Summary
Objective
To determine the incidence of meningitis caused by Haemophilus influenzae, Neisseria meningitidis, and Streptococcus pneumoniae in the North American Arctic during 2000–2010.
Methods
Surveillance data were obtained from the International Circumpolar Surveillance network. We defined a case of bacterial meningitis caused by H. influenzae, N. meningitidis, or S. pneumoniae as a culture-positive isolate obtained from a normally sterile site in a resident with a meningitis diagnosis.
Results
The annual incidence/100,000 persons for meningitis caused by H. influenzae, N. meningitidis, and S. pneumoniae among all North American Arctic residents was: 0.6, 0.5, and 1.5, respectively; the meningitis incidence among indigenous persons in Alaska and Canada (indigenous status not recorded in Greenland) for those three bacteria was: 2.1, 0.8, and 2.4, respectively. The percentage of pneumococcal isolates belonging to a 7-valent pneumococcal conjugate vaccine serotype declined from 2000–2004 to 2005–2010 (31% to 2%, p-value <0.01). During 2005–2010, serotype a caused 55% of H. influenzae meningitis and serogroup B caused 86% of meningococcal meningitis.
Conclusions
Compared with all North American Arctic residents, indigenous people suffer disproportionately from bacterial meningitis. Arctic residents could benefit from the development of a H. influenzae serotype a vaccine and implementation of a meningococcal serogroup B vaccine.
Keywords: bacterial meningitis, epidemiology, Haemophilus influenzae, Neisseria meningitidis, Streptococcus pneumoniae
INTRODUCTION
Historically, populations in the Arctic region of North America, especially indigenous people, have suffered disproportionately from bacterial meningitis compared with non-Arctic populations in the United States and Canada. During 1978–81, the annual incidence of bacterial meningitis in the overall U.S. population was 3/100,000 persons overall and the highest rates were observed among infants aged <1 year (77/100,000 persons).(1) By comparison, the annual incidence of bacterial meningitis among Alaska Native people in southwest Alaska during 1971–74 was 94/100,000 persons overall and 3,242/100,000 among infants aged <1 year.(2) In Northern Canada, the annual bacterial meningitis incidence during 1972–77 was 19/100,000 persons among non-indigenous residents compared with 200/100,000 among indigenous people.(3) Conjugate vaccines to protect against certain serotypes/serogroups of Haemophilus influenzae, Neisseria meningitidis, and Streptococcus pneumoniae, three common causes of bacterial meningitis, are now available and part of routine childhood immunization schedules. (See Table 1 for the specific years that the North American Arctic regions implemented these vaccines). Since the introduction of those vaccines, however, the incidence of meningitis in Arctic populations has not been reevaluated.
Table 1.
Characteristics of North American Arctic Countries
| Alaska | Northern Canada | Greenland | |
|---|---|---|---|
| Populationa | 668 662 | 136 921 | 56 550 |
| % Indigenous | 19% | 60% | Unknown |
| Region size, km2 | 1 518 807 | 4 506 600 | 2 131 863 |
| Year vaccine introduced or recommended in regionb | |||
| 7-valent pneumococcal conjugate vaccine | 2001 | 2002–2006 | N/A |
| 10-valent pneumococcal conjugate vaccine | N/A | 2010–2011 | N/A |
| 13-valent pneumococcal conjugate vaccine | 2010 | 2010–2011 | 2010 |
| 23-valent pneumococcal polysaccharide vaccine | 1983 | 1983 | 1996 |
| Quadrivalent meningococcal conjugate vaccine | 2006 | 2005–2006 | N/A |
| Serogroup C meningococcal conjugate vaccine | N/A | 2002–2007 | N/A |
| Haemophilus influenzae serotype b vaccine | 1991 | 1986–1997 | 1996 |
Abbreviation: N/A, not available during 2000–2010.
Mean population 2000–2010.
Northern Canadian territories introduced vaccines over different time periods.
Populations in Alaska, Northern Canada, and Greenland share certain unique social and environmental risk factors for infectious diseases.(4) The Arctic region is sparsely populated with limited health care/public health infrastructure.(5) The risk for infectious diseases is not uniform within North American Arctic populations. In particular, indigenous people (e.g., Eskimo people in Alaska and Inuit people of Greenland, Northern Canada, and Alaska), who comprise varying proportions of the population in each region (Table 1), experience a greater burden of infectious diseases compared with non-indigenous people.(3, 6) Indigenous people are at higher risk for infectious diseases than non-indigenous people because they have greater exposure to conditions that facilitate disease transmission such as household crowding and inadequate access to water/sanitation services.(7, 8) In order to better understand the distinct epidemiology of infectious diseases in the Arctic that result from these risk factors, public health agencies in countries with populations residing in the Arctic collaboratively operate the International Circumpolar Surveillance (ICS) network.(9) ICS methods allow participating countries to use existing infrastructure to collect and share public health surveillance data.(5) All three North American countries with Arctic populations – United States, Canada, and Greenland – participate in ICS and have shared surveillance data for certain invasive bacterial diseases since 2000.(6) This study uses ICS data to describe the epidemiology of meningitis caused by S. pneumoniae, H. influenzae, and N. meningitidis among persons living in the Arctic region of North America during 2000–2010.
METHODS
Surveillance Methods
ICS defines a case of bacterial meningitis caused by H. influenzae, N. meningitidis, or S. pneumoniae as a culture-positive isolate obtained from a normally sterile site (e.g., blood, cerebrospinal fluid, pleural fluid, peritoneal fluid, synovial fluid) in a resident of the surveillance areas with a diagnosis of meningitis recorded in their medical record. Case-isolates were identified through population-based surveillance by 52 participating laboratories (23 in Alaska, 14 in Northern Canada [Yukon, Northwest Territories and Nunavut], 15 in Greenland).(5) Laboratories reported case-patients to public health personnel and forwarded sterile site specimens to regional reference laboratories for additional testing to confirm organism identity and to determine serotype/serogroup. Public health personnel obtained case-patients’ demographic characteristics, clinical characteristics, risk factor information, and immunization history from reviewing their medical records. These epidemiologic data were recorded on a standard, organism-specific data collection form.
The reference laboratories confirm the identity of S. pneumoniae, H. influenzae, or N. meningitidis by colony morphology/biochemical characteristics using standard laboratory methods (10); however, methods used for determining serotype/serogroup are not uniform across reference laboratories so an interlaboratory quality control program exists to ensure comparability of results.(11, 12) The methods for determining serotype/serogroup have been described previously.(11, 12) For S. pneumoniae, serotype testing was done in all three regions by the Quellung reaction.(13) H. influenzae serotype testing was performed by either slide agglutination or polymerase chain reaction capsule typing; some laboratories employed both methods. N. meningitidis serogroup testing was performed by slide agglutination, latex agglutination together with staphylococcal coagglutination, or polymerase chain reaction; some laboratories employed more than one method.
Additionally, the reference laboratories performed antibiotic susceptibility testing on S. pneumoniae isolates. Antibiotic susceptibility testing was performed by the broth microdilution method in Alaska and Northern Canada and by Etest (AB Biodisk, Solna, Sweden) for isolates from Greenland.(11) Antibiotics tested included penicillin, third generation cephalosporins (cefotaxime and ceftriaxone), chloramphenicol, clindamycin, erythromycin, fluoroquinolones (ofloxacin and levofloxacin), trimethoprim-sulfamethoxazole, and vancomycin. Per ICS protocol, minimal inhibitory concentrations (MICs) were interpreted according to Clinical and Laboratory Standards Institute standards for parenteral nonmeningitis treatment.(14)
Data Analysis
Epidemiologic and laboratory data from the ICS countries were forwarded annually to an ICS coordinator in Anchorage, Alaska. All data were double entered into Paradox version 10.0 (Corel, Ottawa, Ontario, Canada) and analyzed by using SAS version 9.3 (SAS Institute, Cary, NC, USA). Population estimates for incidence calculations were obtained from Statistics Canada website (www.statcan.ca), the Alaska Department of Labor and Workforce Development website (www.labor.state.ak.us), and the Statistics Greenland website (www.statgreen.gl). Census data from Alaska and Canada indicate whether persons belong to an indigenous group. Census data from Greenland do not indicate the indigenous status of persons; therefore, case-patients from Greenland were excluded from analyses by indigenous status. Crude incidence and 95% confidence interval (CI) were calculated by assuming a Poisson distribution. Age-adjusted incidence rates were calculated by using the World Health Organization 2000 standard population and the age groups of <1, 2–19, 20–64, and >65 years.
RESULTS
Descriptive Epidemiology
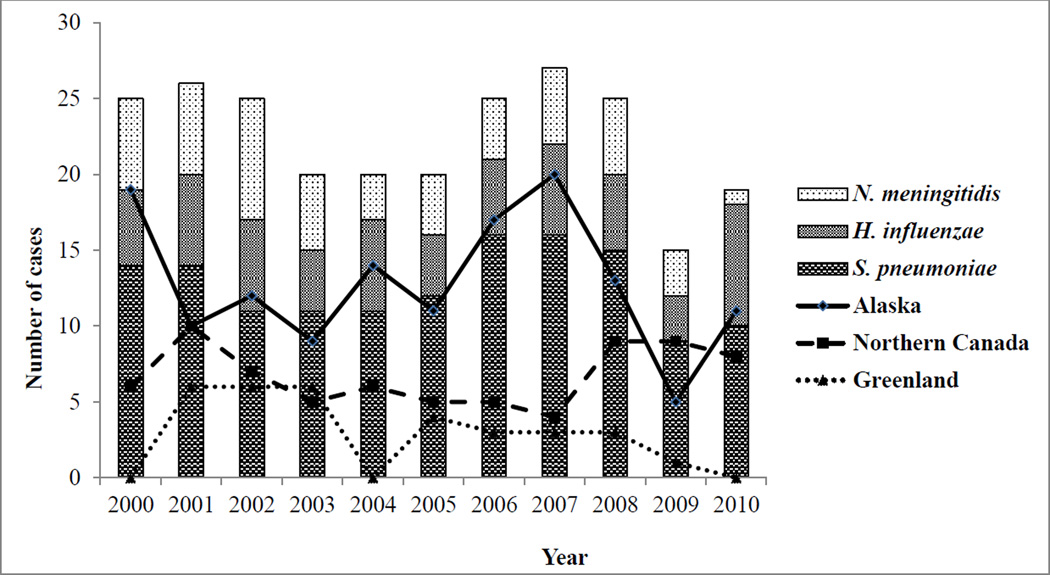
We identified 247 meningitis cases in the North American Arctic during our study period (Figure 1). Of those cases, 139 were caused by S. pneumoniae (median: 12/year; range: 9–16/year), 58 by H. influenzae (median: 5/year; range: 3–8/year), and 50 by N. meningitidis (median: 5/year; range: 1–8/year). By ICS region, 141 cases occurred in Alaska (median: 12/year; range: 5–20/year), 74 in Northern Canada (median: 6/year; range: 4–10/year), and 32 in Greenland (median: 3/year; range: 0–6/year). No cases of H. influenzae meningitis were detected in Greenland. Of the 58 H. influenzae cases, 30 were caused by serotype a (21 cases in Northern Canada and 9 cases in Alaska).
Figure 1.
Number of meningitis cases by bacterial etiology and country, 2000–2010. (N = 247)
Meningitis case-patients were younger in Northern Canada (median age: 0.8 years) compared with Alaska (median age: 17.3 years) and Greenland (median age: 36.6 years) (Table 2). Case-patients infected with S. pneumoniae, the most common cause of meningitis among the three organisms evaluated, were older in Alaska (median age: 38.3 years) and Greenland (median age: 46.2 years) than in Northern Canada (median age: 1.2 years). Meningitis case-patients infected with N. meningitidis were also older in Alaska (median age: 12.6 years) and Greenland (median age: 5.6 years) compared with Northern Canada (median age: 1.8 years). Case-patients with H. influenzae were young in Alaska (median age: 0.8 years) and Northern Canada (median age: 0.5 years). Among case-patients infected with H. influenzae, 83% were aged <2 years compared with 32% and 31% of case-patients infected with S. pneumoniae and N. meningitidis, respectively. Meningitis case-patients were more likely to be indigenous in Northern Canada (86%) than in Alaska (43%). Among case-patients with H. influenzae meningitis whose indigenous status was known, 30 persons (68%) in Northern Canada and 17 persons (97%) in Alaska were indigenous (data not shown). Almost all meningitis case-patients were hospitalized. A total of 20 (14%), 6 (9%), and 6 (21%) case-patients from Alaska, Northern Canada, and Greenland, respectively, died. Meningitis in case-patients was most frequently associated with pneumonia, followed by cellulitis, endocarditis, and pericarditis.
Table 2.
Demographic and Clinical Characteristics of Meningitis Case-Patients by Country, 2000–2010
| Characteristic | Alaska | Northern Canada | Greenland | |
|---|---|---|---|---|
| Number of meningitis case-patients | 141 | 74 | 32 | |
| Number aged <2 years (%)a | 48 (34) | 52 (70) | 9 (28) | |
| Median age (range)a | 17.3 (0.2–87.5) | 0.8 (0–72) | 36.6 (0–70.9) | |
| Number males (%)b | 72 (51) | 38 (52) | 21 (68) | |
| Number indigenous (%)c | 61 (43) | 59 (86) | N/A | |
| Number hospitalized (%)d | 135 (96) | 68 (96) | 32 (100) | |
| Number deaths (%)e | 20 (14) | 6 (9) | 6 (21) | |
| Top 4 associated syndromes | ||||
| Pneumonia | 31 (22) | 6 (8) | 3 (9) | |
| Cellulitis | 4 (3) | 3 (4) | 0 (0) | |
| Endocarditis | 4 (3) | 0 (0) | 1 (3) | |
| Pericarditis | 1 (1) | 2 (3) | 0 (0) | |
Abbreviations: N/A, data not available
Age unknown for 1 case-patient from Greenland.
Sex unknown for 1 case-patient from Northern Canada and 1 case from Greenland.
Indigenous status unknown for 5 case-patients from Northern Canada.
Hospitalization status unknown for 1 case-patient from Alaska and 3 case-patients from Northern Canada.
Death status unknown for 1 case-patient from Alaska, 9 case-patients from Northern Canada, 3 case-patients from Greenland.
Vaccination status was known for 79 case-patients from Alaska and 65 case-patients from Northern Canada. Among case-patients with a known vaccination status in Alaska, 62% (16/26) of persons aged <17 years with pneumococcal meningitis had received ≥1 dose of a pneumococcal conjugate vaccine, 35% (8/23) of persons aged ≥17 years with pneumococcal meningitis had received the 23-valent protein polysaccharide pneumococcal vaccine, and 83% (15/18) of persons aged <10 years with H. influenzae meningitis had received the H. influenzae type b vaccine; 0% (0/12) of case-patients with meningococcal meningitis had received the quadrivalent meningococcal conjugate vaccine. Among case-patients with a known vaccination status in Northern Canada, 36% (5/14) of person aged <17 years with pneumococcal meningitis had received a pneumococcal conjugate vaccine, 18% (2/11) of persons aged ≥17 years with pneumococcal meningitis had received the 23-valent protein polysaccharide pneumococcal vaccine, and 83% (25/30) of persons aged <10 years with H. influenzae meningitis had received the H. influenzae type b vaccine; 38% (3/8) of case-patients with meningococcal meningitis aged <17 years and 0% (0/2) aged ≥17 years had received the quadrivalent meningococcal conjugate vaccine. In Greenland, only the H. influenzae serotype b vaccine was used as part of a routine immunization program during our study period and no case-patients with H. influenzae meningitis were reported (Table 1).
Incidence Rates
The overall annualized crude incidence of meningitis/100,000 persons caused by S. pneumoniae, H. influenzae, and N. meningitidis in the three North American ICS regions during our study period was 2.6 (95% CI: 2.3–2.9) and the age-standardized incidence was 2.7 (Table 3). The overall crude incidence of meningitis among indigenous persons in Alaska and Northern Canada was 5.2 (95% CI: 4.3–6.3), among non-indigenous persons in Alaska and Northern Canada was 1.5 (95% CI: 1.2–1.8), and among all children aged <2 years was 34.9 (95% CI: 28.7–42.1). The overall annualized crude incidence of meningitis hospitalizations was 2.5 (95% CI: 2.2–2.8) and the age-standardized hospitalization incidence was 2.6. The overall annualized crude incidence of meningitis deaths was 0.3 (95% CI: 0.2–0.5) and the age-standardized death incidence was 0.4.
Table 3.
Annualized Incidence (95% Confidence Interval) of Meningitis Cases, Hospitalizations, and Deaths By Country and Bacterial Etiology, 2000–2010.
| Alaska (N = 141) |
Northern Canada (N = 74) |
Greenland (N = 32) |
Streptococcus pneumoniae (N = 139) |
Haemophilus influenzae (N = 58) |
Neisseria meningitidis (N = 50) |
Overall (N = 247) |
|
|---|---|---|---|---|---|---|---|
| Number of meningitis cases, hospitalizations, or deaths per 100 000 persons | |||||||
| Crude meningitis case incidence (all ages) | 1.9 (1.6–2.3) | 4.9 (3.9–6.2) | 5.0 (3.4–7.1) | 1.5 (1.2–1.7) | 0.6 (0.5–0.8) | 0.5 (0.4–0.7) | 2.6 (2.3–2.9) |
| Indigenous | 4.3 (3.3–5.5) | 6.8 (5.2–8.8) | N/A | 2.4 (1.8–3.1) | 2.1 (1.5–2.7) | 0.8 (0.5–1.3) | 5.2 (4.3–6.3) |
| Non-indigenous | 1.4 (1.1–1.7) | 2.4 (1.3–3.9) | N/A | 1.2 (0.9–1.5) | 0.2 (0.1–0.3) | 0.4 (0.3–0.6) | 1.5 (1.2–1.8) |
| Crude meningitis case incidence (<2 years) | 20.1 (14.8–26.7) | 94.7 (70.7–124.2) | 47.8 (21.9–90.8) | 14.4 (10.5–19.3) | 15.4 (11.3–20.4) | 5.1 (2.9–8.3) | 34.9 (28.7–42.1) |
| Indigenous | 46.7 (31.7–66.4) | 110.2 (80.0–148.0) | N/A | 22.6 (14.5–33.6) | 41.4 (30.0–55.6) | 6.6 (2.6–13.6) | 70.6 (55.5–88.5) |
| Non-indigenous | 9.9 (5.8–15.8) | 53.3 (23.0–105.1) | N/A | 10.2 (6.3–15.6) | 1.9 (0.5–5.0) | 4.4 (2.0–8.3) | 13.4 (8.7–19.7) |
| Age-standardized meningitis case incidence | 2.0 | 4.8 | 5.2 | 1.5 | 0.7 | 0.5 | 2.7 |
| Indigenous | 3.8 | 5.7 | N/A | 2.2 | 1.6 | 0.6 | 4.5 |
| Non-indigenous | 1.4 | 3.1 | N/A | 1.2 | 0.2 | 0.5 | 1.5 |
| Crude hospitalization incidence (all ages) | 1.8 (1.5–2.2) | 4.5 (3.5–5.7) | 5.0 (3.4–7.1) | 1.4 (1.2–1.6) | 0.6 (0.5–0.8) | 0.5 (0.4–0.6) | 2.5 (2.2–2.8) |
| Indigenous | 4.0 (3.0–5.2) | 6.6 (5.0–8.5) | N/A | 2.2 (1.6–2.9) | 2.1 (1.5–2.7) | 0.7 (0.4–1.2) | 5.0 (4.1–6.0) |
| Non-indigenous | 1.3 (1.0–1.6) | 1.7 (0.9–3.1) | N/A | 1.1 (0.9–1.4) | 0.1 (0.1–0.2) | 0.4 (0.3–0.6) | 1.4 (1.1–1.7) |
| Age-standardized hospitalization incidence | 1.9 | 4.4 | 5.2 | 1.4 | 0.6 | 0.5 | 2.6 |
| Crude death incidence (all ages) | 0.3 (0.2–0.4) | 0.4 (0.2–0.9) | 1.0 (0.4–2.1) | 0.3 (0.2–0.4) | 0.0a (0.0–0.1)a | 0.1 (0.0–0.1)b | 0.3 (0.2–0.5) |
| Indigenous | 0.6 (0.3–1.2) | 0.4 (0.1–1.0) | N/A | 0.4 (0.2–0.7) | 0.1 (0.0–0.3)b | 0.1 (0.0–0.3) | 0.5 (0.3–0.9) |
| Non-indigenous | 0.2 (0.1–0.3) | 0.5 (0.1–1.4) | N/A | 0.2 (0.1–0.4) | 0.0 | 0.1 (0.0–0.1)b | 0.2 (0.1–0.4) |
| Age-standardized death incidence | 0.3 | 0.4 | 1.0 | 0.3 | 0.02 | 0.1 | 0.4 |
Abbreviation: N/A, data not available.
Incidence estimate rounded down to 0.0.
Lower estimate for 95% confidence rounded down to 0.0.
The annualized crude incidence of meningitis caused by S. pneumoniae, H. influenzae, or N. meningitidis in Alaska was 1.9 (95% CI: 1.6–2.3), in Northern Canada was 4.9 (95% CI: 3.9–6.2), and in Greenland was 5.0 (95% CI: 3.4–7.1) (Table 3). The annualized crude incidence of meningitis hospitalizations in Alaska was 1.8 (95% CI: 1.5–2.2), in Northern Canada was 4.5 (95% CI: 3.5–5.7), and in Greenland was 5.0 (95% CI: 3.4–7.1). The annualized crude incidence of meningitis deaths in Alaska was 0.3 (95% CI: 0.2–0.4), in Northern Canada was 0.4 (95% CI: 0.2–0.9), and in Greenland was 1.0 (95% CI: 0.4–2.1).
The annualized crude incidence of meningitis in all three regions caused by S. pneumoniae was 1.5 (95% CI: 1.2–1.7), by H. influenzae was 0.6 (95% CI: 0.5–0.8), and by N. meningitidis was 0.5 (95% CI: 0.4–0.7) (Table 3). The annualized crude incidence of hospitalizations caused by S. pneumoniae was 1.4 (95% CI: 1.2–1.6), by H. influenzae was 0.6 (95% CI: 0.5–0.8), and by N. meningitidis was 0.5 (95% CI: 0.4–0.6). The overall crude incidence of deaths caused by S. pneumoniae was 0.3 (95% CI: 0.2–0.4), by H. influenzae was 0.0 (95% CI: 0.0–0.1), and by N. meningitidis was 0.1 (95% CI: 0.0–0.1).
Serotype Distribution and Antibiotic Resistance
Among case-patients in Alaska, the percentage of pneumococcal isolates belonging to a serotype included in the 7-valent pneumococcal conjugate vaccine declined from 2000–2004 to 2005–2010 (31% to 2%, p-value <0.01) and the percentage belonging to one of the 6 additional serotypes in the 13-valent pneumococcal conjugate vaccine increased (14% to 43%, p-value = 0.01) (Table 4). The percentage of pneumococcal isolates belonging to the 7-valent pneumococcal conjugate vaccine or one of the 6 additional serotypes in the 13-valent pneumococcal conjugate vaccine did not change between 2000–2004 and 2005–2010 among persons with meningitis in Northern Canada and Greenland. In Alaska and Northern Canada, the percentage of H. influenzae isolates belonging to serotype a or serotype b was similar between 2000–2004 and 2005–2010; by 2005–2010, serotype a was the predominant cause of H. influenzae meningitis. In all three ICS regions, there was no difference in the percentage of meningococcal isolates belonging to a serogroup included in the quadrivalent meningococcal vaccine (serogroups A, C, Y, W135) or to serogroup B between 2000–2004 and 2005–2010; by 2005–2010, the majority of meningococcal meningitis was caused by serogroup B.
Table 4.
Serogroup/Serotype of Bacteria Isolated from Meningitis Cases by Country, 2000–2010.a
| Alaska | Northern Canada | Greenland | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2000– 2004 N (%) |
2005– 2010 N (%) |
p- value |
2000– 2004 N (%) |
2005– 2010 N (%) |
p- value |
2000– 2004 N (%) |
2005– 2010 N (%) |
p- value |
|
| Streptococcus pneumoniae | 35 | 44 | 0.9 | 11 | 14 | 0.9 | 6 | 9 | 0.7 |
| PCV7 serotypeb | 11 (31) | 1 (2) | <0.001 | 5 (46) | 2 (14) | 0.1 | 3 (50) | 3 (33) | 0.8 |
| PCV6+ serotypec | 5 (14) | 19 (43) | 0.01 | 3 (27) | 3 (21) | 0.7 | 1 (17) | 2 (22) | 0.7 |
| Non-PCV serotype | 19 (54) | 24 (55) | 0.9 | 3 (27) | 9 (64) | 0.2 | 2 (33) | 4 (44) | 0.6 |
| Haemophilus influenzae | 10 | 15 | 0.7 | 17 | 16 | 0.3 | N/A | N/A | N/A |
| Serotype a | 2 (20) | 7 (47) | 0.2 | 11 (65) | 10 (62) | 0.4 | N/A | N/A | N/A |
| Serotype b | 4 (40) | 2 (13) | 0.3 | 5 (29) | 3 (19) | 0.3 | N/A | N/A | N/A |
| Other serotyped | 4 (40) | 6 (40) | 0.8 | 1 (6) | 3 (19) | 0.5 | N/A | N/A | N/A |
| Neisseria meningitidis | 16 | 13 | 0.2 | 3 | 6 | 0.3 | 3 | 2 | 0.5 |
| Serogroup A, C, Y, W135 | 3 (19) | 1 (8) | 0.2 | 2 (67) | 2 (33) | 0.8 | 1 (33) | 0 (0) | 0.3 |
| Serogroup B | 13 (81) | 12 (92) | 0.4 | 1 (33) | 4 (67) | 0.3 | 2 (67) | 2 (100) | 0.9 |
| Other serogroup | 0 (0) | 0 (0) | --- | 0 (0) | 0 (0) | --- | 0 (0) | 0 (0) | --- |
Abbreviations: N/A, data not available; PCV, pneumococcal conjugate vaccine.
Data shown for subset of meningitis cases from whom isolates were available for laboratory serotype/serogroup determination.
Includes the 7 serotypes included in the 7-valent pneumococcal conjugate vaccine (4, 6B, 9V, 14,18C, 19F, 23F).
Includes the 6 additional serotypes included in the 13 -valent pneumococcal conjugate vaccine (1, 3, 5, 6A, 7F, 19A).
Includes non-typeable Haemophilus influenza.
Pneumococcal isolates susceptibility to penicillin, ceftriaxone, and vancomycin was 77%, 90%, and 100%, respectively, in Alaska and 86%, 94%, and 100% in Northern Canada (Table 5). In Greenland, all pneumococcal isolates were susceptible to the three antibiotics tested for in that region (penicillin, ceftriaxone, and erythromycin).
Table 5.
Proportion of Pneumococcal Meningitis Case Isolates Susceptible to Selected Antibiotics in Alaska, Northern Canada, and Greenland, 2000–2010.a
| Alaska | Northern Canada | Greenland | ||||
|---|---|---|---|---|---|---|
| Isolates tested (N) | % susceptible | Isolates tested (N) | % susceptible | Isolates tested (N) | % susceptible | |
| Penicillinb | 79 | 77 | 22 | 86 | 14 | 100 |
| Ceftriaxoneb | 79 | 90 | 17 | 94 | 10 | 100 |
| Erythromycin | 79 | 84 | 19 | 95 | 4 | 100 |
| Chloramphenicol | 79 | 99 | 20 | 100 | -- | -- |
| Vancomycin | 79 | 100 | 21 | 100 | -- | -- |
| Trimethoprim-sulfamethoxazole | 79 | 78 | 20 | 85 | -- | -- |
| Levofloxacin | 79 | 100 | 12 | 100 | -- | -- |
| Clindamycin | 73 | 95 | 18 | 100 | -- | -- |
Source: Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Seventeenth informational supplement M100-S17. Wayne, PA: Clinical and Laboratory Standards Institute, 2007.
Minimal inhibitory concentration (MIC) cut-points to predict antibiotic susceptibility (µg/mL): penicillin ≤0.2, ceftriaxone ≤0.1, chloramphenicol ≤4, erythromycin ≤0.25, vancomycin ≤1, trimethoprim-sulfamethoxazole ≤0.5/9.5, levofloxacin ≤2, clindamycin ≤0.25.
Susceptibility interpreted according to Clinical and Laboratory Standards Institute standards for nonmeningitis indications.
DISCUSSION
Previous studies evaluating the epidemiology of bacterial meningitis in the North American Arctic had certain limitations. The incidence estimates from those earlier studies were either imprecise (because the populations under surveillance were small) or unrepresentative (because the surveillance regions encompassed large populations living outside the Arctic) (2, 3, 15). We were able to overcome those limitations by using population-based surveillance data from the International Circumpolar Surveillance network to estimate the incidence of bacterial meningitis caused by S. pneumoniae, H. influenzae, and N. meningitidis in the North American Arctic during 2000–2010.
The meningitis incidence in the North American Arctic is likely much lower now compared with historical estimates. In southwest Alaska, the annual incidence of bacterial meningitis among children aged <5 years was 474/100,000 during 1971–1974.(2) In northern central Canada, the annual incidence of meningitis among all ages during a four-year period in the mid-1970s was 128/100,000.(3) Although the estimates of bacterial meningitis from these older studies are not directly comparable with our results, the greater than ten-fold difference in the magnitude of the incidence increases the possibility that the decline over the previous four decades is likely a real trend.
A substantial proportion of the decline in meningitis rates can be attributed to implementation of vaccines to protect against H. influenzae serotype b (Table 1), the leading cause of bacterial meningitis during the 1970s.(2, 3) Our results indicate that H. influenzae meningitis has been virtually eradicated among non-indigenous people in the Arctic. However, the rates among indigenous people in Alaska and Northern Greenland, especially children aged <2 years, remain high. The high incidence among indigenous people is partly explained by the emergence of H. influenzae serotype a infections in that population. Culture-confirmed invasive H. influenzae serotype a isolates were first identified in Alaska in 2002,(16) and an increase in H. influenzae serotype a disease has also been observed in Canada.(17, 18) According to our results, serotype a is now the predominant cause of H. influenzae meningitis in Alaska and Northern Canada. Thus, the development of a new vaccine against H. influenzae serotype a could be uniquely beneficial to Arctic residents, especially in Alaska and Northern Canada. Additionally, we demonstrated that S. pneumoniae is now the leading cause of meningitis in our population. Therefore, we expect the incidence of meningitis to decrease with increased coverage of pneumococcal conjugate vaccines (Table 1). Similarly, the majority of meningococcal meningitis cases belonged to serogroup B and implementation of a vaccine against that serogroup could result in further reductions in the incidence of meningitis.
Despite the decline in meningitis incidence, it remains elevated in the North American Arctic compared with the general U.S. and Canadian populations. The most recent estimate of bacterial meningitis incidence in Canada comes from a retrospective review of all hospitalized bacterial meningitis cases during 1994–2001; that study determined an annual incidence of 3/100,000.(15) In the United States, the annual incidence of meningitis caused by S. pneumoniae, H. influenzae, N. meningitidis, group B streptococcus, or Listeria monocytogenes for all ages declined from 2.0/100,000 to 1.4/100,000 over a similar time period (1998–2007).(19) The incidence of meningitis caused by S. pneumoniae, H. influenzae, and N. meningitidis among non-indigenous people in our study was similar to that of the general Canadian and US populations. However, the incidence among indigenous people was approximately three-times higher than non-indigenous people. The median age of meningitis case-patients was substantially lower in Northern Canada than in Alaska and Greenland. The lower age of meningitis case-patients in Northern Canada could be because H. influenzae was the predominant cause of meningitis in Northern Canada and the majority of case-patients with H. influenzae meningitis were aged <2 years. In contrast, the predominant cause of meningitis in Alaska and Greenland was S. pneumoniae and the median age for case-patients with pneumococcal meningitis was higher than those with H. influenzae meningitis.
The disparity in the incidence of meningitis cases and hospitalization for meningitis between indigenous people and non-indigenous people could result from differences in social and environmental risk factors. For example, household crowding and inadequate access to in-home piped water are more common among indigenous than non-indigenous persons;(5) these factors have been demonstrated to facilitate transmission of respiratory infections.(8) Additionally, indigenous persons are more likely to live in small communities that are inaccessible by roads.(5) The geographic isolation of these communities combined with limited access to healthcare can be a barrier to identification and provision of prophylactic treatment to contacts of persons with H. influenzae and N. meningitidis meningitis to prevent secondary meningitis cases.
Bacterial meningitis is a serious illness with potential to result in neurologic sequelae or death if appropriate treatment is delayed.(20) Antibiotic susceptibility data was available to us for case-patients with pneumococcal meningitis, the most common cause of meningitis among the three bacteria we evaluated. When pneumococcal meningitis is considered, treatment guidance for children and adults recommend a third generation cephalosporin for empiric therapy; in addition, vancomycin is recommended in Alaska and Canada.(21, 22) Our data demonstrates high susceptibility to those antibiotics among pneumococcal isolates from meningitis case-patients.
Our study had limitations. First, the small population size in the Arctic resulted in insufficient meningitis cases to determine incidence stratified by year, bacterial etiology and region. Aggregating by country alone precluded evaluation of the impact of the different country vaccine programs (Table 1) on the incidence of meningitis caused by bacterial etiology. Similarly, aggregating by bacterial etiology alone masked potential regional differences in the incidence of meningitis that result from the unique geography and environment of each region. Our study encompassed a broad time period and aggregating meningitis cases across all years prevented us from correlating trends in meningitis incidence within our study period with other changes such as improvements in sanitation or implementation of vaccines. Second, certain ICS data elements were not consistently collected across all regions. Information on risk factors for meningitis, such as case-patient’s history of immunosuppressing conditions, was not available for many cases. Thus, some of the differences in meningitis incidence might be the result of differences in the prevalence of those predisposing factors. Also, antibiotic resistance testing was not routinely performed in the participating ICS countries for H. influenzae and N. meningitidis. Therefore, we were unable to comment on the effectiveness of commonly recommended antibiotics for the empiric treatment of bacterial meningitis caused by H. influenzae and N. meningitidis.(21, 22) Finally, we likely underestimated the meningitis incidence by including only culture-confirmed cases in our analysis. For example, a patient with bacterial meningitis might not be reported to our surveillance system if the sterile site culture was obtained after administering antibiotics. Furthermore, any differences between regions in the clinical/laboratory diagnostic capabilities for bacterial meningitis could have affected our comparisons of the incidence.
In this study, we provide updated estimates of the incidence of meningitis in the North American Arctic. Although the incidence of meningitis among Arctic people caused by these three bacteria has declined in recent decades, we demonstrated that a disparity continues to exist between Arctic (especially Indigenous) and non-Arctic populations. Wider implementation of vaccines against S. pneumoniae, H. influenzae, and N. meningitidis can help address that disparity. Our results provide a basis for determining the future impact of the implementation of those vaccines on the incidence of meningitis. Finally, our results can inform empiric treatment decisions of providers caring for persons in the Arctic who present with suspected bacterial meningitis.
Acknowledgements
The authors thank the following individuals for their assistance with the data collection for this study: Christine Cash (Department of Health and Social Services, YK, Canada), Heather Hannah (Government of Northwest Territories, NWT, Canada), Angela Mullen (Department of Health, Nunavut, Canada), Jean-Francois Proulx (Nunavik Board of Health and Social Service – Direction de santé publique du Nunavik, QC, Canada), Kianoush Deghani (Cree Board of Health and Social Service of James Bay - Public Health Department, QC, Canada), Brigitte Lefebvre (Laboratoire de santé publique du Québec, QC, Canada), Y. Anita Li (Centre for Immunization and Respiratory Infectious Diseases, ON, Canada), Walter Demczuk, Irene Martin (National Microbiology Laboratory), and the personnel of the Arctic Investigations Program and the Office of Greenland’s chief medical officer.
Funding: This work was supported by the Centers for Disease Control and Prevention (in-kind support only, no grant).
Footnotes
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official positions of the Centers for Disease Control and Prevention.
Potential conflicts of interest: None of the authors have any conflicts of interests to declare.
Preliminary results from this analysis were presented on August 19, 2014 at the 20th IEA World Congress of Epidemiology held in Anchorage, AK.
References
- 1.Schlech WF, Ward JI, Band JD, Hightower A, Fraser DW, Broome CV. Bacterial meningitis in the united states, 1978 through 1981: The national bacterial meningitis surveillance study. JAMA. 1985;253(12):1749–1754. [Google Scholar]
- 2.Gilsdorf JR. Bacterial meningitis in southwestern Alaska. Am J Epidemiol. 1977 Nov;106(5):388–391. doi: 10.1093/oxfordjournals.aje.a112480. [DOI] [PubMed] [Google Scholar]
- 3.Wotton KA, Stiver HG, Hildes JA. Meningitis in the central Arctic: a 4-year experience. Canadian Medical Association journal. 1981 Apr 1;124(7):887–890. [PMC free article] [PubMed] [Google Scholar]
- 4.AHDR (Arctic Human Development Report) Akureyri, Iceland: Stefansson Arctic Institute; 2004. [Google Scholar]
- 5.Parkinson AJ, Bruce MG, Zulz T. International Circumpolar Surveillance, an Arctic network for the surveillance of infectious diseases. Emerg Infect Dis. 2008 Jan;14(1):18–24. doi: 10.3201/eid1401.070717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruce MG, Deeks SL, Zulz T, Bruden D, Navarro C, Lovgren M, et al. International Circumpolar Surveillance System for invasive pneumococcal disease, 1999–2005. Emerg Infect Dis. 2008 Jan;14(1):25–33. doi: 10.3201/eid1401.071315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bulkow LR, Singleton RJ, Karron RA, Harrison LH. Alaska RSVSG. Risk factors for severe respiratory syncytial virus infection among Alaska native children. Pediatrics. 2002 Feb;109(2):210–216. doi: 10.1542/peds.109.2.210. [DOI] [PubMed] [Google Scholar]
- 8.Hennessy TW, Ritter T, Holman RC, Bruden DL, Yorita KL, Bulkow L, et al. The relationship between in-home water service and the risk of respiratory tract, skin, and gastrointestinal tract infections among rural Alaska natives. Am J Public Health. 2008 Nov;98(11):2072–2078. doi: 10.2105/AJPH.2007.115618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parkinson AJ, Bell AA, Butler JC. International circumpolar surveillance of infectious diseases: monitoring community health in the Arctic. International journal of circumpolar health. 1999 Oct;58(4):222–225. [PubMed] [Google Scholar]
- 10.World Health Organization, Centers for Disease Control and Prevention. World Health Organization Manual. 2nd ed. Geneva, Switzerland: World Health Organization; 2011. Laboratory manual for the diagnosis of meningitis caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae. [Google Scholar]
- 11.Reasonover A, Zulz T, Bruce MG, Bruden D, Jette L, Kaltoft M, et al. The International Circumpolar Surveillance interlaboratory quality control program for Streptococcus pneumoniae, 1999 to 2008. J Clin Microbiol. 2011 Jan;49(1):138–143. doi: 10.1128/JCM.01238-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsang RS, Rudolph K, Lovgren M, Bekal S, Lefebvre B, Lambertsen L, et al. International circumpolar surveillance interlaboratory quality control program for serotyping Haemophilus influenzae and serogrouping Neisseria meningitidis, 2005 to 2009. J Clin Microbiol. 2012 Mar;50(3):651–656. doi: 10.1128/JCM.05084-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austrian R. The quellung reaction, a neglected microbiologic technique. Mt Sinai J Med. 1976 Nov-Dec;43(6):699–709. [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. Seventeenth informational supplement M100-S17. Wayne, PA: Clinical and Laboratory Standards Institute; 2007. Performance standards for antimicrobial susceptibility testing. [Google Scholar]
- 15.Bacterial meningitis in Canada: hospitalizations (1994–2001) Canada communicable disease report = Releve des maladies transmissibles au Canada. 2005 Dec 1;31(23):241–247. [PubMed] [Google Scholar]
- 16.Bruce MG, Zulz T, DeByle C, Singleton R, Hurlburt D, Bruden D, et al. Haemophilus influenzae serotype a invasive disease, Alaska, USA, 1983–2011. Emerg Infect Dis. 2013 Jun;19(6):932–937. doi: 10.3201/eid1906.121805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shuel M, Hoang L, Law DK, Tsang R. Invasive Haemophilus influenzae in British Columbia: non-Hib and non-typeable strains causing disease in children and adults. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2011 Mar;15(3):e167–e173. doi: 10.1016/j.ijid.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Tsang RS, Sill ML, Skinner SJ, Law DK, Zhou J, Wylie J. Characterization of invasive Haemophilus influenzae disease in Manitoba, Canada, 2000–2006: invasive disease due to non-type b strains. Clin Infect Dis. 2007 Jun 15;44(12):1611–1614. doi: 10.1086/518283. [DOI] [PubMed] [Google Scholar]
- 19.Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, Hadler JL, et al. Bacterial meningitis in the United States, 1998–2007. N Engl J Med. 2011 May 26;364(21):2016–2025. doi: 10.1056/NEJMoa1005384. [DOI] [PubMed] [Google Scholar]
- 20.Baraff LJ, Lee SI, Schriger DL. Outcomes of bacterial meningitis in children: a meta-analysis. Pediatr Infect Dis J. 1993 May;12(5):389–394. doi: 10.1097/00006454-199305000-00008. [DOI] [PubMed] [Google Scholar]
- 21.American Academy of Pediatrics. Pneumococcal infection. In: Pickering LK, editor. Red Book: 2012 Report of the Committee on Infectious Diseases. Elk Grove Village, IL: American Academy of Pediatrics; 2012. [Google Scholar]
- 22.Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004 Nov 1;39(9):1267–1284. doi: 10.1086/425368. [DOI] [PubMed] [Google Scholar]