Abstract
PURPOSE
To evaluate the reliability of MRE using a spin-echo echo-planar imaging (SE-EPI) renal MRE technique in healthy volunteers
MATERIALS AND METHODS
Institutional review board approved prospective study in which all participants provided written informed consent. Sixteen healthy volunteers comprising seven males and nine females with a median age of 35 years (age range: 23 to 59 years) were included. Coronal 90-Hz and 60-Hz MRE acquisitions were performed twice within a 30-minute interval between examinations. Renal MRE reliability was assessed by i) test-retest repeatability, and ii) inter-rater agreement between two independent readers. The MRE-measured averaged renal stiffness values were evaluated using: intraclass correlation coefficient (ICC), Bland-Altman and the within-subject coefficient of variation (COV).
RESULTS
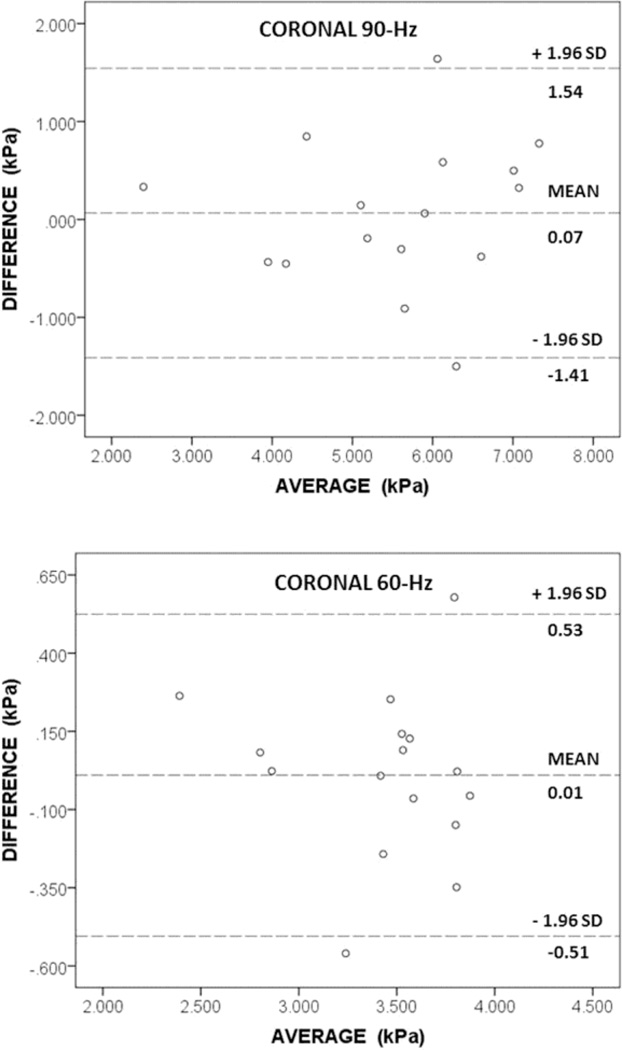
For test-retest repeatability, Bland-Altman showed a mean stiffness difference between examinations of 0.07 kPa (95% limits of agreement: −1.41, 1.54) at 90-Hz and 0.01 kPa (95% limits of agreement: −0.51, 0.53) at 60-Hz. Coefficient of repeatability was 1.47 kPa and 0.52 kPa at 90-Hz and 60-Hz, respectively. The within-subject COV was 13.6% and 7.7% at 90-Hz and 60-Hz, respectively. ICC values were 0.922 and 0.907 for test-retest repeatability and 0.998 and 0.989 for inter-rater agreement, respectively (p < 0.001).
CONCLUSION
SE-EPI renal MRE is a reliable technique
Keywords: Magnetic resonance elastography, Kidneys, Diagnostic reliability
INTRODUCTION
Conventional imaging, including ultrasound, computed tomography and magnetic resonance imaging (MRI), relies predominantly on morphology for detecting renal pathology such as obstructive uropathy but has limited ability to differentiate between pre-renal and intrinsic renal impairment. These techniques may be normal in early disease when renal dysfunction is potentially reversible (1). The capabilities of conventional imaging are further diminished in patients with moderate renal dysfunction as the use of iodinated CT contrast media is restricted due to the risk of contrast-induced nephropathy (2, 3). Furthermore, MR contrast agents such as gadodiamide, gadopentetate dimeglumine and gadoversetamide have been implicated in the development of nephrogenic systemic fibrosis (NSF) – this occurring mainly in patients with pre-existing severe renal impairment particularly those on dialysis (3–10). The risk of NSF is generally centred on the linear gadolinium chelates - rather than the macrocyclic gadolinium chelates – as these less stable chelates may undergo transmetallation with potential release of free gadolinium ions into tissues (6, 9, 10). The Contrast Media Safety Committee of the European Society of Urogenital Radiology (ESUR) suggests that ‘high-risk’ non-ionic linear gadolinium chelates should be contraindicated in patients with a glomerular filtration rate (GFR) < 30 while ‘medium-risk’ linear ionic chelates and the ‘low-risk’ cyclic chelates may be used under caution (4). In contrast, the American College of Radiologists (ACR) MRI safety group suggests that in patients with a GFR < 30, if a risk-benefit assessment warrants the use of a gadolinium contrast agent, then consideration should be given to prescribing the lowest dose that would achieve the diagnostic benefit that was sought, with a half dose, if clinically appropriate, being the default standard (5, 11).
MAGNETIC RESONANCE ELASTOGRAPHY (MRE) is an emerging diagnostic imaging test that characterises tissues based on their intrinsic biomechanical properties. These properties of living tissues, including between physiologic and pathologic states, vary over a wider dynamic range compared with conventional imaging modalities. Dynamic MRE was first described by Muthupillai and colleagues at the Mayo Clinic in 1995 (12). The examination can be performed on most contemporary MRI systems (1.5-T or higher) with the addition of a hardware and software upgrade. The MRE technique is a three step process involving i) the generation of mechanical waves in soft tissues, ii) imaging of tissue wave motion using a modified phase contrast MRI sequence, and iii) transforming the wave image using an inversion algorithm into an elastogram. The largest volume of collective experience on MRE is in the investigation of chronic liver disease. Multiple studies have confirmed the value of MRE for the non-invasive evaluation of liver fibrosis (13–19). The liver stiffness on MRE has been reported to show a consistent and systematic increase with increasing fibrosis scores (13). Using a stiffness threshold of 2.93 kPa at 60-Hz, Yin et al. found that MRE had 98% sensitivity, 99% specificity and 97% negative predictive value for liver fibrosis (13). Studies have also confirmed that liver MRE is a reliable test with good reproducibility and inter-rater agreement (20–23). Only a few exploratory studies, three in animals (24–26), and three in humans (27–29) have reported on the use of renal MRE. Preliminary studies have suggested that MRE is sensitive to pathophysiologic changes such as renal fibrosis and renal hypoperfusion (24–26, 28).
Renal MRE may be performed using a variety of MRI sequences and technical specifications. A spin-echo echo-planar imaging sequence (SE-EPI) is less susceptible to T2*-induced signal loss from iron deposition (in affected organs) compared with a gradient-echo sequence (18). To our knowledge, multislice 2D SE-EPI employing 3D inversion reconstruction for renal MRE has not previously been reported in human investigations. Given that renal MRE may have potential for clinical applications, it is appropriate that its reliability be evaluated. The purpose of this study was to evaluate the reliability of SE-EPI renal MRE in healthy volunteers as assessed by i) the test-retest repeatability and ii) inter-rater agreement.
MATERIALS AND METHODS
Institutional review board approval was granted for this prospective study and all participants provided written informed consent. Study duration was seven months from 1 October 2013 to 30 April 2014.
INCLUSION CRITERIA
-
▪
Healthy volunteers with no relevant medical co-morbidities
EXCLUSION CRITERIA
-
▪
Age < 18 years or > 85 years
-
▪
Pregnancy
-
▪
A recognised contraindication to MRI
EQUIPMENT
Examinations were performed on a 1.5-T whole body MRI scanner (Discovery MR450, General Electric, Waukesha, WI, USA) using an 8-channel receive-only phased-array coil. The MRE equipment consisted of an active driver unit [FIGURE 1A] and a passive driver for the kidneys [FIGURE 1B]. The active driver (Mayo Clinic MRE Prototype System, Rochester, MN, USA) was programmed to generate acoustic waves at 90-Hz and 60-Hz in separate acquisitions. A hollow polyvinyl chloride tube (7.62-m long, 2.5-cm inner diameter) was used to transmit the acoustic waves from the active driver to the passive driver. Renal MRE was performed using a prototype passive driver designed by the Mayo Clinic that converted the acoustic waves into mechanical vibrations. This consisted of two rectangular cushioned pads (each measuring 14.5 × 13.5 cm) connected to the polyvinyl chloride tube using a Y-connector. The cushioned pads were placed on the torso posteriorly – over the kidneys – with the patient lying supine on the table and orientated feet first in the scanner.
FIGURE 1.
(A) Photograph shows the active driver unit used for generating acoustic waves for the MRE examination. The vibration frequency is selected by turning the knob (arrow) on the control panel. (B) Photograph shows the custom designed Y-shaped renal passive driver. The cushioned pads (*) are placed over the kidneys with the patient positioned supine on the MRI table.
RENAL MRE SEQUENCES
Coronal 90-Hz and 60-Hz MRE acquisitions were performed twice, with a 30-minute interval between examinations – from the end of the first examination to the start of the second. Participants came off the MRI scanner after the completion of the first examination and then returned to be repositioned on the scanner for the second examination. Each examination was approximately 15 to 20 minutes in duration. Anatomical localization was performed using a single-shot fast- spin-echo (SSFSE) sequence and a steady-state free precession (SSFP) sequence. Satisfactory positioning of the driver pads over the kidneys was confirmed using markers built into the pads. A multislice, flow-compensated, SE-EPI sequence incorporating alternating motion-sensitising gradients (MSGs) along all three orthogonal directions of motion was performed to capture 3D vector wave data. Trigger pulses transmitted from the MRI scanner synchronised the active driver with the MSGs. A typical MRE sequence consisted of approximately 15 to 25 sections through the kidneys. All examinations were performed during suspended respiration, in expiration. The acquisition time per MRE sequence was between 60 to 70 seconds and involved six or seven breath holds of 11 seconds each. Typical imaging parameters included: repetition time (TR) / echo time (TE) = 1333 – 1584 ms / 37 – 40 ms; field of view = 36 × 36 cm; acquisition matrix = 72 × 72; slice thickness = 3.5 mm; slice spacing = 0 mm; number of excitations = 1; parallel imaging with acceleration factor = 2; 6 motion encoding directions (± X, ± Y, ± Z); motion encoding sensitivity 62.9 µm/(π radians); number of phase offsets = 3 and receiver bandwidth = ± 125 kHz.
IMAGING ANALYSIS
First, the 3D vector curl of the measured 3D vector wave field was calculated to remove longitudinal waves for the data and isolate the shear wave motion. Then, a 3D local frequency estimation (LFE) inversion algorithm was performed on the 3D vector curl data to generate quantitative stiffness maps, termed elastograms. Imaging analysis was performed on a 27-inch 3.4-GHz iMac desktop computer (Cupertino, CA, USA) using OsiriX (Pixmeo, Geneva, Switzerland). Two board certified radiologists acted as independent readers. The radiologists had seven months’ (Reader A, G.L.) and 19 years’ (Reader B, D.J.L.) experience performing MRE. On the magnitude images, each reader manually performed region of interest (ROI) tracings of the outline of the kidneys, enclosing the renal cross-sectional area [FIGURE 2]. The following were excluded from the ROI tracings: renal sinus, focal cysts and extreme boundary slices susceptible to partial volume effects. The ROIs were transposed onto matching elastogram images. The mean stiffness for each kidney was derived from the cumulative ROIs – i.e. the mean stiffness of each slice was summated and a cumulative mean value was then obtained. An averaged stiffness for the kidneys was then calculated as follows:
FIGURE 2.
Corresponding coronal images of the kidneys in a 40-year old male healthy volunteer. (A) Magnitude image, (B) Greyscale stiffness elastogram (90–Hz), and (C) RGB stiffness elastogram (90–Hz). Regions of interest (highlighted in green), enclosing the renal cross-sectional area, are illustrated. The elastograms show a stiffness of 6.1 kPa for the right kidney and 6.7 kPa for the left kidney.
STATISTICAL ANALYSIS
Continuous variables were expressed as median (interquartile range, IQR) or mean ± standard deviation (SD), as appropriate. The stiffness of right and left kidneys were assessed for significant differences using the Wilcoxon signed rank test. In addition, the MRE-measured averaged renal stiffness was subjected to the following statistical techniques:
-
▪
Bland-Altman (a graphical method to plot the difference scores of two measurements against the mean for each subject) (30, 31)
-
▪
Intraclass correlation coefficient (ICC)
-
▪
Within-subject coefficient of variation (COV)
-
▪
Shapiro-Wilk test
-
▪
Paired samples t-test
All analyses were performed on commercially available statistical software (IBM SPSS Statistics, version 21.0, NY, USA). A p-value < 0.05 was considered to be statistically significant.
RESULTS
The 16 healthy volunteers included seven males (43.8%) and nine females with a median age of 35 (20) years or mean age of 36 ± 11.1 years (range: 23 to 59 years). Anatomic assessment of the kidneys on the SSFSE and SSFP localizer sequences showed that the kidneys of all volunteers were of normal size and parenchymal thickness, symmetrical and there was no hydronephrosis or overt abnormality. The only incidental finding was a 2 cm simple cortical cyst in a 33-year old male participant. Renal stiffness was 6.0 (3.4) kPa for the right and 5.4 (1.9) kPa for the left at 90-Hz, and 3.5 (0.5) kPa for the right and 3.5 (0.5) kPa for the left at 60-Hz. No significant differences were noted between right or left renal stiffness (p = 0.098 and 0.148 at 90-Hz and 60-Hz, respectively). The non-significant higher stiffness for the right kidney compared to the left kidney at 90-Hz (but not 60-Hz) is likely due to random variation.
TEST-RETEST REPEATABILITY
The averaged renal stiffness and within-subject COV were 5.6 (1.9) kPa and 13.6% at 90-Hz and 3.4 (0.52) kPa and 7.7% at 60-Hz. Further information is presented in TABLE 1A and FIGURES 3 & 4. On Bland-Altman, mean test-retest difference for averaged renal stiffness was 0.07 kPa (95% limits of agreement: −1.41, 1.54) and 0.01 kPa (95% limits of agreement: −0.51, 0.53) at 90-Hz and 60-Hz, respectively. The coefficient of repeatability was 1.47 kPa and 0.52 kPa at 90-Hz and 60-Hz, respectively. A Shapiro-Wilk test of normality showed that the test-retest data was normally distributed. As such, the parametric paired samples t-test was performed on this data. For test 1 vs. test 2 at 90-Hz, the mean difference was 0.07 kPa with a standard deviation of 0.75 and a p-value of 0.7 suggesting no significant differences between the tests. For test 1 vs. test 2 at 60-Hz, the mean difference was 0.01 kPa with a standard deviation of 0.26 and a p-value of 0.8 suggesting no significant differences between the tests.
TABLE 1.
| (A). ICC VALUES FOR THE TEST-RETEST REPEATABILITY | |||
|---|---|---|---|
| Coronal | ICC | 95% CI | p-value |
| 90-Hz | 0.922 | 0.777 to 0.973 | < 0.001 |
| 60-Hz | 0.907 | 0.731 to 0.968 | < 0.001 |
| (B). ICC VALUES FOR THE INTER-RATER AGREEMENT | |||
|---|---|---|---|
| Coronal | ICC | 95% CI | p-value |
| 90-Hz | 0.998 | 0.995 to 0.999 | < 0.001 |
| 60-Hz | 0.989 | 0.969 to 0.996 | < 0.001 |
ICC – Intraclass Correlation Coefficient; CI – Confidence Interval
FIGURE 3.
Bland-Altman plots demonstrate the test-retest repeatability for the MRE technique at 90-Hz and 60-Hz
FIGURE 4.
Boxplots show the test-retest difference at 90-Hz and 60-Hz
INTER-RATER AGREEMENT
The ICC values between Reader A and B [TABLE 1B] were 0.998 and 0.989 at 90-Hz and 60-Hz, respectively (p < 0.001). The distribution of data for the averaged renal stiffness of the sample population, derived by each reader at 90-Hz and 60-Hz, is illustrated in FIGURE 5. On Bland-Altman [FIGURE 6], the mean difference for the averaged renal stiffness was 0.07 kPa (95% limits of agreement: −0.11, 0.25) and 0.02 kPa (95% limits of agreement: −0.12, 0.16) at 90-Hz and 60-Hz, respectively. The coefficient of repeatability was 0.18 kPa and 0.14 kPa at 90-Hz and 60-Hz, respectively.
FIGURE 5.
Boxplots show the distribution of data for the averaged renal stiffness of the sample population for each reader (A & B) at 90-Hz and 60-Hz
FIGURE 6.
Bland-Altman plots demonstrate the inter-rater agreement for the MRE technique at 90-Hz and 60-Hz
DISCUSSION
The analysis showed that renal MRE has excellent inter-rater agreement as judged by ICC. Test-retest repeatability was acceptable as judged by the same statistical measures. In addition, within-subject COV was 7.7% at 60-Hz and 13.6% at 90-Hz. Rouviere et al. evaluated renal MRE repeatability in ten healthy volunteers, who were each scanned twice (27). They measured the renal stiffness as 4.9 ± 0.5 kPa with a mean variation of 6% (2 to 16%) at 45-Hz and 9.4 ± 0.8 kPa with a mean variation of 6% (1 to 14%) at 76-Hz. There are several differences between Rouviere’s study and our study. Participant numbers and mean ages differed with ten participants (mean age 26 ± 2 years) in Rouviere’s study compared with 16 participants (mean age 36 ± 11.1 years) in our study. The time interval between examinations also differed with a 4 to 5 week interval in Rouviere’s study compared to a 30 minute interval in our study. The physiologic states of the subjects differed as participants in Rouviere et al.’s study underwent dietary preparation whereas our participants did not. The examination parameters were also different. Rouviere performed a gradient echo MRE acquisition of three sagittal sections of the left kidney with 5-mm slice thickness at 45-Hz and 76-Hz, calculated a 3D curl of median- and mean-filtered wave data, and used 2D LFE inversion with directional filtering to estimate tissue stiffness. We performed a multislice SE-EPI MRE with 10 to 20 sections of both kidneys in the coronal plane with 3.5-mm slice thickness at 90-Hz and 60-Hz, calculated a 3D curl of the wave field with no additional filtering, and used 3D LFE inversion without directional filtering to estimate tissue stiffness. Finally, the MRE metric varied as Rouviere evaluated renal shear velocity while we evaluated renal shear stiffness (which is the square of the shear velocity when using an LFE inversion algorithm). While tissue stiffness is expected to increase with frequency due to the dispersive and viscoelastic properties of tissue, the renal stiffness values from Rouviere et al. are systematically higher than the stiffness values reported here. This may be a consequence of the extra filtering and the use of 2D LFE inversion in their study that may overestimate the stiffness of tissue if waves travel obliquely to the imaging plane. In a cohort of 11 renal transplant recipients – the only reported clinical renal MRE study to our knowledge - Lee et al found that the mean renal stiffness was 5.5 ± 1.7 kPa at 90-Hz (similar to our findings), 7.5 kPa ± 2.1 kPa at 120-Hz and 7.5 ± 2.1 kPa at 150-Hz (28). Notwithstanding the renal stiffness variations between Rouviere’s study and ours, both show satisfactory repeatability for renal MRE. In clinical practice, a repeatability of < 20% is likely to be acceptable although human studies of the abnormal kidney will be needed to confirm this (32). Exploratory animal studies have already shown that renal perfusion (25, 33) and urinary pressure (33) are factors that can exert a substantial influence on renal stiffness. We speculate that the test-retest variation for renal MRE in this study is due to the combination of intrinsic measurement error and physiologic variability from renal haemodynamics and urinary pressure.
The study has some limitations. The study cohort comprised healthy volunteers rather than patients. However, this is an exploratory proof of concept study and it is envisaged that this experiments will be conducted next in patients. The small sample size and the influence of anisotropy on the renal stiffness measurements were not considered. On ultrasound elastography, Gennisson et al. showed that the anisotropic properties of the kidneys impacted the elasticity measurements (33). They found renal stiffness varied depending on whether the ultrasound beam was either parallel or perpendicular to the main axis of the medullary pyramids. The renal stiffness was 7.7 ± 2.3 kPa (cortex) and 8.7 ± 2.5 kPa (medulla) when the ultrasound beam was perpendicular to the main pyramid axis compared with 6.9 ± 1.4 kPa (cortex) and 6.6 ± 2.3 kPa (medulla) for a parallel orientation. Given these findings, it is conceivable that anisotropy may at least partly account for some of the variation in our stiffness measurements since our inversion algorithms assume isotropic tissue properties and we cannot control the polarization of the wave field as it enters and propagates through the kidneys. Further studies are required to evaluate the influence of anisotropy on MRE measurements of renal stiffness. In addition, our MRE examination was performed as a multiple breath-hold technique, which could cause data misregistration and may not be well tolerated by some patients. In the future, it may be possible to implement a rigid-body registration algorithm to register the kidney data from each breath hold before subsequent processing, introduce a visual feedback system to help guide subjects in performing more reproducible breath holds, or modify the acquisition and perform it as a respiratory-gated technique instead.
In conclusion, this study shows that SE-EPI MRE measurement of renal stiffness is a reliable technique as determined by test-retest repeatability and inter-rater agreement.
ACKNOWLEDGEMENTS
The authors wish to thank Dr. Jun Chen and Dr. Richard Ehman of the Mayo Clinic, for technical advice on the project and assistance with both hardware and software for the elastography examinations. Thanks also to the MR radiographers of our unit –Ms. Sally Hunter, Ms. Laura McCartney, Ms. Soraia Sousa and Mr. Dario Prudencio, for technical assistance with performing the MRE examinations. Finally, we also wish to acknowledge NIHR CBRC, Addenbrooke’s Charitable Trust and NIH grant EB001981 for support with the project.
REFERENCES
- 1.Vivier PH, Dolores M, Le Cloirec J, et al. Imaging evaluation of renal function: principles and limitations. Journal de radiologie. 2011;92(4):280–290. doi: 10.1016/j.jradio.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Andreucci M, Solomon R, Tasanarong A. Side effects of radiographic contrast media: pathogenesis, risk factors, and prevention. BioMed research international. 2014;2014:741018. doi: 10.1155/2014/741018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stacul F, van der Molen AJ, Reimer P, et al. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. European radiology. 2011;21(12):2527–2541. doi: 10.1007/s00330-011-2225-0. [DOI] [PubMed] [Google Scholar]
- 4.Thomsen HS, Morcos SK, Almen T, et al. Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR Contrast Medium Safety Committee guidelines. European radiology. 2013;23(2):307–318. doi: 10.1007/s00330-012-2597-9. [DOI] [PubMed] [Google Scholar]
- 5.Thomsen HS. How to avoid nephrogenic systemic fibrosis: current guidelines in Europe and the United States. Radiologic clinics of North America. 2009;47(5):871–875. vii. doi: 10.1016/j.rcl.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Vitti RA. Gadolinium-based contrast agents and nephrogenic systemic fibrosis. Radiology. 2009;250(3):959. doi: 10.1148/radiol.2503081497. author reply-60. [DOI] [PubMed] [Google Scholar]
- 7.Shellock FG, Spinazzi A. MRI safety update 2008: part 1, MRI contrast agents and nephrogenic systemic fibrosis. AJR American journal of roentgenology. 2008;191(4):1129–1139. doi: 10.2214/AJR.08.1038.1. [DOI] [PubMed] [Google Scholar]
- 8.Swaminathan S, Horn TD, Pellowski D, et al. Nephrogenic systemic fibrosis, gadolinium, and iron mobilization. The New England journal of medicine. 2007;357(7):720–722. doi: 10.1056/NEJMc070248. [DOI] [PubMed] [Google Scholar]
- 9.Idee JM, Port M, Dencausse A, Lancelot E, Corot C. Involvement of gadolinium chelates in the mechanism of nephrogenic systemic fibrosis: an update. Radiologic clinics of North America. 2009;47(5):855–869. vii. doi: 10.1016/j.rcl.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Prince MR, Zhang HL, Roditi GH, Leiner T, Kucharczyk W. Risk factors for NSF: a literature review. Journal of magnetic resonance imaging : JMRI. 2009;30(6):1298–1308. doi: 10.1002/jmri.21973. [DOI] [PubMed] [Google Scholar]
- 11.Kanal E, Barkovich AJ, Bell C, et al. ACR guidance document for safe MR practices: 2007. AJR American journal of roentgenology. 2007;188(6):1447–1474. doi: 10.2214/AJR.06.1616. [DOI] [PubMed] [Google Scholar]
- 12.Muthupillai R, Lomas DJ, Rossman PJ, Greenleaf JF, Manduca A, Ehman RL. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science. 1995;269(5232):1854–1857. doi: 10.1126/science.7569924. [DOI] [PubMed] [Google Scholar]
- 13.Yin M, Talwalkar JA, Glaser KJ, et al. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol. 2007;5(10):1207 e2–1213 e2. doi: 10.1016/j.cgh.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rouviere O, Yin M, Dresner MA, et al. MR elastography of the liver: preliminary results. Radiology. 2006;240(2):440–448. doi: 10.1148/radiol.2402050606. [DOI] [PubMed] [Google Scholar]
- 15.Huwart L, Peeters F, Sinkus R, et al. Liver fibrosis: non-invasive assessment with MR elastography. NMR Biomed. 2006;19(2):173–179. doi: 10.1002/nbm.1030. [DOI] [PubMed] [Google Scholar]
- 16.Huwart L, Sempoux C, Salameh N, et al. Liver fibrosis: noninvasive assessment with MR elastography versus aspartate aminotransferase-to-platelet ratio index. Radiology. 2007;245(2):458–466. doi: 10.1148/radiol.2452061673. [DOI] [PubMed] [Google Scholar]
- 17.Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135(1):32–40. doi: 10.1053/j.gastro.2008.03.076. [DOI] [PubMed] [Google Scholar]
- 18.Huwart L, Salameh N, ter Beek L, et al. MR elastography of liver fibrosis: preliminary results comparing spin-echo and echo-planar imaging. European radiology. 2008;18(11):2535–2541. doi: 10.1007/s00330-008-1051-5. [DOI] [PubMed] [Google Scholar]
- 19.Godfrey EM, Patterson AJ, Priest AN, et al. A comparison of MR elastography and 31P MR spectroscopy with histological staging of liver fibrosis. European radiology. 2012;22(12):2790–2797. doi: 10.1007/s00330-012-2527-x. [DOI] [PubMed] [Google Scholar]
- 20.Hines CD, Bley TA, Lindstrom MJ, Reeder SB. Repeatability of magnetic resonance elastography for quantification of hepatic stiffness. Journal of magnetic resonance imaging : JMRI. 2010;31(3):725–731. doi: 10.1002/jmri.22066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hines CD, Lindstrom MJ, Varma AK, Reeder SB. Effects of postprandial state and mesenteric blood flow on the repeatability of MR elastography in asymptomatic subjects. Journal of magnetic resonance imaging : JMRI. 2011;33(1):239–244. doi: 10.1002/jmri.22354. [DOI] [PubMed] [Google Scholar]
- 22.Shire NJ, Yin M, Chen J, et al. Test-retest repeatability of MR elastography for noninvasive liver fibrosis assessment in hepatitis C. Journal of magnetic resonance imaging : JMRI. 2011;34(4):947–955. doi: 10.1002/jmri.22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motosugi U, Ichikawa T, Sano K, et al. Magnetic resonance elastography of the liver: preliminary results and estimation of inter-rater reliability. Jpn J Radiol. 2010;28(8):623–627. doi: 10.1007/s11604-010-0478-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah NS, Kruse SA, Lager DJ, et al. Evaluation of renal parenchymal disease in a rat model with magnetic resonance elastography. Magn Reson Med. 2004;52(1):56–64. doi: 10.1002/mrm.20101. [DOI] [PubMed] [Google Scholar]
- 25.Warner L, Yin M, Glaser KJ, et al. Noninvasive In vivo assessment of renal tissue elasticity during graded renal ischemia using MR elastography. Invest Radiol. 2011;46(8):509–514. doi: 10.1097/RLI.0b013e3182183a95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korsmo MJ, Ebrahimi B, Eirin A, et al. Magnetic resonance elastography noninvasively detects in vivo renal medullary fibrosis secondary to swine renal artery stenosis. Invest Radiol. 2013;48(2):61–68. doi: 10.1097/RLI.0b013e31827a4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rouviere O, Souchon R, Pagnoux G, Menager JM, Chapelon JY. Magnetic resonance elastography of the kidneys: feasibility and reproducibility in young healthy adults. Journal of magnetic resonance imaging : JMRI. 2011;34(4):880–886. doi: 10.1002/jmri.22670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee CU, Glockner JF, Glaser KJ, et al. MR elastography in renal transplant patients and correlation with renal allograft biopsy: a feasibility study. Acad Radiol. 2012;19(7):834–841. doi: 10.1016/j.acra.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bensamoun SF, Robert L, Leclerc GE, Debernard L, Charleux F. Stiffness imaging of the kidney and adjacent abdominal tissues measured simultaneously using magnetic resonance elastography. Clinical imaging. 2011;35(4):284–287. doi: 10.1016/j.clinimag.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 31.Bland JM, Altman DG. Measuring agreement in method comparison studies. Statistical methods in medical research. 1999;8(2):135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 32.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 33.Gennisson JL, Grenier N, Combe C, Tanter M. Supersonic shear wave elastography of in vivo pig kidney: influence of blood pressure, urinary pressure and tissue anisotropy. Ultrasound Med Biol. 2012;38(9):1559–1567. doi: 10.1016/j.ultrasmedbio.2012.04.013. [DOI] [PubMed] [Google Scholar]