Abstract
Objective
To examine the effectiveness of genetic testing in a real-world setting and to assess its impact on clinician treatment decisions.
Method:
This was a naturalistic, unblinded, prospective analysis of psychiatric patients and clinicians who utilized a commercially available genetic test (between April and October of 2013), which incorporates 10 genes related to pharmacokinetics and pharmacodynamics of psychiatric medications. Each patient’s genetic results were provided to participating clinicians, who completed a baseline survey including patient medications, history, and severity of illness. Clinicians were prompted to complete surveys within 1 week of receiving the genetic results and again 3 months later. Patients likewise completed assessments of depression, anxiety, medication side effects, and quality of life at baseline, 1 month, and 3 months.
Results:
Data from 685 patients were collected. Approximately 70% and 29% of patients had primary diagnoses of either a mood or anxiety disorder, respectively. Clinician-reported data, as measured by the Clinical Global Impressions–Improvement scale, indicated that 87% of patients showed clinically measurable improvement (rated as very much improved, much improved, or minimally improved), with 62% demonstrating clinically significant improvement. When analysis was restricted to the 69% of individuals with ≥ 2 prior treatment failures, 91% showed clinically measurable improvement. Patients also reported significant decreases in depression (P < .001), anxiety (P < .001), and medication side effects (P < .001) and increases in quality of life (P < .001).
Conclusions
These results suggest that a substantial proportion of individuals receiving pharmacogenetic testing showed clinically significant improvements on multiple measures of symptoms, adverse effects, and quality of life over 3 months. In the absence of a treatment-as-usual comparator, the proportion of improvement attributable to the test cannot be estimated.
Trial Registration:
ClinicalTrials.gov identifier: NCT01507155
Clinical Points
■ New evidence for the use of genetic testing to improve psychiatric patient care is emerging.
■ Clinicians who utilize genetic testing overwhelmingly find it influenced their medication decisions and/or their confidence in those decisions.
■ Future randomized control trials are needed to confirm the utility of genetic testing to improve care for psychiatric patients.
Approximately 30% of US adults have a mental illness,1 and almost half will develop one within their lifetime.2 Global costs for mental health conditions are projected to be over $6 trillion by 2030.3 In addition to depression (the most common psychiatric disorder), approximately 16% of the population had an anxiety disorder in 2009,4 accounting for more than 16% of annual health care costs in the United States.5 Only 50% of patients with depression or anxiety will respond to first-line therapies,6,7 resulting in poor quality of life and significant impairment in functioning.8 Additionally, patients classified with treatment-resistant depression have higher medical expenditures9 and inferior overall health.10 Moreover, the presence of anxiety-related disorders in patients with depression has additive effects on indirect costs, impacting overall function, quality of life, and absenteeism.11
A trial-and-error approach to prescribing has traditionally been utilized in psychiatry,12 contributing to the high costs of treatment and poor outcomes.13 Insight into a patient’s genetic background may help clinicians identify appropriate treatment options by predicting the likelihood of drug response or adverse events.14 A number of studies have demonstrated the cost effectiveness of pharmacogenetic testing within psychiatry.15–20 In addition, a recent report indicated that genetic testing increased medication adherence and decreased outpatient costs among psychiatric patients.21
The present study collected data from both patients and clinicians who utilized a commercially available genetic test, analyzing 10 genes involved in treatment response and side effect risk. The specific aims of the study were to determine the effectiveness of the test based on both clinician-rated and patient-rated measures, and to assess its influence on clinician treatment decisions.
METHOD
Design and Procedures
This study was a 3-month naturalistic unblinded trial (ClinicalTrials.gov identifier: NCT01507155). Recruitment targeted clinicians and patients who utilized a genetic test, the Genecept Assay (Genomind, King of Prussia, Pennsylvania), by directing them to an online portal containing information about the study. Deoxyribonucleic acid (DNA) sample collection occurred as part of the patient’s standard treatment, regardless of study participation. Patients and clinicians consented online using a secure portal; study staff were available by phone to support the consent process. Patient subjects were asked to complete 4 questionnaires at 3 time points: baseline (saliva collection), 1 month, and 3 months. Clinician subjects also completed online questionnaires at 3 time points: at baseline, at the receipt of the patient’s genetic test results, and approximately 3 months later. Subjects could opt out of the study at any time. All study procedures were approved by an independent institutional review board (Chesapeake Research Review, Inc, Columbia, Maryland).
Participants
Subjects included clinicians who ordered the Genecept Assay and the psychiatric patients for whom the test was ordered between April and October of 2013. Clinicians and patients could participate independently of each other, and all participants were compensated for their time.
Clinicians were required to have a valid national provider identifier number, to have the ability to submit a signed electronic informed consent document, and to have ordered the assay for a patient indicated as having a psychiatric condition. Clinicians practiced in both private and group practices across the United States. Patients were eligible if they were over 18 years of age, had the ability to complete the electronic informed consent, and had a psychiatric diagnosis. Although clinicians were instructed to enroll patients with a primary diagnosis of depression and/or anxiety, patients with other diagnoses were not excluded. Patients were excluded from the study if they had an inability to complete surveys online and/or were younger than 18 years old at the time of DNA collection.
Patient-Reported Measures
Four patient scales were used to measure outcomes: the Quick Inventory of Depressive Symptoms (QIDS-SR[16]),22 the Quality of Life Enjoyment and Satisfaction Questionnaire Short Form (Q-LES-Q-SF),23 the Zung Self-Rated Anxiety Scale (SAS),24 and the Udvalg for Kliniske Undersøgelser Side Effect Rating Scale (UKU).25 Patients also completed a demographic questionnaire at baseline and a satisfaction questionnaire at 3 months.
Clinician-Reported Outcomes
Baseline.
Clinicians completed the Clinical Global Impressions—Severity of Illness (CGI-S) scale for disease severity.26 The CGI-S ranks the patient from 1 or “normal/not at all ill” to 7 or “extremely ill.”26 The clinical global impression scales are well-established tools that are applicable to all psychiatric disorders.26
Clinicians also identified the patients’ medication regimen, presenting symptoms, diagnoses, and previous number of failed medication trials. Clinicians described their hypothetical plans for medication changes before receiving the patient’s genetic data. This information was used to assess if medication changes were influenced by the genetic test results.
Results received.
Clinicians completed a form indicating the treatment plan after review of the genetic test results. This form assessed the influence of the assay on the clinicians’ medication choice and confidence in medication choice, as well as the effect on the clinicians’ diagnostic impression.
Month 3.
Clinicians completed the Clinical Global Impressions—Improvement (CGI-I) scale. This scale is also a 7-point scale, wherein 1 indicates “very much improved” and 7 indicates “very much worse.”26 Clinicians also reported any changes to the patients’ medication regimen, symptoms, and diagnosis that occurred since the previous time point.
Genetic Analysis
Genetic variations were analyzed using TaqMan single nucleotide polymorphism (SNP) genotyping assays (Life Technologies, Grand Island, New York). The Genecept Assay tests for variations in 3 cytochrome P450 genes, 2D6 (CYP2D6), 2C19 (CYP2C19), and 3A4 (CYP3A4), as well as in serotonin transporter protein (SLC6A4), serotonin receptor subtype 2C (5HT2C), dopamine 2 receptor (DRD2), L-type voltage-gated calcium channel (CACNA1C), ankyrin g (ANK3), catechol-O-methyltransferase (COMT), and methylenetetrahydrofolate reductase (MTHFR). In total, 22 SNPs, as well as the copy number of CYP2D6, were assessed. These genes have been previously linked to psychiatric presentation, treatment efficacy, and/or risk for adverse reactions.13,18,27–31 A detailed summary of the results and clinical interpretations based on peer-reviewed literature was available to clinicians to view on a secure Internet portal. The cost of testing was charged to the patients or their insurance.
Congruence Between Genetic Results and Medication Change
A set of rules was applied to assess whether or not the changes made by the clinician were congruent with assay results. Changes were deemed congruent if the clinicians specifically indicated that the assay influenced their treatment decisions and if they made a change supported by the genetic report. In general, a medication change was considered congruent if the clinician removed a medication with an indicated risk, initiated a nonrisk medication, did not initiate a medication with an associated risk, or initiated an indicated therapy.
Statistical Analysis
Patient-reported data were analyzed via repeated-measures analysis of variance (ANOVA) models, with Tukey post hoc tests performed where appropriate. In addition, Pearson correlations were computed between CGI-S and patient scales at baseline and CGI-I and patient scales at 3 months.
RESULTS
Subject Characteristics
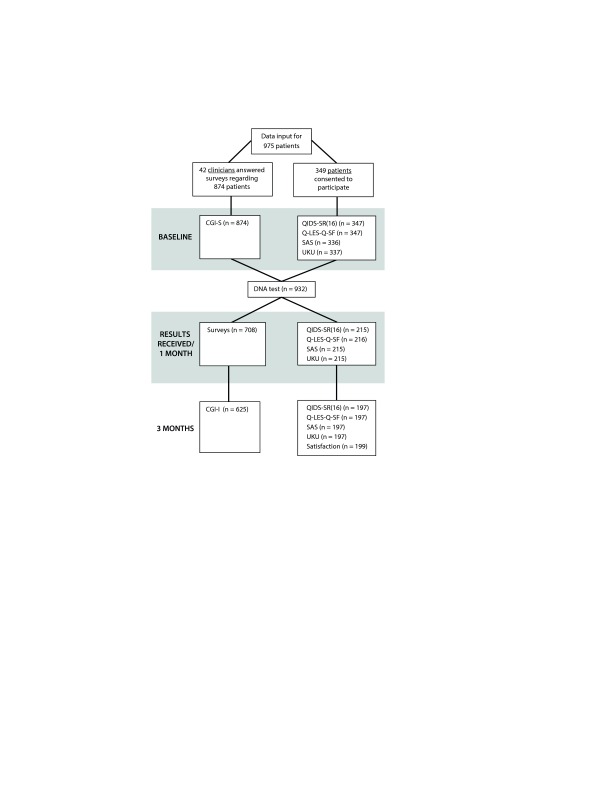
The data collection process is summarized in Figure 1. Following attrition, 42 clinicians completed assessments regarding 625 patients. A total of 197 patients also completed assessments, and, of these, 137 had corresponding clinician assessments. In total, data for all time points were collected on 685 unique patients. Demographic information captured at baseline is presented in Table 1. The majority of patients had a primary mood disorder (70.0%) including 42.6% with major depression and 17.2% with bipolar disorder. Patients with primary anxiety disorder represented 28.9% of the population. The remaining patients’ diagnoses included attention-deficit disorder or attention-deficit/hyperactivity disorder, schizophrenia or schizoaffective disorder, cognitive disorder, substance-related disorder, developmental disorders, and personality disorder. In addition, only 34% of patients had a single psychiatric diagnosis, while 66% had at least 1 comorbid psychiatric condition, including 40% of patients with comorbid mood and anxiety disorders (Supplemental Figure 1).
Figure 1.
Consort Diagrama
aThese data are generated from clinician and patient participants who were able to participate together or independently.
Abbreviations: CGI-I = Clinical Global Impressions–Improvement scale, CGI-S = Clinical Global Impressions–Severity of Illness scale, DNA = deoxyribonucleic acid, QIDS-SR(16) = Quick Inventory of Depressive Symptoms, Q-LES-Q-SF = Quality of Life Enjoyment and Satisfaction Questionnaire–Short Form, SAS = Zung Self-Rated Anxiety Scale, UKU = Udvalg for Kliniske Undersøgelser Side Effect Rating Scale.
Table 1.
Patient Demographic Informationa
| Demographic | Patients |
| Gender | |
| Women | 412 (65.9) |
| Men | 213 (34.1) |
| Age, mean, y | 40.5 |
| Ethnicity | |
| White | 305 (88) |
| Hispanic | 25 (7) |
| Black | 10 (3) |
| Current smoker | 36 (14) |
| Marital status | |
| Married | 177 (51) |
| Divorced/separated | 53 (15) |
| Single | 118 (34) |
| Widowed | 1 (< 1) |
| Education | |
| ≥ College degree | 145 (42) |
| Some college | 140 (40) |
| High school diploma | 50 (14) |
| < High school diploma | 6 (4) |
| Employment status | |
| Full-time | 182 (52) |
| Part-time | 39 (11) |
| Unemployed/disability/leave of absence | 83 (24) |
| Student | 33 (10) |
| Retired | 12 (3) |
| Annual income | |
| > $75,000 | 62 (18) |
| $25,000–75,000 | 152 (44) |
| < $25,000 | 135 (38) |
| No. of failed treatment trials, median (interquartile range) | 3 (1–5) |
Data are presented as n (%) unless otherwise specified.
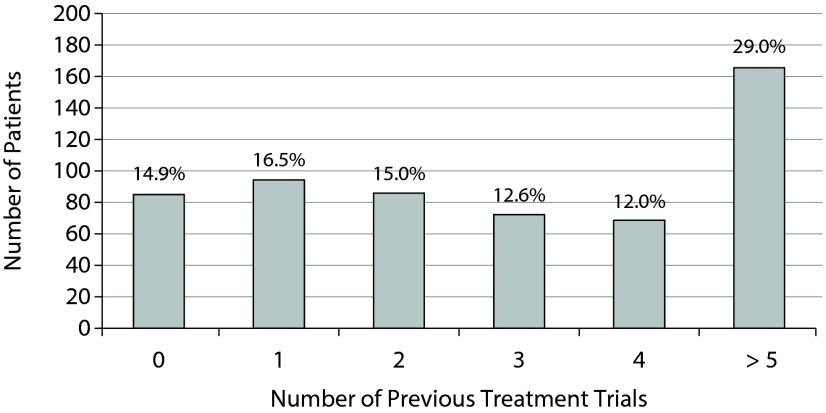
The majority of patients were women (65.9%), and the mean age was 40.5 years. Most patients were white (88%), 3% were black, and 7% were Hispanic. Only 36% indicated that they smoked cigarettes. Over half of participants were married (51%), 63% were employed, and 42% indicated at least a Bachelor’s degree. The gene frequencies were in line with expected population values (data not shown). Finally, 69% of patients had ≥ 2 previous failed treatment trials (Figure 2), with a mean number of 3.3, indicating a treatment-resistant population. This analysis was also restricted by diagnosis, demonstrating that patients with a mood disorder had on average more failed treatment trials compared to patients with anxiety or other diagnosis (4.1, 2.2, and 2.4, respectively, Supplemental Figure 2).
Figure 2.
Number of Previous Treatment Trialsa
aData labels show percent of patients.
Clinician-Reported Measures
CGI-S.
At baseline, 86% of patients were described as being at least “mildly ill” (≥ 3). The mean CGI-S score was 3.6, which falls in the range of moderately ill. No single gene variant was associated with CGI-S score (data not shown). Further, significant correlations were observed between CGI-S scores and baseline scores on all 4 patient scales (QIDS-SR[16]: r = 0.49, Q-LES-Q-SF: r = –0.41, UKU: r = 0.35, and SAS: r = 0.32; all P values < .0001).
Medication changes.
Surveyed clinicians reported that the assay frequently influenced what medication decisions they made as well as the confidence in those decisions (Supplemental Figure 3). Clinicians indicated that the results influenced their medication decisions for 93% of patients and their confidence in those decisions for 94% of patients. Additionally, clinicians made a change to the medication regimen congruent with the assay report for 94% of patients. SSRIs were the most frequently discontinued class of medication (8% of patients with a risk variant of SLC6A4 had an SSRI discontinued compared to only 2% of patients without that variant). l-methylfolate was the most frequently added therapy (44% of patients with a variant in the MTHFR had l-methylfolate added compared to only 4% of patients without a variant). As most of the clinicians made a change that aligned with the results, there were not enough instances in which clinicians did not make a congruent change for comparison.
CGI-I.
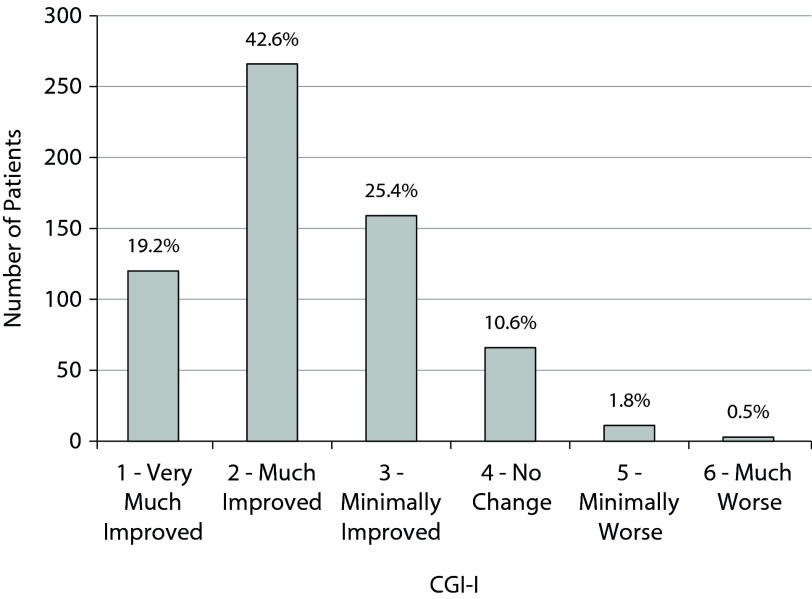
The CGI-I data from month 3 are presented in Figure 3. Eighty-seven percent of patients were judged to show measurable clinical improvement (1, 2, or 3), and 62% demonstrated significant clinical improvement deemed as “very much improved” or “much improved” (1 or 2). A separate analysis excluded patients who were not ill or only borderline mentally ill at baseline (1 or 2 on the CGI-S). Ninety percent of these patients (n = 537) showed measurable clinical improvement, and 63% were much improved or very much improved. In addition, response rates were comparable for patients regardless of number of failed treatment trials. Statistically significant correlations were seen between CGI-I and month 3 scores of all patient scales (QIDS-SR[16]: r = 0.41, Q-LES-Q-SF: r = −0.39, UKU: r = 0.36, and SAS: r = 0.23; all P values < .01).
Figure 3.
CGI-I Distribution at 3 Monthsa
aData labels show percent of patients.
Abbreviation: CGI-I = Clinical Global Impressions–Improvement scale.
Patient-Reported Scales
QIDS-SR(16): depression.
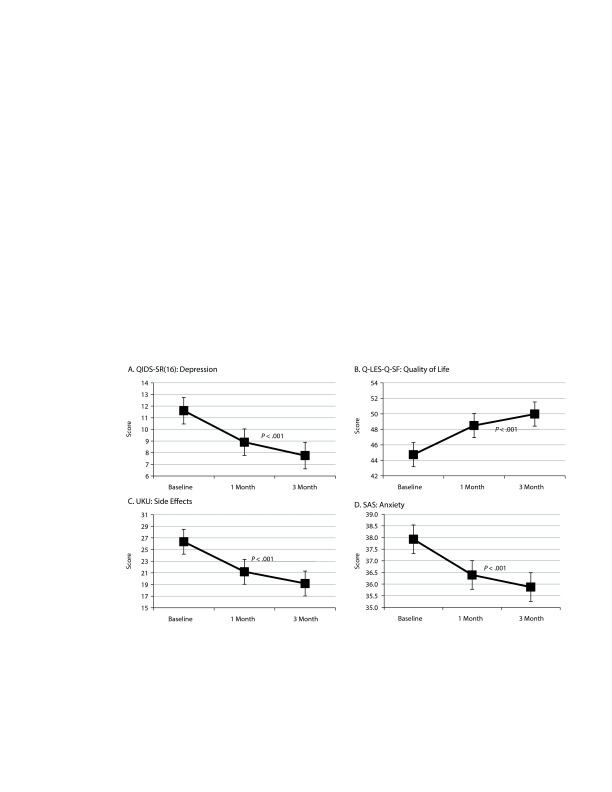
The QIDS-SR(16) data are presented in Figure 4A. At baseline, the mean QIDS-SR(16) score was 11.6, indicating mild to moderate depression. The score decreased to 8.9 by month 1 and to 7.8 by month 3, indicating milder depression. ANOVA verified that the severity of depressive symptoms fell over time (F2,590 = 91.41, P < .001). Tukey post hoc tests revealed that all 3 time points significantly differed from each other (P values < .05). When analysis was restricted to patients with a primary mood disorder, the baseline mean for these patients was 11.9 and decreased to 9.6 at month 1 and 7.9 at month 3 (F2,320 = 47.57, P < .001). Tukey post hoc tests again revealed that all 3 time points significantly differed from each other (P values < .05). In addition, 38% of these patients achieved remission (score < 5), and 39% showed a treatment response (≥ 50% reduction in score), indicating clinical efficacy for 77% of patients with a mood disorder.
Figure 4.
Self-Reported Patient Scales
Abbreviations: QIDS-SR(16) = Quick Inventory of Depressive Symptoms, Q-LES-Q-SF = Quality of Life Enjoyment and Satisfaction Questionnaire–Short Form, SAS = Zung Self-Rated Anxiety Scale, UKU = Udvalg for Kliniske Undersøgelser Side Effect Rating Scale.
Q-LES-Q-SF: quality of life.
The Q-LES-Q-SF data are presented in Figure 4B. The mean Q-LES-Q-SF score for all patients increased from 44.7 at baseline to 48.5 at month 1 and 50.0 at month 3 (F2,590 = 52.92, P < .001). Tukey post hoc tests again revealed that all 3 time points significantly differed from each other (P values < .05).
UKU: side effects.
The UKU data are presented in Figure 4C. The total mean UKU score for all patients decreased from 26.4 at baseline to 21.2 at month 1 and 19.2 at month 3 (F2,590 = 49.20, P < .001). Tukey post hoc tests again revealed that all 3 time points significantly differed from each other (P values < .05). When diagnoses were analyzed separately, patients with a mood disorder had the highest mean total UKU score at baseline (28.68), followed by other and anxiety diagnoses (22.35 and 20.95, respectively). A student T test showed that patients with a mood disorder or with an anxiety disorder had statistically significant decreases in total UKU score from baseline compared to month 3 (P < .001 and P = .009, respectively, Supplemental Figure 4).
SAS: anxiety scale.
The SAS data are presented in Figure 4D. At baseline, the mean anxiety score for all patients was 37.9 and decreased to 36.4 at month 1 and 35.9 at month 3 (F2,590 = 11.4, P < .001). Tukey post hoc tests revealed that the SAS score significantly decreased from baseline to month 1 (P < .05) but did not significantly change from month 1 to month 3 (P > .05). When the analysis was restricted to patients with a primary anxiety disorder, the mean at baseline was 36.5 and decreased to 35.8 at month 1 and 34.1 at month 3. An ANOVA on this group was marginally significant (F2,128 = 3.22, P = .052), in part due to the much lower number of subjects.
DISCUSSION
The clinician- and patient-rated data suggest that patients receiving pharmacogenetic testing were likely to improve in the 3-month period following receipt of test results. In all, 87% of the patients were judged by their clinicians as improved after testing. Notably, the percentage of patients reported to improve was similar regardless of the number of previous failed medication trials. The observed improvement was seen across diagnoses, treatment status, and baseline severity. The patient data demonstrated statistically significant decreases in depression and anxiety symptoms and medication side effects and a significant increase in quality of life. While these data show overall patient improvement following pharmacogenetic testing, it cannot be attributed to a specific benefit of testing in the absence of an untested comparison group.
At baseline, this population demonstrated relative treatment resistance and a significant side effect burden, as well as a high percent of comorbidities; in general, individuals with treatment resistance demonstrate poorer placebo or placebo-like response in randomized trials.33 Identifying effective treatment becomes more difficult and less likely with each additional failed treatment trial, history of medication side effects, and comorbid diagnoses.34–36 The presentation of patients with these characteristics most likely contributed to the clinicians’ decision to use genetic testing to identify more effective treatments for these challenging patients.
Patients reported significant reductions in depression and anxiety. Corresponding with previous literature, the mean QIDS-SR(16) score at baseline was in the mild-to-moderate depression range,37,38 and the remission/response rates also aligned with prior reports.38,39 The present study demonstrated patients with anxiety to have lower than previously reported SAS scores at baseline.40 Future work will need to focus on a population of patients who better meet the criteria for anxiety disorders to better ascertain the effect of genetic testing. Additionally, it is well known that psychotropic medication side effects play a large role in medication adherence.36 In the present study, patients reported a decrease in the total number of side effects following genetic testing.41 As there was no control group, we cannot determine if this reduction was a direct result of the assay. However, this observation is consistent with our previously reported finding of an increase in medication adherence and lower overall health care costs in patients whose therapies were informed by testing.21 Finally, the mean Q-LES-Q-SF score at the conclusion of the study was similar to a population of control subjects,42 indicating good quality of life after treatment.
Following testing, 89% of patients indicated that they understood their results “somewhat” or “completely.” Appropriate patient education has been shown to be an important factor in increased adherence, with patients demonstrating increased likelihood to remain on medications if they perceive a logical reason for the selection.43,44 Along with increasing patient education, testing may help to provide a biological rationale for treatment, aiding in the patients’ positive beliefs and expectations of treatment, which could also increase patient adherence, leading to improved treatment outcomes.43,44
Recent studies have provided support for the utility of genetic testing in psychiatry, demonstrating improvements in depression after the use of genetic testing.45,46 In concordance with previous data, these data demonstrate that patients traditionally less likely to respond to treatments show improved treatment outcomes, an improvement in depressive and anxiety symptoms and quality of life, as well as fewer side effects following genetic testing.
As the current research was a naturalistic study with no control group or blinding, it is challenging to estimate the specific effectiveness of genetic testing as distinct from placebo-like effect. The patient population was predominately white, well-educated, and nonsmokers, which may impact generalizability. Although the population was moderately ill at baseline, there is no a priori reason to suspect that assay-guided treatment would be any less effective in a more severely ill population, but that awaits empirical confirmation. In addition, patient and clinician subjects self-selected to utilize the assay, creating a possible risk of bias toward positive outcomes (ie, expectancy effects). Another limitation may be the reliability of the self-report methodology; however, significant correlations between patient and clinician scales indicate reliability of these measures.
Future studies are needed to estimate the magnitude of clinical utility of genetic testing in the general psychiatric population as compared to treatment as usual. To minimize risk of confounding and placebo-like effects, randomized controlled designs will be needed. Nonetheless, the present results add to a growing body of literature suggesting the utility of pharmacogenetic testing incorporating pharmacokinetic and pharmacodynamic variants to guide treatment of at least a subset of psychiatric patients.
Potential conflicts of interest: Drs Brennan, Lombard, and Scott and Ms Gardner were employees of Genomind at the time of this study. Dr Perlis has served as a consultant to RID Ventures, Pfizer, and Perfect Health and has served on the advisory boards of Genomind, PsyBrain, and Healthrageous. Dr Fava has served as a speaker for or published with Adamed, Advanced Meeting Partners, American Psychiatric Association, American Society of Clinical Psychopharmacology, AstraZeneca, Belvoir Media Group, Boehringer Ingelheim GmbH, Bristol-Myers Squibb, Cephalon, CME Institute/Physicians Postgraduate Press, Eli Lilly, Forest, GlaxoSmithKline, Imedex, MGH Psychiatry Academy/Primedia, MGH Psychiatry Academy/Reed Elsevier, Novartis AG, Organon, Pfizer, PharmaStar, United Biosource, and Wyeth-Ayerst; has equity holdings in Compellis and PsyBrain; holds a patent for Sequential Parallel Comparison Design, which are licensed by MGH to Pharmaceutical Product Development, and has a patent application for a combination of ketamine plus scopolamine in major depressive disorder; and receives copyright royalties for the MGH Cognitive & Physical Functioning Questionnaire, Sexual Functioning Inventory, Antidepressant Treatment Response Questionnaire, Discontinuation-Emergent Signs & Symptoms, and SAFER and from Lippincott Williams & Wilkins, Wolters Kluwer, and World Scientific Publishing.
Funding/support: This study was funded by Genomind, Inc, King of Prussia, Pennsylvania.
Role of the sponsor: The funding organization played a role in the design and conduct of the study; the collection, management, and analysis of data; and the preparation, review, and approval of the manuscript.
Previous presentations: These data were previously presented at 2013 Neuroscience Education Institute Annual Congress; November 14–16, 2013; Colorado Springs, Colorado ▪ 2014 US Psychiatric and Mental Health Congress; September 20, 2014; Orlando, Florida ▪ 2014 Neuroscience Education Institute Annual Congress; November 13–16, 2014; Colorado Springs, Colorado ▪ 2014 American Society for Experimental NeuroTherapeutics; February 20–22, 2014; Bethesda, Maryland.
Supplementary material: See accompanying pages.
Footnotes
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
References
- 1.Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reeves WC, Strine TW, Pratt LA, et al. Centers for Disease Control and Prevention. Mental Illness Surveillance Among Adults in the United States. Morbidity and Mortality Weekly Report. September 2, 2011;60(suppl). http://www.cdc.gov/mmwr/pdf/other/su6003.pdf. Accessed February 3, 2015. [Google Scholar]
- 3.Bloom DE, Cafiero ET, Jané-Llopis E, et al. The Global Economic Burden of Noncommunicable Diseases. Geneva, Switzerland: World Economic Forum; 2011. [Google Scholar]
- 4.Kessler RC, Aguilar-Gaxiola S, Alonso J, et al. The global burden of mental disorders: an update from the WHO World Mental Health (WMH) surveys. Epidemiol Psichiatr Soc. 2009;18(1):23–33. doi: 10.1017/s1121189x00001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shirneshan E, Relyea G, Brown LLB. Incremental health care costs of anxiety disorders in the ambulatory adult population of the United States. Presented at the 35th Annual Meeting of the Society for Medical Decision Making; October 19–23, 2013; Baltimore, MD.
- 6.Stein DJ, Stein MB, Pitts CD, et al. Predictors of response to pharmacotherapy in social anxiety disorder: an analysis of 3 placebo-controlled paroxetine trials. J Clin Psychiatry. 2002;63(2):152–155. doi: 10.4088/jcp.v63n0211. [DOI] [PubMed] [Google Scholar]
- 7.Trivedi MH, Rush AJ, Wisniewski SR, et al. STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 8.Fava M, Rush AJ, Wisniewski SR, et al. A comparison of mirtazapine and nortriptyline following two consecutive failed medication treatments for depressed outpatients: a STAR*D report. Am J Psychiatry. 2006;163(7):1161–1172. doi: 10.1176/ajp.2006.163.7.1161. [DOI] [PubMed] [Google Scholar]
- 9.Olchanski N, McInnis Myers M, Halseth M, et al. The economic burden of treatment-resistant depression. Clin Ther. 2013;35(4):512–522. doi: 10.1016/j.clinthera.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Rubio JM, Markowitz JC, Alegría A, et al. Epidemiology of chronic and nonchronic major depressive disorder: results from the national epidemiologic survey on alcohol and related conditions. Depress Anxiety. 2011;28(8):622–631. doi: 10.1002/da.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein MB, Roy-Byrne PP, Craske MG, et al. Functional impact and health utility of anxiety disorders in primary care outpatients. Med Care. 2005;43(12):1164–1170. doi: 10.1097/01.mlr.0000185750.18119.fd. [DOI] [PubMed] [Google Scholar]
- 12.Mrazek DA. Psychiatric pharmacogenomic testing in clinical practice. Dialogues Clin Neurosci. 2010;12(1):69–76. doi: 10.31887/DCNS.2010.12.1/dmrazek. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dervieux T, Meshkin B, Neri B. Pharmacogenetic testing: proofs of principle and pharmacoeconomic implications. Mutat Res. 2005;573(1–2):180–194. doi: 10.1016/j.mrfmmm.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Vegter S, Boersma C, Rozenbaum M, et al. Pharmacoeconomic evaluations of pharmacogenetic and genomic screening programs: a systematic review on content and adherence to guidelines. Pharmacoeconomics. 2008;26(7):569–587. doi: 10.2165/00019053-200826070-00005. [DOI] [PubMed] [Google Scholar]
- 15.Herbild L, Andersen SE, Werge T, et al. Does pharmacogenetic testing for CYP450 2D6 and 2C19 among patients with diagnoses within the schizophrenic spectrum reduce treatment costs? Basic Clin Pharmacol Toxicol. 2013;113(4):266–272. doi: 10.1111/bcpt.12093. [DOI] [PubMed] [Google Scholar]
- 16.Chou WH, Yan FX, de Leon J, et al. Extension of a pilot study: impact from the cytochrome P450 2D6 polymorphism on outcome and costs associated with severe mental illness. J Clin Psychopharmacol. 2000;20(2):246–251. doi: 10.1097/00004714-200004000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Ruaño G, Szarek BL, Villagra D, et al. Length of psychiatric hospitalization is correlated with CYP2D6 functional status in inpatients with major depressive disorder. Biomarkers Med. 2013;7(3):429–439. doi: 10.2217/bmm.13.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altar CA, Hornberger J, Shewade A, et al. Clinical validity of cytochrome P450 metabolism and serotonin gene variants in psychiatric pharmacotherapy. Int Rev Psychiatry. 2013;25(5):509–533. doi: 10.3109/09540261.2013.825579. [DOI] [PubMed] [Google Scholar]
- 19.Murphy DL, Moya PR. Human serotonin transporter gene (SLC6A4) variants: their contributions to understanding pharmacogenomic and other functional G × G and G × E differences in health and disease. Curr Opin Pharmacol. 2011;11(1):3–10. doi: 10.1016/j.coph.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winner J, Allen JD, Altar CA, et al. Psychiatric pharmacogenomics predicts health resource utilization of outpatients with anxiety and depression. Transl Psychiatry. 2013;3(3):e242. doi: 10.1038/tp.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fagerness JF, Fonseca E, Hess GP, et al. Pharmacogenetic-guided psychiatric intervention associated with increased adherence and cost savings. Am J Manag Care. 2014;20(5):e146–e156. [PubMed] [Google Scholar]
- 22.Rush AJ, Gullion CM, Basco MR, et al. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26(3):477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 23.Stevanovic D. Quality of Life Enjoyment and Satisfaction Questionnaire–Short Form for quality of life assessments in clinical practice: a psychometric study. J Psychiatr Ment Health Nurs. 2011;18(8):744–750. doi: 10.1111/j.1365-2850.2011.01735.x. [DOI] [PubMed] [Google Scholar]
- 24.Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12(6):371–379. doi: 10.1016/S0033-3182(71)71479-0. [DOI] [PubMed] [Google Scholar]
- 25.Lingjaerde O, Ahlfors UG, Bech P, et al. The UKU Side Effect Rating Scale: a new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand suppl. 1987;76(s334):1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- 26.Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont) 2007;4(7):28–37. [PMC free article] [PubMed] [Google Scholar]
- 27.Kato M, Serretti A. Review and meta-analysis of antidepressant pharmacogenetic findings in major depressive disorder. Mol Psychiatry. 2010;15(5):473–500. doi: 10.1038/mp.2008.116. [DOI] [PubMed] [Google Scholar]
- 28.Porcelli S, Fabbri C, Serretti A. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with antidepressant efficacy. Eur Neuropsychopharmacol. 2012;22(4):239–258. doi: 10.1016/j.euroneuro.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Zhang JP, Lencz T, Malhotra AK. D2 receptor genetic variation and clinical response to antipsychotic drug treatment: a meta-analysis. Am J Psychiatry. 2010;167(7):763–772. doi: 10.1176/appi.ajp.2009.09040598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Psychiatric GWAS Consortium Bipolar Disorder Working Group Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet. 2011;43(10):977–983. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baune BT, Hohoff C, Berger K, et al. Association of the COMT val158met variant with antidepressant treatment response in major depression. Neuropsychopharmacology. 2008;33(4):924–932. doi: 10.1038/sj.npp.1301462. [DOI] [PubMed] [Google Scholar]
- 32.Papakostas GI, Shelton RC, Zajecka JM, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry. 2012;169(12):1267–1274. doi: 10.1176/appi.ajp.2012.11071114. [DOI] [PubMed] [Google Scholar]
- 33.Sonawalla SB, Rosenbaum JF. Placebo response in depression. Dialogues Clin Neurosci. 2002;4(1):105–113. doi: 10.31887/DCNS.2002.4.1/ssonawalla. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cacciola JS, Alterman AI, Rutherford MJ, et al. The relationship of psychiatric comorbidity to treatment outcomes in methadone maintained patients. Drug Alcohol Depend. 2001;61(3):271–280. doi: 10.1016/s0376-8716(00)00148-4. [DOI] [PubMed] [Google Scholar]
- 35.Warden D, Rush AJ, Trivedi MH, et al. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9(6):449–459. doi: 10.1007/s11920-007-0061-3. [DOI] [PubMed] [Google Scholar]
- 36.Dibonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12(1):20. doi: 10.1186/1471-244X-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown ES, Murray M, Carmody TJ, et al. The Quick Inventory of Depressive Symptomatology–Self-report: a psychometric evaluation in patients with asthma and major depressive disorder. Ann Allergy Asthma Immunol. 2008;100(5):433–438. doi: 10.1016/S1081-1206(10)60467-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaynes BN, Rush AJ, Trivedi MH, et al. Primary versus specialty care outcomes for depressed outpatients managed with measurement-based care: results from STAR*D. J Gen Intern Med. 2008;23(5):551–560. doi: 10.1007/s11606-008-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez JM, Katon W, Greist JH, et al. A pragmatic 12-week, randomized trial of duloxetine versus generic selective serotonin-reuptake inhibitors in the treatment of adult outpatients in a moderate-to-severe depressive episode. Int Clin Psychopharmacol. 2012;27(1):17–26. doi: 10.1097/YIC.0b013e32834ce11b. [DOI] [PubMed] [Google Scholar]
- 40.Zung Self-Rating Anxiety Scale (SAS) https://www.mnsu.edu/comdis/isad16/papers/therapy16/sugarmanzunganxiety.pdf. Accessed February 20, 2015.
- 41.Lindström E, Lewander T, Malm U, et al. Patient-rated versus clinician-rated side effects of drug treatment in schizophrenia: clinical validation of a self-rating version of the UKU Side Effect Rating Scale (UKU-SERS-Pat) Nord J Psychiatry. 2001;55(suppl 44):5–69. doi: 10.1080/080394801317084428. [DOI] [PubMed] [Google Scholar]
- 42.Schechter D, Endicott J, Nee J. Quality of life of “normal” controls: association with lifetime history of mental illness. Psychiatry Res. 2007;152(1):45–54. doi: 10.1016/j.psychres.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 44.Fenton WS, Blyler CR, Heinssen RK. Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophr Bull. 1997;23(4):637–651. doi: 10.1093/schbul/23.4.637. [DOI] [PubMed] [Google Scholar]
- 45.Winner JG, Carhart JM, Altar CA, et al. A prospective, randomized, double-blind study assessing the clinical impact of integrated pharmacogenomic testing for major depressive disorder. Discov Med. 2013;16(89):219–227. [PubMed] [Google Scholar]
- 46.Hall-Flavin DK, Winner JG, Allen JD, et al. Using a pharmacogenomic algorithm to guide the treatment of depression. Transl Psychiatry. 2012;2(10):e172. doi: 10.1038/tp.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]