Abstract
PURPOSE
To determine neurocognitive, educational and psychological functioning during childhood and early-adolescence among survivors of early-life meningitis who are apparently healthy.
METHODS
In the general population-based Avon Longitudinal Study of Parents and Children birth cohort, meningitis exposure was determined at age 18 months. The outcomes of IQ, short-term memory, working memory, reading and spelling abilities, psychological and behavioural problems, depressive and anxiety symptoms, and psychotic experiences at ages 9 to 13 years were compared between those exposed and unexposed to meningitis. Individuals with special educational needs were excluded.
RESULTS
By age 18 months, 67 out of 11,035 children were reported to have suffered from meningitis (0.61%). These children, compared with the unexposed, performed worse on all neurocognitive and educational measures; mean difference in total IQ 7.36 (95% CI 1.60-13.11). Meningitis was associated with higher depressive and anxiety symptoms (p=0.02), psychological and behavioural problems (p=0.09), and increased risk of psychotic experiences; risk ratio 2.22 (95% CI 1.12-4.38).
CONCLUSIONS
Exposure to meningitis in the early-life is associated with neurocognitive, educational and psychological difficulties during childhood and early-adolescence among survivors who are apparently healthy. Therefore, focusing only on serious neurologic disabilities may underestimate the true impact of early-life meningitis.
Keywords: Meningitis; Central Nervous System Infections; Intelligence; Memory; Educational Achievement; Affective Symptoms; Behavioural Problems; Psychotic Experience; Depression, Anxiety; Psychosis
INTRODUCTION
Meningitis is inflammation of the membranes (meninges) that surrounds and protects the brain and spinal cord. It is most often caused by an infection (bacterial, viral, or fungal) but can also result from non-infectious causes (1-3). Meningitis is associated with considerable death and disability: yearly an estimated 171,000 deaths and 9.8 million disability adjusted life years (DALYs) worldwide according to the World Health Organization (WHO) (4). Early life meningitis, particularly during the neonatal period, is associated with high mortality and morbidity (5-8).
Major sequelae of childhood meningitis have been studied extensively, which in case of bacterial meningitis includes, permanent neurologic disability, sensory and motor impairment, intellectual disability, or loss of a limb (9-14). However, with improved antimicrobial therapy serious complications from early-life meningitis are increasingly less common in the Western countries (13). According to a recent systematic review and meta-analysis of global data on childhood bacterial meningitis, the risk of at least one major sequela is 9% in the WHO European region, compared with over 20% in Africa and South Asia (13). Although relatively less studied, meningitis is associated with poorer neurocognitive and educational performance. Studies have reported deficits in IQ, memory, increased emotional symptoms during childhood and adolescence among survivors of early-life meningitis (10-12, 15, 16). This suggests serious complications, which are often used to measure the impact of childhood meningitis, may only represent the tip of the iceberg in terms of disability associated with the illness.
Previous studies have commonly included meningitis survivors with or without serious complications as a single group, but there is evidence that cognitive impairments are greater in those with a neurologic disability, such as hearing impairment, than those without (11). Thus, the true burden of neurocognitive and psychological difficulties in meningitis survivors who are apparently neurologically healthy remains to be determined. Many studies have employed short follow-up; tests administered at the time of discharge from hospital may not reflect long-term neurocognitive performance (13). Therefore, studies with long duration of follow-up based on representative general population samples are necessary to accurately measure the associations between early-life meningitis and long-term neurocognitive abilities.
In order to determine long-term functioning in survivors of early-life meningitis who are apparently healthy, we have carried out a 12-year follow-up study of meningitis occurring in the first 18 months of life in the Avon Longitudinal Study of Parents and Children (ALSPAC), a general population birth cohort. Focusing exclusively on individuals without a serious neurologic disability, we have compared neurocognitive and educational performance assessed as IQ, short-term memory, working memory, reading and spelling abilities between ages 9 and 11 years, psychological and behavioural problems at age 10 years, symptoms of depression and anxiety at age 11 years, and risk of developing psychotic experiences (PE) at age 13 years between those exposed and unexposed to meningitis. We predicted that early-life meningitis will be associated with poorer performance in neurocognitive, educational and psychological tasks during childhood and early-adolescence.
METHODS
Ethical approval
Ethical approval for the study was obtained from ALSPAC Ethics and Law Committee and the Local Research Ethics Committees. Parents of all participants provided informed written consent.
Description of cohort
The ALSPAC birth cohort is based on all pregnant women resident in the county of Avon, a geographically defined region in the southwest of England, with expected dates of delivery between April 1991 and December 1992 (www.alspac.bris.ac.uk). The initial ALSPAC cohort consisted of 14,062 live births and 13,988 infants still alive at 12 months (17, 18). Avon included both urban and rural areas, and the population was broadly representative of all children in the UK. The parents completed regular postal questionnaires about all aspects of their child’s health and development since birth. Since the age of 7, the children attended an annual assessment clinic during which they participated in a range of face-to-face interviews and physical tests.
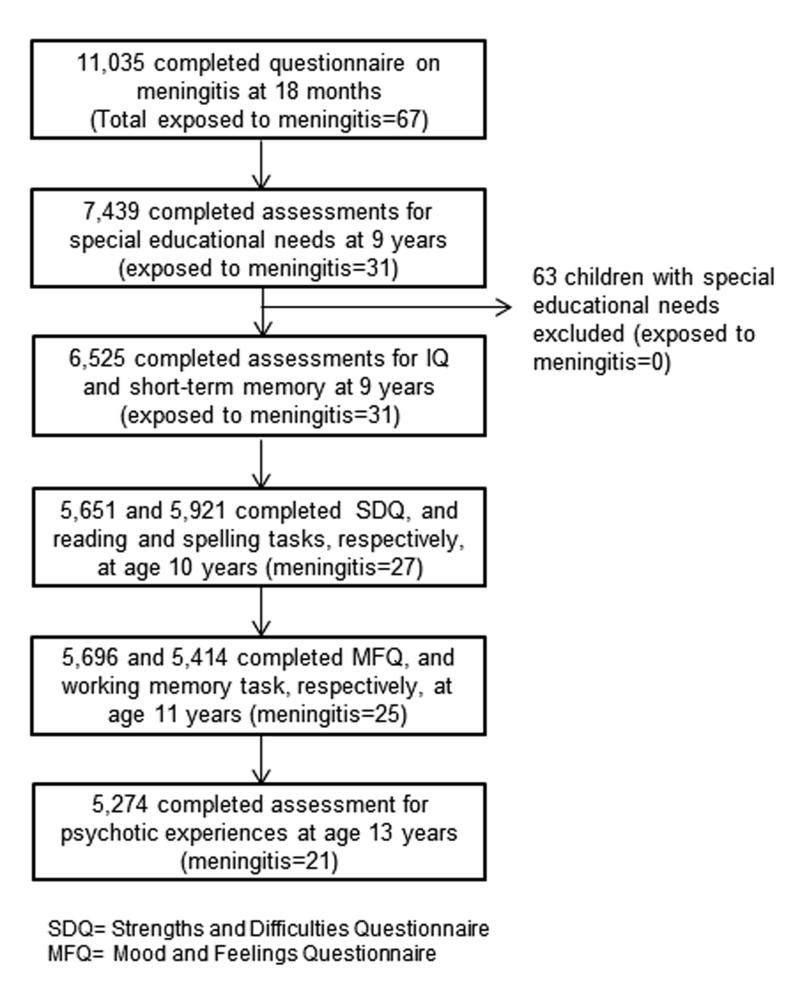
The current study is based on 11,035 participants whose parents provided data on meningitis when the study child was on average 18.3 months old. The number of individuals with available information for both meningitis and specific outcome measures vary, as the latter were completed by different number of individuals (Figure 1). At age 9 years, information on special educational needs were gathered by trained interviewers for the entire cohort that identified 63 children with special needs (20 learning disabilities, 11 ADHD, 8 dyslexia, 7 visual or hearing impairments, and 17 with motor or other impairments). None of these children was reported by their parents to be exposed to meningitis. Children with special educational needs were excluded from analyses in order to improve comparability between exposed and unexposed groups, i.e. meningitis-exposed children without special educational needs were compared with meningitis-unexposed children without special educational needs.
Figure 1. Flow chart of participants in the study of early-life meningitis in the ALSPAC cohort.
Assessment of meningitis
Data on exposure to meningitis were gathered using a questionnaire completed by parents when the ALSPAC participants were on average 18.3 months old. Parents were asked whether the child suffered from meningitis anytime since birth. They could respond either ‘yes’ or ‘no’, which was used to determine whether the child suffered from meningitis in the first 18 months of life. At the time of reporting, the age range of the child was 18 to 31 months; however, 95.8% of the children were 18 or 19 months old.
Assessment of neurocognitive and educational performance
General intelligence and short-term memory (average age 9 years)
IQ was measured by the Wechsler Intelligence Scale for Children (WISC III, 3rd UK edition) (19). A shortened version of the test was applied by trained psychologists, whereby only alternate items were used for all subtests with the exception of the coding subtest which was administered in its standard form. Digit span subtest of WISC III was used as a measure of short-term memory. The WISC has been reported to have very good internal consistency and inter rater reliability. Inter rater reliability coefficients for verbal, performance, and full scale IQ are all greater than 0.9, which is excellent (20, 21).
Reading and spelling abilities (average age 10 years)
Two reading tasks and a spelling task were administered by trained psychologists and speech therapists. Reading was assessed by asking the child to read out loud ten real words, followed by ten non-words. The tester recorded whether the child read each word correctly or incorrectly or whether the child did not attempt the task. For the spelling task, the child was given a series of fifteen words to spell. For each word, the tester first read the word out loud on its own to the child, then within a specific sentence incorporating the word and finally alone again. The child was asked to write down the spelling of the word even if s/he thought they were just guessing. The tester recorded whether the child got each spelling correct or incorrect or whether the child didn’t attempt the task. The two sets of words for the reading tasks and one set of words for the spelling task were specifically chosen for use in ALSPAC after consulting the developers of these tasks (22). Final score for each task was used. These tasks have good validity and reliability for assessing reading and spelling abilities in children. The test-retest reliability coefficient for the word reading task is 0.80. It has a correlation of 0.85 with the Schonell Word Reading Task, and 0.81 with the word spelling given 4 months later (23).
Working memory (average age 11 years)
Computerized Counting Span Task was used (24), which tests information processing and storage abilities simultaneously. A child’s working memory span was calculated automatically by the computer program. The maximum score a child could achieve was five (i.e. all correct). We used the span score, which is the main outcome measure from this task.
Assessment of psychological and behavioural problems
The parental version of the Strengths and Difficulties Questionnaire (SDQ) was completed by mothers when the study child was on average 10 years old. The SDQ is an age appropriate, valid and reliable tool for measuring psychological and behavioural problems in young children (25). In a nationwide epidemiological study of over 10,000 British 5 to 15-year-olds, it has been reported to have good reliability for measuring psychological and behavioural problems (mean Cronbach alpha for internal consistency 0.73) (26). The SDQ assesses problems in four domains (emotional symptoms; conduct problem; hyperactivity; peer-relationship), and gives a total difficulties score of 0-40.
Assessment of depressive and anxiety symptoms
The short version of the Mood and Feelings Questionnaire (SMFQ) was completed by the children at average age 11 years. The MFQ is a validated tool widely used in epidemiological studies (27). The SMFQ includes 13 items covering core symptoms of depression and anxiety experienced in the past two weeks. Each item is scored zero (not true), one (sometimes true) or two (true) giving a total score of 0-26. Analyses of psychometric properties of the SMFQ in a community sample of 7 to 11-year-old British children suggests that it is a valid tool for measuring depressive and anxiety symptoms (28).
Assessment of psychotic experiences
Psychotic experiences were assessed by the semi-structured Psychosis-like Symptoms interview (PLIKSi) at a mean age of 12.9 years (SD 0.23). The PLIKSi comprised 12 ‘core’ questions derived from the Diagnostic Interview Schedule for Children–IV (DISC–IV) (29), and the Schedules for Clinical Assessment in Neuropsychiatry version 2.0 (SCAN 2.0) (30). They include key symptoms covering the three main domains of positive psychotic symptoms: hallucinations (visual and auditory); delusions (delusions of being spied on, persecution, thoughts being read, reference, control, grandiose ability, and other unspecified delusions); experiences of though interference (thought broadcasting, insertion, and withdrawal). This allowed an observer rating for the presence of any psychotic experiences in the past six months. Children with psychotic experiences were compared with the rest of the cohort. The PLIKSi has good inter-rater reliability (kappa=0.7) (31). Details of the interview and coding process, and training and reliability of the interview procedure have been reported elsewhere (31).
Assessment of covariates
Age at the time of testing (in days), sex, ethnicity, and father’s social class were included as potential confounders. As per the UK Office of National Statistics classification system social class was recorded in six categories: I, II, III non-manual, III manual, IV, and V (in descending order, with professionals and higher managerial workers representing social class I).
Statistical analysis
Linear regression was used to compare mean scores of IQ, memory, reading and spelling tasks between those with and without meningitis. Mean difference (95% CI) between groups was calculated for each task. Regression models were adjusted for age at the time of testing (in days), sex, social class and ethnicity. Since SDQ and SMFQ scores were not normally distributed, their distributions were compared between meningitis-exposed and unexposed groups using the independent samples Kruskal Wallis test.
We examined whether deficit in total IQ in the meningitis group was due to a sub-group of individuals with very low IQ, or low IQ in all cases of meningitis (i.e. left shift of entire distribution of IQ scores). Linearity of association between exposure to meningitis and the outcome of total IQ score was examined by including a quadratic term (square of IQ score) within the linear regression model.
We calculated the relative risk or risk ratio (RR) for psychotic experiences at age 13 years in the meningitis-exposed, compared with the unexposed group. Age at the time of assessment psychotic experiences (in days), sex, ethnicity, social class and childhood IQ were included as potential confounders.
RESULTS
In the first 18 months of life, 67 children were reported to have suffered from meningitis out of the total 11,035 (0.61%). Table 1 presents baseline characteristics of children exposed and unexposed to meningitis.
Table 1. Baseline characteristics of those exposed and unexposed to early-life meningitis in the ALSPAC cohort.
| Group characteristics | Meningitis group | Unexposed group | χ2 statistic; p-value |
|---|---|---|---|
| Number | 67 | 10,968 | - |
| Mean age (SD) in years† | 12.82 (0.16) | 12.88 (0.23) | 1.87; 0.18‡ |
| Male (%) | 64.2 | 51.6 | 4.24; 0.03 |
| British White (%) | 98.3 | 97.9 | 2.05; 0.97 |
| Social class (%) | 11.65; 0.07 | ||
| I | 4.0 | 11.6 | |
| II | 30.0 | 35.1 | |
| III non manual | 6.0 | 11.3 | |
| III manual | 36.0 | 30.1 | |
| IV | 20.0 | 9.0 | |
| V | 4.0 | 2.7 |
Mean age at the time of assessment of psychotic experiences
Independent sample t-test statistic and p value
χ2= Chi-squared
SD= Standard deviation
Neurocognitive and educational performance at ages 9 to 11 years
General Intelligence
Analysis of meningitis and IQ was based on 6,525 individuals (after excluding those with special educational needs): 31 exposed to meningitis (46% of all children exposed to meningitis) and 6,494 unexposed (59% of all children unexposed to meningitis. Children exposed to meningitis performed worse on all measures of IQ compared with the unexposed (Table 2). Mean differences between groups were slightly attenuated after adjusting for age at the time of IQ testing, sex, social class and ethnicity; however, still remained significant for full scale IQ.
Table 2. Childhood neurocognitive performance and educational abilities between those exposed and unexposed to early-life meningitis in the ALSPAC cohort.
| Cognitive ability and average age of testing |
Meningitis group | Unexposed group | Mean difference (95% CI) between groups |
|||
|---|---|---|---|---|---|---|
|
| ||||||
| n | Mean (SD) | n | Mean (SD) | Unadjusted | Adjusted | |
| Age 9 years | ||||||
| Total IQ | 31 | 97.32 (20.39) | 6494 | 104.68 (16.29) | 7.36 (1.60- 13.11) | 6.28 (0.19- 12.37) |
| Verbal IQ | 31 | 101.09 (18.90) | 6522 | 107.60 (16.62) | 6.50 (0.63- 12.37) | 5.91 (−0.03- 12.12) |
| Performance IQ | 31 | 93.58 (20.15) | 6511 | 100.11 (16.92) | 6.52 (0.55- 12.50) | 5.02 (-1.38- 11.44) |
| Short-term memory | 31 | 9.25 (3.59) | 6467 | 10.38 (3.07) | 1.13 (0.05- 2.22) | 1.07 (−0.09- 2.24) |
| Age 10 years | ||||||
| Reading | 27 | 6.89 (2.83) | 5894 | 7.64 (2.37) | 0.74 (−0.15- 1.64) | 0.46 (−0.49- 1.40) |
| Non-word reading | 27 | 4.26 (1.91) | 5888 | 5.32 (2.47) | 1.05 (0.12- 1.99) | 1.01 (0.01- 2.02) |
| Spelling | 27 | 9.18 (3.18) | 5883 | 10.37 (3.38) | 1.19 (−0.08- 2.47) | 0.59 (−0.75- 1.94) |
| Age 11 years | ||||||
| Working memory | 28 | 3.03 (0.99) | 5386 | 3.43 (0.85) | 0.40 (0.09- 0.72) | 0.37 (0.02- 0.71) |
SD= Standard deviation
CI= Confidence interval
Adjusted analyses included age at the time of testing, sex, social class and ethnicity as potential confounders
Memory, reading and spelling abilities
Compared with the unexposed, children exposed to meningitis performed worse on all measures. Mean difference between groups were slightly attenuated after adjusting for potential confounders, although still remained significant for working memory and reading ability (Table 2).
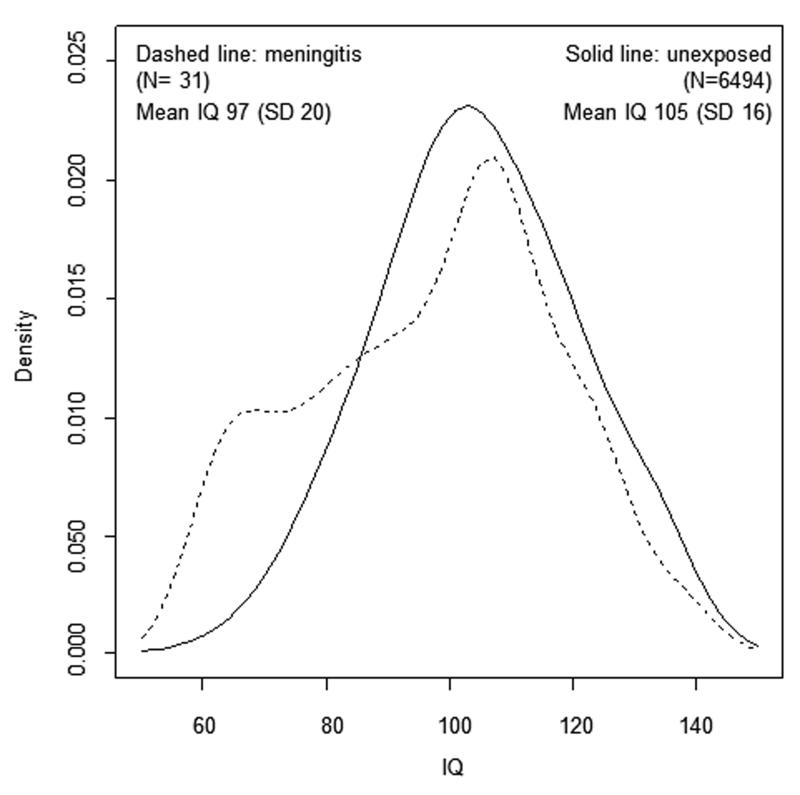
Distribution of IQ scores between those with and without meningitis
Compared with the unexposed, the proportion of children exposed to meningitis gradually decreased with increasing IQ (Figure 2). Overall the association between IQ and meningitis was non-linear (regression coefficient for the quadratic term=0.005; SE <0.001; p <0.001).
Figure 2. Distribution of total IQ scores at age 9 years between groups exposed and unexposed to meningitis in the ALSPAC cohort.
Psychological and behavioural problems, depressive and anxiety symptoms at ages 10 to 11 years
Median values of total SDQ and SMFQ score at ages 10 to 11 years were higher in the meningitis, compared with the unexposed group (Table 3).
Table 3. Childhood psychological and behavioural problems, depressive and anxiety symptoms between those exposed and unexposed to early-life meningitis in ALSPAC.
| Test score and age | Meningitis group | Unexposed group |
p-value† |
|---|---|---|---|
| SDQ score at age 10 years | |||
| Total number | 27 | 5625 | |
| Median (IQR) | 6.00 (5.00- 11.00) | 6.00 (3.00- 9.00) | 0.09 |
| SMFQ score at age 11 years | |||
| Total number | 25 | 5671 | |
| Median (IQR) | 5.00 (3.00- 6.50) | 3.00 (1.00- 6.00) | 0.02 |
Distributions of SDQ and SMFQ scores were compared between meningitis exposed and unexposed groups by the independent sample Kruskal-Wallis test
SDQ= Strengths and difficulties questionnaire
SMFQ= Short version of the Mood and feelings questionnaire
IQR= Interquartile range
Risk of psychotic experiences at age 13 years
Data on both meningitis and PE were available for 5,274 individuals; 21 exposed to meningitis. In the meningitis group, 6 (28.6%) reported PE at age 13 years compared with 676 (12.9%) in the unexposed group. This equated to over two-fold increased risk of PE at age 13 years in the meningitis, compared with the unexposed group. Adjusting for age at the time of assessment of PE, sex, social class, ethnicity and total IQ at age 9 years had minimal influence on the risk ratios (Table 4).
Table 4. Early-life meningitis and risk of psychotic experiences (PE) at age 13 years in the ALSPAC cohort.
| Adjustment for confounding | Sample | Risk ratio (95% CI) for PE |
|---|---|---|
| Unadjusted | 5274 | 2.22 (1.12-4.38) |
| Age at assessment of PE | 5274 | 2.24 (1.13-4.42) |
| Sex | 5274 | 2.19 (1.11-4.32) |
| Social class | 4818 | 2.01 (0.95-4.27) |
| Ethnicity | 5172 | 1.94 (0.91-4.17) |
| Total IQ at age 9 years | 5222 | 2.32 (1.18-4.58) |
| Age, sex, social class, ethnicity, and IQ | 4760 | 2.09 (0.98- 4.46) |
PE= Psychotic experiences
CI= Confidence interval
DISCUSSION
To our knowledge, this is the first study to focus exclusively on meningitis survivors without serious neurologic complications. We have examined a variety of neurocognitive, educational and behavioural outcomes in a general population birth cohort. The findings demonstrate that meningitis in the first 18 months of life is associated with deficits in childhood IQ, working memory, and reading ability in exposed individuals who are apparently healthy. As well as higher scores on measures for psychological problems, depression or anxiety, meningitis-exposed children are twice as likely to report psychotic experiences in early adolescence, compared with the unexposed. None of the 31 meningitis survivors included in this study was noted to have any special educational needs. It has been reported that risk of major sequelae from meningitis is higher in children aged less than five years compared with those aged five years or more (13). Thus, survivors of early-life meningitis included in our analyses would be considered to have ‘good outcome’ from the illness by most standards. Even in this group, we show significant deficits in neurocognitive and educational domains to be present, as well as higher psychological and behavioural manifestations on 8 to 12 years follow-up.
These findings have important implications. Intelligence and memory deficits would interfere with a child’s academic achievement. This is reflected by poor reading ability among children with a history of meningitis in our sample. Compared with their same age peers who did not have meningitis, many meningitis-exposed children have long-term neurocognitive deficits although they are by and large within the normal range of intellectual ability. Therefore, awareness and attention within family and school are necessary to enable these children to reach their full academic potential. Meningitis is a leading cause of disability (32), which is associated with an annual 9.8 million DALYs worldwide according to the WHO (4). Estimation of the burden of meningitis has most commonly involved data on mortality and, in some cases, the cost of disability from serious complications (33, 34). However, there is little data on the indirect cost of long-term so called ‘soft-neurological’ impairments reported in this study. The findings suggest loss of productivity as a result of cognitive or educational deficits in survivors of meningitis who are apparently healthy might be considerable.
To our knowledge, this is the first study to examine the association between adolescent PE and early-life meningitis. Population-based longitudinal studies suggest children who report PE are 12-16 times more likely to develop a psychotic disorder that resembles schizophrenia by early adulthood (35, 36). Childhood meningitis has been also linked with the risk of adult schizophrenia (37). Higher risk of depressive and anxiety symptoms among meningitis survivors is consistent with previous studies (11, 12). Childhood psychological and behavioural problems are associated with an increased risk of depression in adult life (38). Thus, individuals exposed to meningitis in the early-life may have a high burden of neuropsychiatric disorders in adulthood.
Limitations of the study include the use of parent report at 18 months as a means for determining exposure to meningitis. Reliability of parent-report data on meningitis is unknown. We did not have access to hospital records so cross-validation of parent reported data were not possible including sensitivity and specificity of reporting. However, meningitis is a serious illness requiring urgent medical intervention. Therefore, the diagnosis of meningitis would have been made by a clinician. It is also unlikely that a parent would forget such a recent major event. Therefore, it is likely that children who were reported by their parents to have suffered from meningitis in deed had the illness. As this information was collected prospectively this also minimizes any chance of recall bias.
There are differences in the causative organisms and covariates for neonatal and childhood meningitis. Lack of detailed clinical information precluded any comment on the causative agent for meningitis. In the future studies should include detailed information on the causative agents and covariates from hospital records. Neurologic outcomes of childhood bacterial meningitis are worse than aseptic meningitis. The bimodal distribution of IQ scores may reflect different types of meningitis (Figure 2). Regardless of specific causes for meningitis, the findings suggest that survivors of early-life meningitis, who are apparently neurologically well, as a group, show long-term difficulties in various neurocognitive and educational domains as well as increased risk of psychological and behavioural problems. This is an important message.
It is likely that the small number of children with meningitis has contributed to low statistical power. Meningitis-exposed children performed worse on all domains compared with the unexposed, but the difference was not statistically significant for some of the cognitive and behavioural tasks. However, it is worth noting that the direction of association was consistent for all domains, i.e. meningitis-exposed group performed worse than the unexposed group. Another potential limitation is bias due to attrition. Data on cognitive and educational outcomes were available for 31 out of 67 children with meningitis. If meningitis-exposed children who did not attend neurocognitive assessments had a more favorable outcome, such as higher IQ, this would lead to spurious over-estimation of the IQ deficit in meningitis-exposed group in our analysis. However, there was no reason to believe that this was the case. Individuals from lower social classes were over-represented in the missing sample. In the ALPAC cohort, lower social class was associated with the lower IQ, higher risk of psychotic experiences, and, overall, poorer health outcomes. Thus, it is more likely that those who developed more severe adverse sequelae from meningitis were absent from follow up. This assumption is consistent with the observation that none of the meningitis-exposed children included in our sample was noted to have any special educational needs at age 9 years.
In summary, we report that survivors of early-life meningitis without serious complications, as a group, show deficits in a range of neurocognitive domains, experience more psychological and behavioural issues including increased risk of psychotic experiences in childhood and early-adolescence. Further studies on this population involving larger samples and longer follow-up are necessary. Studies that focus only on serious complications may under-estimate the true burden of meningitis. Findings from this study are consistent with a substantial long-term health and economic impact of early-life meningitis among survivors who are apparently healthy.
ACKNOWLEDGEMENTS
Financial support
This work was supported by a doctoral clinical research training grant from the Wellcome Trust to GMK (094790/Z/10/Z); Wellcome Trust (095844/Z/11/Z & 088869/Z/09/Z), and National Institute for Health Research (NIHR) (RP-PG-0606-1335) grants to PBJ, that also supports JS; Wellcome Trust grant for studying depression (084268/Z/07/Z), and the UK Medical Research Council (MRC) grant for studying psychosis (G0701503) in the Avon Longitudinal Study of Parents and Children (ALSPAC) birth cohort. The MRC (Grant ref: 74882), the Wellcome Trust (Grant ref: 092731) and the University of Bristol provide core support for ALSPAC. The funding bodies had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
We thank all the ALSPAC families, the midwives for their help in recruiting them, and the whole ALSPAC team, including interviewers, computer and laboratory technicians, clerical workers, research scientists, statisticians, volunteers, managers, receptionists and nurses. We thank Professor Robert Booy and Dr. Gulam Khandaker from the University of Sydney for their helpful comments on an earlier version of this manuscript.
Abbreviations
- ALSPAC
Avon Longitudinal Study of Parents and Children
- PE
Psychotic experiences
- IQ
Intelligence quotient
- CI
Confidence interval
- WHO
World Health Organization
- DALYs
Disability adjusted life years
- WISC
Wechsler intelligence scale for children
- SDQ
Strengths and difficulties questionnaire
- MFQ
Mood and feelings questionnaire
- SMFQ
Short version of the mood and feelings questionnaire
- PLIKSi
Psychotic-like symptoms interview
- SCAN
Schedules for clinical assessment in neuropsychiatry
- SD
Standard deviation
Footnotes
Conflict of interest
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Youssef FG, El-Sakka H, Azab A, Eloun S, Chapman GD, Ismail T, et al. Etiology, antimicrobial susceptibility profiles, and mortality associated with bacterial meningitis among children in Egypt. Annals of epidemiology. 2004;14(1):44–8. doi: 10.1016/s1047-2797(03)00075-9. Epub 2003/12/11. [DOI] [PubMed] [Google Scholar]
- 2.Dominguez A, Munoz P, Cardenosa N, Martinez A, Cayla J, Meningococcal Disease Study G Time-series analysis of meningococcal disease in Catalonia. Annals of epidemiology. 2007;17(9):654–62. doi: 10.1016/j.annepidem.2007.03.006. Epub 2007/06/09. [DOI] [PubMed] [Google Scholar]
- 3.Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;27(Suppl 2):B51–63. doi: 10.1016/j.vaccine.2009.04.063. Epub 2009/05/30. [DOI] [PubMed] [Google Scholar]
- 4.WHO . World Health Report. World Health Organization; 2000. [Google Scholar]
- 5.Harvey D, Holt DE, Bedford H. Bacterial meningitis in the newborn: a prospective study of mortality and morbidity. Seminars in perinatology. 1999;23(3):218–25. doi: 10.1016/s0146-0005(99)80066-4. Epub 1999/07/15. [DOI] [PubMed] [Google Scholar]
- 6.de Louvois J, Halket S, Harvey D. Neonatal meningitis in England and Wales: sequelae at 5 years of age. European journal of pediatrics. 2005;164(12):730–4. doi: 10.1007/s00431-005-1747-3. Epub 2005/09/02. [DOI] [PubMed] [Google Scholar]
- 7.Levent F, Baker CJ, Rench MA, Edwards MS. Early outcomes of group B streptococcal meningitis in the 21st century. The Pediatric infectious disease journal. 2010;29(11):1009–12. doi: 10.1097/INF.0b013e3181e74c83. Epub 2010/06/18. [DOI] [PubMed] [Google Scholar]
- 8.Polin RA, Harris MC. Neonatal bacterial meningitis. Seminars in neonatology : SN. 2001;6(2):157–72. doi: 10.1053/siny.2001.0045. Epub 2001/08/03. [DOI] [PubMed] [Google Scholar]
- 9.Hristeva L, Booy R, Bowler I, Wilkinson AR. Prospective surveillance of neonatal meningitis. Archives of disease in childhood. 1993;69(1 Spec No):14–8. doi: 10.1136/adc.69.1_spec_no.14. Epub 1993/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viner RM, Booy R, Johnson H, Edmunds WJ, Hudson L, Bedford H, et al. Outcomes of invasive meningococcal serogroup B disease in children and adolescents (MOSAIC): a case-control study. Lancet neurology. 2012;11(9):774–83. doi: 10.1016/S1474-4422(12)70180-1. Epub 2012/08/07. [DOI] [PubMed] [Google Scholar]
- 11.Christie D, Viner RM, Knox K, Coen PG, Wang H, El Bashir H, et al. Long-term outcomes of pneumococcal meningitis in childhood and adolescence. European journal of pediatrics. 2011;170(8):997–1006. doi: 10.1007/s00431-010-1390-5. [DOI] [PubMed] [Google Scholar]
- 12.Borg J, Christie D, Coen PG, Booy R, Viner RM. Outcomes of meningococcal disease in adolescence: prospective, matched-cohort study. Pediatrics. 2009;123(3):e502–9. doi: 10.1542/peds.2008-0581. Epub 2009/03/04. [DOI] [PubMed] [Google Scholar]
- 13.Edmond K, Clark A, Korczak VS, Sanderson C, Griffiths UK, Rudan I. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. The Lancet infectious diseases. 2010;10(5):317–28. doi: 10.1016/S1473-3099(10)70048-7. Epub 2010/04/27. [DOI] [PubMed] [Google Scholar]
- 14.Ladhani S, Heath PT, Aibara RJ, Ramsay ME, Slack MP, Hibberd ML, et al. Long-term complications and risk of other serious infections following invasive Haemophilus influenzae serotype b disease in vaccinated children. Vaccine. 2010;28(10):2195–200. doi: 10.1016/j.vaccine.2009.12.057. Epub 2010/01/09. [DOI] [PubMed] [Google Scholar]
- 15.Clark LJ, Glennie L, Audrey S, Hickman M, Trotter CL. The health, social and educational needs of children who have survived meningitis and septicaemia: the parents’ perspective. BMC public health. 2013;13:954. doi: 10.1186/1471-2458-13-954. Epub 2013/10/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimwood K, Anderson P, Anderson V, Tan L, Nolan T. Twelve year outcomes following bacterial meningitis: further evidence for persisting effects. Archives of disease in childhood. 2000;83(2):111–6. doi: 10.1136/adc.83.2.111. Epub 2000/07/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, et al. Cohort Profile: The ‘Children of the 90s’--the index offspring of the Avon Longitudinal Study of Parents and Children. International journal of epidemiology. 2013;42(1):111–27. doi: 10.1093/ije/dys064. Epub 2012/04/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, Davey Smith G, et al. Cohort Profile: The Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. International journal of epidemiology. 2013;42(1):97–110. doi: 10.1093/ije/dys066. Epub 2012/04/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wechsler D, Golombok S, Rust J. Weschler Intelligence Scale for Children. 3rd Edition. The Psychological Corporation; 1992. (WISC–III UK) [Google Scholar]
- 20.Levinson EM, Folino L. Correlations of scores on the Gifted Evaluation Scale with those on WISC-III and Kaufman Brief Intelligence Test for students referred for gifted evaluation. Psychological Reports. 1994;74:419–24. doi: 10.2466/pr0.1994.74.2.419. [DOI] [PubMed] [Google Scholar]
- 21.Sandoval J. The Twelfth Mental Measurements Yearbook. Third Edition University of Nebraska Press; Linclon, Nebraska: 1995. Review of the Wechsler Intelligence Scale for Children. [Google Scholar]
- 22.Nunes T, Bryant P, Olsson J. Learning morphological and phonological spelling rules: An intervention study. Scientific Studies of Reading. 2003;7:289–307. [Google Scholar]
- 23.Schonell F, Goodacre E. The psychology and teaching of reading. Oliver and Boyd; London: 1971. [Google Scholar]
- 24.Case R, Kurland DM, Goldberg J. Operational efficiency and the growth of short-term memory span. Journal of Experimental Child Psychology. 1982;33:386–404. [Google Scholar]
- 25.Goodman R. The Strengths and Difficulties Questionnaire: a research note. Journal of child psychology and psychiatry, and allied disciplines. 1997;38(5):581–6. doi: 10.1111/j.1469-7610.1997.tb01545.x. Epub 1997/07/01. [DOI] [PubMed] [Google Scholar]
- 26.Goodman R. Psychometric properties of the strengths and difficulties questionnaire. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(11):1337–45. doi: 10.1097/00004583-200111000-00015. Epub 2001/11/09. [DOI] [PubMed] [Google Scholar]
- 27.Angold A, Costello EJ, Messer SC. Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. International Journal of Methods in Psychiatric Research. 1995;5:237–49. [Google Scholar]
- 28.Sharp C, Goodyer IM, Croudace TJ. The Short Mood and Feelings Questionnaire (SMFQ): a unidimensional item response theory and categorical data factor analysis of self-report ratings from a community sample of 7-through 11-year-old children. Journal of abnormal child psychology. 2006;34(3):379–91. doi: 10.1007/s10802-006-9027-x. Epub 2006/05/02. [DOI] [PubMed] [Google Scholar]
- 29.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. Epub 2000/01/19. [DOI] [PubMed] [Google Scholar]
- 30.WHO . SCAN: Schedules for Clinical Assessment in Neuropsychiatry Version 2.0. Psychiatric Publishers International/American Psychiatric Press Inc; Geneva, Switzerland: 1994. [Google Scholar]
- 31.Horwood J, Salvi G, Thomas K, Duffy L, Gunnell D, Hollis C, et al. IQ and non-clinical psychotic symptoms in 12-year-olds: results from the ALSPAC birth cohort. The British journal of psychiatry : the journal of mental science. 2008;193(3):185–91. doi: 10.1192/bjp.bp.108.051904. Epub 2008/09/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray CJ, Lopez AD. Measuring the global burden of disease. The New England journal of medicine. 2013;369(5):448–57. doi: 10.1056/NEJMra1201534. Epub 2013/08/02. [DOI] [PubMed] [Google Scholar]
- 33.Huang SS, Johnson KM, Ray GT, Wroe P, Lieu TA, Moore MR, et al. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine. 2011;29(18):3398–412. doi: 10.1016/j.vaccine.2011.02.088. Epub 2011/03/15. [DOI] [PubMed] [Google Scholar]
- 34.O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374(9693):893–902. doi: 10.1016/S0140-6736(09)61204-6. Epub 2009/09/15. [DOI] [PubMed] [Google Scholar]
- 35.Poulton R, Caspi A, Moffitt TE, Cannon M, Murray R, Harrington H. Children’s self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Archives of general psychiatry. 2000;57(11):1053–8. doi: 10.1001/archpsyc.57.11.1053. Epub 2000/11/14. [DOI] [PubMed] [Google Scholar]
- 36.Zammit S, Kounali D, Cannon M, David AS, Gunnell D, Heron J, et al. Psychotic Experiences and Psychotic Disorders at Age 18 in Relation to Psychotic Experiences at Age 12 in a Longitudinal Population-Based Cohort Study. The American journal of psychiatry. 2013;170(7):742–50. doi: 10.1176/appi.ajp.2013.12060768. [DOI] [PubMed] [Google Scholar]
- 37.Khandaker GM, Zimbron J, Dalman C, Lewis G, Jones PB. Childhood infection and adult schizophrenia: a meta-analysis of population-based studies. Schizophrenia research. 2012;139(1-3):161–8. doi: 10.1016/j.schres.2012.05.023. Epub 2012/06/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harrington R, Fudge H, Rutter M, Pickles A, Hill J. Adult outcomes of childhood and adolescent depression. I. Psychiatric status. Archives of general psychiatry. 1990;47(5):465–73. doi: 10.1001/archpsyc.1990.01810170065010. Epub 1990/05/01. [DOI] [PubMed] [Google Scholar]