Abstract
The pathological accumulation of abnormally hyperphosphorylated and aggregated tau, a neuronal microtubule (MT)-associated protein that functions to maintain MT stability, is implicated in a number of hereditary and sporadic neurodegenerative diseases including frontotemporal dementia and Alzheimer's disease. Targeting tau for the treatment of these diseases is an area of intense interest and toward that end, modulation of cellular molecular chaperones is a potential therapeutic target. In particular, the constitutive Hsp70 isoform, Hsc70, seems highly interconnected with tau, preserving tau protein levels and synergizing with it to assemble MTs. But the relationship between tau and Hsc70, as well as the impact of this interaction in neurons and its therapeutic implications remain unknown. Using a human dominant negative Hsc70 that resembles isoform selective inhibition of this important chaperone, we found for the first time that Hsc70 activity is required to stimulate MT assembly in cells and brain. However, surprisingly, active Hsc70 also requires active tau to regulate MT assembly in vivo, suggesting that tau acts in some ways as a co-chaperone for Hsc70 to coordinate MT assembly. This was despite tau binding to Hsc70 as substrate, as determined biochemically. Moreover, we show that while chronic Hsc70 inhibition damaged MT dynamics, intermittent treatment with a small molecule Hsp70 inhibitor lowered tau in brain tissue without disrupting MT integrity. Thus, in tauopathies, where MT injury would be detrimental to neurons, the unique relationship of tau with the Hsc70 machinery can be exploited to deplete tau levels without damaging MT networks.
Introduction
Cellular protein metabolism relies on a network of the heat shock protein (Hsp) family of chaperones whose variety of functions ensures proper proteostasis: refolding, sorting and degradation of client proteins. The vital role of these proteins in many aspects of cellular functions has led to the development of small molecule inhibitors targeting chaperones for use as anti-cancer agents (1–4) and therapeutics for neurodegenerative disorders (5–8).
While molecules targeting Hsp90, the major chaperone ATPase, have been in development for more than a decade and are in advanced clinical stages, inhibitors of Hsp70, the other major ATPase chaperone family, have only recently been described and none have yet entered the clinic based on this mechanism of action. One of the reasons for this slower development of Hsp70 inhibitors has been that there are 10 isoforms in mammalian cells and selective inhibitors are currently not available (9–13). Moreover, Hsp70 proteins are very promiscuous, affecting many proteins in the cell, while Hsp90 proteins are slightly more discriminating in regard to their clientele. In particular, the constitutively expressed isoform of Hsp70, Hsc70/Hsp73/HSPA8, accounts for 2% of total protein in cells and affects a number of processes including cytoskeletal function and clathrin-mediated endocytosis (14). Hsc70 associates with both intact MTs (15–19) and tubulin directly through its nucleotide-binding domain (NBD), suggesting functional regulation of tubulin by Hsc70 beyond proteostasis (20). Hsc70 also interacts with the microtubule (MT)-associated protein (MAP) tau, a putative ‘typical’ client of Hsc70 (6,21–23) further suggesting that Hsc70 uniquely regulates MT dynamics (24,25). In addition to its role in MT stability, abnormal tau accumulation is linked to the pathology of a number of neurodegenerative diseases including Alzheimer's disease (AD), frontotemporal dementia (FTD), Pick's disease and chronic traumatic encephalopathy (26). Several of these tauopathies are characterized by mutations in the MAPT gene resulting in tau protein mutations that can compromise tau-mediated MT stabilization (27,28).
A growing body of evidence indicates that tau has a unique relationship with chaperone proteins despite its intrinsically disordered structure. For example, tau was recently shown to interact directly with Hsp90, showing for the first time how a client binds to the large Hsp90 homodimer (29). Tau is also triaged differently by distinct Hsp70 isoforms: tau degradation is facilitated by the stress-inducible Hsp72, whereas the constitutively expressed Hsc70 stabilizes tau levels and promotes MT assembly (30). Hsc70-mediated stabilization of tau levels may be relevant to tauopathies, as high levels of Hsc70 are found in brains of AD patients relative to other Hsp70 isoforms (30). Compounds that inhibit Hsp90 and Hsp70 family proteins reduce tau levels (6,11,31–33) and rescue synaptic plasticity defects in tauopathy mouse models (33). Based on this evidence, we sought to determine why Hsc70 and tau are so intimately involved with each other and perhaps determine the most effective way to exploit this interaction for therapeutic intervention to treat tauopathies.
Results
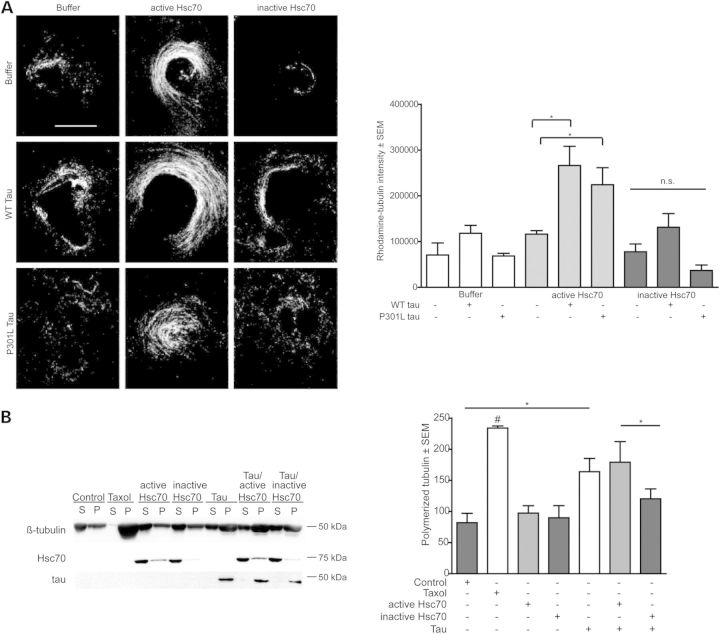
Our previous work showed that the primary isoform of Hsp70 that contributes to tau preservation in cells is Hsc70 (30). We also discovered that wild-type (WT) Hsc70 and tau could synergistically stimulate MT assembly in Xenopus cell-free oocyte extracts (22), but it remained unclear whether the enzymatic activity of Hsc70 was necessary for this mechanism and whether this relationship was relevant in vivo. We examined the effects of an engineered variant of human Hsc70 that lacks both ATPase and refolding activity (34) on MT assembly in the Xenopus system (22). As previously shown, active, WT Hsc70 stimulated tubulin assembly (30), but the inactive Hsc70 variant did not (Fig. 1A), showing for the first time a clear connection between Hsc70 activity and MT assembly. When active and inactive Hsc70 were administered with recombinant WT tau to the same Xenopus extracts, tubulin polymerization was significantly increased in lysates having WT tau and active Hsc70, but not with WT tau and inactive Hsc70 (Fig. 1A), indicating that tau cooperates with active Hsc70 to stimulate MT assembly in this cell-free system. Interestingly, active Hsc70 was unable to effectively polymerize MTs when combined with the FTD-associated P301L mutant tau (Fig. 1A), further suggesting that these two proteins, Hsc70 and tau, are interdependent on each other's functionality to stimulate MT assembly as a machine (Fig. 1A). These results were then confirmed using a reconstituted purified bovine tubulin polymerization assay—together, active Hsc70 and WT tau were able to assemble tubulin more efficiently than inactive Hsc70 (Fig. 1B). These data suggest that Hsc70 activity was indeed required for it to synergize with tau to enhance MT assembly, and disease-associated tau mutations can impair this function, further suggesting a functional link between these two proteins with regard to MT dynamics.
Figure 1.
Hsc70 activity and tau are required to stimulate tubulin assembly in vitro. (A) Polymerization of rhodamine-labeled tubulin in Xenopus oocyte extracts in the presence of WT tau or P301L tau and active or inactive Hsc70. Quantification of tubulin intensity, *P < 0.05, n = 10 one-way ANOVA (P = 0.0001, F = 7.0746 with Tukey's post-hoc test for pairwise comparisons). Scale bar is 30 µm. (B) Representative western blot showing polymerization of 5 µg purified bovine tubulin in the presence of active or inactive Hsc70 with and without tau. S, supernatant, P, pellet. Error bars are ± SEM n = 4. Data were tested by one-way ANOVA (P = 0.0100, F = 5.945 with Tukey's post-hoc test for pairwise comparisons, *P < 0.05).
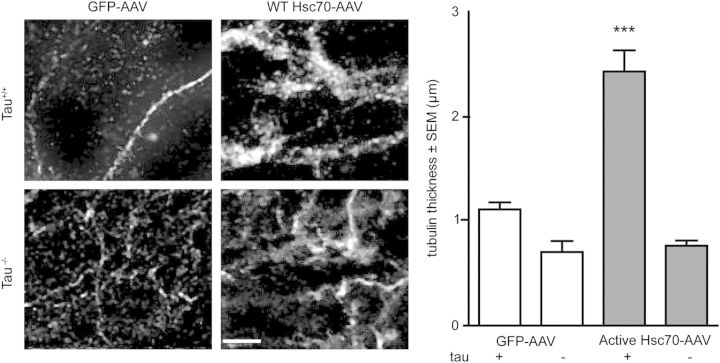
We suspected that this could explain the evolutionary advantage of the interaction between Hsc70 and tau; that tau functions as a co-regulator with Hsc70 to control MT dynamics (35–37). To confirm that the tau-tubulin association was enhanced by Hsc70, we performed live-cell imaging with GFP-tubulin and RFP-tau in the presence of either active or inactive Hsc70 (Fig. 2A). We found that live cells overexpressing GFP-tubulin and active Hsc70 contained less diffuse tubulin signal, suggesting more tubulin is assembled into MTs in cells overexpressing active Hsc70 (Fig. 2A and B). Conversely, cells expressing inactive Hsc70 had much more diffuse tubulin signal (Fig. 2A). These results further suggested that ATPase activity was required for Hsc70-mediated regulation of MTs, even in human cells. We then sought to evaluate the importance of tau to this mechanism, as well as quantify the extent of MT bundling, a process involving tau (34–36). We performed live-cell imaging in cells expressing active or inactive Hsc70 in the presence of WT tau over-expression or tau knockdown using small interfering RNA (siRNA) and performed a randomized tubulin morphology analysis, where cellular tubulin phenotype was classed as diffuse or bundled signal (Fig. 2B). Then, we performed densitometry to quantify bundled MTs, using a masking step to exclude diffuse signal. Interestingly, tau knockdown significantly abrogated active Hsc70 from assembling and bundling MTs, whereas tau over-expression did not rescue the disassembly of MTs caused by inactive Hsc70 over-expression (Fig. 2B). Tau over-expression increased active Hsc70-mediated MT bundling, an effect that was abrogated in the presence of the P301L mutant tau (Fig. 2). We then examined the effects of WT Hsc70 on MT stabilization in neurons from both wild-type and Mapt−/− mice (tau knockout mice) to confirm the relevance of this mechanism in the brain. Expression of active Hsc70-FLAG AAV9 in WT organotypic slice cultures significantly increased axonal MT thickness compared with tau knockout slices expressing active Hsc70-AAV9 or slices from WT mice expressing GFP-AAV9 (Fig. 3). As expected, the inactive Hsc70 variant completely abolished any acetylated-tubulin signal (data not shown; see Fig. 3 legend), confirming that active Hsc70 requires tau to stabilize MTs, but the inactive Hsc70 takes on a dominant negative property that can disrupt MT integrity independent of tau. Collectively, these findings indicate that a hierarchy exists in the chaperone-mediated MT assembly pathway, where Hsc70 activity acts upstream of tau in controlling MT assembly and bundling, but tau is necessary for Hsc70 to regulate MT kinetics, perhaps explaining the exquisitely controlled and dynamic relationship between Hsc70 and tau.
Figure 2.
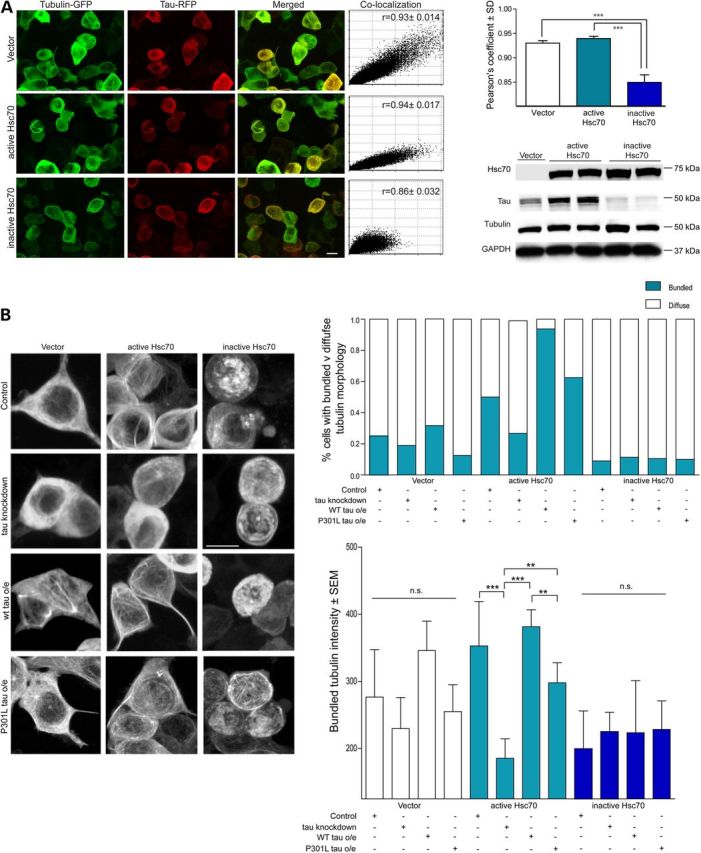
Activity of both Hsc70 and tau are required for synergistic MT assembly and bundling. (A) Live cell confocal microscopy of HEK293T cells overexpressing GFP-tubulin and RFP-tau in the presence of vector, WT Hsc70 or E175S Hsc70. Confocal z-stack images at ×60; scale bar is 10 µm. Colocalization plots [mean ± SEM, n = 3 repeats, 10 independent z-stacks per repeat by one-way ANOVA (P = < 0.0001, F = 18.08; ***P < 0.001 with Tukey's post-hoc test)] are shown with average Pearson's constants; data are shown in bar graph below. (B) Live cell confocal microscopy of HEK293T cells overexpressing GFP-tubulin and either vector, active Hsc70 or inactive Hsc70. Cells were transfected with MAPT siRNA, or WT or P301L 4R0N tau. Confocal z-stack images were taken at ×60 over 10 fields, scale bar represents 10 µm. Tubulin morphology in cells was characterized in a randomized, blind analysis by a naïve tester and represented as % cells with diffuse or bundled signal. Quantification of bundled/ordered tubulin intensity is shown below. Data are mean ± SEM, n = 3 (10 cells per experiment); ns by ANOVA (P = < 0.0001, F = 38.86; ***P < 0.001, **P < 0.01 with Tukey's post-hoc test).
Figure 3.
Active Hsc70 can only promote MT bundling in neurons when tau is present. Microtubule thickness in WT or Mapt−/− organotypic hippocampal slices expressing AAV9-GFP, WT Hsc70-Flag or inactive Hsc70-Flag immunostained with anti-acetylated-tubulin. Scale bar is 10 µm (×60). Thickness data are mean diameter ± SEM, n = 4 animals, three slices per animal by one-way ANOVA (P = < 0.0001, F = 40.14; ***P < 0.001 by Tukey's post-hoc analysis). Results from inactive Hsc70 transduced slices are not shown because no acetylated-tubulin signal was visible.
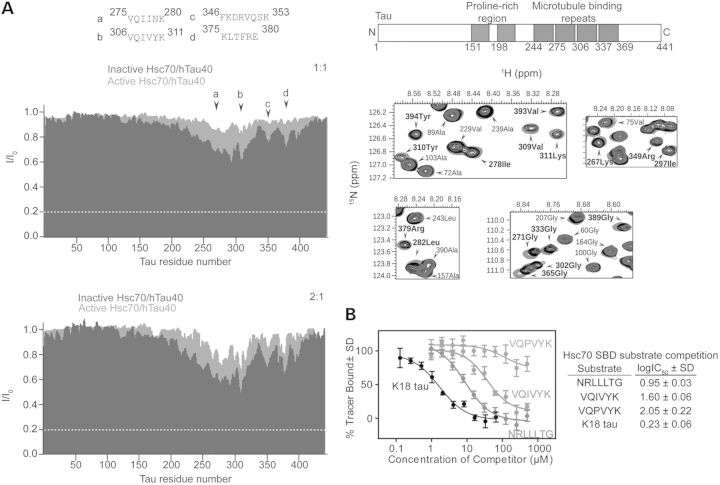
These results suggested the unexpected possibility that tau, a putative client/substrate of Hsc70, could also be working with Hsc70 to coordinate MT assembly. To further explore this possibility, we used 2D nuclear magnetic resonance spectroscopy with 15N labeled recombinant tau and active or inactive Hsc70 to evaluate if they both bound to the same peptide motifs in tau with similar affinities. Binding specificity between either Hsc70 variant to tau was similar, with both active and inactive Hsc70 binding within the MT repeat region of tau. Thus, inhibition of Hsc70 activity does not preclude tau binding (Fig. 4A). To definitively determine that tau was a bona-fide client of Hsc70, we developed a fluorescence polarization assay using a LVEALY tracer, which binds specifically to the substrate-binding domain of Hsc70 with low µm affinity (Supplementary Material, Fig. S1A and B), and is unaffected by the presence of nucleotide (Supplementary Material, Fig. S1C and D). The tau 306VQIVYK311 peptide, which most strongly interacted with Hsc70 (Fig. 4B) (29,30), competed off the LVEALY tracer with an IC50 of 1.60 ± 0.06 µm, while a mutant tau peptide VQPVYK did not (Fig. 4B). Moreover, the K18 tau fragment, which spans the entire MT-binding region of tau (amino acids 246–369), was also very effective at competing off the LVEALY tracer with an IC50 of 0.23 ± 0.06 µm, even surpassing the IC50 of the NRLLLTG model substrate peptide (Fig. 4B). Taken together, these data confirm that tau does bind to Hsc70 as a substrate regardless of nucleotide binding or dynamics, but tau works functionally with Hsc70 to coordinate MT assembly.
Figure 4.
Tau acts as a bona-fide Hsc70 client. (A) Active and inactive Hsc70 bind with the same specificity to tau. NMR intensity ratios I/I0 (I, intensity of tau resonances in presence of active or inactive Hsc70; I0, peak intensities of free tau) obtained from 2D 1H–15N HSQC spectra of tau in the absence and presence of active Hsc70 (cyan) and inactive Hsc70 (blue). Molar ratios indicated. Superposition of absence (green) and presence of equimolar (black) and 2-fold excess (blue) ratios of inactive Hsc70 in highlighted resonances. (B) FP-LVEALY shows tau peptides VQIVYK (red), K18 tau (black) and model substrate NRLLTG (blue) compete for tracer binding in the SBD of Hsc70, whereas VQPVYK (gray) does not. Data are mean ± SD.
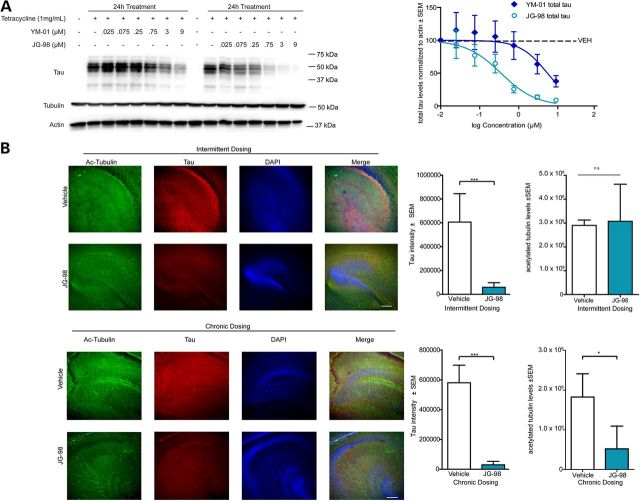
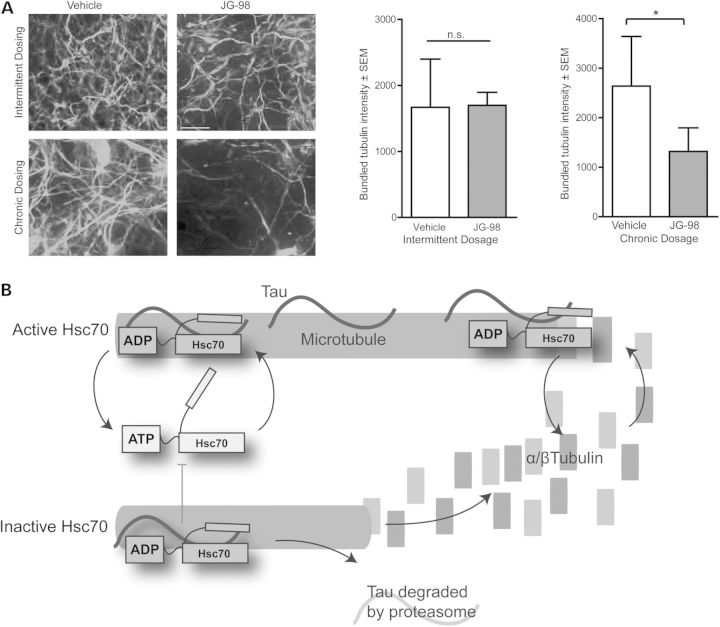
Since the Hsc70/tau complex is essential for proper MT control, chronic inhibition of their interaction would be detrimental to neurons. However, we hypothesized that the highly specialized relationship between tau and Hsc70 that can lead to such rapid changes in tau metabolism could ultimately be exploited in a way to avoid toxicity but still promote tau clearance. First, we examined the anti-tau efficacy and potency of a recently described rhodacyanine derivative, JG-98 (3). Indeed, this compound had superior anti-tau activity compared with other previously described Hsp70 inhibitors (Fig. 5A) (6,11,32,33). Importantly, tubulin levels were unchanged by Hsp70 inhibition. Using this new test compound, we sought to determine an Hsp70 inhibitor treatment schedule that could lower tau in neurons without affecting MT integrity. Organotypic slices from a tau transgenic mouse model were treated daily for 1 week (chronic) or twice over 1 week (intermittent) with 1 µm of JG-98. Immunofluorescence microscopy analysis show the chronic and intermittent treatment schedules both significantly reduced tau levels compared with vehicle control after 1 week (Fig. 5B). Immunofluorescence staining for acetylated tubulin as a marker of stabilized MTs revealed that the density and extent of MT bundling was significantly reduced only in the chronically treated slices, not in the slices receiving the intermittent dosing schedule of JG-98 (Figs 5B and 6A). Moreover, the tau remaining in the slices treated intermittently with JG-98 was co-localized with tubulin to a similar extent as seen in control slices. These findings suggest that the remaining tau following intermittent Hsp70 inhibition was associated with MTs (Fig. 5B), consistent with previous findings that active chaperone proteins primarily target malfunctioning tau species that are not associated with MTs (22). These data suggest that while Hsp70 inhibition may be deleterious for neurons using a traditional treatment strategy (i.e. once daily), intermittent or occasional dosing strategies (i.e. twice weekly) may promote the clearance of abnormal tau without affecting MT integrity.
Figure 5.
Intermittent and chronic Hsp70 inhibition reduce tau levels similarly in brain. (A) Representative western blot of tetracycline-inducible HEK293 cells overexpressing P301L 4R0N tau. Cells were induced to overexpress tau for 24h and treated with indicated doses of YM-01 or JG-98 for 24h. Quantification shown is tau levels (mean ± SEM, n = 3 experiments) compared with vehicle control and acetylated-tubulin levels (mean ± SEM, n = 3 experiments). (B) Hippocampus of organotypic slices from tau transgenic mice treated chronically (daily for 5 days) or intermittently (every 2.5 days for 5 days) with vehicle or 1µm JG-98 and immunostained for acetylated tubulin (green) and total tau (red). Nuclei are visualized by DAPI. Quantification graphs are tau intensity ± SEM, n = 3 animals and two slices per animal, eight images per slice. ***P = 0.0012 (intermittent) and ***P =< 0.0001 (chronic) by Student's t-test. Acetylated-tubulin intensity ± SEM, n = 3 animals and two slices per animal, eight images per slice. *P = 0.0316 (chronic) by Student's t-test. Scale bar is 20µm at ×10.
Figure 6.
Intermittent treatment with an Hsp70 inhibitor does not impair MT integrity. (A) Acetylated tubulin in tau transgenic mice organotypic slices treated chronically or intermittently with 1 µm JG-98. Quantification of bundled tubulin ± SEM, n = 10, by one-way ANOVA (P = 0.0012, F = 10.47, *P < 0.05, with Tukey's post-hoc test for multiple comparisons). Scale bar is 10 µm at ×60. (B) Schematic representation illustrating that Hsc70 ATPase activity, with tau, is required for proper MT dynamics.
Discussion
We show for the first time that MT dynamics are controlled by an active Hsc70/tau complex. Hsc70 must be functionally active to promote MT assembly. However, Hsc70 required functional tau to promote MT dynamics, suggesting the novel concept that tau binds to Hsc70 as a client, but functions with Hsc70 to facilitate MT assembly, an idea supported by the fact that tau binds the substrate-binding domain of Hsc70. This newly described function may help to explain why tau is so sensitive to chaperone modulation despite its intrinsically disordered structure: Tau is used by the chaperone network to regulate MT stability (35,36,38,39). But interestingly, if tau has a disease-causing mutation, it can still interact with Hsc70, yet prevents MT assembly, adding Hsc70 as a new player to this pathogenic mechanism for tauopathy-causing mutations. This important function of the Hsc70/tau complex introduces new challenges with regard to the therapeutic potential of Hsp70 modulators for neurodegenerative diseases, particularly in the case of developing Hsc70-mediated therapeutics for use in tauopathies with known tau mutations, such as P301L, which alter tau-MT binding. However, the exquisite sensitivity of tau to Hsc70 modulation that is required for this dynamic regulation of MTs could be exploited when designing therapeutic strategies around this complex. In fact, intermittent Hsc70 inhibition in brain tissue reduced tau levels without damaging MTs. Thus, the potency of Hsp70 inhibitors against tau could be successfully exploited as a therapeutic strategy in tauopathies, but only if administered in a carefully monitored regimen which may require fine-tuning, particularly if the patient possesses a tau mutation, such as P301L, that may already have some MT deficiencies.
These findings indicate Hsc70, tau and tubulin function as a tripartite complex governed by nucleotide dynamics. For this to be the case, tau and tubulin require distinct binding sites on Hsc70. Previous reports indicate tubulin has a non-canonical binding site located near subdomain IIB in the NBD (40), and we now show that tau binds Hsc70 within the substrate-binding domain; thus, both tau and tubulin are able to interact with Hsc70 simultaneously. Tubulin assembly requires both tau and Hsc70 activity, suggesting that tubulin binding is responsive to the conformational changes in Hsc70 associated with the steps of substrate binding and nucleotide exchange (41,42). Together, our data propose a model wherein Hsc70 is able to bind tau both on, and as it is just disengaged, from the MT (Fig. 6B) (22). Moreover, based on the state of MT dynamics, Hsc70 either triages tau for degradation or restores it to the MT to promote assembly. When Hsc70 activity is inhibited, the decision of tau fate is always shifted towards that of degradation, regardless of the state of the MT.
These data have further therapeutic implications beyond neurodegenerative diseases. It has been previously shown that small molecule Hsp70 inhibitors based on the MKT-077 scaffold (i.e. YM-01 and JG-98), as well as other scaffolds, are extremely effective against cancer cell lines (3,43–48). The MKT-077-based compounds have been shown to trap all Hsp70 isoforms into an ADP bound conformer, which can disrupt a number of downstream cancer signaling pathways that regulate transactivation, cell invasion and adhesion (48–50). Because the induction of the Hsp72 isoform in oncogenic cell lines is known to be cyto-protective, most of the mechanistic studies regarding Hsp70 inhibitors in cancer have been focused on how these compounds influence this inducible Hsp70 isoform. However, our findings now provide another mechanism through which general Hsp70 inhibitors might be effective anti-cancer agents; by inhibiting Hsc70-mediated regulation of MT stability. Indeed, compounds that alter MT dynamics are an area of extensive study and several are currently used in the clinic (51). Moreover, recent work even implicates tau in the pathogenesis and prognosis of cancer (52–56), further supporting this mechanistic link between tau, Hsc70 and MTs. Thus, while isoform selective Hsp70 inhibitors could be best suited for neurodegenerative diseases, it is possible that compounds capable of targeting multiple Hsp70 isoforms could be most advantageous for cancer treatment.
In conclusion, these data provide new insights into how a discreet Hsp70 isoform, Hsc70, can impact MT regulation through a unique interaction with its client tau. By understanding this mechanism, implementation of Hsp70-based therapeutics in neurodegenerative diseases with abnormal tau accumulation could be realized while avoiding a potential major on-target side effect. Our findings finally explain the evolutionary advantage of the chaperone interaction with the intrinsically disordered protein tau, which was a mystery until now. More broadly, these results also show that the chaperone/client interface can have a functional role in essential cellular processes beyond that of proteostasis and protein metabolism.
Materials and Methods
Reagents
Unless stated otherwise, all chemical reagents were purchased from Sigma-Aldrich (St Louis, MO, USA).
Plasmids and antibodies
The following antibodies were purchased commercially: Hsc70 (Stressgen, Enzo Life Sciences, Farmingdale, NY, USA), tauH150 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), β-actin, acetylated tubulin, β-tubulin and M2-Flag (Sigma-Aldrich), DnaJC7, DnaJA1 and beclin-1(Abcam, Cambridge, MA, USA) and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Cell Signaling Technologies, Danvers, MA, USA). The PHF1 antibody was a generous gift from Dr Peter Davies.
For mammalian expression experiments, full-length human Hsc70 was cloned into pCMV6 (Origene, Rockville, MD, USA) or pAAV9 vectors with an N-terminal flag tag to distinguish between endogenous and overexpressed isoforms. The Hsc70 E175S (inactive) mutation and P301L tau mutations were introduced via site-directed mutagenesis (Stratagene, La Jolla, CA, USA) and all sequences were verified before use. WT 4R0N tau was cloned into pCDNA3.1+ as previously described (30). For recombinant protein preparations, active and inactive Hsc70 were cloned into the pET28a vector (Midwest Center for Structural Genomics, Bethesda, MD, USA).
Expression and purification of recombinant proteins
Wild-type 4R0N Tau, Hsc70 (active and inactive), Hsc70 SBD and Hsc70 NBD fragments, were purified as described previously (30,57).
Fluorescence polarization assays
Fluorescence polarization (FP) experiments using the HLA-FAM and ATP-FAM reporter were performed as described previously (57). For the LVEALY reporter, please see Supplementary Material, Experimental Procedures.
Nuclear magnetic resonance spectroscopy
2D 15N–1H HSQC NMR analysis for the interaction of WT-tau with active Hsc70 and inactive Hsc70 was performed as described in Jinwal et al. (30).
In vitro MT assays
Microtubule assays with recombinant WT or P301L 4R0N tau and active or inactive Hsc70 in Xenoupus egg extracts were performed as described previously (30). Purified bovine brain tubulin (generously provided by Dr Jared Cochran) was incubated at 34°C in the presence of recombinant active or inactive Hsc70 and WT 4R0N tau for 1 h and then centrifuged to pellet polymerized tubulin. Supernatants and pellets were then assayed by western blot.
Cell culture, lysis and immunoblotting
HEK293T cells were maintained and transfected as described previously (30).
siRNA-mediated knockdown
siRNAs for human MAPT were purchased from Qiagen (Buffalo Grove, IL, USA). Knockdowns were performed for 72 h on HEK293T with 40 nm siRNA using silentFECT (BioRad, Hercules, CA, USA).
Ex-vivo slice cultures, transduction, drug treatment and immunofluorescent staining
All procedures involving experimentation on animal subjects were done in accord with the guidelines set forth by the Institutional Animal Care and Use Committee of the University of South Florida. Organotypic slice cultures were prepared from post-natal Day 5 wild-type, P301L tau or Mapt(−/−) mice and maintained in complete culture medium [DMEM/F12, 2 mm Glutamax, pencillin/streptomycin and 25% (v/v) heat-inactivated horse serum (Life Technologies)]. At DIV2, slices were transduced with activeHsc70-Flag, AAV9 or AAV9-GFP at 1010 titer. At DIV15, slices were fixed, permeablized and blocked as described previously (58). Slices were incubated in the appropriate primary antibodies for 2 days followed by anti-goat Alexa-Fluor conjugated secondary antibodies (Life Technologies). Nuclei were visualized with 4′,6-diamidino-2-phenylindole (DAPI) staining, then coverglass applied using ProLong antifade reagent (Life Technologies). For JG-98 treatments, 1 µm JG-98 or the equivalent amount of vehicle (DMSO) was added to the culture media of P301L tau organotypic slices at DIV5 for 6 h before returning to complete media without treatment. Slices were treated either daily (chronic) or every 2.5 days (intermittent), then processed for immunofluorescence as described earlier. Acute slice cultures from rTg4510 mice were performed as described (33).
Microscopy and image analysis
All imaging was performed on an Olympus FV1000 MPE multiphoton laser scanning microscope. For both fixed slices and live cells, confocal z-stack images (1 µm slices) were captured using a ×10 or ×60 objective from a minimum of four fields containing at least 15–20 cells per condition over three experimental repeats. Image analysis was performed using Image J. Briefly, z-stacks were background subtracted, despeckled and thresholds set in each channel to include regions of interest. For organotypics, masking was applied to include only neurons that expressed virus. Pearson's coefficients and mean red and green intensities were calculated. Data are means of at least five cells per field and eight z-stacks each from three experimental repeats for cells and eight fields from each of two slices per animal, n = 3 animals per treatment.
For bundled tubulin imaging from live cells, only particles corresponding to bundled MTs (calculated in the vector only control condition) were analyzed for intensity by applying a minimum threshold to exclude diffuse, non-bundled signal and a masking analyses applied to avoid bright but unbundled, unincorporated tubulin signal. These thresholds were applied equally across conditions and intensity density was used to quantify bundled tubulin. Data are means of at least 10-slice z-stacks of five cells per field, eight fields per experiment. Experiments were repeated three times. Microtubule thickness was measured using the line tool in ImageJ. Colocalization was analyzed using the Colocalization Indices plugin in ImageJ.
Statistical analysis
Statistical analyses were performed by one-way ANOVA tests with Tukey's post-hoc analyses as indicated in the figure legends, using GraphPad Prism 5.0 software. In the instance of only a pairwise comparison in Figure 5, Student’s t-tests were performed.
Supplementary Material
Authors’ Contributions
S.N.F. and C.A.D. designed experiments and interpreted results. S.N.F., M.D.M. and M.C. performed cell-culture experiments. S.N.F. and J.J.S. performed organotypic experiments. J.E.G. provided chemicals and insights regarding results. S.N.F. performed microscopy and image analysis. S.N.F., B.A.N., V.A.A. and S.B. performed biochemical experiments. E.A. and M.Z. performed and analyzed NMR experiments. M.Z. and J.J.S. contributed to manuscript edits. S.N.F. and C.A.D. wrote manuscript.
Funding
This work was supported by NS073899 (NIH) and BX001637 (VA) to C.A.D; 2R01NS059690 and AG042813 to J.E.G. M.Z. was supported by Deutsche Forschungsgemeinschaft through project ZW 71/7-1 and ZW 71/8-1.
Supplementary Material
Acknowledgements
This material is the result of work supported with resources and the use of facilities at the James A. Haley Veterans' Hospital. The contents of this publication do not represent the views of the Department of Veterans Affairs or the US Government. The authors wish to thank Dr Byeong Cha for assistance with microscopy and Dr Jared Cochran for purified bovine tubulin.
Conflict of Interest statement. None declared.
References
- 1.Ma L., Sato F., Sato R., Matsubara T., Hirai K., Yamasaki M., Shin T., Shimada T., Nomura T., Mori K., et al. (2014) Dual targeting of heat shock proteins 90 and 70 promotes cell death and enhances the anticancer effect of chemotherapeutic agents in bladder cancer. Oncol. Rep., 31, 2482–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zitzmann K., Ailer G., Vlotides G., Spoettl G., Maurer J., Goke B., Beuschlein F., Auernhammer C.J. (2013) Potent antitumor activity of the novel HSP90 inhibitors AUY922 and HSP990 in neuroendocrine carcinoid cells. Int. J. Oncol., 43, 1824–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X., Srinivasan S.R., Connarn J., Ahmad A., Young Z.T., Kabza A.M., Zuiderweg E.R., Sun D., Gestwicki J.E. (2013) Analogs of the allosteric heat shock protein 70 (Hsp70) inhibitor, MKT-077, as anti-cancer agents. ACS Med. Chem. Lett., 4, 1042–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodina A., Vilenchik M., Moulick K., Aguirre J., Kim J., Chiang A., Litz J., Clement C.C., Kang Y., She Y., et al. (2007) Selective compounds define Hsp90 as a major inhibitor of apoptosis in small-cell lung cancer. Nat. Chem. Biol., 3, 498–507. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y., Wang B., Liu D., Li J.J., Xue Y., Sakata K., Zhu L.Q., Heldt S.A., Xu H., Liao F.F. (2014) Hsp90 chaperone inhibitor 17-AAG attenuates Abeta-induced synaptic toxicity and memory impairment. J. Neurosci, 34, 2464–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyata Y., Li X., Lee H.F., Jinwal U.K., Srinivasan S.R., Seguin S.P., Young Z.T., Brodsky J.L., Dickey C.A., Sun D., et al. (2013) Synthesis and initial evaluation of YM-08, a blood-brain barrier permeable derivative of the heat shock protein 70 (Hsp70) inhibitor MKT-077, which reduces tau levels. ACS Chem. Neurosci., 4, 930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ansar S., Burlison J.A., Hadden M.K., Yu X.M., Desino K.E., Bean J., Neckers L., Audus K.L., Michaelis M.L., Blagg B.S. (2007) A non-toxic Hsp90 inhibitor protects neurons from Abeta-induced toxicity. Bioorg. Med. Chem. Letters, 17, 1984–1990. [DOI] [PubMed] [Google Scholar]
- 8.Putcha P., Danzer K.M., Kranich L.R., Scott A., Silinski M., Mabbett S., Hicks C.D., Veal J.M., Steed P.M., Hyman B.T., et al. (2010) Brain-permeable small-molecule inhibitors of Hsp90 prevent alpha-synuclein oligomer formation and rescue alpha-synuclein-induced toxicity. J. Pharm. Exp. Ther., 332, 849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang Y., Taldone T., Patel H.J., Patel P.D., Rodina A., Gozman A., Maharaj R., Clement C.C., Patel M.R., Brodsky J.L., et al. (2014) Heat shock protein 70 inhibitors. 1. 2,5′-thiodipyrimidine and 5-(phenylthio)pyrimidine acrylamides as irreversible binders to an allosteric site on heat shock protein 70. J. Med. Chem., 57, 1188–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodina A., Patel P.D., Kang Y., Patel Y., Baaklini I., Wong M.J., Taldone T., Yan P., Yang C., Maharaj R., et al. (2013) Identification of an allosteric pocket on human hsp70 reveals a mode of inhibition of this therapeutically important protein. Chem. Biol., 20, 1469–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rousaki A., Miyata Y., Jinwal U.K., Dickey C.A., Gestwicki J.E., Zuiderweg E.R. (2011) Allosteric drugs: the interaction of antitumor compound MKT-077 with human Hsp70 chaperones. J. Mol. Biol., 411, 614–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taldone T., Kang Y., Patel H.J., Patel M.R., Patel P.D., Rodina A., Patel Y., Gozman A., Maharaj R., Clement C.C., et al. (2014) Heat shock protein 70 inhibitors. 2. 2,5′-thiodipyrimidines, 5-(phenylthio)pyrimidines, 2-(pyridin-3-ylthio)pyrimidines, and 3-(phenylthio)pyridines as reversible binders to an allosteric site on heat shock protein 70. J. Med. Chem., 57, 1208–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlecht R., Scholz S.R., Dahmen H., Wegener A., Sirrenberg C., Musil D., Bomke J., Eggenweiler H.M., Mayer M.P., Bukau B. (2013) Functional analysis of Hsp70 inhibitors. PLoS one, 8, e78443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young J.C., Agashe V.R., Siegers K., Hartl F.U. (2004) Pathways of chaperone-mediated protein folding in the cytosol. Nat. Rev. Mol. Cell. Biol., 5, 781–791. [DOI] [PubMed] [Google Scholar]
- 15.Agueli C., Geraci F., Giudice G., Chimenti L., Cascino D., Sconzo G. (2001) A constitutive 70 kDa heat-shock protein is localized on the fibres of spindles and asters at metaphase in an ATP-dependent manner: a new chaperone role is proposed. Biochem. J., 360, 413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gache V., Louwagie M., Garin J., Caudron N., Lafanechere L., Valiron O. (2005) Identification of proteins binding the native tubulin dimer. Biochem. Biophys. Res. Comm., 327, 35–42. [DOI] [PubMed] [Google Scholar]
- 17.Hughes J.R., Meireles A.M., Fisher K.H., Garcia A., Antrobus P.R., Wainman A., Zitzmann N., Deane C., Ohkura H., Wakefield J.G. (2008) A microtubule interactome: complexes with roles in cell cycle and mitosis. PLoS Biol., 6, e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rattner J.B. (1991) hsp70 is localized to the centrosome of dividing HeLa cells. Exp. Cell Res., 195, 110–113. [DOI] [PubMed] [Google Scholar]
- 19.Yuan A., Mills R.G., Chia C.P., Bray J.J. (2000) Tubulin and neurofilament proteins are transported differently in axons of chicken motoneurons. Cell. Mol. Neurobiol., 20, 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez C., Padilla R., Paciucci R., Zabala J.C., Avila J. (1994) Binding of heat-shock protein 70 (hsp70) to tubulin. Arch. Biochem. Biophys., 310, 428–432. [DOI] [PubMed] [Google Scholar]
- 21.Dou F., Netzer W.J., Tanemura K., Li F., Hartl F.U., Takashima A., Gouras G.K., Greengard P., Xu H. (2003) Chaperones increase association of tau protein with microtubules. Proc. Natl Acad. Sci. USA, 100, 721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jinwal U.K., O'Leary J.C., III, Borysov S.I., Jones J.R., Li Q., Koren J., III, Abisambra J.F., Vestal G.D., Lawson L.Y., Johnson A.G., et al. (2010) Hsc70 rapidly engages tau after microtubule destabilization. J. Biol. Chem., 285, 16798–16805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarkar M., Kuret J., Lee G. (2008) Two motifs within the tau microtubule-binding domain mediate its association with the hsc70 molecular chaperone. J. Neurosci. Res., 86, 2763–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmad S., Ahuja R., Venner T.J., Gupta R.S. (1990) Identification of a protein altered in mutants resistant to microtubule inhibitors as a member of the major heat shock protein (hsp70) family. Mol. Cell. Biol., 10, 5160–5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sconzo G., Palla F., Agueli C., Spinelli G., Giudice G., Cascino D., Geraci F. (1999) Constitutive hsp70 is essential to mitosis during early cleavage of Paracentrotus lividus embryos: the blockage of constitutive hsp70 impairs mitosis. Biochem. Biophys. Res. Comm., 260, 143–149. [DOI] [PubMed] [Google Scholar]
- 26.Johnson V.E., Stewart W., Smith D.H. (2012) Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain Pathol., 22, 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasegawa M., Smith M.J., Goedert M. (1998) Tau proteins with FTDP-17 mutations have a reduced ability to promote microtubule assembly. FEBS Lett., 437, 207–210. [DOI] [PubMed] [Google Scholar]
- 28.Fischer D., Mukrasch M.D., von Bergen M., Klos-Witkowska A., Biernat J., Griesinger C., Mandelkow E., Zweckstetter M. (2007) Structural and microtubule binding properties of tau mutants of frontotemporal dementias. Biochemistry, 46, 2574–2582. [DOI] [PubMed] [Google Scholar]
- 29.Karagoz G.E., Duarte A.M., Akoury E., Ippel H., Biernat J., Moran Luengo T., Radli M., Didenko T., Nordhues B.A., Veprintsev D.B., et al. (2014) Hsp90-Tau complex reveals molecular basis for specificity in chaperone action. Cell, 156, 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jinwal U.K., Akoury E., Abisambra J.F., O'Leary J.C., III, Thompson A.D., Blair L.J., Jin Y., Bacon J., Nordhues B.A., Cockman M., et al. (2013) Imbalance of Hsp70 family variants fosters tau accumulation. FASEB J., 27, 1450–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dickey C.A., Kamal A., Lundgren K., Klosak N., Bailey R.M., Dunmore J., Ash P., Shoraka S., Zlatkovic J., Eckman C.B., et al. (2007) The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J. Clin. Invest., 117, 648–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jinwal U.K., Miyata Y., Koren J., III, Jones J.R., Trotter J.H., Chang L., O'Leary J., Morgan D., Lee D.C., Shults C.L., et al. (2009) Chemical manipulation of hsp70 ATPase activity regulates tau stability. J. Neurosci., 29, 12079–12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abisambra J., Jinwal U.K., Miyata Y., Rogers J., Blair L., Li X., Seguin S.P., Wang L., Jin Y., Bacon J., et al. (2013) Allosteric heat shock protein 70 inhibitors rapidly rescue synaptic plasticity deficits by reducing aberrant tau. Biol. Psychiatry, 74, 367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flaherty K.M., Wilbanks S.M., DeLuca-Flaherty C., McKay D.B. (1994) Structural basis of the 70-kilodalton heat shock cognate protein ATP hydrolytic activity. II. Structure of the active site with ADP or ATP bound to wild type and mutant ATPase fragment. J. Biol. Chem., 269, 12899–12907. [PubMed] [Google Scholar]
- 35.Breuzard G., Hubert P., Nouar R., De Bessa T., Devred F., Barbier P., Sturgis J.N., Peyrot V. (2013) Molecular mechanisms of Tau binding to microtubules and its role in microtubule dynamics in live cells. J. Cell Sci., 126, 2810–2819. [DOI] [PubMed] [Google Scholar]
- 36.Choi M.C., Raviv U., Miller H.P., Gaylord M.R., Kiris E., Ventimiglia D., Needleman D.J., Kim M.W., Wilson L., Feinstein S.C., et al. (2009) Human microtubule-associated-protein tau regulates the number of protofilaments in microtubules: a synchrotron x-ray scattering study. Biophys. J., 97, 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panda D., Samuel J.C., Massie M., Feinstein S.C., Wilson L. (2003) Differential regulation of microtubule dynamics by three- and four-repeat tau: implications for the onset of neurodegenerative disease. Proc. Natl Acad. Sci. USA, 100, 9548–9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weingarten M.D., Lockwood A.H., Hwo S.Y., Kirschner M.W. (1975) A protein factor essential for microtubule assembly. Proc. Natl Acad. Sci. USA, 72, 1858–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goode B.L., Denis P.E., Panda D., Radeke M.J., Miller H.P., Wilson L., Feinstein S.C. (1997) Functional interactions between the proline-rich and repeat regions of tau enhance microtubule binding and assembly. Mol. Biol. Cell, 8, 353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanchez I., Cohen W.D. (1994) Assembly and bundling of marginal band microtubule protein: role of tau. Cytoskeleton, 29, 57–71. [DOI] [PubMed] [Google Scholar]
- 41.Mayer M.P., Schroder H., Rudiger S., Paal K., Laufen T., Bukau B. (2000) Multistep mechanism of substrate binding determines chaperone activity of Hsp70. Nat. Struct. Biol., 7, 586–593. [DOI] [PubMed] [Google Scholar]
- 42.Kityk R., Kopp J., Sinning I., Mayer M.P. (2012) Structure and dynamics of the ATP-bound open conformation of Hsp70 chaperones. Mol. Cell, 48, 863–874. [DOI] [PubMed] [Google Scholar]
- 43.Koya K., Li Y., Wang H., Ukai T., Tatsuta N., Kawakami M., Shishido, Chen L.B. (1996) MKT-077, a novel rhodacyanine dye in clinical trials, exhibits anticarcinoma activity in preclinical studies based on selective mitochondrial accumulation. Cancer Res., 56, 538–543. [PubMed] [Google Scholar]
- 44.Modica-Napolitano J.S., Koya K., Weisberg E., Brunelli B.T., Li Y., Chen L.B. (1996) Selective damage to carcinoma mitochondria by the rhodacyanine MKT-077. Cancer Res., 56, 544–550. [PubMed] [Google Scholar]
- 45.Tikoo A., Shakri R., Connolly L., Hirokawa Y., Shishido T., Bowers B., Ye L.H., Kohama K., Simpson R.J., Maruta H. (2000) Treatment of ras-induced cancers by the F-actin-bundling drug MKT-077. Cancer J., 6, 162–168. [PubMed] [Google Scholar]
- 46.Wadhwa R., Sugihara T., Yoshida A., Nomura H., Reddel R.R., Simpson R., Maruta H., Kaul S.C. (2000) Selective toxicity of MKT-077 to cancer cells is mediated by its binding to the hsp70 family protein mot-2 and reactivation of p53 function. Cancer Res., 60, 6818–6821. [PubMed] [Google Scholar]
- 47.Koren J., III, Miyata Y., Kiray J., O'Leary J.C., III, Nguyen L., Guo J., Blair L.J., Li X., Jinwal U.K., Cheng J.Q., et al. (2012) Rhodacyanine derivative selectively targets cancer cells and overcomes tamoxifen resistance. PLoS one, 7, e35566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colvin T.A., Gabai V.L., Gong J., Calderwood S.K., Li H., Gummuluru S., Matchuk O.N., Smirnova S.G., Orlova N.V., Zamulaeva I.A., et al. (2014) Hsp70-Bag3 interactions regulate cancer-related signaling networks. Cancer Res., 74, 4731–4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iwasaki M., Homma S., Hishiya A., Dolezal S.J., Reed J.C., Takayama S. (2007) BAG3 regulates motility and adhesion of epithelial cancer cells. Cancer Res., 67, 10252–10259. [DOI] [PubMed] [Google Scholar]
- 50.Akihisa T., Kikuchi T., Nagai H., Ishii K., Tabata K., Suzuki T. (2011) 4-Hydroxyderricin from Angelica keiskei roots induces caspase-dependent apoptotic cell death in HL60 human leukemia cells. J. Oleo Sci., 60, 71–77. [DOI] [PubMed] [Google Scholar]
- 51.Parker A.L., Kavallaris M., McCarroll J.A. (2014) Microtubules and their role in cellular stress in cancer. Front. Oncol., 4, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baquero M.T., Lostritto K., Gustavson M.D., Bassi K.A., Appia F., Camp R.L., Molinaro A.M., Harris L.N., Rimm D.L. (2011) Evaluation of prognostic and predictive value of microtubule associated protein tau in two independent cohorts. Breast Cancer Res., 13, R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Z.H., Xiong Q.Y., Tu J.H., Gong Y., Qiu W., Zhang H.Q., Wei W.S., Hou Y.F., Cui W.Q. (2013) Tau proteins expressions in advanced breast cancer and its significance in taxane-containing neoadjuvant chemotherapy. Med. Oncol., 30, 591. [DOI] [PubMed] [Google Scholar]
- 54.Smoter M., Bodnar L., Grala B., Stec R., Zieniuk K., Kozlowski W., Szczylik C. (2013) Tau protein as a potential predictive marker in epithelial ovarian cancer patients treated with paclitaxel/platinum first-line chemotherapy. J. Exp. Clin. Can. Res., 32, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pentheroudakis G., Kalogeras K.T., Wirtz R.M., Grimani I., Zografos G., Gogas H., Stropp U., Pectasides D., Skarlos D., Hennig G., et al. (2009) Gene expression of estrogen receptor, progesterone receptor and microtubule-associated protein Tau in high-risk early breast cancer: a quest for molecular predictors of treatment benefit in the context of a Hellenic Cooperative Oncology Group trial. Breast Cancer Res. Treat., 116, 131–143. [DOI] [PubMed] [Google Scholar]
- 56.Pusztai L., Jeong J.H., Gong Y., Ross J.S., Kim C., Paik S., Rouzier R., Andre F., Hortobagyi G.N., Wolmark N., et al. (2009) Evaluation of microtubule-associated protein-Tau expression as a prognostic and predictive marker in the NSABP-B 28 randomized clinical trial. J. Clin. Oncol., 27, 4287–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rauch J.N., Gestwicki J.E. (2014) Binding of human nucleotide exchange factors to heat shock protein 70 (Hsp70) generates functionally distinct complexes in vitro. J. Biol. Chem., 289, 1402–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gogolla N., Galimberti I., DePaola V., Caroni P. (2006) Staining protocol for organotypic hippocampal slice cultures. Nat. Protoc., 1, 2452–2456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.