter mediate HIF-1 binding and transcriptional activation of BMP4 expression in hypoxic PASMCs.
Figure 3.
Promoter-luciferase reporter, EMSA, and ChIP assays reveal the presence of functional hypoxia responsive elements (HREs) in rat Bmp4 gene promoter. (A) Diagram illustrating the sequence of wild-type rat Bmp4 promoter (WT) and Bmp4 promoter with a 4-bp deletion in HRE1 (dHRE1) or HRE2 (dHRE2). (B and C) Rat PASMCs were transfected with renilla luciferase reporter plasmids and firefly luciferease vectors pGL3-Enhancer (control), pGL3-Enhancer with wild-type Bmp4 promoter (WT), or pGL3-Enhancer with Bmp4 promoter containing a deletion in HRE1 (dHRE1) or HRE2 (dHRE2). The transfected cells were exposed to normoxia (Nor) or hypoxia (Hyp, 4% O2) for 24 h (B), or infected with AdCA5 or AdLacZ for 24 h (C), firefly luciferase activity was determined, normalized to renilla luciferase activity, and presented as percentage of that in Nor or AdLacZ groups. Bar values are mean ± SEM, n = 3 for each group; *P < 0.05 vs. respective control, and **P < 0.001 vs. WT. (D) Representative EMSA image from three independent experiments demonstrating the binding of nuclear proteins from hypoxic rat PASMCs (4% O2 for 60 h) to the HRE1 and HRE2 probes; the arrow indicates specific DNA- HIF-1α antibody complexes. (E and F) ChIP assay of specific binding of HIF-1α to HRE1 and HRE2 in rat PASMCs exposed to Nor or Hyp (4% O2) for 60 h. (E) Representative images of the PCR products of ChIP-enriched DNA fragments containing HRE1 (230 bp) and HRE2 (182 bp). PCR products amplified with total DNA from hypoxic PASMCs (Input) were utilized as positive control. (F) Sheared chromatin from Nor or Hyp exposed rat PASMCs was immunoprecipitated with HIF-1α mAb, and HRE1 and HRE2 sequences were quantified by real-time PCR and presented as fold to IgG control. Values are mean ± SEM, n = 3 for each group; *P < 0.05 vs. respective Nor.
3.4. BMP4 antagonism or knockdown prevents the hypoxic increases of TRPC expression and basal [Ca2+]i in rat distal PASMCs
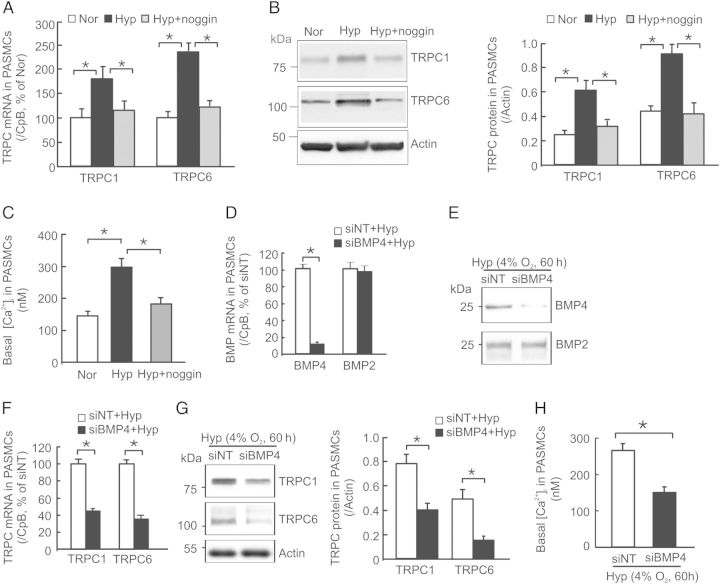
Previously we demonstrated that exposure to BMP4 treatment up-regulated TRPC1 and TRPC6 expression and thus led to increases of basal [Ca2+]i via enhanced SOCE in rat distal PASMCs.23 To determine the functional influence of hypoxia-induced BMP4 expression on TRPC expression and basal [Ca2+]i, we employed BMP4 antagonist noggin to block BMP4 signalling and BMP4-specific siRNA (siBMP4) to eliminate endogenous BMP4 expression. Similar to the results we reported before,8 we observed significant increases of TRPC1 and TRPC6 mRNA (Figure 4A) and protein (Figure 4B) expression and basal [Ca2+]i (Figure 4C) in rat PASMCs exposed to prolonged hypoxia. These hypoxic responses were completely blocked by pre-treatment with BMPs antagonist noggin (200 ng/mL) (Figure 4A–C). In comparison with siNT, treatment of rat PASMCs with siBMP4 caused ∼89% reduction in BMP4 mRNA levels (Figure 4D), and marked reduction in the level of secreted BMP4 protein (Figure 4E), but did not alter BMP2 mRNA expression and protein secretion (Figure 4D and E). Consequently, specific knockdown of BMP4 by siBMP4 treatment led to a significant reduction in the levels of TRPC1 and TRPC6 mRNA (Figure 4F) and protein (Figure 4G), and basal [Ca2+]i (Figure 4H) in rat PASMCs under hypoxic conditions comparing with siNT-treated cells. These results indicate that the up-regulated BMP4 expression is required for the increases of TRPC expression and basal [Ca2+]i in PASMCs under hypoxic condition.
Figure 4.
Effects of noggin and BMP4 knockdown on TRPC1 and TRPC6 expression, and basal [Ca2+]i in hypoxic rat PASMCs. (A–C) cells were exposed to Nor, prolonged hypoxia (Hyp; 4% O2 for 60 h) or Hyp plus 200 ng/mL noggin (Hyp+noggin). (D–H) PASMCs were treated with non-targeting control siRNA (siNT), or siRNA targeted to BMP4 (siBMP4) for 24 h, then exposed to Hyp for 60 h. (A, D and F) Levels of TRPC1, TRPC6, BMP2 or BMP4 mRNA determined by real-time quantitative RNA and normalized to CpB; n = 4 for each group. (B and G) TRPC1 and TRPC6 western blots were quantified and normalized to actin; n = 4 for each group. (E) Representative image of four independent experiments of BMP2 and BMP4 western blots for cultured media of siNT- and siBMP4-treated PASMCs under prolonged hypoxia exposure. (C and H) Basal [Ca2+]i in PASMCs treated with Nor (n = 4 for 109 cells), Hyp (n = 4 for 117 cells), Hyp + noggin (n = 4 in 115 cells), siNT + Hyp (n = 4 for 108 cells), or siBMP4 + Hyp (n = 4 for 110 cells). Values in each bar are mean ± SEM. *P < 0.05.
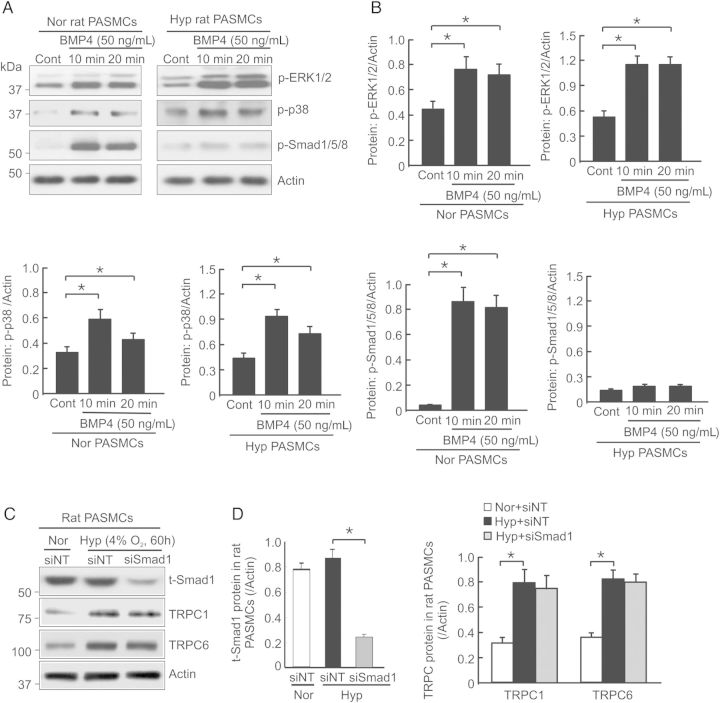
3.5. Hypoxia induces TRPC1 and TRPC6 up-regulation independent of Smad1 in rat distal PASMCs
In normoxic PASMCs, BMP4 can activate both Smad and non-Smad signalling molecules, such as Smad1/5/8, ERK1/2, and p38 MAPK.26 We previously demonstrated that BMP4 up-regulated TRPC1 and TRPC6 expression dependent on ERK1/2 and p38 MAPK activation in rat PASMCs.27 Here we determined the relationship between Smad1 and the increased expression of TRPC1 and TRPC6 in hypoxic rat PASMCs. As seen in Figure 5A and B, although BMP4 treatment (50 ng/mL) for 10 min and 20 min caused significant phosphorylation of ERK1/2, p38 MAPK, and Smad1/5/8 in normoxic rat PASMCs, it only led to activation of ERK1/2 and p38 MAPK, but not Smad1/5/8, in cells exposed to prolonged hypoxia (4% O2 for 60 h; Figure 5A and B). Treatment of hypoxic PASMCs with Smad1 siRNA caused ∼80% reduction in total Smad1 protein (Figure 5C and D), compared with siNT-treated cells. However, the knockdown of Smad1 did not influence the induction of TRPC1 and TRPC6 expression in hypoxic PASMCs (Figure 5C and D), indicating that Smad1 activation is not required for up-regulation of TRPC1 and TRPC6 under hypoxic condition.
Figure 5.
Effect of Smad1 knockdown on TRPC1 and TRPC6 expression in rat PASMCs exposed to prolonged hypoxia. (A) Representative images of western blots for phosphorylated ERK1/2 (p-ERK1/2), p38 MAPK (p-p38), Smad1/5/8 (p-Smad1/5/8), and actin in rat PASMCs exposed to normoxia (Nor) or hypoxia (Hyp; 4% O2) for 60 h, and then stimulated with or without BMP4 (50 ng/mL) for 10 or 20 min (n = 4 for each group of cells). (B) Bar graphs show the intensity changes of p-ERK1/2, p-p38 and p-Smad1/5/8 normalized to actin in rat PASMCs exposed to Nor or Hyp with or without BMP4 stimulation for 10 or 20 min; values are mean ± SEM, n = 4 for each group of cells, *P < 0.05. (C) Representative western blot images of total smad1 (t-Smad1), TRPC1, TRPC6, and actin in PASMCs treated with non-targeting siRNA (siNT) + Nor, siNT + Hyp or Smad 1 targeting siRNA (siSmad1) + Hyp. (D) Bar graphs show the intensity changes in t-smad1, TRPC1 and TRPC6 normalized to actin; values are mean ± SEM, n = 4 for each group of cells, *P < 0.05.
3.6. CH induces BMP4 expression dependent of HIF-1α in mouse distal PA
In HIF-1α+/+ mice that were exposed to CH (10% O2 for 21 days), the levels of BMP4 mRNA (Figure 6A), pro-, and mature protein (Figure 6B and C) were, respectively, increased by 88, 97, and 226% in distal PA when compared with control mice under normoxia. However, these responses were significantly impaired in their HIF-1α+/− littermates (Figure 6). These results confirm that hypoxia up-regulates BMP4 expression in mouse distal PA in an HIF-1α-dependent manner.
Figure 6.
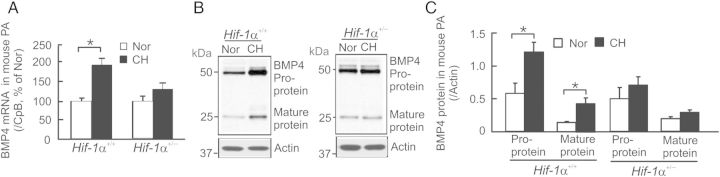
Effects of chronic hypoxia (10% O2 for 21 days) on BMP4 mRNA and protein expression in distal PA from HIF-1α+/+ and HIF-1α+/− mice. (A) Levels of BMP4 mRNA determined by real-time PCR in distal PA from CH-exposed HIF-1α+/+ and HIF-1α+/− mice were normalized to CpB and presented as percentage of respective Nor control. Values are mean ± SEM, n = 5 for each group of animals, *P < 0.05. (B) Representative western blots of BMP4 proteins and actin in distal PA from Nor or CH-exposed HIF-1α+/+ and HIF-1α+/− mice. (C) Intensity changes of BMP4 pro- and mature protein expression normalized to actin. Values are mean ± SEM, n = 5 for each group of animals, *P < 0.05.
3.7. Partial deficiency of BMP4 attenuates TRPC1 and TRPC6 expression leading to normalized SOCE and basal [Ca2+]i in distal PASMCs from CHPH mice
As seen in Figure 7, BMP4+/+ mice exposed to CH developed CHPH (Figure 7A–C), reflected by increases of RVSP for 99.3% (Figure 7A), Fulton index (RV/LV+S) for 49.2% (Figure 7B), and small pulmonary vessel wall thickness for 125.5% (Figure 7C). In association with these haemodynamic and histopathological changes, CH-exposed BMP4+/+ mice exhibited increased expression of BMP4 pro- and mature, TRPC1 and TRPC6 proteins (Figure 7D) in the distal PA. However, in BMP4+/− mice, the induction of BMP4 by CH were significantly reduced, and the hypoxic increases of TRPC1 and TRPC6 protein were essentially abolished (Figure 7D); these responses were accompanied with attenuated changes in RVSP, RVH, and small pulmonary vessel wall thickening comparing with that in BMP4+/+ mice (Figure 7A–C). Moreover, in PASMCs isolated from CH BMP4+/+ mice, SOCE (Figure 7E) and basal [Ca2+]i (Figure 7F) were significantly increased comparing with normoxic BMP4+/+ mice; these hypoxic increases were both attenuated in cells from BMP4+/− mice (58.2 ± 15.3 vs. 92.6 ± 18.9% for basal [Ca2+]i, n = 6 each, P < 0.05; 37.5 ± 18.8 vs. 115.3 ± 17.4% for SOCE in BMP4+/+ mice, n = 5 each, P < 0.01). These data suggest that BMP4 plays an important role in the pathogenesis of mouse CHPH likely by up-regulating TRPC1 and TRPC6 expression, which lead to elevated SOCE and basal [Ca2+]i in PASMCs.
Figure 7.
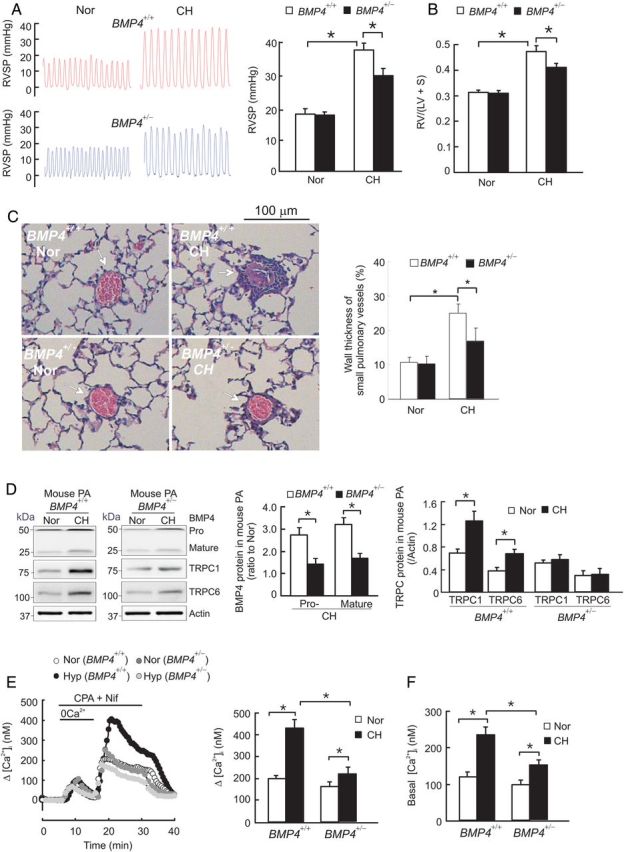
Effects of partial deficiency of BMP4 on TRPC1 and TRPC6 protein expression in distal PA, and on SOCE and basal [Ca2+]i in PASMCs from CHPH mice. (A) Representative traces of RVSP in BMP4+/+ and BMP4+/− mice exposed to normoxia (Nor) or chronic hypoxia (CH; 10% O2 for 21 days); bar graph shows the average RVSP in each group of animals (n = 6). (B) the mass ratio of right ventricle (RV) to left ventricle plus septum (LV+S), presented as RV/(LV + S) in each group of animals (n = 6). (C) Representative images of H&E staining of lung tissue showing small pulmonary vessels in BMP4+/+ and BMP4+/− mice exposed to Nor or CH; bar graph shows the wall thickness of small pulmonary vessels in each group of animals (n = 6). (D) Levels of BMP4 pro- and mature protein, TRPC1 and TRPC6 proteins in distal PA from Nor or CH exposed BMP4+/+ and BMP4+/− mice, determined by western blotting and normalized to actin (n = 5 for each group). (E) Left, traces of SOCE in PASMCs from Nor or CH exposed BMP4+/+ and BMP4+/− mice; right, bar graph shows maximum Δ[Ca2+]i in response to Ca2+ restoration; n = 5 for each group. (F) Basal [Ca2+]i in PASMCs from Nor or CH exposed BMP4+/+ and BMP4+/− mice; n = 6 for each group. Bar values are mean ± SEM, *P < 0.05.
3.8. Effects of HIF-1α-mediated up-regulation of BMP4 on TRPC1 and TRPC6 expression, and on increases in SOCE and basal [Ca2+]i in human PASMCs under hypoxic condition
To determine the clinical relevance of the above signalling events discovered in animals, we analysed distal PA from human COPD subjects with or without PH, and in human PASMCs exposed to prolonged hypoxia (4% O2 for 60 h). The clinical characteristics of the control, COPD, and COPD–PH patients were described in Supplementary material online, Table S1. As shown in Supplementary material online, Figure S2, compared with healthy control subjects, the levels of HIF-1α (Supplementary material online, Figure S2A and B), BMP4, TRPC1, and TRPC6 (Supplementary material online, Figure S2C and D) were all greater in distal PA from COPD patients without PH, but even greater in that from COPD patients with PH (Supplementary material online, Figure S2A and B). In human PASMCs exposed to prolonged hypoxia, the level of nuclear HIF-1α protein was increased for ∼4.5-fold compared with nomoxic cells; treatment with HIF-1α siRNA (siHIF-1) but not BMP4 siRNA (siBMP4) blunted the hypoxic induction of HIF-1α nucleus accumulation (Supplementary material online, Figure S3A and B). Hypoxia exposure increased the levels of BMP4 mature protein, TRPC1 and TRPC6 proteins, SOCE, and basal [Ca2+]i for about 103, 52, 81, 55, and 191%, respectively, in human PASMCs treated with siNT; treatment with either HIF-1α (siHIF-1) or BMP4 siRNA (siBMP4) abolished these hypoxic-inductions (Supplementary material online, Figure S3A–E). These results confirmed that signalling of HIF-1 → BMP4 → TRPC1 and TRPC6 → SOCE → basal [Ca2+]i occurs in hypoxic human PASMCs, thus likely contributing to the pathogenesis of PH in COPD patients.
4. Discussion
Elucidation of the molecular mechanisms by which hypoxia elevates basal [Ca2+]i in PASMCs is critical for understanding the pathogenesis of CHPH. The present study provides evidence, demonstrating that HIF-1 mediates the hypoxic induction of BMP4 expression, which is required for increased TRPC expression and basal [Ca2+]i in PASMCs.
BMP4 is widely expressed in endothelium, vascular smooth muscle, and epithelium of adult mouse lung.22,28 Here, in mouse and rat lungs exposed to CH, and in lung tissue from COPD patients, we localized BMP4 up-regulation to the distal PA, which is the site of hypoxic pulmonary vasoconstriction and PH-associated vascular remodelling. Moreover, we found that cultured PASMCs exposed to prolonged hypoxia exhibited higher levels of BMP4 expression and secretion. In rat PASMCs under hypoxia, HIF-1α was necessary to induce BMP4 expression through the binding of HIF-1 to two sites in the 5′-flanking region of rat Bmp4 gene, leading to transcriptional activation. Furthermore, we demonstrated that BMP4 played a similar role to HIF-1 in up-regulating TRPC1 and TRPC6 expression as well as elevating basal [Ca2+]i in hypoxic PASMCs.8 BMP4 antagonism or knockdown blocked the hypoxia-induced TRPC1, TRPC6 expression, and increases of basal [Ca2+]i in rat PASMCs. Moreover, BMP4 heterozygous mutant mice exhibited attenuated CHPH, lowered TRPC levels, decreased SOCE, and basal [Ca2+]i.
According to a previous report,29 the expression of TRPC exhibits plasticity between cultured and native tissues: while the levels of TRPC1 and TRPC6 mRNA in cultured rat cerebral arteries increased more than five-fold greater than that in the native tissue, the expression of TRPC3 was decreased. Thus, it is likely that the up-regulatory effects of BMP4 on TRPC expression in cultured PASMCs were different from that in native cells. To clarify this presumption, we further utilized BMP4 heterozygous mutant mice to investigate the influence of BMP4 on hypoxic induction of TRPC1 and TRPC6 in vivo. As a result, we demonstrated that the BMP4 mutant mice exhibited attenuated CHPH, lowered TRPC levels, as well as decreased SOCE and basal [Ca2+]i comparing with their wild-type littermates. In this study, we focused on TRPC1 and TRPC6, as they are the most abundantly expressed TRPC members and selectively up-regulated by hypoxia in both cultured rat PASMCs and native rat and mouse PA isolates.8,14,16
Like other TGF-β family members, BMP4 transduces signals by binding to type II serine–threonine kinase receptors (i.e. BMPRII, ActRIIa, and/or ActRIIb), which subsequently causes recruitment and phosphorylation of type I receptors (i.e. ALK2, BMPRIa, and/or BMPRIb), leading to activation of Smad and non-Smad signalling molecules (i.e. ERK1/2 and p38 MAPK).26,27,30,31 We previously demonstrated the dependence of ERK1/2 and p38 MAPK activation on BMP4-induced up-regulation of TRPC1 and TRPC6 expression, elevation of basal [Ca2+]i, and increases of PASMC proliferation and migration.27 In this study, we found that the BMP4-stimulated Smad1/5/8 activation in rat PASMCs was impaired, whereas the activations of ERK1/2 and p38 MAPK were enhanced under hypoxic condition. Moreover, we found Smad1 was not required for hypoxia-induced TRPC expression in PASMCs. Notably, previous reports demonstrated that BMP4-induced Smad1/5 phosphorylation was primarily dependent on BMPRII.32,33 In addition to the increased expression of BMP4, CHPH animals display reduced expression of BMPRII protein in PA.34–36 In PASMCs from idiopathic pulmonary arterial hypertension patients or PH animals, decreased BMPRII correlates with reduced Smad1/5 phosphorylation and suppression of a number of target genes involved in the development of PH.33 Therefore, in the context of BMPRII deficiency and Smad1/5/8 dysfunction under hypoxia, it is reasonable to propose that the pro-proliferative responses of PASMCs to BMP4 are likely stimulated by MAPK-mediated up-regulation of TRPC1 and TRPC6 expression and subsequent elevation of basal [Ca2+]i.37 Consistent with this presumption, we recently identified that BMPRII was not responsible for BMP4-induced activation of p38 and ERK1/2, and subsequent increases of TRPC1 and TRPC6 expression and elevation of basal [Ca2+]i in rat PASMCs.38 To date, a number of studies indicated that TAK1 and MKK3/6 might be involved in bridging BMP receptors and BMP ligands-induced p38 MAPK and ERK1/2 activation.27 Nevertheless, the role of the other two type II receptors ActRIIa and ActRIIb in BMP4-induced MAPK activation, and the downstream mechanisms linking BMPs receptors and MAPK activation are largely unknown, which warrant future investigation.
Changes in pulmonary vessels are common in patients with COPD.2,39 COPD has two distinct phenotypes: emphysema and chronic bronchitis. In the case of emphysema, the associated PH mostly results from vascular bed destruction; whereas, in chronic bronchitis, the increased PA pressure is likely caused by vascular remodelling, which is characterized of intimal fibrosis and smooth muscle hypertrophy in the muscularized pulmonary vessels.2 Interestingly, while one study reported an increased level of HIF-1α in PA from chronic bronchitis patients,40 another one demonstrated decreased HIF-1α in lung tissue from emphysema patients.41 In former case, the increase of HIF-1α was presumed to be the result of chronic hypoxemia, and/or inflammation, as both conditions could stabilize HIF-1α and contribute to the aetiology of pulmonary vascular abnormalities associated with COPD.24,42,43 To determine the clinical significance of our results obtained from animals, we selected chronic bronchitis-type COPD subjects, in which the associated pulmonary vascular abnormalities are similar to those seen in CHPH animal models. Consistent with the report described above with chronic bronchitis patients,40 we found the level of HIF-1α in nuclear extract of distal PA from COPD patients without PH was increased compared with healthy control subjects. In conjunction with this increase, the levels of BMP4, TRPC1, and TRPC6 proteins were also greater in these COPD patients. In PA from COPD patients with PH, the protein levels of HIF-1α, BMP4, TRPC1, and TRPC6 protein were even greater comparing with COPH subjects without PH.
To elucidate the cause–effect relationships between HIF-1α and BMP4, and between BMP4 and TRPC up-regulations, we next utilized human PASMCs cultured under hypoxic condition. As a result, we found knockdown of either HIF-1α or BMP4 abolished hypoxia-induced TRPC expression; knockdown of HIF-1α attenuated hypoxia-induced BMP4 expression, indicating that the induction of TRPC by hypoxia requires HIF-1α-mediated up-regulation of BMP4. These results suggest that HIF-1α, BMP4, and TRPC signalling potentially contribute to the development of PH associated with chronic bronchitis-type COPD.
In summary, we found that CH up-regulated BMP4 expression in PASMCs in an HIF-1α-dependent manner, leading to increased TRPC1, TRPC6 expression, SOCE and basal [Ca2+]i (Hypoxia → HIF-1α → BMP4 → ERK1/2 and p38 MAPK → TRPC1/TRPC6 → SOCE → basal [Ca2+]i; Supplementary material online, Figure S4). These findings provide further mechanistic evidence that both HIF-1α and BMP4 play indispensable roles in the pathogenesis of CHPH. Our results also provide additional information on how abnormal BMP signalling influences the development of CHPH.
Supplementary material
Supplementary Material is available at Cardiovascular Research online.
Funding
This work was supported by NIH (R01-HL093020), National Natural Science Foundation of China (81173112, 81470246, 81170052, 81220108001), Guangdong Natural Science Foundation Team Grant (1035101200300000), Guangdong Province Universities and Colleges Science and Technology Innovation Key Project (B01), the Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2014), Guangzhou Department of Education Yangcheng Scholarships (12A001S), Guangzhou Department of Natural Science (2014Y2-00167) and Guangzhou Department of Education Team Grant for Innovation (13C08). G.L.S. is the C. Michael Armstrong Professor at the Johns Hopkins University School of Medicine.
Acknowledgments
Conflict of interest: none declared.
References
- 1.Sylvester JT, Shimoda LA, Aaronson PI, Ward JP. Hypoxic pulmonary vasoconstriction. Physiol Rev 2012;92:367–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shujaat A, Bajwa AA, Cury JD. Pulmonary hypertension secondary to COPD. Pulm Med 2012;2012:203952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 2006;99:675–691. [DOI] [PubMed] [Google Scholar]
- 4.Meyrick BO, Perkett EA. The sequence of cellular and hemodynamic changes of chronic pulmonary hypertension induced by hypoxia and other stimuli. Am Rev Respir Dis 1989;140:1486–1489. [DOI] [PubMed] [Google Scholar]
- 5.Rabinovitch M, Gamble W, Nadas AS, Miettinen OS, Reid L. Rat pulmonary circulation after chronic hypoxia: hemodynamic and structural features. Am J Physiol 1979;236:H818–203827. [DOI] [PubMed] [Google Scholar]
- 6.Oka M, Morris KG, McMurtry IF. NIP-121 is more effective than nifedipine in acutely reversing chronic pulmonary hypertension. J Appl Physiol 1993;75:1075–1080. [DOI] [PubMed] [Google Scholar]
- 7.Shimoda LA, Wang J, Sylvester JT. Ca2+ channels and chronic hypoxia. Microcirculation 2006;13:657–670. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res 2006;98:1528–1537. [DOI] [PubMed] [Google Scholar]
- 9.Karaki H, Ozaki H, Hori M, Mitsui-Saito M, Amano K, Harada K, Miyamoto S, Nakazawa H, Won KJ, Sato K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev 1997;49:157–230. [PubMed] [Google Scholar]
- 10.Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res 2004;95:496–505. [DOI] [PubMed] [Google Scholar]
- 11.Trebak M. STIM/Orai signalling complexes in vascular smooth muscle. J Physiol 2012;590:4201–4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parekh AB, Putney JW., Jr. Store-operated calcium channels. Physiol Rev 2005;85:757–810. [DOI] [PubMed] [Google Scholar]
- 13.Remillard CV, Yuan JX. TRP channels, CCE, and the pulmonary vascular smooth muscle. Microcirculation 2006;13:671–692. [DOI] [PubMed] [Google Scholar]
- 14.Lu W, Wang J, Shimoda LA, Sylvester JT. Differences in STIM1 and TRPC expression in proximal and distal pulmonary arterial smooth muscle are associated with differences in Ca2+ responses to hypoxia. Am J Physiol Lung Cell Mol Physiol 2008;295:L104–L113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu W, Ran P, Zhang D, Peng G, Li B, Zhong N, Wang J. Sildenafil inhibits chronically hypoxic upregulation of canonical transient receptor potential expression in rat pulmonary arterial smooth muscle. Am J Physiol Cell Physiol 2010;298:C114–C123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia Y, Yang XR, Fu Z, Paudel O, Abramowitz J, Birnbaumer L, Sham JS. Classical transient receptor potential 1 and 6 contribute to hypoxic pulmonary hypertension through differential regulation of pulmonary vascular functions. Hypertension 2014;63:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev 2000;14:1983–1991. [PubMed] [Google Scholar]
- 18.Prabhakar NR, Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev 2012;92:967–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, Semenza GL. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest 1999;103:691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eickelberg O, Morty RE. Transforming growth factor beta/bone morphogenic protein signaling in pulmonary arterial hypertension: remodeling revisited. Trends Cardiovasc Med 2007;17:263–269. [DOI] [PubMed] [Google Scholar]
- 21.Sopory S, Nelsen SM, Degnin C, Wong C, Christian JL. Regulation of bone morphogenetic protein-4 activity by sequence elements within the prodomain. J Biol Chem 2006;281:34021–34031. [DOI] [PubMed] [Google Scholar]
- 22.Frank DB, Abtahi A, Yamaguchi DJ, Manning S, Shyr Y, Pozzi A, Baldwin HS, Johnson JE, de Caestecker MP. Bone morphogenetic protein 4 promotes pulmonary vascular remodeling in hypoxic pulmonary hypertension. Circ Res 2005;97:496–504. [DOI] [PubMed] [Google Scholar]
- 23.Lu W, Ran P, Zhang D, Lai N, Zhong N, Wang J. Bone morphogenetic protein 4 enhances canonical transient receptor potential expression, store-operated Ca2+ entry, and basal [Ca2+]i in rat distal pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol 2010;299:C1370–C1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Chen Y, Lin C, Jia J, Tian L, Yang K, Zhao L, Lai N, Jiang Q, Sun Y, Zhong NS, Ran P, Lu W. Effect of chronic exposure to cigarette smoke on canonical transient receptor potential expression in rat pulmonary arterial smooth muscle. Am J Physiol Cell Physiol 2014;306:C364–C373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res 2003;93:1074–1081. [DOI] [PubMed] [Google Scholar]
- 26.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003;425:577–584. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Lu W, Fu X, Zhang Y, Yang K, Zhong N, Ran P, Wang J. BMP4 increases canonical transient receptor potential protein expression by activating p38 MAPK and ERK1/2 signaling pathways in pulmonary arterial smooth muscle cells. Am J Respir Cell Mol Biol 2013;49:212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellusci S, Henderson R, Winnier G, Oikawa T, Hogan BL. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development 1996;122:1693–1702. [DOI] [PubMed] [Google Scholar]
- 29.Bergdahl A, Gomez MF, Wihlborg AK, Erlinge D, Eyjolfson A, Xu SZ, Beech DJ, Dreja K, Hellstrand P. Plasticity of TRPC expression in arterial smooth muscle: correlation with store-operated Ca2+ entry. Am J Physiol Cell Physiol 2005;288:C872–C880. [DOI] [PubMed] [Google Scholar]
- 30.Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem 2010;147:35–51. [DOI] [PubMed] [Google Scholar]
- 31.Yang X, Long L, Southwood M, Rudarakanchana N, Upton PD, Jeffery TK, Atkinson C, Chen H, Trembath RC, Morrell NW. Dysfunctional Smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circ Res 2005;96:1053–1063. [DOI] [PubMed] [Google Scholar]
- 32.Upton PD, Long L, Trembath RC, Morrell NW. Functional characterization of bone morphogenetic protein binding sites and Smad1/5 activation in human vascular cells. Mol Pharmacol 2008;73:539–552. [DOI] [PubMed] [Google Scholar]
- 33.Yang J, Davies RJ, Southwood M, Long L, Yang X, Sobolewski A, Upton PD, Trembath RC, Morrell NW. Mutations in bone morphogenetic protein type II receptor cause dysregulation of Id gene expression in pulmonary artery smooth muscle cells: implications for familial pulmonary arterial hypertension. Circ Res 2008;102:1212–1221. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi H, Goto N, Kojima Y, Tsuda Y, Morio Y, Muramatsu M, Fukuchi Y. Downregulation of type II bone morphogenetic protein receptor in hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2006;290:L450–L458. [DOI] [PubMed] [Google Scholar]
- 35.Murakami K, Mathew R, Huang J, Farahani R, Peng H, Olson SC, Etlinger JD. Smurf1 ubiquitin ligase causes downregulation of BMP receptors and is induced in monocrotaline and hypoxia models of pulmonary arterial hypertension. Exp Biol Med (Maywood) 2010;235:805–813. [DOI] [PubMed] [Google Scholar]
- 36.Long L, Crosby A, Yang X, Southwood M, Upton PD, Kim DK, Morrell NW. Altered bone morphogenetic protein and transforming growth factor-beta signaling in rat models of pulmonary hypertension: potential for activin receptor-like kinase-5 inhibition in prevention and progression of disease. Circulation 2009;119:566–576. [DOI] [PubMed] [Google Scholar]
- 37.Nasim MT, Ogo T, Chowdhury HM, Zhao L, Chen CN, Rhodes C, Trembath RC. BMPR-II deficiency elicits pro-proliferative and anti-apoptotic responses through the activation of TGFbeta-TAK1-MAPK pathways in PAH. Hum Mol Genet 2012;21:2548–2558. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Wang Y, Yang K, Tian L, Fu X, Sun Y, Jiang Q, Lu W, Wang J. BMP4 increases the expression of TRPC and basal [Ca2+]i via the p38MAPK and ERK1/2 pathways independent of BMPRII in PASMCs. PLoS One 2014;9:e112695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peinado VI, Pizarro S, Barbera JA. Pulmonary vascular involvement in COPD. Chest 2008;134:808–814. [DOI] [PubMed] [Google Scholar]
- 40.Lee SH, Kim CH, Yang KS, Lee EJ, Min KH, Hur GY, Lee SY, Kim JH, Shin C, Shim JJ, In KH, Kang KH. Increased expression of vascular endothelial growth factor and hypoxia inducible factor-1alpha in lung tissue of patients with chronic bronchitis. Clin Biochem 2014;47:552–559. [DOI] [PubMed] [Google Scholar]
- 41.Yasuo M, Mizuno S, Kraskauskas D, Bogaard HJ, Natarajan R, Cool CD, Zamora M, Voelkel NF. Hypoxia inducible factor-1alpha in human emphysema lung tissue. Eur Respir J 2011;37:775–783. [DOI] [PubMed] [Google Scholar]
- 42.Barbera JA, Riverola A, Roca J, Ramirez J, Wagner PD, Ros D, Wiggs BR, Rodriguez-Roisin R. Pulmonary vascular abnormalities and ventilation–perfusion relationships in mild chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1994;149:423–429. [DOI] [PubMed] [Google Scholar]
- 43.Wright JL, Churg A. Effect of long-term cigarette smoke exposure on pulmonary vascular structure and function in the guinea pig. Exp Lung Res 1991;17:997–1009. [DOI] [PubMed] [Google Scholar]