Abstract
Antibody gene transfer, which involves the delivery of genes that encode potent, broadly neutralizing anti-HIV antibodies, is a promising new strategy to prevent HIV infection. A satellite symposium at the AIDS Vaccine 2012 conference brought together many of the groups working in this field.
Despite nearly thirty years of intense study, efforts to develop a safe and effective HIV vaccine by conventional means have either failed or provided only modest, short-lived protection. While the tantalizing results of the HIV vaccine trial RV144 in Thailand continue to guide efforts to improve the efficacy and duration of a vaccine that harnesses the natural immune system, it remains uncertain when or if such efforts will succeed.
Recent studies describing the discovery of more potent, broadly neutralizing antibodies targeting HIV from chronically infected patients have revealed the potential for the humoral response to produce protective antibodies during the course of natural infection1,2. However, it is unclear whether immunogens can be designed that will elicit these rare antibodies efficiently.
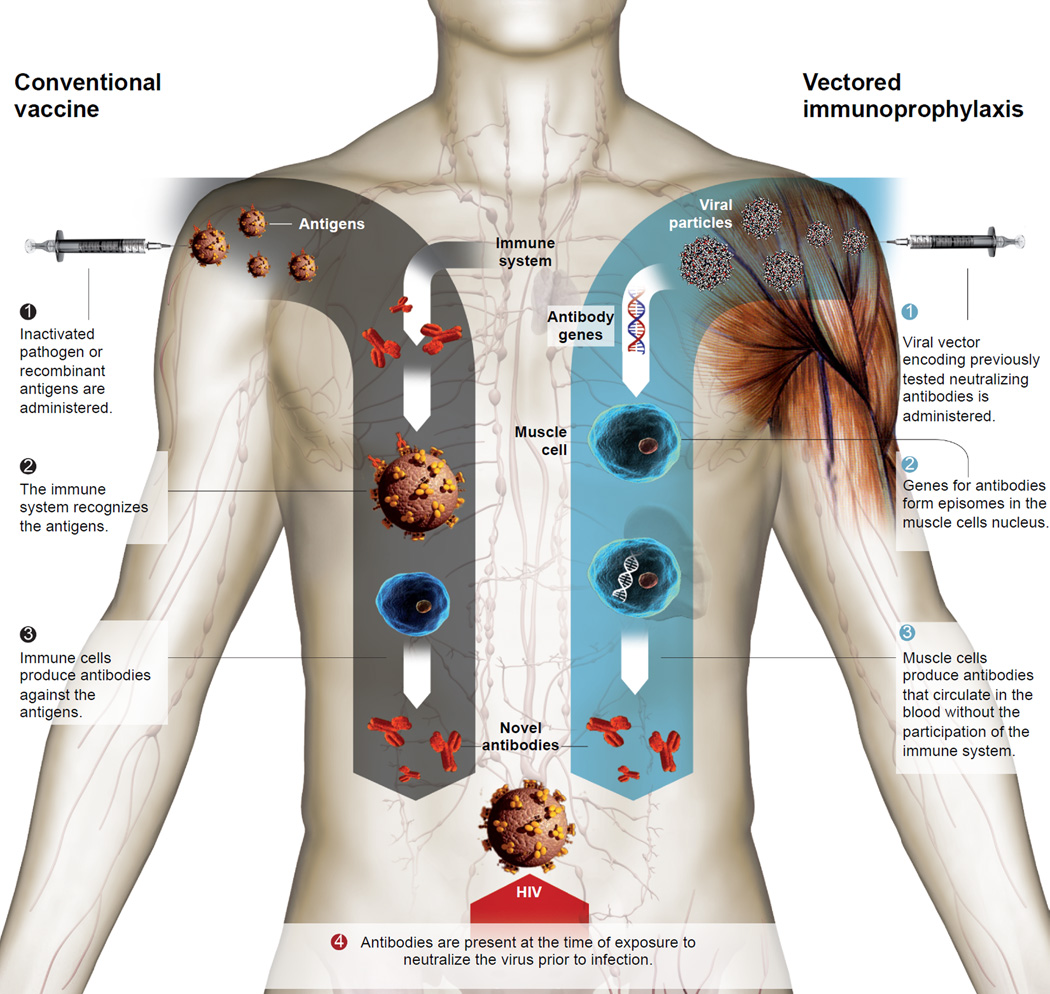
Antibody gene transfer is a novel protective strategy that bypasses the natural immune response, which has been the central focus of previous attempts to develop an HIV vaccine, by directing the production of antibodies from non-hematopoietic tissues, such as muscle (Figure 1). Because this approach skips many of the steps in the usual path of vaccine development, it has been described as a leapfrog strategy. Recent advances in the use of gene transfer for the correction of genetic deficiencies3,4 – particularly the successful expression of factor IX in a small group of Hemophilia B patients – have bolstered the intriguing possibility of utilizing adeno-associated virus (AAV) vectors as a vehicle for antibody gene delivery in humans. Two recent studies have demonstrated the feasibility of this approach against both SIV in macaques5 as well as HIV in humanized mice6.
Figure 1. Comparison of prophylaxis approaches.
Traditional vaccines work by engaging the adaptive immune system to produce a response that recognizes the administered antigen. Vectored immunoprophylaxis employs a viral vector, such as Adeno-Associated Virus (AAV), to deliver the genes encoding for a given antibody into muscle cells that express the desired antibodies - and secrete them into the circulation – without employing the immune system. Adapted from an illustration prepared by La Vanguardia, Barcelona.
The Foundation for Vaccine Research (FVR) organized a special satellite symposium at the AIDS Vaccine 2012 conference in September in Boston to discuss the latest developments in this promising area of translational research.
AAV-Mediated Delivery of Broadly Neutralizing Antibodies
To provide a framework for the session, Phil Johnson (Children's Hospital of Philadelphia) delivered a comprehensive introduction to the biology and history of adeno-associated virus (AAV) vectors. A member of the parvoviridae family, AAV is a ubiquitous commensal virus in humans that has never been associated with any disease. It consists of a protein capsid shell that surrounds a single-strand of genomic DNA that encodes just two viral genes (Rep and Cap), flanked on either side by inverted-terminal repeat sequences (ITRs). These ITRs form unique hairpin structures that, in conjunction with Rep and Cap, mediate both DNA replication and packaging during virus production. Natural AAV propagation is entirely dependent upon co-infection with adenovirus to deliver necessary helper factors in trans. To produce AAV vectors for immunoprophylaxis, a desired antibody transgene is inserted between two ITR sequences and can be packaged into viral particles by co-transfection with plasmid expressing the desired Rep and Cap genes as well as a separate plasmid to provide adenoviral helper functions. The serotype of the AAV vector produced is the result of the capsid gene, which impacts both the cellular tropism of the vector as well as biological properties such as intracellular trafficking. AAV serotypes 1 and 8 have both been found to efficiently infect muscle tissues to form episomal head-to-tail concatamers of their genome in the nucleus, which result in very long-lived, high-level gene expression in animals models and have demonstrated excellent safety and tolerability in previous human trials. Presenting follow-up data from his 2009 study of anti-SIV immunoadhesins-based protection of macaques given AAV1 in macaques5, Johnson showed that animals expressing the 4L6 immunoadhesin have sustained circulating concentrations of approximately 20µg/mL of protein for the past five years with no adverse health effects following the four intramuscular injections given at the initiation of the study. These results are consistent with previous AAV studies in macaques that demonstrated stable erythropoietin expression for over 6 years7 and raise the hope that similar longevity of immunoprophylaxis might be achieved in human patients.
Animal models of Immunoprophylaxis
Following the first descriptions of broadly neutralizing antibodies 2F5, 4E10, b12 and 2G12, a number of passive transfer studies were undertaken to determine their efficacy in preventing infection with SIV-HIV-1 hybrid virus (SHIV) in macaques. Since this time, newer neutralizing antibodies have been described with considerably higher potency in vitro, raising the possibility that lower in vivo concentrations might be sufficient to provide protection. Dennis Burton (Scripps) confirmed this possibility during the session by presenting early results of experiments in which PGT121 exhibited remarkable protection in macaques. Animals given 5mg/kg, 1mg/kg or 0.2mg/kg doses of PGT121 exhibited approximately 100µg/mL, 15µg/mL or 2µg/mL of antibody in circulation one day after administration and just prior to intravaginal challenge with 300 TCID50 of SHIV162P3. At the vaginal surface, PGT121 was detected at 0.9µg/mL and 0.2µg/mL respectively in animals receiving 5mg/kg and 1mg/kg doses and was undetectable in animals that received 0.2mg/kg. Following challenge, animals in the 5mg/kg and 1mg/kg groups remained uninfected, while 3/5 of animals receiving 0.2mg/kg PGT121 were protected from challenge, despite a lack of detectable antibody at the vaginal surface. These results represent an improvement over original studies of b12 in which 25mg/kg protected 8/9 animals from a similar challenge8.
Human-to-human mucosal transmission of HIV requires the virus to mobilize across significant host barriers, resulting in only one or a handful of viruses initiating most infections9,10. Substantial effort has been directed towards understanding the unique characteristics of such transmitted founder strains of HIV that have succeeded in this process11. While enhanced resistance to neutralizing antibodies has not been observed for these strains10,11, it was unclear whether infection by such strains in vivo would exhibit neutralization resistance. In follow up work to his earlier study demonstrating robust protection of humanized mice against the CXCR4-tropic NL4-3 strain of HIV by vectored immunoprophylaxis (VIP)6, David Baltimore (California Institute of Technology) presented results in which humanized mice given VIP expressing b12 or VRC01 antibodies were challenged with a transmitted founder strain, REJO.c. In this experiment, significant protection against REJO.c infection was seen in mice expressing VRC01, but not b12, consistent with results obtained in vitro for these antibody/strain combinations. These results suggest that transmitted founder strains may not necessarily be more difficult to neutralize than non-founder strains in vivo, lending further support to translation of VIP in humans.
As most transmission of HIV occurs across mucosal surfaces, there has been substantial effort to model this process in macaques and more recently, humanized BLT mice12,13. In addition, recent macaque studies have implemented low-dose repetitive challenge to better simulate low-probability HIV transmission in humans14. During the session, Baltimore presented data utilizing a novel, modified humanized BLT mouse model incorporating repetitive low-dose intravaginal challenge with either the CCR5-tropic laboratory strain JR-CSF, or the transmitted founder strain REJO.c. Using this model, he demonstrated that BLT mice given VIP expressing VRC01 or a more potent VRC01-like antibody (VRC07G54W) were highly resistant to intravaginal HIV challenge. Challenge of mice expressing VRC01 or VRC07G54W resulted in 5/8 or 12/12 animals exhibiting undetectable viral load respectively, despite at least 15 exposures to virus. Interestingly, he observed limited CD4-cell depletion in the peripheral blood, but substantial CD4-cell depletion in mucosal tissues of control animals analogous to observations in recently infected human patients15. Viral load assayed throughout the experiment by qPCR revealed that control mice were infected within six challenges with JR-CSF while the two mice expressing VRC01 that became infected did so only at very late time points, suggesting that VRC01 expression provided substantial protection from mucosal transmission. More strikingly, all of the mice expressing VRC07G54W were protected against as many as 20 consecutive weekly challenges with the REJO.c transmitted molecular founder strain and showed no signs of viral load by a commercial viral load assay sensitive to 200 copies/mL.
Challenges for Prophylactic Proteins
Multiple studies have described the potential for proteins with different antibody-based architectures to neutralize HIV. Among these architectures, immunoadhesins, consisting of the fusion of a single-chain Fv to an IgG Fc domain, have demonstrated potent activity in a macaque SIV-challenge model5. This architecture has the advantage of a single, compact coding region that enables it to be delivered by self-complementary AAV vectors, which have limited carrying capacity5. However, recent studies have reported that conversion of full-length antibodies into immunoadhesins can negatively impact neutralization potency16. Phil Johnson presented results comparing an immunoadhesin form of PG9 to the native IgG architecture in which he found that the immunoadhesin exhibited 10 fold reduced neutralization potency. As a result of this, Johnson suggested that natural antibody architectures might be preferred for further clinical development.
The PG9 antibody recognizes a V2/V3 epitope on the HIV envelope trimer and its activity is highly dependent on the presence of specific glycans17. The original description of this family of antibodies noted that for certain viral strains, complete neutralization activity was not observed, regardless of antibody concentration1. This was subsequently ascribed, at least in part, to glycan heterogeneity of the Env spike. To determine the extent of this behavior, Burton presented data from experiments testing a variety of antibodies against large virus panels. He concluded that the behavior, which was limited, was most apparent for PG9-like and MPER antibodies but was relatively rare for many PGT antibodies recognizing glycans as well as CD4 binding site antibodies. Ultimately, the significance of viral glycan heterogeneity for the antibody gene transfer approach is uncertain, but it probably should be factored into the choice of selecting antibody combinations for prophylaxis.
Improved Antibodies
Prior to the latest patient-derived bNAbs that reinvigorated the field, soluble bi- or tetra-valent forms of CD4 were among the most potent protein-based anti-HIV reagents. Michael Farzan (Harvard University) presented studies on the fusion of such a reagent with a co-receptor mimetic peptide. HIV-1 co-receptors (CCR5 and CXCR4) share amino-terminal domains that include sulfotyrosines, which bind to a conserved site on gp120. The heavy-chain CDR3 regions of several antibodies targeting the co-receptor binding site of gp120 also include sulfotyrosines, and sulfopeptides based on these CDR3 regions can bind gp120 and neutralize HIV-1. A fusion of one such peptide with the CD4-Ig, dubbed “eCD4-Ig”, was at least as potent as the current generation of anti-HIV Abs and neutralized not only all neutralization resistant (all Tier 2 and 3) HIV-1 strains, but also both HIV-2 and SIV isolates. Additionally, it significantly outperformed CD4-Ig and IgGb12 in an assay of antibody-dependent cell-mediated cytotoxicity (ADCC).
The dramatic breadth of eCD4-Ig presumably relates to its narrow targeting of HIV’s conserved receptor and co-receptor binding sites, in contrast to antibodies, which generally rely on contacts with less conserved regions of Env. In the context of AAV-based gene delivery, eCD4-Ig is small enough to be delivered by self-complementary AAV vectors. Macaque studies are now being undertaken to determine the utility of eCD4-Ig in prophylactic and therapeutic contexts.
Pamela Bjorkman (California Institute of Technology) presented studies using structure-based design to improve the potency of CD4 binding site antibodies, as well as to overcome common escape mutations the virus acquires to evade these antibodies. Antibody NIH45-46, a more potent variant of VRC01, contains an insertion that contacts the inner domain of gp120 and contributes to its greater activity compared to VRC01. Noticing that both NIH45-46 and VRC01 failed to fill a conserved hydrophobic pocket on gp120 that is filled by Phe43 of CD4, a mutant of NIH45-46 that substituted a Glycine for Tryptophan at position 54 was designed and found to be about 10-fold more potent than the parent antibody against a cross-clade panel of difficult to neutralize viruses18.
Highly potent VRC01-like antibodies, which have been isolated from at least 5 individuals, derive from the same germline gene, VH1-2*02. Bjorkman described key shared features of these antibodies, including a short CDR L3 loop and characteristic heavy chain residues (Trp50, Asn58, Arg71, and Trp100B). These residues are critical for initial binding of the VH1-2*02 germline to its HIV-1 target and explain the restricted VH gene segment usage of this class of antibodies19. The lack of genes with these features in laboratory animals suggests that attempts to test vaccines designed to induce these antibodies may need to be performed in mice with human antibodies genes.
Viruses resistant to VRC01-like antibodies often have mutations at sites contacted by characteristic residues. This observation was used to design antibody variants that could neutralize such viruses. One such antibody, 45–46m2, neutralizes 96% of HIV strains in a cross-clade panel and neutralizes a set of viral isolates resistant to all other known bNAbs20. A second mutant, 45–46m7, designed to thwart resistance from NIH45-46G54W, restores neutralization of consensus escape mutants, thus effectively targeting a common route of HIV escape. Bjorkman emphasized that finding a few well-chosen substitutions capable of dramatically improving natural bNAbs demonstrates that such antibodies are not necessarily optimal as isolated, raising the possibility of continued improvements using structure-based design methods. In addition, raising the percent of viral isolates neutralized from ~90% (VRC01 and related antibodies) to 96% suggests achieving nearly100% breadth may be possible.
Antibodies as a Treatment?
Although the meeting primarily dealt with the potential of antibody gene delivery to prevent HIV-1 infection, Michel Nussenzweig (The Rockefeller University) presented studies exploring the possibility that antibodies might also be used to treat established infections21. Anti-retroviral therapy has been a resounding success, but daily dosing, side effects, and resistance to antiretroviral drugs suggest that alternatives should be investigated.
Earlier studies demonstrated that the first generation of bNAbs (b12, 2G12, 4E10 and 2F5) were of limited efficacy as therapeutics in humans or in humanized mice because escape variants emerged within a short period of time. To examine the potential of more potent antibodies to function as therapeutic agents, the Nussenzweig laboratory made use of humanized-mice engrafted with hematopoietic stem cells and then infected with a CCR5-tropic HIV-1YU222.
Groups of mice were treated with either a single antibody (among five different bNAbs) or combinations of three or five antibodies. Antibodies targeting several different epitopes were utilized: NIH45-46G54W, PG16, PGT128, 10-1074 (a more potent variant of PGT121), and 3BC176, which recognizes a conformational, but yet to be defined epitope.
Nussenzweig reported that as in previous studies, HIV-1 escaped rapidly from treatment with a single antibody for all of the antibodies tested. However the viruses that escaped showed a limited number of genetic alterations in sites targeted directly by the antibodies. Moreover, combinations of five broadly neutralizing antibodies (bNAbs) were found to effectively control HIV-1 infection and suppress viral load to levels below detection during the entire therapy period of up to 60 days. In contrast to antiretroviral therapy (ART)23–25, the longer half-life of antibodies led to viremic control for an average of 60 days after cessation of therapy. Nussenzweig concluded that combinations of potent monoclonal antibodies can effectively control HIV-1 replication in humanized mice, and he suggested that they should be re-examined as a therapeutic modality in HIV-1-infected individuals.
Prospects for Human Trials
In light of the encouraging results being reported by multiple groups in animal models of HIV transmission, there is substantial interest in accelerating translation of these findings to both humans as well as animals through clinical trials (Box 1). However, this promising approach carries with it a number of unique challenges that must be addressed before proof of principle can be achieved in humans. Given the seemingly permanent nature of AAV transduction in animal models, there are heightened concerns surrounding the choice of antibody transgene for immunoprophylaxis. Several groups have reported natural poly- or auto-reactivity of some of the broadly neutralizing antibodies, and certain improvements in potency via structure-based design have resulted in enhanced auto-reactivity. Though poly-reactivity is not an uncommon feature of natural anti-HIV antibodies26, there are concerns regarding off-target toxicity and effect on antibody half-life. To address these concerns, evaluation of antibody proteins in humans by passive transfer to confirm safety and activity prior to delivery by viral vectors may be warranted. Despite such testing, the possibility of rare adverse events may spur the development of immunoprophylaxis vectors capable of regulating, or eliminating antibody expression in such cases. Ultimately, adverse events may be minimized as a result of improvements in antibody potency, which substantially reduce the serum concentrations necessary to achieve sterilizing protection in animals. Potency improvements would have the added benefit of reducing the potential costs of VIP by enabling lower vector doses to be administered to patients.
An AIDS Vaccine for Wild Chimpanzees?
Beatrice Hahn (Univ. of Pennsylvania) described the negative impact that simian immunodeficiency virus (SIVcpz) has on wild chimpanzee populations. Although some SIV strains do not cause pathology in their natural hosts, Hahn presented evidence that chimpanzees infected with SIVcpz have a 10 to 16 fold increased risk of death, and can develop pathology similar to end-stage AIDS. In wild chimpanzee populations already jeopardized by habitat loss and poaching, SIVcpz could pose a serious additional threat. Indeed, one chimpanzee community in Gombe National Park, Tanzania, where SIVcpz prevalence has consistently been over 40%, has suffered dramatic population decline over the past four decades. Accompanying the loss of wild populations, such as those in Gombe, will be the loss of decades of behavioral and other research performed at these unique sites.
HIV infection can be controlled by anti-retroviral therapy; however, treating wild chimpanzees with daily medication is infeasible. While there is no effective SIV vaccine, AAV-mediated antibody gene transfer offers a potential approach. This method might be practical for wild chimpanzees because a single dose of AAV administered by dart could potentially be sufficient to induce long-lasting antibody expression. The success of VIP could be monitored non-invasively, with fecal screening for secreted antibodies and by testing for new SIVcpz infections.
AAV-mediated gene delivery to control SIVcpz infection would require highly potent and broadly neutralizing anti-SIVcpz reagents. To identify such reagents, Hahn and colleagues utilized a panel of 11 SIVcpz infectious molecular clones (IMCs), 1 SIVgor IMC, and 3 HIV-1 control IMCs to test for antibody neutralization. They screened nearly 50 monoclonal antibodies known to broadly and potently neutralize HIV-1. Generally, these antibodies had little neutralizing activity on the SIVcpz IMC panel. The only effective neutralizers were CD4 D1–D2-containing reagents, the best of which included a fused sulfopeptide as described in the presentation by Farzan. These reagents may be considered for an antibody-mediated vaccine for wild chimpanzees that could protect certain endangered populations from simian AIDS, and the outcome of VIP in chimpanzees may also inform human studies.
Two separate Phase I trials are currently under development involving AAV for the expression of antibody from muscle tissues. Phil Johnson, who is leading one of these trials has completed discussions with the FDA to conduct a trial of AAV1 expressing PG9 antibody from muscle in high risk, seronegative patients. Clinical grade manufacturing of this vector has been completed and the trial is expected to commence in the near future. David Baltimore, in partnership with the Vaccine Research Center at the NIH, is planning a separate trial of AAV8 expressing a CD4 binding site antibody from muscle in infected patients receiving treatment with antiretroviral drugs. During the session he revealed that efforts are currently underway to identify manufacturing capacity for vector production and to finalize the design of the trial.
In spite of the challenges, a successful demonstration of gene transfer-based immunoprophylaxis could alter the landscape of vaccine development and provide a new pathway for tackling challenging vaccine targets like Hepatitis C, pandemic Influenza and Malaria. The ever-expanding universe of antibodies targeting not only infectious diseases, but also aberrant forms of endogenous proteins may lead to the development of entirely new prophylactic and therapeutic interventions that could have a major impact on protecting patients from disease.
Acknowledgments
The authors wish to thank the session presenters for allowing the description of their unpublished work and Peter Hale for helpful discussions. We also thank S.A. Plotkin and B.H. Hahn for organizing the scientific program and the Foundation for Vaccine Research for sponsoring the symposium.
The Foundation wishes to acknowledge and thank the Wellcome Trust for their generous support, as well as the International AIDS Vaccine Initiative (IAVI).
Footnotes
Competing Financial Interests
The authors declare no competing financial interests.
References
- 1.Walker LM, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu X, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathwani AC, et al. Adenovirus-Associated Virus Vector–Mediated Gene Transfer in Hemophilia B. N Engl J Med. 2011 doi: 10.1056/NEJMoa1108046. 111210080018000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maguire AM, et al. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson PR, et al. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nature Medicine. 2009;15:901–906. doi: 10.1038/nm.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balazs AB, et al. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2011 doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivera VM, et al. Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood. 2005;105:1424–1430. doi: 10.1182/blood-2004-06-2501. [DOI] [PubMed] [Google Scholar]
- 8.Hessell AJ, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 9.Salazar-Gonzalez JF, et al. Deciphering human immunodeficiency virus type 1 transmission and early envelope diversification by single-genome amplification and sequencing. Journal of Virology. 2008;82:3952–3970. doi: 10.1128/JVI.02660-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keele BF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci USA. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilen CB, et al. Phenotypic and immunologic comparison of clade B transmitted/founder and chronic HIV-1 envelope glycoproteins. Journal of Virology. 2011;85:8514–8527. doi: 10.1128/JVI.00736-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Z, et al. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J Exp Med. 2007;204:705–714. doi: 10.1084/jem.20062411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denton PW, et al. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 2008;5:e16. doi: 10.1371/journal.pmed.0050016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDermott AB, et al. Repeated low-dose mucosal simian immunodeficiency virus SIVmac239 challenge results in the same viral and immunological kinetics as high-dose challenge: a model for the evaluation of vaccine efficacy in nonhuman primates. Journal of Virology. 2004;78:3140–3144. doi: 10.1128/JVI.78.6.3140-3144.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guadalupe M, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. Journal of Virology. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.West AP, Galimidi RP, Gnanapragasam PNP, bjorkman PJ. Single-chain Fv-based anti-HIV proteins: potential and limitations. Journal of Virology. 2012;86:195–202. doi: 10.1128/JVI.05848-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doores KJ, Burton DR. Variable loop glycan dependency of the broad and potent HIV-1-neutralizing antibodies PG9 and PG16. Journal of Virology. 2010;84:10510–10521. doi: 10.1128/JVI.00552-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diskin R, et al. Increasing the Potency and Breadth of an HIV Antibody by Using Structure-Based Rational Design. Science. 2011 doi: 10.1126/science.1213782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West AP, Diskin R, Nussenzweig MC, bjorkman PJ. Structural basis for germ-line gene usage of a potent class of antibodies targeting the CD4-binding site of HIV-1 gp120. Proc Natl Acad Sci USA. 2012;109:E2083–E2090. doi: 10.1073/pnas.1208984109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sather DN, et al. Broadly neutralizing antibodies developed by an HIV+ elite neutralizer exact replication fitness cost to the contemporaneous virus. Journal of Virology. 2012 doi: 10.1128/JVI.01893-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein F, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012 doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baenziger S, et al. Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2−/−gamma c−/− mice. Proc Natl Acad Sci USA. 2006;103:15951–15956. doi: 10.1073/pnas.0604493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choudhary SK, et al. Suppression of human immunodeficiency virus type 1 (HIV-1) viremia with reverse transcriptase and integrase inhibitors, CD4+ T-cell recovery, and viral rebound upon interruption of therapy in a new model for HIV treatment in the humanized Rag2−/−{gamma}c−/− mouse. Journal of Virology. 2009;83:8254–8258. doi: 10.1128/JVI.00580-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nischang M, et al. Humanized mice recapitulate key features of HIV-1 infection: a novel concept using long-acting anti-retroviral drugs for treating HIV-1. PLoS ONE. 2012;7:e38853. doi: 10.1371/journal.pone.0038853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denton PW, et al. Generation of HIV Latency in Humanized BLT Mice. Journal of Virology. 2012;86:630–634. doi: 10.1128/JVI.06120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mouquet H, et al. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature. 2010;467:591–595. doi: 10.1038/nature09385. [DOI] [PMC free article] [PubMed] [Google Scholar]