Abstract
Background
Cytosolic aldehyde dehydrogenase, or ALDH1A1, functions in ethanol detoxification, metabolism of neurotransmitters, and synthesis of retinoic acid. Because the promoter region of a gene can influence gene expression, the ALDH1A1 promoter regions were studied to identify polymorphism, to assess their functional significance, and to determine whether they were associated with a risk for developing alcoholism.
Methods
Sequence analysis was performed in the promoter region by using Asian, Caucasian, and African American subjects. The resulting polymorphisms were assessed for frequency in Asian, Caucasian, Jewish, and African American populations and tested for associations with alcohol dependence in Asian and African American populations of alcoholics and controls. The functional significance of each polymorphism was determined through in vitro expression analysis by using HeLa and HepG2 cells.
Results
Two polymorphisms, a 17 base pair (bp) deletion (−416/−432) and a 3 bp insertion (−524), were discovered in the ALDH1A1 promoter region: ALDH1A1*2 and ALDH1A1*3, respectively. ALDH1A1*2 was observed at frequencies of 0.035, 0.023, 0.023, and 0.012 in the Asian, Caucasian, Jewish, and African American populations, respectively. ALDH1A1*3 was observed only in the African American population, at a frequency of 0.029. By using HeLa and HepG2 cells for in vitro expression, the activity of the luciferase reporter gene was significantly decreased after transient transfection of ALDH1A1*3-luciferase compared with the wild-type construct ALDH1A1*1-luciferase. In an African American population, a trend for higher frequencies of the ALDH1A1*2 and ALDH1A1*3 alleles was observed in a population of alcoholics (p = 0.03 and f = 0.12, respectively) compared with the control population.
Conclusions
ALDH1A1*2 and ALDH1A1*3 may influence ALDH1A1 gene expression. Both ALDH1A1*2 and ALDH1A1*3 produce a trend in an African American population that may be indicative of an association with alcoholism; however, more samples are required to validate this observation. The underlying mechanisms contributing to these trends are still unknown.
Keywords: ALDH1A1, Alcoholism, Polymorphism, Human, Alcohol Metabolism
Human aldehyde dehydrogenase 1 (ALDH1A1) functions as an important enzyme in both the metabolism of acetaldehyde and the synthesis of retinoic acid (Elizondo et al., 2000; Ueshima et al., 1993). ALDH1A1 also has been implicated in several alcohol-related phenotypes, including alcoholism, alcohol-induced flushing, and alcohol sensitivity (Chan, 1986; Yoshida, 1992). Studies suggest that low ALDH1A1 activity may contribute to alcohol sensitivity and alcohol-induced flushing in Caucasians and some Asians (Ward et al., 1994; Yoshida et al., 1989). Adverse reactions resulting from reduced ALDH1A1 function may be influencing the predisposition for alcoholism in non-Asian populations (Eriksson, 2001). In fact, a study has identified a polymorphism in the coding region of ALDH1A1 that contributes to ethanol preference in high alcohol-preferring (HAP)/low alcohol-preferring (LAP) rats, suggesting that a functionally altered ALDH1A1 influences alcohol consumption in an animal model (Negoro et al., 1997; Nishiguchi et al., 2002). Due to its involvement in ethanol metabolism, ALDH1A1 is an interesting candidate for alcohol research.
Multiple aldehyde dehydrogenase isozymes have been characterized that exhibit similar functional properties implicated in ethanol detoxification, including ALDH1A1, ALDH1B1, ALDH2, and ALDH3A1 (Vasiliou and Pappa, 2000; Yoshida, 1992). The mitochondrial form of aldehyde dehydrogenase, or ALDH2, has been associated with a reduced incidence of alcoholism in certain Asian populations (Higuchi et al., 1995). In these populations, a functional polymorphism in ALDH2 leads to acetaldehyde accumulation, resulting in alcohol-induced flushing (Takeshita et al., 1994), but the underlying mechanism influencing alcoholic predisposition is still unknown (Li, 1997). The ALDH2 enzyme exhibits a higher affinity for acetaldehyde and primarily oxidizes acetaldehyde in humans (Klyosov et al., 1996); however, the functions of the ALDH isozymes in the central nervous system remain unclear (Stewart et al., 1996; Tank et al., 1986).
The promoter region contains regulatory binding sites that are involved in gene expression and tissue specificity (Mitchell and Tjian, 1989). Mutations in regulatory binding sites can substantially affect gene regulation, altering enzyme levels that can ultimately contribute to phenotypic variability throughout a population. Polymorphism in the ALDH1A1 promoter region could affect the steady-state levels of ALDH1A1 and alter acetaldehyde and retinoid metabolism. Thus far, variants of the regulatory region in the promoter of the ALDH1A1 gene have not yet been studied.
Although previous studies indicate that ALDH1A1 may contribute to alcoholism, alcohol sensitivity, and alcohol-induced flushing, no definitive evidence has been provided to adequately link ALDH1A1 to these phenotypes. The purpose of this study was to identify human ALDH1A1 promoter polymorphisms, to determine their functional significance, and to screen for associations between these polymorphisms and alcoholism.
METHODS
Sequence Analysis
The ALDH1A1 promoter region was sequenced by using genomic DNA from 10 Caucasians, 10 Asians, and 10 African Americans. On the basis of the human ALDH1A1 promoter sequence (accession number U28416), three primer pairs were designed: A1-forward (5′-ATGCTGGAGCACTGGTTTCTT-3′) and A1-reverse (5′-CAAAGCGGTGAGTAGGACAGG-3′), A2-forward (5′-CAGGGTTCTCTCCTCACCAG-3′) and A2-reverse (5′-GGCAGGAAGCCTTTGACTTT-3′), and A3-forward (5′-TGGTGATTGTGTGTGACAGTG-3′) and A3-reverse (5′-AGAATTTGAGGATTGAAAAGAGTC-3′). These primers were used to amplify three overlapping DNA fragments, extending from −690 to +100 with respect to the transcriptional start point (+1). The resulting polymerase chain reaction (PCR) products were purified (GenElute PCR Cleanup Kit, Sigma, St. Louis, MO) and sequenced (Thermo Sequenase Sequencing Kit, USB, Cleveland, OH).
Subjects
A general screen for frequency distribution of the ALDH1A1*1, ALDH1A1*2, and ALDH1A1*3 alleles was conducted by genotyping subjects in several populations, including Asian (n = 71), Caucasian (n = 239), African American (n = 85), and Jewish (n = 171). The Asian population consisted of Asian American men and women between the ages of 21 and 26 years recruited from the University of California, San Diego (Luczak et al., 2002), and the African American population included men and women from San Diego county who were between 18 and 25 years old (Ehlers et al., 2003). Both the Asian American and African American populations were sampled from the general population. The Caucasian population was selected as a control population and contained both men and women recruited from the Helsinki affiliation of the State Alcohol Company; all subjects were excluded who displayed a previous history of alcoholism. The Jewish population consisted of men and women ranging in age from 18 to 82 years and were all of Eastern European descent (Carr et al., 2002).
To screen for associations between these polymorphisms and alcoholism, alcoholic and control populations were studied that included Hans (n = 70), Koreans (n = 83), Mongolians (n = 42), and African Americans (n = 116). In the Asian populations, all subjects were males between the ages of 18 and 70 years, without serious current physical disorders, and with no cross-ethnic marriages; alcohol dependence was classified according to International Classification of Diseases, 10th revision, diagnostic criteria (Shen et al., 1997). The African American population consisted of males and females who were acquired from Howard University in Washington, DC; these subjects were classified for alcohol dependence according to DSM-IV criteria. The DNA used for genotyping was obtained from the DNA bank maintained by the Molecular Biology Core of the Indiana University Alcohol Research Center, and Fisher’s exact test was used to calculate the significance of differences in allele frequencies between populations.
Genotyping
Human genomic DNA was isolated from dried blood spots (Truett et al., 2000). The primers A4-forward (5′-GCACTGAAAATACACAAGACTGAT-3′) and A4-reverse (5′-AGAATTTGAGGATTGAAAAGAGTC-3′) were designed on the basis of the human ALDH1A1 promoter sequence (accession number U28416) and used in PCR reactions to obtain [α-33P]deoxycytidine triphosphate-radiolabeled fragments. Products were electrophoresed on 6% acrylamide denaturing gels and scored on the basis of the mobility of each resulting PCR fragment.
Luciferase Reporter Constructs
DNA samples were selected from subjects testing positive for each allelic variant: ALDH1A1*1, ALDH1A1*2, and ALDH1A1*3. Two primers were designed – A5-forward (5′-ATTAGAGCTCCTGCTGGCTTTTCTGTTC-3′) and A5-reverse (5′-CTTGAGATCTGGAACACAGGTGACTGGC-3′) – to encompass each polymorphism and contained flanking SstI and BglII restriction enzyme sites. Resulting PCR fragments were ligated into the pCR2.1-TOPO vector (Invitrogen, Carlsbad, CA) and sequenced to confirm that there were no errors. The plasmids were digested with SstI and BglII (Promega, Madison, WI). The resulting fragments were gel-isolated with the GenElute Agarose Spin Column (Sigma) and ligated to a linearized pGL-3-Basic vector upstream of the luciferase gene (Promega). Plasmids were isolated by using the EndoFree Plasmid Maxi Kit (Qiagen, Valencia, CA).
Transient Transfection and Luciferase Assays
Human liver HepG2 cells and human HeLa cells were cultured in Eagle’s minimal essential medium containing 7.5% NaHCO3, 2 mM Glutmax, 0.1 mM nonessential amino acids, 1 mM pyruvate, and 10% fetal bovine serum (Invitrogen) and maintained at 37°C in a humidified 5% CO2 incubator. Twenty-four hours before transfection, 5.0 × 104 cells were plated into each well of a 24-well plate. Then 0.5 μg of each pGL-3 luciferase test plasmid was transfected per well by using Tfx 50 reagent (Promega). Cytomegalovirus (2.5 ng) Renilla vector was co-transfected with each pGL-3-luciferase test plasmid to serve as an internal control for transfection efficiency. The cells were incubated at 37°C for 24 hr, washed with phosphate-buffered saline, and then harvested by using passive lysis buffer (Promega). The cell extracts were assayed for firefly and Renilla luciferase activities in a TD-20/20 Luminometer by using the Dual-Luciferase Reporter Assay System according to the manufacturer’s instructions (Promega). Luciferase assays were performed three to six times in triplicate by using plasmids that were independently purified at least twice.
RESULTS
Polymorphism in the Human ALDH1A1 Promoter
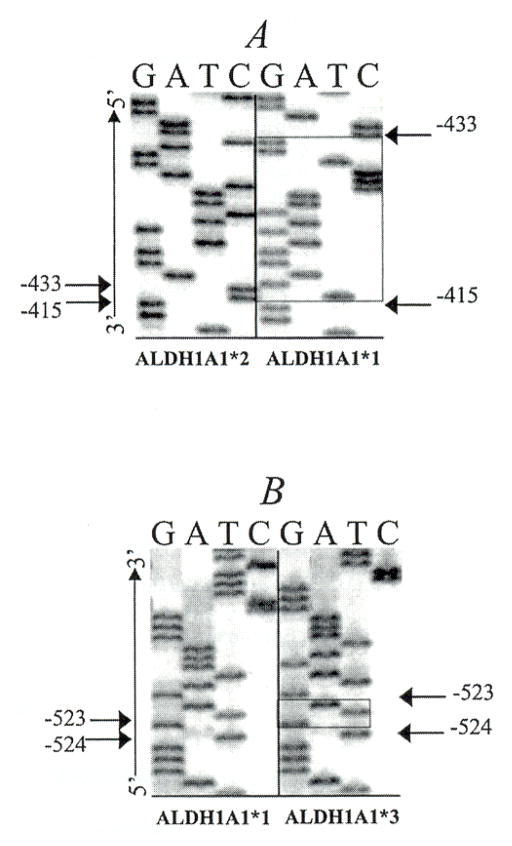
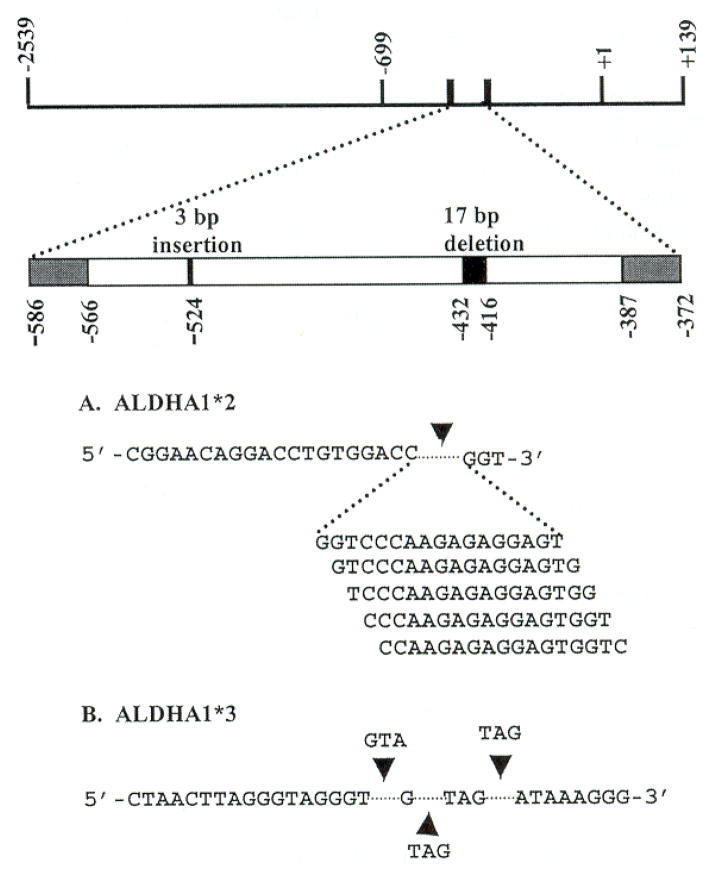
Genomic DNA from Asians, Caucasians, and African Americans was sequenced to screen for polymorphisms in the ALDH1A1 promoter region. Two polymorphisms, ALDH1A1*2 and ALDH1A1*3, were discovered in the promoter region of ALDH1A1: a 17 base pair (bp) deletion from position −416 to −432 relative to the transcriptional start site and a 3 bp insertion at −524, respectively (Fig. 1). Because a 4 bp repeat exists on either side of the deletion, five possible sequences could be deleted that would result in the same ALDH1A1*2 sequence (Fig. 2). There are three possible insertion sites (−524, −523, or −519) that would result in the same ALDH1A1*3 sequence (Fig. 2).
Fig. 1.
Sequence analysis of polymorphism in the human ALDH1A1 promoter region. (A) ALDH1A1*2: the box highlights the 17-bp sequence (−416/−432) that was deleted from ALDH1A1*1 to generate ALDH1A1*2. (B) ALDH1A1*3: the box designates the 3-bp insertion (−524). Arrows indicate common flanking nucleotides, and G, A, T, and C represent guanine, adenine, thiamine, and cytosine, respectively.
Fig. 2.
The human ALDH1A1 promoter region. Sequence analysis was performed on the region spanning −699 to +139 with respect to the transcriptional start point (+1). The magnified region extending from −586 to −372 designates the fragment that was amplified during the genotyping assay. Both polymorphisms are distinguished in this diagram: the 3-bp insertion (−524) and the 17-bp deletion (−416/−432). (A) The deletion of five possible fragments results in the same ALDH1A1*2 sequence due to flanking 5′-GGTC-3′ repeats. (B) Three possible insertions at −524, −523, or −519 generate the same ALDH1A1*3 sequence.
Frequency Distribution of ALDH1A1 Polymorphism in Various Ethnic Groups
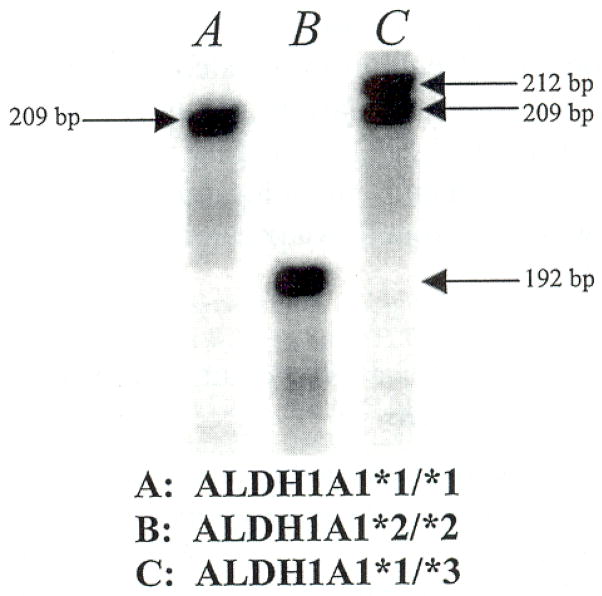
Asian, Caucasian, African American, and Jewish populations were genotyped to determine the frequency of each polymorphism endemic in each population. An example of an autoradiogram depicting the three possible ALDH1A1 alleles is shown in Fig. 3. The frequency of the ALDH1A1*2 allele varied among ethnic groups, with frequencies of 0.035, 0.023, 0.023, and 0.012 in the Asian, Caucasian, Jewish, and African American populations, respectively; ALDH1A1*3 was observed only in the African American population, at a frequency of 0.029 (Table 1).
Fig. 3.
Autoradiogram of ALDH1A1*1, ALDH1A1*2, and ALDH1A1*3 genotypes. PCR was used to generate [α-33P]deoxycytidine triphosphate-radiolabeled fragments that were later separated on a 6% acrylamide gel by using electrophoresis. Each allele, ALDH1A1*1, ALDH1A1*2, and ALDH1A1*3, can be readily identified with this procedure. (A) A homozygous Caucasian with the genotype ALDH1A1*1/*1, the wild-type genotype. (B) A homozygous Asian with the genotype ALDHlA1*2/*2. (C) A heterozygous African American with the genotype ALDH1A1*1/*3.
Table 1.
ALDH1A1 Genotype and Allele Frequencies for the General Population
| Population | Genotype frequency
|
Allele frequency
|
|||||
|---|---|---|---|---|---|---|---|
| *1/*1 | *1/*2 | *2/*2 | *1/*3 | *1 | *2 | *3 | |
| Asian (n = 71) | 0.93 | 0.07 | 0 | 0 | 0.97 | 0.03 | 0 |
| Caucasian (n = 239) | 0.95 | 0.05 | 0 | 0 | 0.98 | 0.02 | 0 |
| Jewish (n = 36) | 0.95 | 0.05 | 0 | 0 | 0.98 | 0.02 | 0 |
| African (n = 85) | 0.92 | 0.02 | 0 | 0.06 | 0.96 | 0.01 | 0.03 |
Increased Frequency of ALDH1A1*2 and ALDH1A1*3 Alleles in a Population of African American Alcoholics
To establish whether the polymorphism was associated with the risk for developing alcoholism, alcoholic and control Asian and African American populations were genotyped. In an African American population recruited from the same region of the United States, a significant association was observed between the ALDH1A1*3 allele and the population of alcoholics compared with the control group (p = 0.03); a trend was also observed for the ALDH1A1*2 allele to be more frequent in the alcoholic group (p = 0.12; Table 2). In Asian populations, ALDH1A1*3 was not observed, and ALDH1A1*2 yielded no significant association with alcoholism when controlling for the ALDH2*2 genotype that was determined in a previous study (Table 2; Shen et al., 1997).
Table 2.
Allele Frequency Distribution for Asian and African American Alcoholics and Controls
| Population | Genotype frequency
|
Allele frequency
|
|||||
|---|---|---|---|---|---|---|---|
| *1/*1 | *1/*2 | *2/*2 | *1/*3 | *1 | *2 | *3 | |
| Asian | |||||||
| Alcoholic (n = 111) | 0.95 | 0.05 | 0 | 0 | 0.97 | 0.03 | 0 |
| Control (n = 84) | 0.91 | 0.08 | 0.01 | 0 | 0.95 | 0.05 | 0 |
| Asiana | |||||||
| Alcoholic (n = 100) | 0.96 | 0.04 | 0 | 0 | 0.98 | 0.02 | 0 |
| Control (n = 49) | 0.96 | 0.02 | 0.02 | 0 | 0.98 | 0.02 | 0 |
| African American | |||||||
| Alcoholic (n = 80) | 0.82 | 0.07 | 0 | 0.11 | 0.91 | 0.03 | 0.06* |
| Control (n = 36) | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
Controlled for ALDH2*2.
The ALDH1A1*3 allele frequency was significantly different from the ALDH1A1*1 allele frequency (p = 0.03) with Fisher’s exact test.
Reduced ALDH1A1*3 Promoter Activity In Vitro With HepG2 and HeLa Cells
To determine the functional effect each polymorphism might have on ALDH1A1 gene expression, PCR was used to generate DNA fragments containing the ALDH1A1*1, ALDH1A1*2, and ALDH1A1*3 alleles. These fragments were cloned into the pGL-3-Basic luciferase vector upstream of the luciferase promoter. All constructs were transiently transfected into HepG2 cells and HeLa cells, which constitutively express the endogenous ALDH1A1 gene (Schnier et al., 1999; Yanagawa et al., 1995). Luciferase expression was expressed in fold-change compared with the ALDH1A1*1-luciferase (luc) construct (Fig. 4). The ALDH1A1*3-luc construct exhibited a significant decrease in expression in both HeLa and HepG2 cells (0.43- and 0.22-fold decreases, respectively) compared with the ALDH1A1*1-luc construct (p < 0.05). The ALDH1A1*2-luc vector did not significantly alter luciferase activity in either HeLa or HepG2 cells.
Fig. 4.
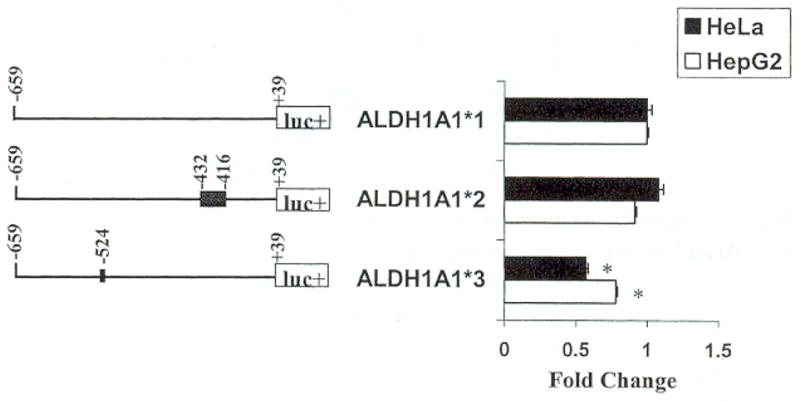
Functional importance of ALDH1A1 promoter polymorphisms. Plasmids ALDH1A1*1, ALDH1A1*2, and ALDH1A1*3 were transfected into HepG2 and HeLa cells; luc+ denotes the luciferase gene. Each respective fragment amplified from the ALDH1A1 promoter extended from −659 to +39 with respect to the transcriptional start point, and the polymorphisms, the deletion (−416/−432) and the insertion (−524), are noted. The activity of each construct was normalized by co-transfection of the internal control plasmid, pRL-CMV, and was expressed as fold change compared with the activity of ALDH1A1*1-luc. The mean ± SEM are representative of the results from three to six independent transfections performed in triplicate with two different plasmid preparations. The significance of the difference between mean values within and between multiple constructs was analyzed with ANOVA. ALDH1A1*3-luc significantly decreased luciferase expression in both Hep G2 and HeLa cells compared with ALDH1A1*1-luc (*p < 0.05). No significant change in expression was associated with ALDH1A1*2-luc.
DISCUSSION
In this study, two polymorphisms, ALDH1A1*2 and ALDH1A1*3, were discovered in the promoter region of human ALDH1A1. ALDH1A1*2, a 17-bp deletion, was identified in various populations, including Asians, Caucasians, and African Americans, whereas ALDH1A1*3, a 3-bp insertion, was observed only in African Americans. In both HeLa and HepG2 cells, ALDH1A1*3 significantly decreased expression of the luciferase reporter gene in transient transfection assays (p < 0.05). In a population of African Americans, the ALDH1A1*3 allele yielded a significant association with alcoholism (p = 0.03), and a trend was also observed between subjects carrying the ALDH1A1*2 allele and alcoholism (p = 0.12). However, unlike the ALDH2*2 allele, which is protective against alcoholism in Asians and is more prevalent in nonalcoholic subjects, the ALDH1A1*2 and ALDH1A1*3 alleles were more frequent in the African American alcoholic population. In fact, none of the control subjects carried the ALDH1A1*2 or ALDH1A1*3 alleles (Table 2). These results suggest that expression of ALDH1A1 may be modulated by the ALDH1A1*2 and ALDH1A1*3 polymorphisms, contributing to alcohol-related effects by a mechanism different from that of ALDH2*2.
In reporter gene assays, the ALDH1A1*3 polymorphism significantly decreased expression of the luciferase gene; therefore, our data suggest that ALDH1A1*3 may be functional. A transcription factor database (Transcription Element Search System; http://www.cbil.upenn.edu/tess/) was used to identify putative protein binding sites in the polymorphic regions of the ALDH1A1 promoter. The ALDH1A1*3 polymorphism (3-bp insertion) is located in close proximity to a predicted GATA binding site (Yanagawa et al., 1995), and the ALDH1A1*2 polymorphism (17-bp deletion) eliminates a predicted c-myb binding site from the promoter region of ALDH1A1. Although in vitro expression analysis provides an indicator of the functional significance associated with regulatory polymorphisms, these assessments may not fully reflect the regulatory mechanisms underlying gene expression in vivo.
A reduction in ALDH1A1 expression could potentially influence the physiology of the central nervous system contributing to documented etiological symptoms of alcoholism. The ALDH1A1 gene is expressed in various tissues throughout the body, including the central nervous system (Stewart et al., 1996), and is known to be involved in two important biochemical functions: ethanol metabolism and cell differentiation. ALDH1A1 eliminates acetaldehyde, a potentially pharmacological and neurotoxic agent, from the nervous system (Brien and Loomis, 1983). ALDH1A1 has also been implicated in the synthesis of retinoic acid, an important agent in many developmental processes and an essential precursor in vitamin A production (Crabb et al., 2001). In fact, it has been suggested that acetaldehyde and retinaldehyde competition may be a contributing factor in vitamin A abnormalities observed in alcoholics (Ambroziak et al., 1999). In addition, ALDH1A1 has been linked to retinoid signaling in the central nervous system, a system integral to complex learned behavior (Denisenko-Nehrbass et al., 2000). In short, ALDH1A1*2 and ALDH1A1*3 may affect various systems of the body, as well as the central nervous system.
A variety of genes have been identified, by using genetic studies of association, that influence an individual’s susceptibility to alcoholism; the most prominent of these genes code for enzymes in the alcohol metabolism pathway (Li, 2000). In this study, both ALDH1A1*2 and ALDH1A1*3 were observed at higher frequencies in African American alcoholics when compared with an African American control population. These associations may reflect cause and effect relationships between these polymorphisms and an increased risk for alcoholism. However, due to the multifactorial nature of alcoholism and the ubiquity and complexity of aldehyde dehydrogenase, it is difficult to postulate a mechanism underlying an association between ALDH1A1*2 and ALDH1A1*3 and alcohol-related phenotypes.
In conclusion, the promoter identified by the ALDH1A1*3 allele is significantly less active in transient transfection assays using both HepG2 and HeLa cells, and a tendency for an increase in ALDH1A1*2 and ALDH1A1*3 allele frequencies was observed in African American alcoholics. After controlling for the ALDH2*2 allele frequency, no association was evident between the ALDH1A1 polymorphisms and alcoholism in the Asian population. The underlying mechanisms associated with these polymorphisms are unknown; however, they seem to be different than that of the ALDH2*2 polymorphism observed in Asian populations. Thus, additional studies are needed to better understand the effects of these promoter polymorphisms on ALDH1A1 expression.
Acknowledgments
Supported by NIAAA Grants AA07611, AA00269, AA11257, and AA05546 and by Alcoholic Beverage Medical Research Foundation Grant U24-AA-11898.
References
- Ambroziak W, Izaguirre G, Pietruszko R. Metabolism of retinaldehyde and other aldehydes in soluble extracts of human liver and kidney. J Biol Chem. 1999;274:33366–33373. doi: 10.1074/jbc.274.47.33366. [DOI] [PubMed] [Google Scholar]
- Brien JF, Loomis CW. Pharmacology of acetaldehyde. Can J Physiol Pharmacol. 1983;61:1–22. doi: 10.1139/y83-001. [DOI] [PubMed] [Google Scholar]
- Carr LG, Foroud T, Stewart T, Castelluccio P, Edenberg HJ, Li T-K. Influence of ADH1B polymorphism on alcohol use and its subjective effects in a Jewish population. Am J Med. 2002;112:138–143. doi: 10.1002/ajmg.10674. [DOI] [PubMed] [Google Scholar]
- Chan AW. Racial differences in alcohol sensitivity. Alcohol Alcohol. 1986;21:93–104. [PubMed] [Google Scholar]
- Crabb DW, Pinairs J, Hasanadka R, Fang M, Leo MA, Lieber CS, Tsukamoto H, Motomura K, Miyahara T, Ohata M, Bosron W, Sanghani S, Kedishvili N, Shiraishi H, Yokoyama H, Miyagi M, Ishii H, Bergheim I, Menzl I, Parlesak A, Bode C. Alcohol and retinoids. Alcohol Clin Exp Res. 2001;25(Suppl 5):207S–217S. doi: 10.1097/00000374-200105051-00034. [DOI] [PubMed] [Google Scholar]
- Denisenko-Nehrbass NI, Jarvis E, Scharff C, Nottebohm F, Mello CV. Site-specific retinoic acid production in the brain of adult song-birds. Neuron. 2000;27:359–370. doi: 10.1016/s0896-6273(00)00043-x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Carr L, Betancourt M, Montane-Jaime K. Association of the ADH2*3 allele with greater alcohol expectancies in African-American young adults. J Stud Alcohol. 2003;64:176–181. doi: 10.15288/jsa.2003.64.176. [DOI] [PubMed] [Google Scholar]
- Elizondo G, Corchero J, Sterneck E, Gonzalez FJ. Feedback inhibition of the retinaldehyde dehydrogenase gene ALDH1 by retinoic acid through retinoic acid receptor alpha and CCAAT/enhancer-binding protein beta. J Biol Chem. 2000;275:39747–39753. doi: 10.1074/jbc.M004987200. [DOI] [PubMed] [Google Scholar]
- Eriksson CJ. The role of acetaldehyde in the actions of alcohol. Alcohol Clin Exp Res. 2001;25(Suppl 5):15S–32S. doi: 10.1097/00000374-200105051-00005. [DOI] [PubMed] [Google Scholar]
- Higuchi S, Matsushita S, Murayama M, Takagi S, Hayashida M. Alcohol and aldehyde dehydrogenase polymorphisms and the risk for alcoholism. Am J Psychiatry. 1995;152:1219–1221. doi: 10.1176/ajp.152.8.1219. [DOI] [PubMed] [Google Scholar]
- Klyosov AA, Rashkovetsky LG, Tahir MK, Keung WM. Possible role of liver cytosolic and mitochondrial aldehyde dehydrogenases in acetaldehyde metabolism. Biochemistry. 1996;35:4445–4456. doi: 10.1021/bi9521093. [DOI] [PubMed] [Google Scholar]
- Li T-K. Research direction. Liver Transpl Surg. 1997;3:296–299. doi: 10.1002/lt.500030316. [DOI] [PubMed] [Google Scholar]
- Li T-K. Pharmacogenetics of responses to alcohol and genes that influence alcohol drinking. J Stud Alcohol. 2000;61:5–12. doi: 10.15288/jsa.2000.61.5. [DOI] [PubMed] [Google Scholar]
- Luczak SE, Elvine-Kreis B, Shea SH, Carr LG, Wall TL. Genetic risk for alcoholism relates to level of response to alcohol in Asian-American men and women. J Stud Alcohol. 2002;63:74–82. [PubMed] [Google Scholar]
- Mitchell PJ, Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989;245:371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Negoro M, Hatake K, Taniguchi T, Ouchi H, Minami T, Hishida S. Liver cytosolic aldehyde dehydrogenase (ALDH1) polymorphism and its inheritance in Wistar rats. Nihon Arukoru Yakubutsu Igakkai Zasshi. 1997;32:182–188. [PubMed] [Google Scholar]
- Nishiguchi M, Kinoshita H, Mostofa J, Taniguchi T, Ouchi H, Minami T, Hatake K, Utsumi T, Motomura H, Hishida S. Different blood acetaldehyde concentration following ethanol administration in a newly developed high alcohol preference and low alcohol preference rat model system. Alcohol Alcohol. 2002;37:9–12. doi: 10.1093/alcalc/37.1.9. [DOI] [PubMed] [Google Scholar]
- Schnier JB, Kaur G, Kaiser A, Stinson SF, Sausville EA, Gardner J, Nishi K, Bradbury EM, Senderowicz AM. Identification of cytosolic aldehyde dehydrogenase 1 from non-small cell lung carcinomas as a flavopiridol-binding protein. FEBS Lett. 1999;454:100–104. doi: 10.1016/s0014-5793(99)00773-5. [DOI] [PubMed] [Google Scholar]
- Shen YC, Fan JH, Edenberg HJ, Li T-K, Cui YH, Wang YF, Tian CH, Zhou CF, Zhou RL, Wang J, Zhao ZL, Xia GY. Polymorphism of ADH and ALDH genes among four ethnic groups in China and effects upon the risk for alcoholism. Alcohol Clin Exp Res. 1997;21:1272–1277. [PubMed] [Google Scholar]
- Stewart MJ, Malek K, Crabb DW. Distribution of messenger RNAs for aldehyde dehydrogenase 1, aldehyde dehydrogenase 2, and aldehyde dehydrogenase 5 in human tissues. J Investig Med. 1996;44:42–46. [PubMed] [Google Scholar]
- Takeshita T, Morimoto K, Mao X, Hashimoto T, Furuyama J. Characterization of the three genotypes of low Km aldehyde dehydrogenase in a Japanese population. Hum Genet. 1994;94:217–223. doi: 10.1007/BF00208273. [DOI] [PubMed] [Google Scholar]
- Tank AW, Deitrich RA, Weiner H. Effects of induction of rat liver cytosolic aldehyde dehydrogenase on the oxidation of biogenic aldehydes. Biochem Pharmacol. 1986;35:4563–4569. doi: 10.1016/0006-2952(86)90779-3. [DOI] [PubMed] [Google Scholar]
- Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, Warman ML. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT) Biotechniques. 2000;29:52–54. doi: 10.2144/00291bm09. [DOI] [PubMed] [Google Scholar]
- Ueshima Y, Matsuda Y, Tsutsumi M, Takada A. Role of the aldehyde dehydrogenase-1 isozyme in the metabolism of acetaldehyde. Alcohol Alcohol Suppl. 1993;1B:15–19. doi: 10.1093/alcalc/28.supplement_1b.15. [DOI] [PubMed] [Google Scholar]
- Vasiliou V, Pappa A. Polymorphisms of human aldehyde dehydrogenases. Consequences for drug metabolism and disease. Pharmacology. 2000;61:192–198. doi: 10.1159/000028400. [DOI] [PubMed] [Google Scholar]
- Ward RJ, McPherson AJ, Chow C, Ealing J, Sherman DI, Yoshida A, Peters TJ. Identification and characterisation of alcohol-induced flushing in Caucasian subjects. Alcohol Alcohol. 1994;29:433–438. [PubMed] [Google Scholar]
- Yanagawa Y, Chen JC, Hsu LC, Yoshida A. The transcriptional regulation of human aldehyde dehydrogenase I gene. The structural and functional analysis of the promoter. J Biol Chem. 1995;270:17521–17527. doi: 10.1074/jbc.270.29.17521. [DOI] [PubMed] [Google Scholar]
- Yoshida A. Molecular genetics of human aldehyde dehydrogenase. Pharmacogenetics. 1992;2:139–147. doi: 10.1097/00008571-199208000-00001. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Dave V, Ward RJ, Peters TJ. Cytosolic aldehyde dehydrogenase (ALDH1) variants found in alcohol flushers. Ann Hum Genet. 1989;53:1–7. doi: 10.1111/j.1469-1809.1989.tb01116.x. [DOI] [PubMed] [Google Scholar]