Abstract
Objective:
To characterize pathogenic effects of antibodies to dipeptidyl-peptidase-like protein 6 (DPPX), a subunit of Kv4.2 potassium channels, on gut and brain neurons.
Methods:
We identified a new patient with anti-DPPX encephalitis and analyzed the effects of the patient's serum and purified immunoglobulin G (IgG), and of serum of a previous patient with anti-DPPX encephalitis, on the activity of enteric neurons by voltage-sensitive dye imaging in guinea pig myenteric and human submucous plexus preparations. We studied the subcellular localization of DPPX by immunocytochemistry in cultured murine hippocampal neurons using sera of 4 patients with anti-DPPX encephalitis. We investigated the influence of anti-DPPX-containing serum and purified IgG on neuronal surface expression of DPPX and Kv4.2 by immunoblots of purified murine hippocampal neuron membranes.
Results:
The new patient with anti-DPPX encephalitis presented with a 2-month episode of diarrhea, which was followed by tremor, disorientation, and mild memory impairment. Anti-DPPX-IgG-containing sera and purified IgG increased the excitability and action potential frequency of guinea pig and human enteric nervous system neurons. Patient sera revealed a somatodendritic and perisynaptic neuronal surface staining that colocalized with the signal of commercial anti-DPPX and Kv4.2 antibodies. Incubation of hippocampal neurons with patient serum and purified IgG resulted in a decreased expression of DPPX and Kv4.2 in neuronal membranes.
Conclusions:
Hyperexcitability of enteric nervous system neurons and downregulation of DPPX and Kv4.2 from hippocampal neuron membranes mirror the clinical phenotype of patients with anti-DPPX encephalitis and support a pathogenic role of anti-DPPX antibodies in anti-DPPX encephalitis.
In 2013, a novel autoimmune encephalitis associated with antibodies to dipeptidyl-peptidase-like protein 6 (DPPX), an auxiliary subunit of Kv4.2 potassium channels, was identified in 4 patients whose clinical presentation included agitation, hallucinations, confusion, myoclonus, tremor, and seizures.1 An additional 3 patients with anti-DPPX antibodies and a distinct syndrome resembling progressive encephalomyelitis with rigidity and myoclonus (PERM) were subsequently described.2 Recently, clinical features and outcomes were characterized in 20 patients with anti-DPPX encephalitis.3 Remarkably, 14 of the 27 patients with anti-DPPX encephalitis reported so far had pronounced gastrointestinal symptoms, including severe diarrhea in 10 and constipation in 4 patients.1–3
DPPX is a membrane glycoprotein involved in increasing the surface expression and channel conductance of Kv4.2 channels.4–6 Although its function and the expression of DPPX in hippocampus, cerebellum, striatum, and myenteric plexus1,7 are compatible with the clinical symptoms of anti-DPPX encephalitis, the pathogenic mechanisms of anti-DPPX antibodies have not been characterized. We report on a new patient with anti-DPPX encephalitis and analyze potential pathogenic effects of anti-DPPX-antibody-containing sera on gut and brain neurons.
METHODS
Standard protocol approvals, registrations, and patient consents.
The study was approved by the ethical committees of the involved institutions. Written informed consent was obtained from all patients participating in this study.
Details of the additional patients with anti-DPPX encephalitis analyzed in this study and the methods employed in this work are provided in appendix e-1 on the Neurology® Web site at Neurology.org.
Case report.
In April 2012, a 68-year-old man developed severe diarrhea, which lasted for about 2 months, was associated with 20-kg weight loss, and remained unexplained on gastrointestinal workup including gastroscopy, colonoscopy, and microbiologic stool examinations (figure 1A). The patient subsequently noted progressive topographical disorientation (e.g., he could not find the way to his local supermarket) and short-term memory problems, starting in September 2012. He also developed tremor of the left more than the right hand, gait unsteadiness, and complained of an increased need for sleep, with up to 4 hours of daytime sleep in addition to regular sleep at night. On admission to the hospital, in October 2013, he had a mild cerebellar syndrome, mild rigidity of the arms, and a resting and postural tremor in his hands. In a detailed neuropsychological examination he was oriented, but had a reduced attention span and impairment of anterograde memory for verbal contents, consistent with amnestic mild cognitive impairment.
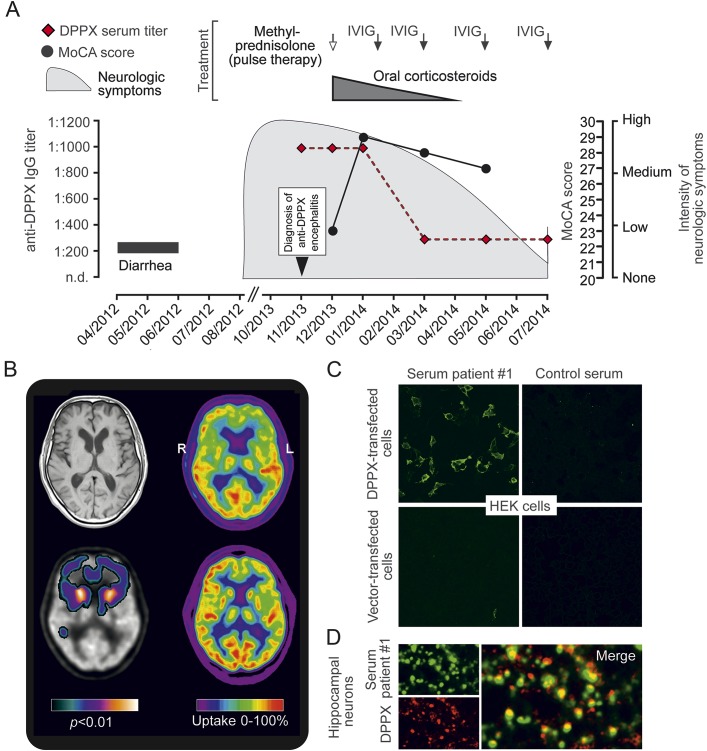
Figure 1. Clinical and paraclinical findings of a novel patient with anti–dipeptidyl-peptidase-like protein 6 encephalitis.
(A) Time course of the disease, intensity of neurologic symptoms, Montreal Cognitive Assessment (MoCA) test scores, anti–dipeptidyl-peptidase-like protein 6 (DPPX) antibody titers, and treatments of a newly identified patient (patient 1) with anti-DPPX encephalitis. (B) T1-weighted axial MRI demonstrates no significant atrophy (upper left). PET with F-18-fluorodeoxyglucose (FDG-PET) as marker of synaptic activity shows strongly reduced activity bilaterally in the heads of the caudate nuclei and mild to moderate reduction in the frontal cortex (upper right); compared with FDG-PET of a healthy person (lower right). Reduction of activity in the heads of the caudate nuclei and frontal cortex was confirmed by voxel-based testing vs a healthy control group, demonstrating statistical significance at the uncorrected α = 0.01 level (lower left; brighter colors indicate lower p values). (C) Binding of patient 1 serum (1:100 dilution) to DPPX-transfected HEK293 cells. Healthy control serum (1:10 dilution) and HEK293 cells transfected with empty vector served as controls. (D) Primary hippocampal neurons were incubated for 2 hours with patient 1 serum (1:100 dilution), fixed, and double-stained for human immunoglobulin G (IgG) and DPPX using a commercial anti-DPPX antibody. The merge demonstrates overlap of both signals (confocal images). IVIG = IV immunoglobulin; n.d. = not detectable.
Cranial MRI was normal except for moderate microangiopathic leukoencephalopathy. CSF examination revealed a normal cell count and protein (data on oligoclonal bands/immunoglobulin G [IgG] synthesis not available). Whole-body fluorodeoxyglucose positron emission CT showed no neoplasia, but demonstrated a markedly reduced uptake in the caudate nuclei bilaterally and a moderately reduced uptake in the frontal cortex (figure 1B). Broad screening for antineuronal as well as gliadin (IgG and immunoglobulin A) serum autoantibodies was negative. However, high titer (1:1,000) IgG serum antibodies to DPPX were independently detected in 2 laboratories (Euroimmun, Lübeck, Germany; Dalmau Laboratory, Barcelona, Spain), using HEK293 cells overexpressing DPPX (figure 1C). The patient's serum staining pattern in cultured murine hippocampal neurons overlapped with that of a commercial monoclonal antibody against DPPX (figure 1D).
The patient was treated with IV methylprednisolone (3 × 1 g/day) followed by tapered oral corticosteroids and IV immunoglobulins. This was associated with a decline of the anti-DPPX antibody titer and marked improvement of the patient's cognitive as well as motor symptoms with almost complete return to his premorbid level of functioning (figure 1A).
RESULTS
Anti-DPPX-antibody-containing sera cause hyperexcitability of enteric nervous system neurons.
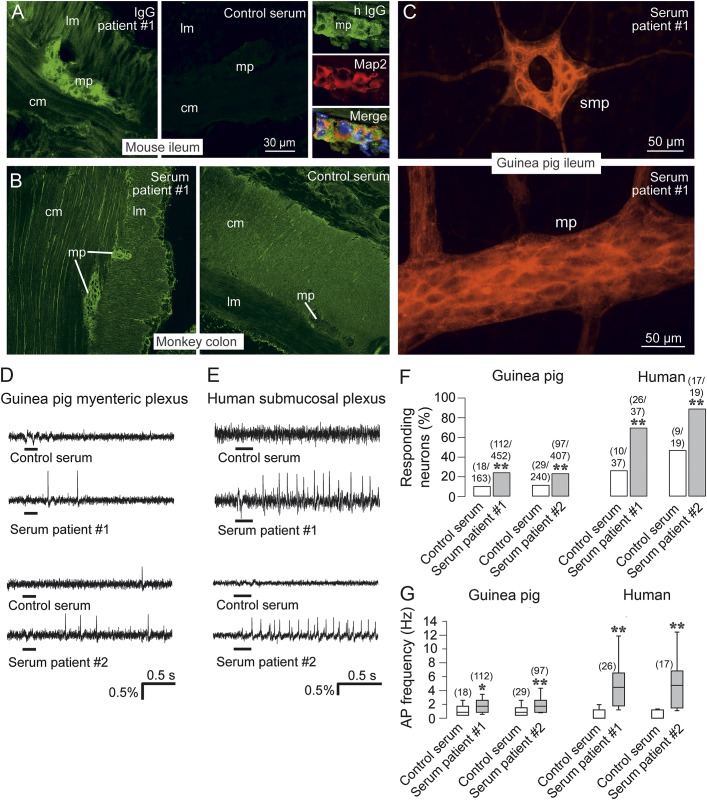
Given the prominent gastrointestinal symptoms in patients with anti-DPPX encephalitis, we analyzed the effects of our patient's (patient 1) serum on enteric neurons. Indirect immunofluorescence on mouse and monkey gut tissue sections and on guinea pig ileum whole-mount preparations confirmed binding of the patient's serum and purified IgG, but not of a healthy control serum, to enteric neurons in the myenteric and submucous plexus (figure 2, A–C). Consistent with this binding, brief application (200–400 ms) of patient 1 serum to guinea pig myenteric or human submucous plexus preparations resulted in increased activity of enteric neurons. The same result was obtained with serum of a previous patient (patient 2) with anti-DPPX encephalitis. In contrast, application of sera from healthy controls (see figure e-1 for additional traces of healthy control sera) was not associated with increased enteric neuron activity (figure 2, D and E). Both the percentage of guinea pig myenteric plexus neurons that fired action potentials as well as the frequencies of these action potentials were significantly higher after stimulation with the 2 anti-DPPX-antibody-containing sera than after stimulation with healthy control serum. Similar, but even more pronounced, effects were observed in experiments using human submucous plexus preparations (figure 2, F and G). Likewise, application of purified IgG from patient 1, but not from a healthy control, to human submucous plexus preparations caused increased enteric neuron activity (figure e-2).
Figure 2. Anti–dipeptidyl-peptidase-like protein 6 sera cause hyperactivity of enteric nervous system neurons.
(A) Indirect immunofluorescence of mouse small intestine with purified patient 1 immunoglobulin G (IgG) (1:100) or control serum (1:100) demonstrates staining of the myenteric plexus by patient IgG. Costaining for microtubule-associated protein 2 (Map2) confirms binding of patient IgG to neuronal structures; nuclei are counterstained in blue (4′,6-diamidino-2-phenylindole [DAPI]). (B) Sections of monkey (Macaca mulatta) gut were incubated with patient 1 or control serum (1:10), revealing staining of the myenteric plexus by the patient's serum. (C) Indirect immunofluorescence of guinea pig ileum whole mount preparations of submucous and myenteric plexus with serum of patient 1 (1:1,000) shows strong staining of neuronal structures. (D) Enteric nervous system neurons of guinea pig myenteric plexus were loaded with the voltage sensitive dye DI-8-ANEPPS and electrical activity was recorded as changes in fluorescence intensity (% ∆F/F) after local application of patient or control serum for 200 ms (horizontal black bar). Top trace shows no response to the control serum. Second trace shows 2 action potentials (spikes) after application of serum from patient 1. Similar results were obtained with serum from another patient with anti–dipeptidyl-peptidase-like protein 6 (DPPX) encephalitis (patient 2). Scale bars apply to all traces. (E) Same experimental setting as in (D) using human submucous plexus. The response to the application of patient sera consists of bursts of action potentials that last throughout the recording period. (F) Percentage of guinea pig and human enteric nervous system neurons that fired action potentials following application of patient or control serum. Numbers above the bars indicate the absolute numbers of responding neurons out of the total number of neurons analyzed. **p < 0.001; χ2-test (guinea pig), McNemar test (human). (G) Frequency of action potentials in guinea pig and human enteric nervous system neurons following application of anti-DPPX or control sera. Numbers of neurons analyzed are indicated above the bars. *p < 0.05, **p < 0.001; guinea pig, Mann-Whitney U test; human, Wilcoxon test (patient 1), paired t test (patient 2). AP = action potential; cm = circular musculature; lm = longitudinal musculature; mp = myenteric plexus; smp = submucous plexus.
Binding of anti-DPPX serum to excitatory and inhibitory synapses of CNS neurons and association with Kv4.2.
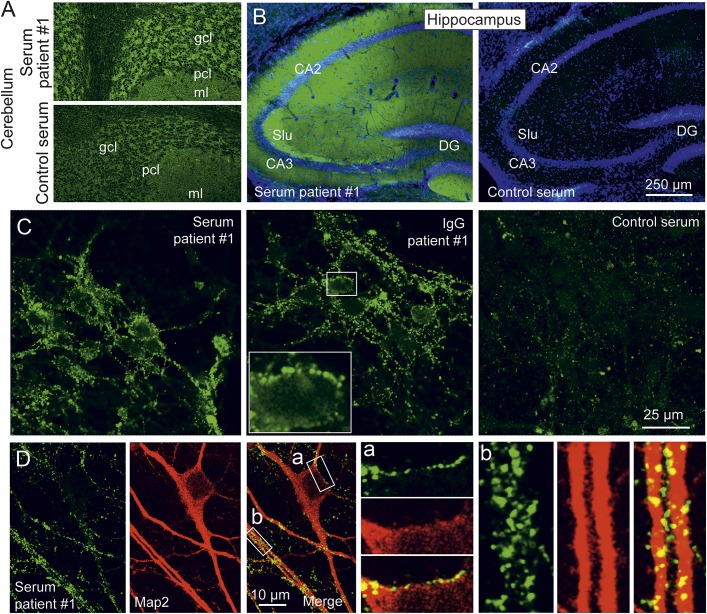
Indirect immunofluorescence with serum from patient 1 on rat cerebellum demonstrated marked staining of the cerebellar glomeruli within the granule cell layer together with a faint staining of the molecular layer, but not of Purkinje cells and white matter (figure 3A). In mouse hippocampus sections, the patient's serum strongly and homogeneously stained the neuropilar regions of the cornu ammonis (most prominent for the mossy fiber terminals in the stratum lucidum) and dentate gyrus (figure 3B). Incubation of living murine hippocampal neurons with serum and purified IgG from patient 1 revealed a punctuate staining pattern at the neuronal surface (figure 3C). Double staining with the microtubular protein Map2 indicated a somatodendritic localization of the signal (figure 3D). Further characterization using synapsin as a pansynaptic marker demonstrated a close, but not complete, colocalization of the patient's serum signal with synaptic structures (figure 4A). Sera of 3 further patients (2–4) with anti-DPPX encephalitis revealed a similar somatodendritic, perisynaptic staining pattern (figure e-3). Double immunofluorescence with serum from patient 1 and antibodies to VGLUT 1 and 2 (markers of excitatory connections) or to VGAT (a marker of inhibitory synapses) indicated that both types of synaptic connections were labeled (figure 4, B and C). Double staining with serum from patient 1 and antibodies to Kv4.2 showed a close association of the antigenic targets (figure 4D).
Figure 3. Staining of CNS neurons by anti–dipeptidyl-peptidase-like protein 6 serum.
(A) Indirect immunofluorescence of rat cerebellum with patient 1 and control serum (both at 1:10) demonstrates prominent staining of the cerebellar cortex, particularly in the granule cell layer glomeruli, by the patient's serum. (B) Indirect immunofluorescence of murine hippocampus sections with patient 1 and control serum (both at 1:100) reveals strong immunoreactivity within the neuropilar regions of the cornu ammonis and dentate gyrus. (C) Staining of living cultured primary hippocampal neurons with patient 1 serum, purified patient 1 immunoglobulin G (IgG), or control serum (all at 1:100) showed a punctuate staining pattern at the cell surface following incubation with patient serum or purified IgG. (D) Hippocampal cultures were incubated with patient 1 serum as before, fixed and counterstained for microtubule-associated protein 2 (Map2) as somatodendritic marker. Immunoreactivity of the patient's serum shows a clear somatodendritic distribution. The boxed areas labeled a and b in the merged image are also shown in higher magnification. All microimages were obtained by confocal imaging. CA = cornu ammonis; DG = dentate gyrus; gcl = granule cell layer; ml = molecular layer; pcl = Purkinje cell layer; Slu = stratum lucidum.
Figure 4. Binding of anti–dipeptidyl-peptidase-like protein 6 serum to excitatory and inhibitory synapses and association with Kv4.2.
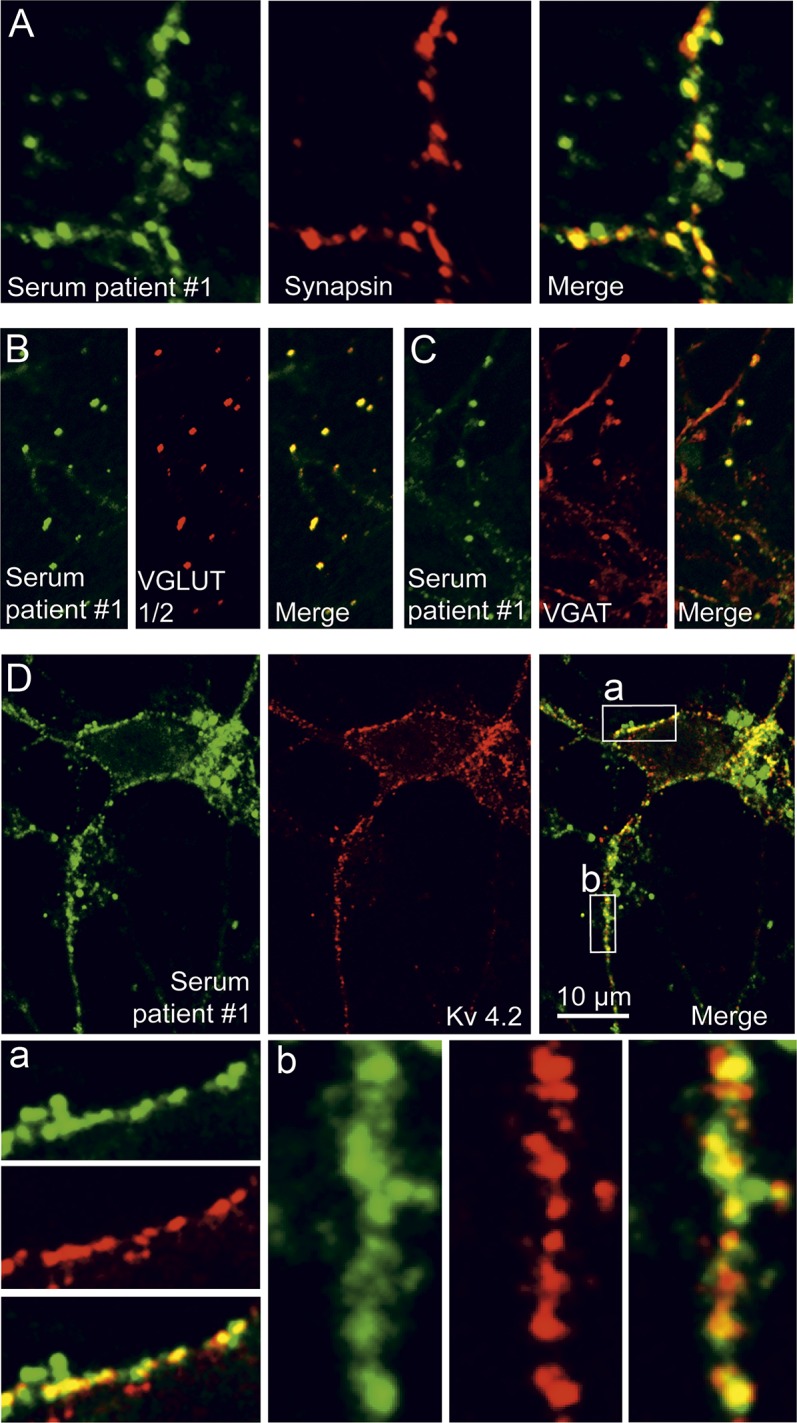
Cultured hippocampal neurons were incubated for 2 hours with patient 1 serum (1:100), fixed, and double-stained for the general synaptic marker synapsin (A), the vesicular glutamate transporters 1 and 2 (VGLUT1/2, B), or the vesicular GABA transporter (VGAT, C) to mark excitatory and inhibitory synapses, respectively. The patient's serum reveals a predominant, though not exclusive, synaptic staining pattern, including both glutamatergic and GABAergic synapses. (D) Hippocampal neurons were incubated for 2 hours with purified patient 1 immunoglobulin G (IgG) (1:100), fixed, and double-stained for human IgG and Kv4.2. Both signals showed a close colocalization at neuronal surfaces. The boxed areas labeled a and b in the merged image are also shown in higher magnification.
Anti-DPPX serum decreases membrane expression of DPPX and Kv4.2 in hippocampal neurons.
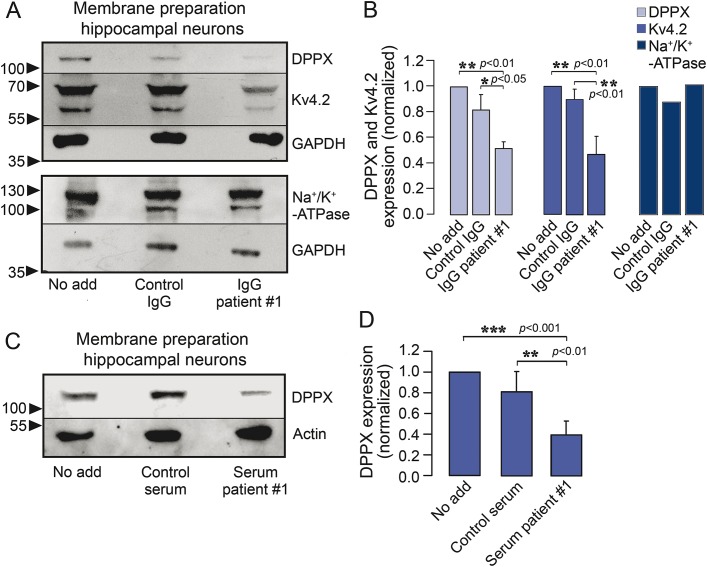
To analyze the effect of anti-DPPX serum on neuronal membrane expression of DPPX and Kv4.2, we incubated hippocampal neurons with patient 1 or healthy control purified IgG for 3 days, isolated neuronal membrane fractions, and subjected these highly enriched fractions to immunoblot analysis for DPPX and Kv4.2. Compared to control IgG, treatment with patient IgG resulted in a significantly lower membrane expression of DPPX and Kv4.2. This effect was not due to a nonspecific effect on cell surface proteins because the ubiquitous plasma membrane protein Na+/K+-ATPase was not decreased by the patient's IgG (figure 5, A and B). Likewise, incubation of hippocampal neurons with serum from patient 1, but not with control serum, caused a decreased neuronal membrane expression of DPPX (figure 5, C and D).
Figure 5. Antibody-mediated downregulation of dipeptidyl-peptidase-like protein 6 and Kv4.2 from neuronal membranes.
(A) Membrane fractions were generated from cultured hippocampal neurons and processed for immunoblotting after preincubation of neurons with patient 1 or control immunoglobulin G (IgG) for 3 days (1:100, daily application). Immunoblots were developed with commercial anti–dipeptidyl-peptidase-like protein 6 (DPPX), Kv4.2, and Na+/K+-ATPase antibodies. Glycerinaldehyde-3-phosphate dehydrogenase (GAPDH) was used as loading control. (B) Quantification of DPPX, Kv4.2, and Na+/K+-ATPase expression in relation to the GAPDH signal and normalized to untreated cultures. Data are means ± SEM from 3 (DPPX, Kv4.2) or 2 (Na+/K+-ATPase) independent experiments. (C) Membrane fractions were generated from cultured hippocampal neurons and processed for immunoblotting after preincubation of neurons with patient 1 or control serum for 3 days (1:100, daily application). Fractions were tested for DPPX expression using a commercial antibody. Actin was used as loading control. (D) Quantification of DPPX expression in relation to the actin signal, normalized to untreated cultures. Data are means ± SEM from 3 independent experiments. Statistical significance was assessed by Mann-Whitney U test.
DISCUSSION
The clinical findings of our newly identified patient with anti-DPPX encephalitis are reminiscent of those reported in the original series1 rather than the PERM-like presentation described in 3 patients with anti-DPPX antibodies.2 A noteworthy feature was transient hypersomnia, consistent with the recent observation of sleep disturbances in 9 of 20 patients with anti-DPPX encephalitis.3 With increasing recognition of new patients, the clinical spectrum of anti-DPPX encephalitis is likely to expand further, similar to other autoimmune encephalitides.8–11 This report confirms that anti-DPPX encephalitis may respond to immunotherapy. Thus, in clinical practice, the combination of prodromal diarrhea with subsequent even mild neurologic and psychiatric symptoms should elicit testing for anti-DPPX antibodies.
We observed a significantly increased activity of enteric neurons after application of anti-DPPX sera to guinea pig and human myenteric plexus preparations. The rapid onset of this effect is remarkable and likely to be due to an immediate modulation of the electrophysiologic properties of gastrointestinal DPPX/Kv4.2 complexes upon binding of anti-DPPX antibodies to their antigenic target, rather than to internalization of DPPX or Kv4.2. Our findings are consistent with the enhanced neuronal excitability observed in DPPX knockouts12 and indicate that genetic ablation or antibody-mediated alteration of DPPX may result in neuronal hyperexcitability. It thus seems conceivable that the initial severe diarrhea of patients with anti-DPPX encephalitis is related to anti-DPPX-antibody-induced hyperexcitability of enteric neurons, leading to gastrointestinal hyperactivity. Nevertheless, the occasional occurrence of gastrointestinal hypomotility2,3 suggests that in certain instances anti-DPPX antibodies could also lead to loss or exhaustion of enteric neurons, perhaps in more chronic stages of the disease.
The staining pattern of our patient's serum in cerebellum and hippocampus corresponded to the known distribution of DPPX in the CNS.1–4,7 Extending previous observations, immunocytochemical characterization of the binding pattern in murine hippocampal neurons demonstrated a somatodendritic, perisynaptic localization in both glutamatergic and GABAergic synapses and a close colocalization with Kv4.2, consistent with DPPX being an auxiliary subunit of Kv4.2.
Receptor internalization in cultured neurons has been identified as a pathogenic mechanism in several encephalitides mediated by antibodies to neuronal surface proteins and is a likely correlate of the reversibility of clinical symptoms if those proteins can be re-expressed at the cellular surface.13–16 Likewise, our patient's purified IgG and serum reduced the expression of DPPX in membranes of hippocampal neurons. Of note, this was accompanied by a similar reduction of Kv4.2. As under physiologic conditions DPPX enhances cell surface expression of Kv4.2,4 it seems plausible that anti-DPPX antibodies interfere with the DPPX-mediated membrane targeting of Kv4.2. Similar to the hyperexcitability of CNS neurons observed in DPPX knockouts,12 downregulation of DPPX and Kv4.2 by anti-DPPX serum is expected to result in CNS hyperexcitability, which may underlie the neurologic manifestations of anti-DPPX encephalitis.
This work identifies potential pathogenic mechanisms of anti-DPPX antibodies in anti-DPPX encephalitis, supporting a pathogenic role of anti-DPPX antibodies in this novel form of autoimmune encephalitis.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Birgit Metze and Suzann Öztürk for technical assistance.
GLOSSARY
- DPPX
dipeptidyl-peptidase-like protein 6
- IgG
immunoglobulin G
- PERM
progressive encephalomyelitis with rigidity and myoclonus
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
M.H., K.R., J.P., K.M., M.S., W.S., J.D., and G.A.-H. designed the study and analyzed data. J.P., C.O., C.D., R.B., B.B., H.-M.M., and K.R. collected and interpreted clinical data. J.P., M.H., C.P., and Q.L. carried out experiments. S.J. was involved in revising the manuscript for important intellectual content. K.R., M.H., G.A.-H., and J.P. drafted the manuscript, which was then revised, edited, and approved by all authors.
STUDY FUNDING
Supported by NIH (RO1NS077851, J.D.), Fondo de Investigaciones Sanitarias (11/01780, J.D.), and the Charité Research Fund.
DISCLOSURE
J. Piepgras, M. Höltje, K. Michel, Q. Li, C. Otto, and C. Drenckhahn report no disclosures relevant to the manuscript. C. Probst is a shareholder and employee of Euroimmun AG. M. Schemann and S. Jarius report no disclosures relevant to the manuscript. W. Stöcker is shareholder of Euroimmun AG and is a member of the board of Euroimmun AG. B. Balint holds a research grant from the Gossweiler Foundation and received travel grants from the Movement Disorder Society and the EFNS-ENS. H. Meinck and R. Buchert report no disclosures relevant to the manuscript. J. Dalmau holds patents for the use of Ma2, NMDAR, and GABAbR as autoantibody tests and has patent applications for DPPX, GABAaR, and IgLON5 as autoantibody tests; receives royalties for the above indicated diagnostic tests; and has a research grant from Euroimmun AG. G. Ahnert-Hilger reports no disclosures relevant to the manuscript. K. Ruprecht received research support from Novartis as well as speaking fees and travel grants from Bayer Healthcare, Biogen Idec, Merck Serono, Sanofi/Genzyme, Teva, and Novartis, and is supported by the German Ministry of Education and Research (BMBF/KKNMS, Competence Network Multiple Sclerosis). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Boronat A, Gelfand JM, Gresa-Arribas N, et al. Encephalitis and antibodies to dipeptidyl-peptidase-like protein-6, a subunit of Kv4.2 potassium channels. Ann Neurol 2013;73:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balint B, Jarius S, Nagel S, et al. Progressive encephalomyelitis with rigidity and myoclonus: a new variant with DPPX antibodies. Neurology 2014;82:1521–1528. [DOI] [PubMed] [Google Scholar]
- 3.Tobin WO, Lennon VA, Komorowski L, et al. DPPX potassium channel antibody: frequency, clinical accompaniments, and outcomes in 20 patients. Neurology 2014;83:1797–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nadal MS, Ozaita A, Amarillo Y, et al. The CD26-related dipeptidyl aminopeptidase-like protein DPPX is a critical component of neuronal A-type K+ channels. Neuron 2003;37:449–461. [DOI] [PubMed] [Google Scholar]
- 5.Kim J, Nadal MS, Clemens AM, et al. Kv4 accessory protein DPPX (DPP6) is a critical regulator of membrane excitability in hippocampal CA1 pyramidal neurons. J Neurophysiol 2008;100:1835–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaulin YA, De Santiago-Castillo JA, Rocha CA, Nadal MS, Rudy B, Covarrubias M. The dipeptidyl-peptidase-like protein DPP6 determines the unitary conductance of neuronal Kv4.2 channels. J Neurosci 2009;29:3242–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark BD, Kwon E, Maffie J, et al. DPP6 localization in brain supports function as a Kv4 channel associated protein. Front Mol Neurosci 2008;1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011;10:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finke C, Mengel A, Pruss H, Stocker W, Meisel A, Ruprecht K. Anti-NMDAR encephalitis mimicking HaNDL syndrome. Cephalalgia 2014;34:1012–1014. [DOI] [PubMed] [Google Scholar]
- 10.Jarius S, Steinmeyer F, Knobel A, et al. GABAB receptor antibodies in paraneoplastic cerebellar ataxia. J Neuroimmunol 2013;256:94–96. [DOI] [PubMed] [Google Scholar]
- 11.Hoftberger R, Titulaer MJ, Sabater L, et al. Encephalitis and GABAB receptor antibodies: novel findings in a new case series of 20 patients. Neurology 2013;81:1500–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun W, Maffie JK, Lin L, Petralia RS, Rudy B, Hoffman DA. DPP6 establishes the A-type K(+) current gradient critical for the regulation of dendritic excitability in CA1 hippocampal neurons. Neuron 2011;71:1102–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai M, Hughes EG, Peng X, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol 2009;65:424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes EG, Peng X, Gleichman AJ, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci 2010;30:5866–5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petit-Pedrol M, Armangue T, Peng X, et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol 2014;13:276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohkawa T, Fukata Y, Yamasaki M, et al. Autoantibodies to epilepsy-related LGI1 in limbic encephalitis neutralize LGI1-ADAM22 interaction and reduce synaptic AMPA receptors. J Neurosci 2013;33:18161–18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.