Despite the epidemiological studies showing that increased levels of high density lipoprotein cholesterol (HDL-C) are associated with a reduced risk of future coronary heart disease (CHD), neither monogenic diseases characterized by extreme HDL-C levels nor genetic variants associated with higher HDL-C levels are associated with CHD risk reduction1, 2. Most importantly, increasing HDL-C levels as a therapeutic approach to reduce cardiovascular risk has been called into question by the recent failure of several randomized trials in which therapies aimed at increasing HDL-C levels such as niacin and cholesteryl ester transferase protein (CETP) inhibitors have failed to improve cardiovascular outcomes3-5.
However, it remains clear that HDL has multiple potential anti-atherogenic functions6, including its well-described role in reverse cholesterol transport (RCT) 7 (figure, panel A). Thus, a novel approach toward CHD prevention and HDL-targeting therapies focuses on HDL function rather than HDL-C levels. That cholesterol efflux capacity of HDL isolated from human subjects is inversely correlated with CHD status8 reinforces the concept that atheroprotection is associated with HDL function rather than HDL-C levels. In this issue of Arteriosclerosis Thrombosis and Vascular Biology, Gille et al. present evidence that in healthy volunteers, infusion of CSL112, a reconstituted HDL (rHDL) containing apolipoprotein A-I (apoA-I) and phospholipids improves biomarkers of RCT and cholesterol efflux when measured ex vivo.
Figure 1.
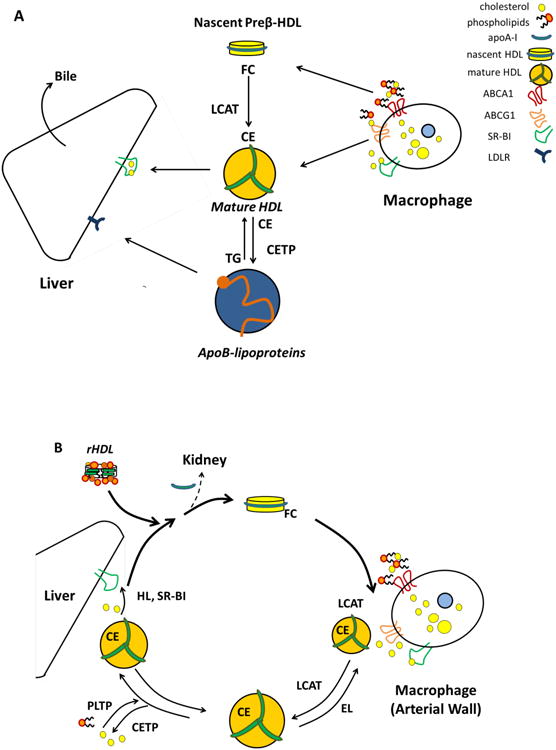
Panel A. Reverse cholesterol transport. ApoA-I (synthesized in the liver and intestines) acquires phospholipid and a small amount of free cholesterol via the ABCA1 transporter to form nascent Preβ-HDL. Preβ-HDL is the preferred acceptor of cholesterol effluxed by ABCA1 and acquires additional lipids from peripheral cells such as macrophages in the arterial wall. LCAT esterifies free cholesterol to hydrophobic cholesteryl esters, which migrate into the HDL core, driving the maturation of HDL into spherical α particles. Mature HDL particles are able to acquire additional lipids via other pathways, such as those mediated by ABCG1 or SR-BI, or by a passive diffusion process. Cholesterol carried on HDL can be delivered directly to the liver via SR-BI. Alternatively, cholesteryl ester present on HDL can be exchanged for triglycerides by CETP and transferred to VLDL and LDL, and delivered to the liver via the LDL-receptor. Once in the liver, cholesterol can be excreted with the bile, either as free cholesterol or after being converted to a bile salt. Panel B. HDL remodeling. HDL is constantly remodeled in the circulation by numerous enzymes and proteins. Nascent pre-β HDL acquires free cholesterol by ABCA1. HDL-free cholesterol is esterified by LCAT, to form mature HDL that can acquire more cholesterol. Mature HDL particles can unload their lipid cargo by several mechanism: via CETP that transfer cholesteryl ester to apoB-containing lipoproteins; via endothelial lipase (EL) and hepatic lipases (HL) that can hydrolyze phospholipid and triglycerides respectively; via liver SR-BI, that can selectively uptake cholesterol. Through the loss of apoA-I and other proteins, HDL particles lose volume, regenerating lipid poor, nascent pre-βHDL. Insufficiently lipidated apoA-I is catabolized by the kidney. Infusion of reconstituted HDL is associated with increased production of nascent, pre-β HDL, possibly favoring HDL remodeling and decreasing renal catabolism.
During the first step in RCT, cholesterol efflux from peripheral tissues, lipid-poor apoA-I particles (also called pre-beta particles or nascent HDL) interact with the membrane-bound ATP-binding cassette transporter ABCA1 to accept free cholesterol from cells, such as macrophages in the arterial wall. The free cholesterol is then esterified by lecithin cholesterol acyltransferase (LCAT)9 to facilitate the formation of mature HDL that in turn can accept cholesterol via other transporters. Animal studies show that either apoA-I overexpression or treatment with a bolus of reconstituted HDL (rHDL) removes cholesterol from plaques reversing the atherosclerotic process10, 11. rHDL containing either apoA-I Milano or wild type apoA-I result in similar plaque regression12, 13. Furthermore, high-dose single bolus rHDL infusions lead to acute plaque stabilization in animals14, suggesting that a rapid, dynamic change in HDL level and efflux may lead to plaque stabilization.
Several rHDL formulations have been used in human studies. In patients with acute coronary syndrome, rHDL infusions composed of either apoA-I Milano15 or wild type apoA-I16,17 reduced atheroma volume assessed by intravascular ultrasound compared to baseline values. However, no significant changes were found as compared with placebo groups. It is possible that in the context of acute coronary syndrome, biomarkers of plaque stability or reverse cholesterol transport may be more relevant than atheroma volume. Interestingly, in patients with peripheral vascular disease, infusion of the CSL-112 predecessor, CSL-111 resulted in decreased lipid content of excised femoral plaque as well as decreased inflammatory markers as compared with saline18. Furthermore, infusion of rHDL containing a recombinant pro-apoA-I has been shown to stimulate fecal steroid secretion in humans, suggesting upregulation of reverse cholesterol transport 19.
The present work by Gille et al. provides strong evidence that infusion of CSL112 markedly improves ex vivo cholesterol efflux capacity, the most accurate measure of the reverse cholesterol transport pathway available today. While total cholesterol efflux capacity is increased, the largest increase occurs in ABCA1-dependent efflux, which likely reflects the significant acute, dose-dependent increases in circulating pre-beta particles, the preferred ABCA1 substrate. The increase in pre-beta particles is likely a consequence of the participation of rHDL in HDL remodeling (figure, panel B). rHDL, with a composition similar to that of CSL112, have been shown to affect the activity of several key proteins in HDL metabolism and promote the production of pre-beta particles20, 21.
As expected, the initial rise in circulating cholesterol occurs as free cholesterol, with a peak reached 2-4 hours after the start of the CSL112 infusion. Interestingly, the peak in cholesteryl esters is observed at 24 hours, suggesting that LCAT may be a limiting factor. As esterification by LCAT enhances the ability of HDL to accept cholesterol and may contribute to reverse cholesterol transport 22, it would be interesting to assess if the concomitant administration of CSL-112 with recombinant LCAT may enhance the amount of cholesterol mobilized over time.
A limitation of this work is that cholesterol efflux capacity measured ex vivo remains a surrogate marker of RCT. Additionally, significant improvements in cholesterol efflux capacity and RCT may or may not result in a reduction in major adverse cardiac events in the months subsequent to an acute coronary syndrome. Studies will need to be performed in patients with acute coronary syndrome to assess the efficacy and safety of this strategy.
It is also important to consider that HDL-based therapies may have therapeutic effects extending beyond cholesterol efflux and RCT. Indeed, anti-inflammatory and nitric oxide promoting functions of HDL as well as improvement in glucose metabolism have been observed in patients receiving rHDL infusion18, 23-25. Alternatively, rHDL infusion has also been shown to have direct cardiac effects and can cause shortening of the QT interval, prolongation of which is associated with sudden cardiac death26.
In summary, unlike other therapies that increased HDL-C levels but failed to improve clinical outcomes in randomized controlled trials, rHDL therapies have the potential to reduce coronary disease and stabilize atherosclerotic plaque by improving cholesterol efflux and RCT. Further studies will determine the safety and efficacy of CSL112 in the acute coronary syndrome population, and whether CSL112 may have a broader role in stable coronary artery disease or diseases such as type II diabetes, arthritis, or heart failure. Though significant work remains to be done, there is reason for optimism about this approach as a promising new therapy.
Acknowledgments
Dr Javaheri's salary is supported by NIH grant 2T32 HL007843-16.
Disclosures: Dr. Cuchel has received grants from CSL Behring.
References
- 1.van Capelleveen JC, Bochem AE, Motazacker MM, Hovingh GK, Kastelein JJ. Genetics of HDL-C: a causal link to atherosclerosis? Current atherosclerosis reports. 2013;15:326. doi: 10.1007/s11883-013-0326-8. [DOI] [PubMed] [Google Scholar]
- 2.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–80. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Group HTC, Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, Armitage J. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–12. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 4.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. The New England journal of medicine. 2011;365:2255–67. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. The New England journal of medicine. 2012;367:2089–99. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 6.Rye KA, Barter PJ. Cardioprotective functions of HDLs. Journal of lipid research. 2014;55:168–79. doi: 10.1194/jlr.R039297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenson RS, Brewer HB, Jr, Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ, Remaley AT, Rothblat GH, Tall AR, Yvan-Charvet L. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–19. doi: 10.1161/CIRCULATIONAHA.111.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–35. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calabresi L, Simonelli S, Gomaraschi M, Franceschini G. Genetic lecithin:cholesterol acyltransferase deficiency and cardiovascular disease. Atherosclerosis. 2012;222:299–306. doi: 10.1016/j.atherosclerosis.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 10.Shah PK, Nilsson J, Kaul S, Fishbein MC, Ageland H, Hamsten A, Johansson J, Karpe F, Cercek B. Effects of recombinant apolipoprotein A-I(Milano) on aortic atherosclerosis in apolipoprotein E-deficient mice. Circulation. 1998;97:780–5. doi: 10.1161/01.cir.97.8.780. [DOI] [PubMed] [Google Scholar]
- 11.Shah PK. Apolipoprotein A-I/HDL infusion therapy for plaque stabilization-regression: a novel therapeutic approach. Current pharmaceutical design. 2007;13:1031–8. doi: 10.2174/138161207780487520. [DOI] [PubMed] [Google Scholar]
- 12.Feng Y, Van Craeyveld E, Jacobs F, Lievens J, Snoeys J, De Geest B. Wild-type apo A-I and apo A-I(Milano) gene transfer reduce native and transplant arteriosclerosis to a similar extent. Journal of molecular medicine. 2009;87:287–97. doi: 10.1007/s00109-008-0427-y. [DOI] [PubMed] [Google Scholar]
- 13.Lebherz C, Sanmiguel J, Wilson JM, Rader DJ. Gene transfer of wild-type apoA-I and apoA-I Milano reduce atherosclerosis to a similar extent. Cardiovascular diabetology. 2007;6:15. doi: 10.1186/1475-2840-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah PK, Yano J, Reyes O, Chyu KY, Kaul S, Bisgaier CL, Drake S, Cercek B. High-dose recombinant apolipoprotein A-I(milano) mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein e-deficient mice. Potential implications for acute plaque stabilization. Circulation. 2001;103:3047–50. doi: 10.1161/hc2501.092494. [DOI] [PubMed] [Google Scholar]
- 15.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2003;290:2292–300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 16.Tardif JC, Ballantyne CM, Barter P, et al. Effects of the high-density lipoprotein mimetic agent CER-001 on coronary atherosclerosis in patients with acute coronary syndromes: a randomized trial. Eur Heart J. 2014 doi: 10.1093/eurheartj/ehu171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tardif JC, Gregoire J, L'Allier PL, Ibrahim R, Lesperance J, Heinonen TM, Kouz S, Berry C, Basser R, Lavoie MA, Guertin MC, Rodes-Cabau J Effect of rHDL on Atherosclerosis-Safety and Efficacy (ERASE) Investigators. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2007;297:1675–82. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 18.Shaw JA, Bobik A, Murphy A, Kanellakis P, Blombery P, Mukhamedova N, Woollard K, Lyon S, Sviridov D, Dart AM. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circulation research. 2008;103:1084–91. doi: 10.1161/CIRCRESAHA.108.182063. [DOI] [PubMed] [Google Scholar]
- 19.Eriksson M, Carlson LA, Miettinen TA, Angelin B. Stimulation of fecal steroid excretion after infusion of recombinant proapolipoprotein A-I. Potential reverse cholesterol transport in humans. Circulation. 1999;100:594–8. doi: 10.1161/01.cir.100.6.594. [DOI] [PubMed] [Google Scholar]
- 20.Nanjee MN, Cooke CJ, Garvin R, Semeria F, Lewis G, Olszewski WL, Miller NE. Intravenous apoA-I/lecithin discs increase pre-beta-HDL concentration in tissue fluid and stimulate reverse cholesterol transport in humans. Journal of lipid research. 2001;42:1586–93. [PubMed] [Google Scholar]
- 21.Kujiraoka T, Nanjee MN, Oka T, Ito M, Nagano M, Cooke CJ, Takahashi S, Olszewski WL, Wong JS, Stepanova IP, Hamilton RL, Egashira T, Hattori H, Miller NE. Effects of intravenous apolipoprotein A-I/phosphatidylcholine discs on LCAT, PLTP, and CETP in plasma and peripheral lymph in humans. Arterioscler Thromb Vasc Biol. 2003;23:1653–9. doi: 10.1161/01.ATV.0000089328.23279.3F. [DOI] [PubMed] [Google Scholar]
- 22.Ahsan L, Ossoli AF, Freeman L, Vaisman B, Amar MJ, Shamburek RD, Remaley AT. Role of Lecithin: Cholesterol Acyltransferase in HDL Metabolism and Atherosclerosis. The HDL Handbook: Biological Functions and Clinical Implications. 2013:159. [Google Scholar]
- 23.Patel S, Drew BG, Nakhla S, Duffy SJ, Murphy AJ, Barter PJ, Rye KA, Chin-Dusting J, Hoang A, Sviridov D, Celermajer DS, Kingwell BA. Reconstituted high-density lipoprotein increases plasma high-density lipoprotein anti-inflammatory properties and cholesterol efflux capacity in patients with type 2 diabetes. Journal of the American College of Cardiology. 2009;53:962–71. doi: 10.1016/j.jacc.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Nieuwdorp M, Vergeer M, Bisoendial RJ, op't Roodt J, Levels H, Birjmohun RS, Kuivenhoven JA, Basser R, Rabelink TJ, Kastelein JJ, Stroes ES. Reconstituted HDL infusion restores endothelial function in patients with type 2 diabetes mellitus. Diabetologia. 2008;51:1081–4. doi: 10.1007/s00125-008-0975-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drew BG, Duffy SJ, Formosa MF, et al. High-density lipoprotein modulates glucose metabolism in patients with type 2 diabetes mellitus. Circulation. 2009;119:2103–11. doi: 10.1161/CIRCULATIONAHA.108.843219. [DOI] [PubMed] [Google Scholar]
- 26.Den Ruijter HM, Franssen R, Verkerk AO, van Wijk DF, Vaessen SF, Holleboom AG, Levels JH, Opthof T, Sungnoon R, Stroes ES. Reconstituted high-density lipoprotein shortens cardiac repolarization. Journal of the American College of Cardiology. 2011;58:40–44. doi: 10.1016/j.jacc.2010.11.072. [DOI] [PubMed] [Google Scholar]


