Abstract
We report herein a 41-year-old female with a tubo-ovarian abscess (TOA), which microbial cultures showed to contain extended-spectrum beta-lactamase (ESBL)-producing E. coli, a causative agent of community-acquired infection. The patient initially presented with acute abdominal pain and back pain. Pelvic computed tomography and transvaginal ultrasonography revealed multiple cystic lesions in the bilateral ovaries that suggested TOA. An emergency laparotomy was therefore performed due to the potential for life-threatening septic shock from the TOA-associated pelvic inflammatory disease. Microbial cultures of postoperative fluid discharge from the placed intra-abdominal catheter, vaginal secretions, urine, blood, and feces detected ESBL-producing E.coli. In summary, we successfully performed emergency surgery for life-threatening septic TOA caused by ESBL-producing E. coli infection.
Keywords: Emergency surgery, Tubo-ovarian abscess, Extended-spectrum beta-lactamase, Surgery
Background
A tubo-ovarian abscess (TOA) can develop in reproductive age women with pelvic inflammatory disease (PID). The cause of TOA is mostly ascending infection through the uterus (mainly sexually transmitted diseases) due to Neisseria gonorrhoeae, Chlamydia, Escherichia coli (E. coli), and/or endogenous bacteria of the vagina and cervix [1, 2]. The management of TOA is a fundamentally conservative treatment with systemic broad-spectrum antibiotics. However, a TOA can have serious and potentially life-threatening consequences when there is a risk of abscess rupture. In such cases, antibiotic therapy is not sufficient for treating the TOA, and surgical drainage must be performed [3–6].
In this case report of a patient with TOA, surgery was indicated after the initial diagnosis due to the immediate threat of clinically dangerous septic shock. Although the abscesses were not ruptured, infections to the vagina, uterus, and bilateral ovaries by extended-spectrum beta-lactamase (ESBL)-producing E. coli had the potential to induce septic shock and disseminated intravascular coagulation, both of which can be life-threatening conditions. ESBL-producing bacteria infect individuals with impaired immunity, have resistance to many antibiotics, and can cause nosocomial infection [7–12]. To our knowledge, this is the first reported case of a patient with TOA caused by ESBL-producing E. coli. and treated with emergency surgery.
Case presentation
A 41-year-old woman presenting with acute abdominal and back pain was admitted into a neighboring hospital. She was initially diagnosed with a urinary tract infection and received systemic antibiotic therapy for 2 days. She complained of worsening abdominal pain and high fever, suggesting PID due to bacterial infection into the bilateral ovaries, and was subsequently referred to our hospital. She was previously healthy with no history of heavy alcohol consumption, smoking, diabetes mellitus, or overseas travel.
Physical examination revealed a body temperature of 40 degrees, mild abdominal pain, and markedly anemic conjunctiva. Her abdomen was soft, but showed mild tenderness and rebound pain. Hematological and biochemical testing showed a red blood cell count of 280 × 104/uL (normal range, 370–490 × 104/uL), hematocrit of 22.5 % (normal range, 34–49 %), hemoglobin (Hb) level of 7.3 g/dL (normal range, 11.2–15.7 g/dL), white blood cell count of 16,500/uL, platelet count of 18,000/uL, C-reactive protein level of 18.0 mg/dL, aspartate transaminase (AST) of 146 IU/L (normal range, 8–38 IU/L), alanine transaminase (ALT) of 79 IU/L (normal range, 4–44 IU/L), alkaline phosphatase (ALP) of 563 IU/L (normal range, 115–359 IU/L), lactate dehydrogenase (LD) of 899 IU/L (normal range, 106–211 IU/L); total protein concentration of 4.6 g/dL (normal range, 6.7–8.3 g/dL), albumin levels of 2.0 g/dL (normal range, 3.8–5.3 g/dL), cholinesterase concentration of 104 IU/L (normal range, 229–521 IU/L), creatinine concentration of 3.1 mg/dL (normal range, 0.4–0.8 mg/dL), prothrombin time of 44.1 % (normal range, 77.0–104.0 %), activated partial thromboplastin time, 47.7 s (normal range, 20.0 to 40.0 s), fibrin/fibrinogen degradation products at 239.4 ug/mL (normal range, 0.0–10.0 ug/mL), D-dimer levels of 75.1 ug/mL (normal range, 0.0–1.0 ug/mL), antithrombin 3 activity of 53 % (normal range, 81–123 %), base excesses of −13.0 mmol/L (normal range, −3.0–3.0 mmol/L), and lactate concentration of 12.9 mmol/L (normal range, 0.5–2.0 mmol/L). Other laboratory tests, including tumor markers (carcinoembryonic antigen, carbohydrate 19–9, and carbohydrate antigen 125) and viral status for hepatitis B status and C status, were normal. Abdominal computed tomography (CT) revealed thick-walled multilocular cystic lesions in the bilateral ovaries, and no ascites in the pelvis (Fig. 1). TOA was strongly suspected, and the patient underwent transvaginal ultrasonography, which demonstrated an 11.1 × 7.9 cm left ovarian cystic mass with low echogenicity, and a 6.3 × 4.4 cm right ovarian mass with a similar echo-texture (Fig. 2). For further definitive diagnosis, ultrasound-guided puncture was conducted in the cystic mass and revealed a purulent content. Together, the clinical presentation and imaging findings provided a diagnosis of TOA. An emergency laparotomy was performed based on the clinical diagnosis of septic shock and already completed DIC due to the TOA.
Fig. 1.
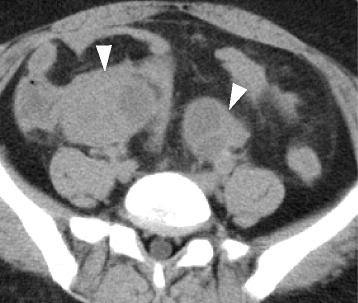
Abdominal computed tomography revealed thick-walled multilocular cystic lesions at the bilateral ovaries (white arrowheads)
Fig. 2.
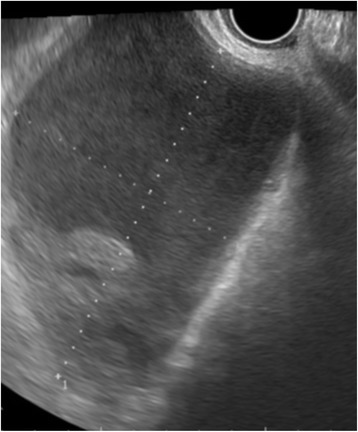
Transvaginal sonography revealed cystic lesions with septum at the bilateral ovary
The gross examination during surgery showed inflammation around the bilateral ovaries that was so marked that it prevented identification of individual organs. The bilateral ovaries and uterus were firm to palpate; however, the vagina in the pelvic cavity was relatively soft on palpitation. The inflammatory lesions including the ovaries and the uterus containing a uterine fibroid were resected to control oozing hemorrhage from the pelvic cavity because the patient showed evidence of DIC. During this procedure, the cystic component was partially damaged and discharged a small amount of purulent fluid, which subsequent cultures showed to contain ESBL-producing E.coli. Resected specimens of the bilateral ovaries showed marked swelling and thick-walled multilocular cystic lesions with intraluminal abscesses (Fig. 3). Microscopic examination of the resected specimen revealed infiltration of foamy cells and neutrophils into the abscess cavity (Fig. 4). No cell types showed atypical mitotic images or pleomorphisms.
Fig. 3.
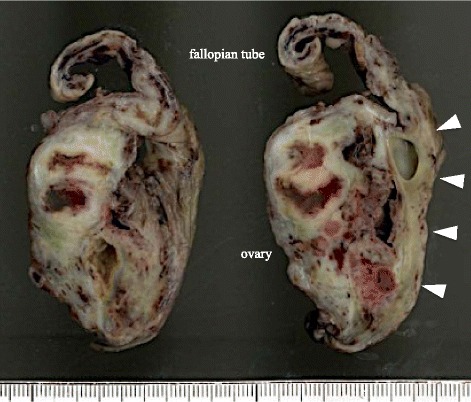
Resected specimen of ovary, showing marked swelling of the bilateral ovaries with intraluminal abscess. Pathological examination revealed the presence of ectopic endometriosis around the ovary (white arrowheads)
Fig. 4.
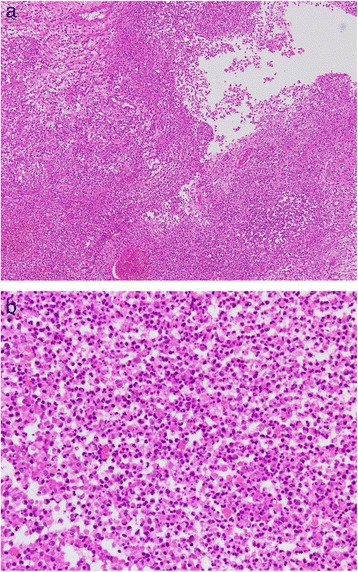
a HE stain ×10. The ovary showed a high degree of neutrophil infiltration with abscess formation. b HE stain ×100. High-power magnification showed a cluster of neutrophils in the ovary
Postoperative microbial cultures of the fluid discharge from the placed intra-abdominal catheter, vaginal secretions, urine, blood, and feces detected ESBL-producing E.coli. The patient suffered further abscess formation due to infection of the postoperative hematoma in the pelvic cavity, and underwent a repeat laparotomy with abdominal drainage for diffuse peritonitis 12 days after the initial surgery. She subsequently suffered intra-abdominal septic complications, and underwent ultrasound-guided percutaneous drainage on the 27th postoperative day. After the drainage, she made a favorable recovery and was discharged from our hospital 72 days after the first emergency surgery.
Discussion
TOA is most frequently induced by ascending infection through the uterus due to Neisseria, gonorrhoeae, Chlamydia, E. coli, or indigenous bacteria of the vagina and cervix, and it usually follows PID [1, 2]. Herein, we present the first reported case of a patient with TOA caused by ESBL-producing E. coli.
ESBL-producing bacteria is well known as one of the multiple-antibiotic-resistant bacterium identified by German Knothe in 1983 [13]. Our case had no comorbidity, including diabetes mellitus, respiratory disease, ischemic heart disease, immune-deficiency disease, and had never been hospitalized following birth. Thus, we considered this case to be a community-acquired infection. Most infections due to ESBL-producing bacteria remain hospital-acquired and are even more common among long-term hospitalized and immunocompromised patients [8]. Recently, however, the incidence of community-acquired infection by ESBL-producing bacteria has gradually increased in Western countries, although it remains rare in Japan, and the increasing numbers reported have been attributed to advances in diagnostic techniques and equipments [12, 14, 15]. In the recent years, it has been reported that the incidence of colonization induced by community-acquired ESBL-producing bacteria from urine and/or feces has been increased, even with patients with no comorbidities in Japan as the West. Therefore, the current study suggested that physicians should pay attention to the community-acquired infection of ESBL-producing bacteria which might have been increased in the future [14, 16, 17].
Treatment of TOA is important to avoid complications such as life-threatening abscess rupture and sepsis, and to preserve fertility, so early detection and treatment of TOA can prevent such adverse outcomes. However, the peculiar clinical signs of TOA are absent, such as lower abdominal tenderness, abnormal vaginal or cervical discharge, fever, abnormal vaginal bleeding, dyspareunia, cervical motion tenderness, and adnexal tenderness [18, 19]. Furthermore, it was also very difficult to detect clinically TOA according to laboratory investigations like following; findings, presence of excess leucocytes, and/or C-reactive protein [19]. The differential diagnosis for TOA includes a wide array, such as reproductive system disease, or gastrointestinal inflammation, urinary tract infection. Therefore the diagnosis of TOA based on clinical criteria alone at the early stage is difficult and inaccurate [18, 19]. In the present case, the diagnosis of potential TOA was suggested strongly by subsequent transvaginal ultrasound. Interestingly, this patient with insufficiency of intravenous antibiotics treatment ran a fatal course due to subsequent septic complication for TOA in only two days. In general, ruptured TOA can rapidly become critical for the patient, whereas unruptured TOA commonly progresses slowly [19, 20]. A rapid onset of endotoxin shock and DIC is commonly inferred with Gram-negative E. coli, especially in this case with detection of the multiple-antibiotic-resistant ESBL-producing bacteria, which produces endotoxin and generally enter the bloodstream and peritoneal pelvic cavity, causing high fever and acute lower abdominal pain.
Therapeutically, when conservative management with antibiotics is ineffective, surgical management is strongly recommended for TOA [4, 7]. As surgical management, surgical drainage, salpingo-oophorectomy, and abdominal hysterectomy in combination with antibiotics is generally performed in women with TOA [21–23], with the aim of being minimally invasive and as conservative as possible [24–27]. In our case, because the TOA could have developed under septic conditions and thus rapidly induce the critical condition of DIC, we immediately proceeded with surgical treatment by salpingo-oophorectomy and hysterectomy.
In conclusion, we successfully performed emergency surgery for life-threatening septic TOA caused by ESBL-producing E. coli, which was not controlled with conservative antibiotic treatment. To ensure the best management of such cases, reporting of cases with similar pathological and bacteriological findings is warranted.
Conclusions
Aggressive surgery is a valid choice for treating TOA in specific life-threatening situations.
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Acknowledgements
This work was supported by the Kochi organization for medical reformation and renewal grants.
Footnotes
Competing interests
The authors declare that they have no competing interests
Authors’ contributions
This work was supported by the Kochi organization for medical reformation and renewal grants. TT wrote the manuscript and researched data. YO, TO, KH, YY, KO, and JI researched data. YS and TO reviewed/edited the manuscript. KH, YY KO, and JI contributed to discussion and reviewed/edited the manuscript. YS researched data and contributed to discussion. All authors read and approved the final manuscript.
Contributor Information
Teppei Tokumaru, Phone: +81-88-837-3000, Email: teppei.1090@gmail.com.
Yasuo Shima, Email: yasuo_shima@khsc.or.jp.
Takehiro Okabayashi, Email: takehiro_okabayashi@khsc.or.jp.
Kazutoshi Hayashi, Email: kazutoshi_hayashi@khsc.or.jp.
Yorito Yamamoto, Email: yorito_yamamoto@khsc.or.jp.
Kazuhide Ozaki, Email: kazuhide_ozaki@khsc.or.jp.
Jun Iwata, Email: jun_iwata@khsc.or.jp.
References
- 1.Granberg S, Gjelland K, Ekerhovd E. The management of pelvic abscess. Best Pract Res Clin Obstet Gynaecol. 2009;23(5):667–78. doi: 10.1016/j.bpobgyn.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Yavuzcan A, Cağlar M, Dilbaz S, Kumru S, Avcioğlu F, Ustün Y. Identification of Clostridium septicum in a tubo-ovarian abscess: a rare case and review of the literature. Vojnosanit Pregl. 2014;71(9):884–8. doi: 10.2298/VSP130406038Y. [DOI] [PubMed] [Google Scholar]
- 3.Garbin O, Verdon R, Fauconnier A. Treatment of the tubo-ovarian abscesses. J Gynecol Obstet Biol Reprod. 2012;41(8):875–85. doi: 10.1016/j.jgyn.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Roberts W, Dockery JL. Operative and conservative treatment of tubo-ovarian abscess due to pelvic inflammatory disease. South Med J. 1984;77(7):860–3. doi: 10.1097/00007611-198407000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Chappell CA, Wiesenfeld HC. Pathogenesis, diagnosis, and management of severe pelvic inflammatory disease and tuboovarian abscess. Clin Obstet Gynecol. 2012;55(4):893–903. doi: 10.1097/GRF.0b013e3182714681. [DOI] [PubMed] [Google Scholar]
- 6.Nakayama K, Ishikawa M, Katagiri H, Katagiri A, Ishibashi T, Iida K, Nakayama N, Miyazaki K. Surgical treatment outcomes of serious chronic tubo-ovarian abscess: a single-center series of 20 cases. Clin Exp Obstet Gynecol. 2013;40(3):377–80. [PubMed] [Google Scholar]
- 7.Ben-Ami R, Rodríguez-Baño J, Arslan H, Pitout JD, Quentin C, Calbo ES, Azap OK, Arpin C, Pascual A, Livermore DM, Garau J, Carmeli Y. A multinational survey of risk factors for infection with extended-spectrum beta-lactamase-producing enterobacteriaceae in nonhospitalized patients. Clin Infect Dis. 2009;49(5):682–90. doi: 10.1086/604713. [DOI] [PubMed] [Google Scholar]
- 8.Shah AA, Hasan F, Ahmed S, Hameed A, Falagas ME, Karageorgopoulos DE. Extended-spectrum beta-lactamase-producing organisms. J Hosp Infect. 2009;73(4):345–54. doi: 10.1016/j.jhin.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 9.Shah AA, Hasan F, Ahmed S, Hameed A. Extended-spectrum beta-lactamases (ESbLs): characterization, epidemiology and detection. Crit Rev Microbiol. 2004;30(1):25–32. doi: 10.1080/10408410490266429. [DOI] [PubMed] [Google Scholar]
- 10.Cantón R, Novais A, Valverde A, Machado E, Peixe L, Baquero F, Coque TM. Prevalence and spread of extended-spectrum beta-lactamase-producing Enterobacteriaceae in Europe. Clin Microbiol Infect. 2008;14(Suppl 1):144–53. doi: 10.1111/j.1469-0691.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- 11.Bush K. Extended-spectrum beta-lactamases in North America, 1987-2006. Clin Microbiol Infect. 2008;14(Suppl 1):134–43. doi: 10.1111/j.1469-0691.2007.01848.x. [DOI] [PubMed] [Google Scholar]
- 12.Hirakata Y, Matsuda J, Miyazaki Y, Kamihira S, Kawakami S, Miyazawa Y, Ono Y, Nakazaki N, Hirata Y, Inoue M, Turnidge JD, Bell JM, Jones RN, Kohno S, SENTRY Asia-Pacific Participants Regional variation in the prevalence of extended-spectrum beta-lactamase-producing clinical isolates in the Asia-Pacific region (SENTRY 1998-2002) Diagn Microbiol Infect Dis. 2005;52(4):323–9. doi: 10.1016/j.diagmicrobio.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Knothe H, Shah P, Krcmery V, Antal M, Mitsuhashi S. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection. 1983;11(6):315–7. doi: 10.1007/BF01641355. [DOI] [PubMed] [Google Scholar]
- 14.Doi Y, Park YS, Rivera JI, Adams-Haduch JM, Hingwe A, Sordillo EM, Lewis JS, 2nd, Howard WJ, Johnson LE, Polsky B, Jorgensen JH, Richter SS, Shutt KA, Paterson DL. Community-associated extended-spectrumβ-lactamase-producing Escherichia coli infection in the United States. Clin Infect Dis. 2013;56(5):641. doi: 10.1093/cid/cis942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev. 2005;18(4):657–86. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawamura I, Ohmagari N, Tsukahara M, Kudo T, Kurai H. Surveillance of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae at a comprehensive cancer center in Japan, 2009-2013. Am J Infect Control. 2015;43(2):185–7. doi: 10.1016/j.ajic.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Chong Y, Shimoda S, Yakushiji H, Ito Y, Miyamoto T, Kamimura T, Shimono N, Akashi K. Community spread of extended-spectrum β-lactamase-producing Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis: a long-term study in Japan. J Med Microbiol. 2013;62(Pt 7):1038–43. doi: 10.1099/jmm.0.059279-0. [DOI] [PubMed] [Google Scholar]
- 18.Terao M, Koga K, Fujimoto A, Wada-Hiraike O, Osuga Y, Yano T, Kozuma S. Factors that predict poor clinical course among patients hospitalized with pelvic inflammatory disease. J Obstet Gynaecol Res. 2014;40(2):495–500. doi: 10.1111/jog.12189. [DOI] [PubMed] [Google Scholar]
- 19.Landers DV, Sweet RL. Tubo-ovarian abscess: contemporary approach to management. Rev Infect Dis. 1983;5(5):876. doi: 10.1093/clinids/5.5.876. [DOI] [PubMed] [Google Scholar]
- 20.Wiesenfeld HC, Sweet RL. Progress in the management of tuboovarian abscesses. Clin Obstet Gynecol. 1993;36(2):433. doi: 10.1097/00003081-199306000-00022. [DOI] [PubMed] [Google Scholar]
- 21.Pedowitz P, Bloomfield RD. Ruptured adnexal abscess (tuboovarian) with generalized peritonitis. Am J Obstet Gynecol. 1964;88:721. doi: 10.1016/0002-9378(64)90604-0. [DOI] [PubMed] [Google Scholar]
- 22.Vermeeren J, Te Linde RW. Intraabdominal rupture of pelvic abscesses. Am J Obstet Gynecol. 1954;68(1):402. doi: 10.1016/0002-9378(54)90498-6. [DOI] [PubMed] [Google Scholar]
- 23.Krivak TC, Cooksey C, Propst AM. Tubo-ovarian abscess: diagnosis, medical and surgical management. Compr Ther. 2004;Summer;30(2):93–100. doi: 10.1007/s12019-004-0003-5. [DOI] [PubMed] [Google Scholar]
- 24.Rosen M, Breitkopf D, Waud K. Tubo-ovarian abscess management options for women who desire fertility. Obstet Gynecol Surv. 2009;64(10):681–9. doi: 10.1097/OGX.0b013e3181b8b0d6. [DOI] [PubMed] [Google Scholar]
- 25.Mizushima T, Yoshida H, Ohi Y, Ishikawa M, Hirahara F. Evaluating the risk factors for developing resistance to parenteral therapy for tubo-ovarian abscess: a case-control study. J Obstet Gynaecol Res. 2013;39(5):1019–23. doi: 10.1111/jog.12018. [DOI] [PubMed] [Google Scholar]
- 26.Buchweitz O, Malik E, Kressin P, Meyhoefer-Malik A, Diedrich K. Laparoscopic management of tubo-ovarian abscesses: retrospective analysis of 60 cases. Surg Endosc. 2000;14(10):948–50. doi: 10.1007/s004640000249. [DOI] [PubMed] [Google Scholar]
- 27.Gjelland K, Granberg S, Kiserud T, Wentzel-Larsen T, Ekerhovd E. Pregnancies following ultrasound-guided drainage of tubo-ovarian abscess. Fertil Steril. 2012;98(1):136–40. doi: 10.1016/j.fertnstert.2012.03.054. [DOI] [PubMed] [Google Scholar]


