Abstract
Focal adhesion kinase (FAK) is a tyrosine kinase that interacts with a multitude of signaling partners and helps cells to survive in the face of various proapoptotic signals. One of the most important interactions for FAK is with the tumor suppressor protein p53. p53 binds not only to the amino-terminal domain of FAK but also to the FAK promoter to inhibit its transcription. A study now reports the biological implications of the kinase-independent interaction of FAK with p53, which opens up future perspectives in cell signaling and cancer research. We focus on FAK and p53 signaling, which link signal transduction pathways from the extracellular matrix and cytoplasm to the nucleus, in human and mouse cells. FAK is proposed to be a critical scaffold protein that sequesters proapoptotic proteins, such as p53, to mediate cell survival.
The sites of contact between cells and the extracellular matrix are called focal adhesions, and these represent areas of intense cellular signaling that is mediated in large part by the cell-surface adhesion receptors known as integrins and intracellular protein complexes. One of the critical focal adhesion signaling proteins is FAK, which was originally described in chick embryo fibroblasts transformed with v-src (1). FAK is a 125-kD protein that is tyrosine-phosphorylated and activated in response to integrin clustering (1). When FAK is activated, Tyr397 is autophosphorylated, which creates a high-affinity binding site for the Src homology 2 domains of Src family kinases. The interaction between Tyr397-phosphorylated FAK and Src leads to a cascade of tyrosine phosphorylation of multiple sites in FAK (residues Tyr576, Tyr577, and Tyr925), as well as in other signaling molecules such as the 130-kD adaptor protein Crk-associated substrate (p130Cas) and paxillin. This cascade results in cytoskeletal changes and activation of other downstream signaling pathways, including those of the guanosine triphosphatase Ras and the mitogen-activated protein kinases extracellular signal–regulated kinase 1 (ERK1) and ERK2. FAK provides critical survival signals for cells to resist apoptosis (2) and is important for cell motility, invasion, metastasis, and angiogenesis. FAK contains a typical central tyrosine kinase domain, which is flanked by long N-terminal and C-terminal regions that enable FAK to interact with various signaling and cytoskeletal molecules (1). By virtue of these scaffolding and kinase domains, a myriad of signaling pathways intersect with FAK, making its role rather enigmatic. Perturbations in FAK abundance and function are highly associated with cancer (3). When FAK is overexpressed in malignant cells, they can survive while they invade tissues or metastasize or while they are exposed to stresses such as cytotoxic chemotherapy or ionizing radiation (4–9). Given these findings, it is not surprising that one way that FAK could promote survival is by interacting with the tumor suppressor protein p53 (10, 11). p53 is the most frequent target for genetic alterations in human cancers, and it is mutated in almost 50% of all tumors (11). Inactivation of the p53 gene is one important step in tumorigenesis. After induction by various cell stresses such as DNA damage, hypoxia, or activated oncogenes, p53 stimulates the transcription of a set of genes that can promote cell death and growth arrest—such as those that encode p21, GADD45 (Growth arrest and DNA-damage-inducible 45), cyclin G, the proapoptotic protein Bax (Bcl-2–associating X protein), and the ubiquitin ligase Mdm2 (mouse double minute 2)—and inhibits the transcription of several cell-survival genes, including those that encode survivin, cell-division–cycle 2 (Cdc2), cyclin B1, Cdc25, stathmin, and the antiapoptotic protein Bcl-2 (11). We have shown not only that FAK and p53 physically interact, but also that FAK inhibits p53-mediated activation of the transcription of p21-, Mdm2-, and Bax-luciferase reporters through physical interaction with p53, which causes cancer cells to survive (10).
The report by Lim et al. now provides important insights into the biological mechanism and importance of this FAK-p53 interaction (12). They have demonstrated that FAK enters the nucleus, where it binds to and causes the degradation of p53 (12). This binding event occurs through a region in the N terminus of FAK known as the FERM (FAK N-terminal band 4.1/ezrin/radixin/moesin homology) domain. The FERM domain contains three lobes, which interact with the cytoplasmic regions of transmembrane receptors. The authors defined that different areas of the FAK FERM domain have their individual functions. The F1 lobe bound to p53, the F2 lobe was required for the nuclear localization of FAK, and the F3 lobe bound to Mdm2 protein (12). In spite of the homology between the FERM domains of FAK and the FAK homolog Pyk2, we did not find binding of Pyk2 with p53 (10).
The binding of FAK to Mdm2, which causes the ubiquitination and degradation of p53, is another important part of this study (12). The authors expressed FAK in reconstituted FAK−/−p21−/− fibroblasts and demonstrated that the FERM domain of FAK resulted in p53 turnover through enhanced Mdm2-dependent p53 ubiquitination. This provides evidence for a nuclear survival function of FAK through ubiquitination and degradation of p53 (12). Furthermore, this function did not require the kinase activity of FAK. This is one of the most provocative findings of this study, reinforcing the concept that FAK’s role in mediating cell survival depends on both kinase-dependent and kinase-independent signaling pathways.
But there is much more to this FAK-p53 story. p53 not only binds to the N-terminal domain of FAK (10, 12), but it also inhibits the transcription of FAK by directly binding to the FAK promoter (3, 11, 13) (Fig. 1). This finding has several implications for cellular survival. First, there must be a delicate cellular balance between the abundances of these proteins to prevent the cell from undergoing apoptotic cell death. Second, the study by Lim et al. suggests the presence of an amplification loop involving FAK that could drive the cell toward survival (12). For example, in a malignant cell in which FAK is more highly abundant that it is in a normal cell, there would be more FAK available to bind to p53 and prevent it from repressing FAK expression, thus leading to the accumulation of FAK in the cell. The balance and feedback loop between FAK and p53 expression have been shown both by (i) introducing adenoviral-expressed p53 into cells and measuring the abundance of FAK mRNA and protein (3), and by (ii) introducing adenoviral-expressed FAK into cells and measuring the abundance of p53 protein (12). This positive feedback loop, taken together with the targeted degradation of p53 mediated by FAK, would be predicted to have broad implications for enhancing cellular survival, independently of the kinase activity of FAK. How would this proposed mechanism work in a cell with mutated p53, which is not capable of repressing FAK transcription (3, 13)? We would predict that the abundance of FAK in these cells would increase because of increased FAK transcription. Indeed, we have shown a correlation between the incidence of mutations of p53 and the abundance of FAK mRNA (3) and protein in a large series (600 cases) of human breast cancers (14).
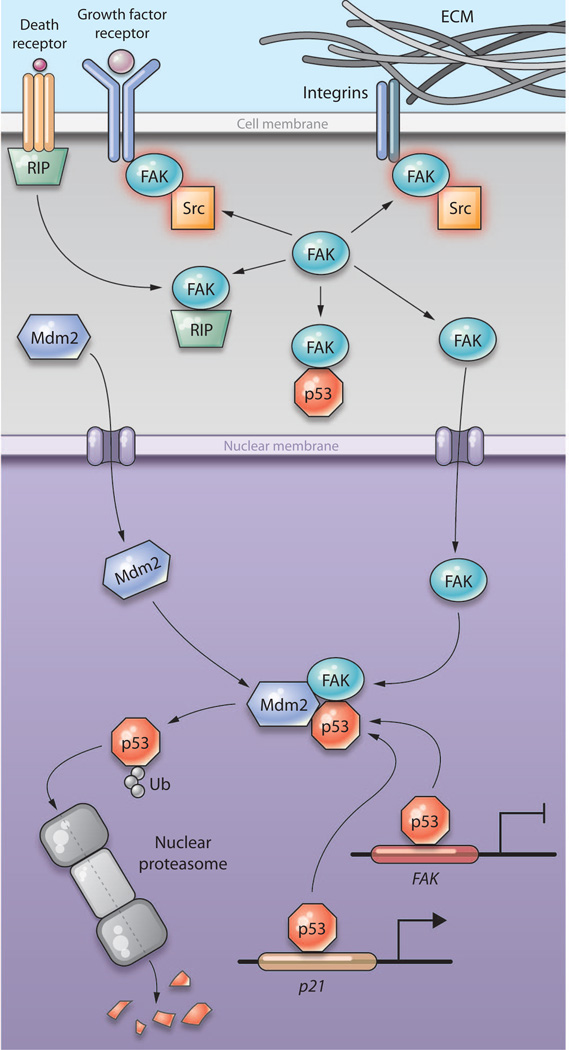
Fig. 1.
A model of FAK and p53 interactions and how they affect cell survival. FAK is normally recruited to activated integrins, and growth factor receptors, such as HER-1 or HER-2, or VEGFR-3, at focal adhesions at the cell surface. Through its kinase activity (shown as a red glow), FAK activates downstream signaling proteins, such as the kinase Src. In the absence of receptor signals, FAK accumulates in the cytoplasm, where, through a kinase-independent mechanism, FAK sequesters the protein kinase RIP. FAK also binds to p53 in the cytoplasm, but the effect of this interaction is unknown. FAK and p53 both shuttle between the cytoplasm and the nucleus to mediate prosurvival or proapoptotic signaling. Whereas p53 stimulates the transcription of the gene encoding p21, which causes cell cycle arrest, p53 binds to the promoter region of FAK to inhibit its transcription. In the nucleus, FAK—through its FERM domain—acts as a scaffold protein to bind to p53 and the E3 ubiquitin ligase Mdm2, causing the ubiquitination and degradation of p53. This results in increased cell survival and also serves as a feedforward mechanism to increase the abundance of FAK, by decreasing the p53-mediated repression of the FAK gene. FAK may also sequester other proapoptotic proteins in the nucleus and cytoplasm in a kinase-independent manner to promote cell survival. ECM, extracellular matrix; Ub, ubiquitin.
However, FAK influences cell survival by mechanisms other than by simply interacting with p53 (11). As a scaffold protein, FAK binds to several other signaling and cytoskeletal molecules. For example, FAK binds to the death domain protein receptor–interacting protein (RIP) (15). Although RIP has been described as an antiapoptotic protein, it is known to provide both prosurvival and proapoptotic signals. RIP is a death domain–containing serine/threonine kinase, which interacts weakly with the death receptors Fas and tumor necrosis factor receptor 1 (TNFR1) (16). RIP may directly link the TNFR1 complex to the caspase cascade (16). Overexpression of RIP protein induces the activation of the transcription factor nuclear factor κB, which is associated with protection from tumor necrosis factor–induced cell death (17). Thus, the RIP complex is capable of transducing signals that either activate or suppress cell death. The detailed mechanism by which such proapoptotic and antiapoptotic functions are controlled and switched in cells remains unknown. However, FAK promotes cell survival by binding to RIP and preventing it from accomplishing its proapoptotic functions (15). This suggests that FAK might sequester other proapoptotic signaling molecules to promote cell survival (Fig. 1). This sequestration model for FAK was originally proposed by S. Frisch (18) and provides one possible way to balance proapoptotic and prosurvival signals in the cell. Shifts in this balance could, on one hand, lead to embryonic lethality or, on the other hand, lead to the resistance to cell death exhibited by metastatic cancer cells. In addition, this scaffolding function of FAK leads to the binding of several receptor tyrosine kinases, including epidermal growth factor receptor 1 or human epidermal receptor 1 (HER-1), (19, 20), platelet-derived growth factor receptor (19), HER-2, c-Met (21), and vascular endothelial growth factor receptor 3 (VEGFR-3) (22). These interactions enhance FAK’s survival function, at least in part, by stabilizing the cytoplasmic localization of FAK as well as co-opting other survival pathways (17). This supports the concept of FAK as a critical integrator of signals from other receptor tyrosine kinases (19).
Both FAK and p53 shuttle between the cytoplasm and the nucleus (10, 12, 23–28). It is not known whether FAK is involved in the regulation of the cytoplasmic functions of p53 or in the binding and regulation of the Bax proteins, which promote apoptosis through increased mitochondrial membrane permeability, cytochrome c release, and caspase-3 activation. Future studies will add additional mechanistic data to identify other downstream pathways along the FAK-p53 signaling axis. Although FAK also binds p53 in the cytoplasm (10), it will be important to establish whether FAK regulates p53 function and expression in the cytoplasm, because p53 is now known to directly bind to Bax in the cytoplasm (28, 29). Thus, there is evidence for an apoptotic, transcription-independent function of p53 in the cytoplasm, which depends on the proline-rich region (PRR) in the N terminus of p53 (28, 29). Does the N-terminal FERM domain of FAK, which binds to p53 (10, 12), play a role only in cell survival by leading to the degradation of p53, or does it also have proapoptotic functions, and if so, how are these two functions balanced? Although a kinase-independent function of FAK adds even more complexity to its signaling pathways, it does provide an explanation of how prosurvival signals are transmitted from the extracellular matrix to the nucleus.
So what are the implications of these findings? The next step will be to determine whether there are any other tumor suppressor proteins that are sequestered and inhibited by FAK. Furthermore, it is possible that there are other kinases that function like FAK in sequestering proapoptotic proteins in a kinase-independent fashion. It will also be critical to determine the exact sites of interaction between FAK and p53. Recently, we identified the seven amino acid–binding site in the PRR of p53 (30), which is known to be involved in apoptotic signaling, but the exact site in FAK that interacts with p53 remains unknown. Because both of these molecules are implicated in cancer, further investigation of the interaction between FAK and p53 could lead to not only a better understanding of cancer biology, but also the development of strategies to regulate the balance between cell survival and apoptosis.
Perhaps the broadest implication of these findings will be in designing therapeutic strategies for targeting tyrosine kinase signaling. To date, most approaches have focused on targeting either the adenosine triphosphate–binding site of the enzyme or the extracellular ligand-receptor interaction. The kinase-independent functions of FAK that are now shown suggest that other approaches may be effective, especially those targeting protein-protein interactions. If the FAK-FERM–p53 interaction could be disrupted, it might be possible to shift the cellular balance one way or the other, and this could provide a new avenue for developing targeted therapeutics designed to induce apoptosis in cancer cells.
References
- 1.Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. pp125FAK, a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc. Natl. Acad. Sci. U.S.A. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu LH, Yang X, Bradham CA, Brenner DA, Baldwin AS, Craven RJ, Cance WG. The focal adhesion kinase suppresses transformation-associated, anchorage-independent apoptosis in human breast cancer cells. J. Biol. Chem. 2000;275:30597–30604. doi: 10.1074/jbc.M910027199. [DOI] [PubMed] [Google Scholar]
- 3.Golubovskaya VM, Finch R, Kweh F, Massoll NA, Campbell-Thompson M, Wallace MR, Cance WG. p53 regulates FAK expression in human tumor cells. Mol. Carcinog. 2008;47:373–382. doi: 10.1002/mc.20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiner TM, Liu ET, Craven RJ, Cance WG. Expression of focal adhesion kinase gene and invasive cancer. Lancet. 1993;342:1024–1025. doi: 10.1016/0140-6736(93)92881-s. [DOI] [PubMed] [Google Scholar]
- 5.Owens LV, Xu L, Dent GA, Yang X, Sturge GC, Craven RJ, Cance WG. Focal adhesion kinase as a marker of invasive potential in differentiated human thyroid cancer. Ann. Surg. Oncol. 1996;3:100–105. doi: 10.1007/BF02409059. [DOI] [PubMed] [Google Scholar]
- 6.Owens LV, Xu L, Craven RJ, Dent GA, Weiner TM, Kornberg L, Liu ET, Cance WG. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995;55:2752–2755. [PubMed] [Google Scholar]
- 7.Lightfoot HM, Lark A, Livasy CA, Moore DT, Cowan D, Dressler L, Craven RJ, Cance WG. Upregulation of focal adhesion kinase (FAK) expression in ductal carcinoma in situ (DCIS) is an early event in breast tumorigenesis. Breast Cancer Res. Treat. 2004;88:109–116. doi: 10.1007/s10549-004-1022-8. [DOI] [PubMed] [Google Scholar]
- 8.Lark AL, Livasy CA, Calvo B, Caskey L, Moore DT, Yang X, Cance WG. Overexpression of focal adhesion kinase in primary colorectal carcinomas and colorectal liver metastases: Immunohistochemistry and real-time PCR analyses. Clin. Cancer Res. 2003;9:215–222. [PubMed] [Google Scholar]
- 9.Cance WG, Harris JE, Iacocca MV, Roche E, Yang X, Chang J, Simkins S, Xu L. Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: Correlation with preinvasive and invasive phenotypes. Clin. Cancer Res. 2000;6:2417–2423. [PubMed] [Google Scholar]
- 10.Golubovskaya VM, Finch R, Cance WG. Direct interaction of the N-terminal domain of focal adhesion kinase with the N-terminal transactivation domain of p53*. J. Biol. Chem. 2005;280:25008–25021. doi: 10.1074/jbc.M414172200. [DOI] [PubMed] [Google Scholar]
- 11.Golubovskaya VM, Cance WG. Focal adhesion kinase and p53 signaling in cancer cells. Int. Rev. Cytol. 2007;263:103–153. doi: 10.1016/S0074-7696(07)63003-4. [DOI] [PubMed] [Google Scholar]
- 12.Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K, Larocque N, Fisher SJ, Schlaepfer DD, Ilic D. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol. Cell. 2008;29:9–22. doi: 10.1016/j.molcel.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golubovskaya V, Kaur A, Cance WG. Cloning and characterization of the promoter region of human focal adhesion kinase gene: Nuclear factor κB and p53 binding sites. Biochim. Biophys. Acta. 2004;1678:111–125. doi: 10.1016/j.bbaexp.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Golubovskaya V, Conway-Dorsey K, Moore D, Millikan R, Cance WG. FAK overexpression and p53 mutations positively correlate in a population-based series of breast cancer tumors. American Association for Cancer Research Proceedings Supplement. 2008;49:44. late-breaking abstract LB-155. [Google Scholar]
- 15.Kurenova E, Xu LH, Yang X, Baldwin AS, Jr, Craven RJ, Hanks SK, Liu Z-g, Cance WG. Focal adhesion kinase suppresses apoptosis by binding to the death domain of receptor-interacting protein. Mol. Cell. Biol. 2004;24:4361–4371. doi: 10.1128/MCB.24.10.4361-4371.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanger BZ, Leder P, Lee TH, Kim E, Seed B. RIP: A novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell. 1995;81:513–523. doi: 10.1016/0092-8674(95)90072-1. [DOI] [PubMed] [Google Scholar]
- 17.Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 18.Frisch S. personal communication [Google Scholar]
- 19.Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD. FAK integrates growth factor and integrin signals to promote cell migration. Nat. Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 20.Golubovskaya V, Beviglia L, Xu LH, Earp HS, III, Craven R, Cance W. Dual inhibition of focal adhesion kinase and epidermal growth factor receptor pathways cooperatively induces death receptor-mediated apoptosis in human breast cancer cells. J. Biol. Chem. 2002;277:38978–38987. doi: 10.1074/jbc.M205002200. [DOI] [PubMed] [Google Scholar]
- 21.Chen SY, Chen HC. Direct interaction of focal adhesion kinase (FAK) with Met is required for FAK to promote hepatocyte growth factor-induced cell invasion. Mol. Cell. Biol. 2006;26:5155–5167. doi: 10.1128/MCB.02186-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garces CA, Kurenova EV, Golubovskaya VM, Cance WG. Vascular endothelial growth factor receptor-3 and focal adhesion kinase bind and suppress apoptosis in breast cancer cells. Cancer Res. 2006;66:1446–1454. doi: 10.1158/0008-5472.CAN-05-1661. [DOI] [PubMed] [Google Scholar]
- 23.Beviglia L, Golubovskaya V, Xu L, Yang X, Craven RJ, Cance WG. Focal adhesion kinase N-terminus in breast carcinoma cells induces rounding, detachment and apoptosis. Biochem. J. 2003;373:201–210. doi: 10.1042/BJ20021846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones G, Machado J, Jr, Merlo A. Loss of focal adhesion kinase (FAK) inhibits epidermal growth factor receptor-dependent migration and induces aggregation of nh(2)-terminal FAK in the nuclei of apoptotic glioblastoma cells. Cancer Res. 2001;61:4978–4981. [PubMed] [Google Scholar]
- 25.Jones G, Stewart G. Nuclear import of N-terminal FAK by activation of the FcεRI receptor in RBL-2H3 cells. Biochem. Biophys. Res. Commun. 2004;314:39–45. doi: 10.1016/j.bbrc.2003.12.055. [DOI] [PubMed] [Google Scholar]
- 26.Lobo M, Zachary I. Nuclear localization and apoptotic regulation of an amino-terminal domain focal adhesion kinase fragment in endothelial cells. Biochem. Biophys. Res. Commun. 2000;276:1068–1074. doi: 10.1006/bbrc.2000.3547. [DOI] [PubMed] [Google Scholar]
- 27.Kadare G, Toutant M, Formstecher E, Corvol JC, Carnaud M, Boutterin MC, Girault JA. PIAS1-mediated sumoylation of focal adhesion kinase activates its autophosphorylation. J. Biol. Chem. 2003;278:47434–47440. doi: 10.1074/jbc.M308562200. [DOI] [PubMed] [Google Scholar]
- 28.Chipuk JE, Green DR. Cytoplasmic p53: Bax and Forward. Cell Cycle. 2004;3:429–431. [PubMed] [Google Scholar]
- 29.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 30.Golubovskaya V, Finch R, Zheng M, Kurenova EV, Cance WG. The 7 amino-acid site in the proline-rich region of the N-terminal domain of p53 is involved in interaction with FAK and is critical for p53 functioning. Biochem. J. 2008;411:151–160. doi: 10.1042/BJ20071657. [DOI] [PubMed] [Google Scholar]