Abstract
OBJECTIVE
Weight regain contributes to the therapeutic failure in 15–20% of type 2 diabetic patients after Roux-en-Y gastric bypass surgery (RYGB), and the mechanism remains largely unknown. This study was conducted to explore the mechanism of weight regain.
RESEARCH DESIGN
Wild-type (WT) diet-induced obese (DIO) mice were used to mimic human obesity, and ob/ob mice were used for leptin deficiency-induced obesity. Two groups of mice were compared in weight regain for 10 months after RYGB. Weight loss, food intake, fecal energy loss and energy expenditure were monitored in the study of weight regain. Fasting insulin, insulin tolerance and homeostatic model assessment-insulin resistance were tested for insulin sensitivity under the weight regain. Weight loss from RYGB and calorie restriction was compared for the impact in insulin sensitivity.
RESULTS
In WT mice, RYGB induced a sustained weight loss and insulin sensitization over the sham operation in this 10-month study. However, RYGB failed to generate the same effects in leptin-deficient ob/ob mice, which suffered a weight regain over the pre-surgery level. In ob/ob mice, body weight was reduced by RYGB transiently in the first week, recovered in the second week and increased over the baseline thereafter. Weight loss was induced by RYGB relative to that of sham mice, but the loss was not sufficient to keep body weight below the pre-surgery levels. In addition, insulin sensitivity was not improved by the weight loss. The response to RYGB was improved in ob/ob mice by 2 weeks of leptin treatment. Weight loss from calorie restriction had an equivalent effect on insulin sensitization compared with that of RYGB.
CONCLUSION
Those data demonstrate that ob/ob mice and DIO mice responded differently to RYGB surgery, suggesting that leptin may be one of the factors required for RYGB to prevent weight regain and diabetes recurrence.
INTRODUCTION
Roux-en-Y gastric bypass surgery (RYGB) is among the most effective bariatric surgeries in producing sustained decrease in body weight and remission of type-2 diabetes.1,2 In addition, RYGB improves most of the deleterious comorbidities associated with severe obesity.2 Despite intensive efforts, the critical mechanisms responsible for these beneficial effects of RYGB have not yet been clearly identified. Ultimately, RYGB must change patterns of humoral and neural signaling between the gastrointestinal system and other organs (such as the brain, liver, adipose tissue and so on) involved in the energy regulation.3,4 Much attention has been given to changes in circulating gut hormones, such as glucagon-like peptide 1 (GLP-1),5,6 peptide tyrosine tyrosine (PYY)7,8 and ghrelin9 in mechanistic studies. However, recent findings in various rodent models have provided relatively little support for their critical roles in the effects of RYGB,10–14 although synergistic actions between these hormones and other factors have not been excluded.
It has also become clear that the negative energy balance after RYGB does not result from an inability to increase energy intake. If RYGB rodents were properly stimulated, food intake can easily be doubled leading to substantial weight regain.10,15,16 Also, 15–20% of RYGB patients return to pre-surgical levels of food intake and regain body weight in spite of their rearranged gut.1 One interpretation of these observations is that successful surgery establishes a new lower level of body weight that is actively defended after RYGB. This function is classically ascribed to the hypothalamus, where leptin and other feedback signals regulate energy balance by orchestrating the necessary changes in energy intake and expenditure.17 It is not clear if leptin contributes to the defense of post-surgery low body weight.
Leptin resistance contributes to hyperphagia and hypometabolism in obesity. Serum leptin is increased by obesity and reduced by RYGB,5,18 suggesting an increase in leptin sensitivity. However, it is largely unknown if leptin has a role in the control of weight regain after RYGB.4 In two early studies of leptin receptor-deficient Zucker obese rats, RYGB induced weight loss and insulin sensitization immediately post surgery.19,20 However, the long-term effect of RYGB was not examined in these studies for weight regain.
The present study was designed to test the impact of leptin in the long-term effects of RYGB with a focus on weight regain and glycemic control. We have recently established a valid mouse RYGB model, characterized by a small gastric pouch, sustained suppression of body weight below the pre-surgical level, and low mortality.10,21 Here, we take advantage of this model to study weight regain and glycemic control for 10 months after RYGB in diet-induced obese (DIO) mice and leptin-deficient ob/ob. In addition, leptin-replacement therapy was tested in ob/ob mice for 2 weeks at 25 weeks after RYGB.
MATERIALS AND METHODS
Obese mice
All experiments were approved by the Institutional Animal Care and Use Committee of Pennington Biomedical Research Center. Animals were housed under conventional conditions at the Pennington Animal Facility. In the study design, wild-type (WT) male C57BL/6J mice were used in DIO group and chow-fed group (lean control). DIO mice were generated by feeding 6-week-old mice with a high-fat diet (HFD, D12331 diet, 58% calories from fat, Research Diets Inc.) for 14 weeks. Then, the DIO mice were divided into three subgroups (n = 7): DIO+RYGB, DIO+sham and DIO+weight matched. The lean control mice (n = 7) were fed regular chow diet (11% calorie from fat, 5001 LabDiet) ad libitum. After surgery, DIO mice were fed a medium-fat diet (25% calorie from fat, 5015 LabDiet) to reflect diet condition in patients. DIO+weight-matched group were subject to food restriction at 14 weeks of HFD feeding to induce an identical weight loss to that of DIO+RYGB group. Forty C57BL/6 mice were used to generate the models and 28 mice were left after excluding unqualified DIO mice and dead mice in surgery. Male ob/ob mice (26 mice) in C57BL/6 gene background were purchased from the Jackson Laboratory at 4–7 weeks in age and fed the regular chow diet ad libitum before and after the surgery.
Surgical procedures
RYGB surgery was performed in DIO mice at 14 weeks on HFD with a body weight of 46 ± 5 g. The surgery was performed in ob/ob mice at 6 week (35 g) and 10 week (50 g) in age. The surgery operation was conducted as described in detail earlier.21 In brief, animals were fasted 4–6 h before the operation and anesthesia was administrated with isoflurane inhalation. In RYGB surgery, the anterior and posterior left gastric vessels, as well as the esophageal vessels, were ligated and cut. A small gastric pouch at 5% of the total gastric volume was generated and anastomosed with the jejunum by 16–18 interrupted stiches with 11–0 nylon suture. The Roux limb and the biliopancreatic limb were ~5–6 cm in length. The intestine was arranged in an ‘S’ position to avoid intestinal obstruction before closing of the abdominal cavity. In sham operation, the perigastric ligaments were cut, and a 3-mm incision was made in the stomach closed with a titanium clip. The jejunum was transected 2 cm distal to the ligament of Treitz, and the two cut ends were anastomosed. Standard aseptic procedures were used throughout. In the first 24 h after the operation, the mice were put in regular shoe box cages on a heating pad at 35 °C. The mice were given 0.8 ml per mouse of saline subcutaneously and carprofen (5 mg kg−1, sc) for analgesia immediately after surgery. The mice had access to water and solid diet right after surgery. Post-surgical survival rates were 90% in DIO mice and 80% in ob/ob mice.
Physiological parameters
Body weight, body composition and food intake were measured in this study as described elsewhere.21 The body weight was determined daily in the first 2 weeks and then weekly after the surgery. Body composition was determined using nuclear magnetic resonance in each cohort at times indicated in Figure 1. Food intake was determined in single-housed mouse manually or using the metabolic chamber for 1–2 weeks.
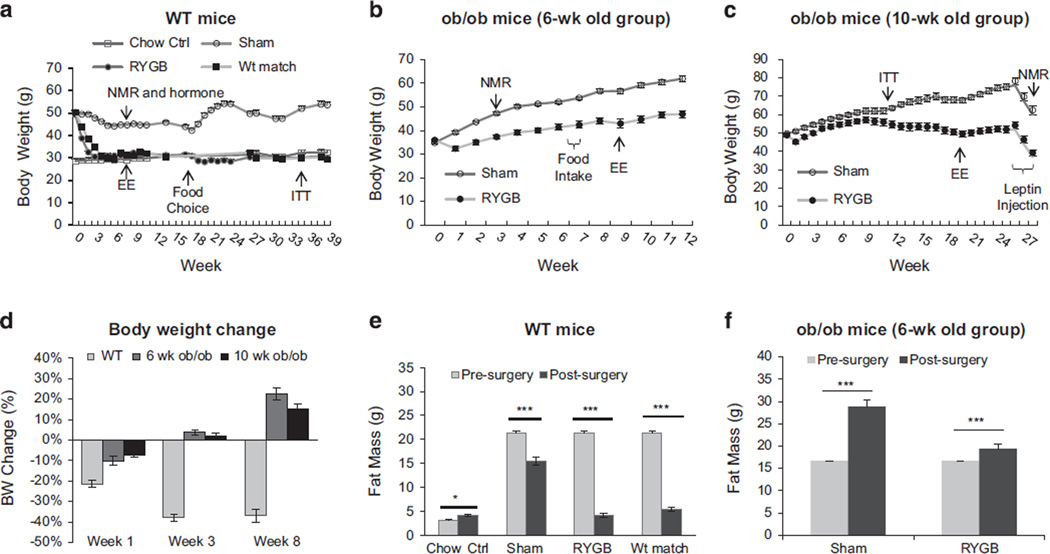
Figure 1.
Body weight and fat content after RYGB. (a) Body weight of WT mice (DIO). The mice were divided into four groups: chow diet control (Chow Ctrl), Sham-operated, RYGB and weight matched (Wt match). The mice were fed HFD before surgery and the breeder chow (medium fat) diet after surgery. RYGB was performed in the mice around 14 wks on HFD. Body weight was monitored weekly in the first 4 wks and then biweekly for 39 wks. Body composition and energy expenditure (EE) were tested at 7 wks. Food choice test was conducted at 16 wks, where the mice had free access to the HFD and the regular chow diet for 6 wks. ITT was tested at 35 wks (N = 7 Chow Ctrl, 7 Sham, 7 RYGB, 7 Wt match). (b) ob/ob mice (6-wk-old group, n = 5). RYGB was performed in the mice at 6 wks in age. Body weight was monitored for 39 wks with composition test at 3 wks, food intake at 6–7 wks, and EE at 8–9 wks post surgery. (c) ob/ob mice (10-wk-old group, n = 6). RYGB was performed in the mice at 10 wks in age. The body weight was monitored for 27 wks with ITT at 11 wks, EE at 19 wks and leptin treatment at 25 wks. (d) Percentage weight loss after RYGB. WT and ob/ob mice were compared in percentage weight loss after RYGB at three time points as indicated. (e) Body fat content of WT mice. Body composition was monitored at 8 wks after RYGB (N = 8 Chow, 7 Sham, 8 RYGB, 7 Wt match). (f) Body fat content of ob/ob mice (6-wk-old group). The body composition was measured at 3 wks after RYGB (n = 5). The data are expressed as mean ± s.e. *P < 0.05, ***P < 0.001 vs before surgery or calorie restriction by t-test.
Energy expenditure
Indirect calorimetry measurement was performed with the Comprehensive Laboratory Animal Monitoring System (Columbus Instruments, Columbus, OH, USA) for individually housed mice as described in detail earlier.22 After 96 h of adaptation, the data for oxygen consumption (VO2), carbon dioxide production (VCO2), respiratory exchange ratio and spontaneous physical activity were simultaneously recorded and analyzed.22 The data of day and night are presented.
Fecal calorie content
Fecal samples were collected for each mouse after surgery and stored in −80 °C. The caloric content was determined using a Parr 1266 Isoperibol Bomb Calorimeter (Parr Instrument Company, Moline, IL, USA). Approximately 1 gm fecal samples were freeze dried to remove all moisture. For each 1 gm fecal sample, an aliquot of ~0.2–0.3 grams were processed in the bomb calorimeter. Hexadecane (Sigma-Aldrich, St Louis, MO, USA), a combustible spiking agent, was added to each sample to ensure complete combustion of the entire fecal sample. A heat of combustion value is obtained for each fecal sample in calories per gram after 8 min. The value was corrected for combustion heat of the spiking agent automatically and a final gross heat value was used for the fecal calorie. Each sample was run in duplicate trials in order to obtain an average value.
Insulin, leptin and insulin tolerance test
Blood was collected from mice through retro-optical bleeding at 6 weeks post surgery after overnight fasting. Serum insulin and leptin were tested using multiplex kit (MMHMAG-44K, Millipore Corporation, 28820 Single Oak Drive, Temecula, California). Insulin tolerance was performed in each cohort at times indicated in Figure 1. The test was conducted with peritoneal injection of insulin at 0.7 U Kg−1 after 4 h fasting. MOHA-IR was calculated with a formula: homeostatic model assessment-insulin resistance (mg dl−1) = glucose × insulin/405.
Leptin injection
For long-term leptin effects on RYGB ob/ob mice, leptin was intraperitoneally injected twice daily over 2 weeks at a dosage 1mg kg−1 per day. Food intake and body weight was monitored daily using the BioDaq cage system.
Statistical analysis
In this study, values are presented as mean ± s.e.m. Student’s t-test was used in most data analysis for body weight, insulin tolerance, energy expenditure, substrate utilization and body temperature. Two-way ANOVA was performed in the study of leptin sensitivity followed by a post hoc analysis. P-values <0.01 were considered significant.
RESULTS
RYGB prevented weight regain in WT, but not ob/ob mice
In the DIO model that was generated in WT C57BL/6 mice, RYGB surgery was performed at 14 weeks on HFD when the body weight was around 50 g. DIO mice exhibited a persistent weight loss after RYGB (Figure 1a). Most weight reduction was observed in the first 2 weeks post surgery and the weight loss was about 35% at the end of 3 weeks. At this time, WT mice lost all of the weight gain on HFD. Thereafter, the RYGB mice shared an identical body weight to the chow diet group in the entire 39-week study without weight regain (Figure 1a). ob/ob mice were tested in RYGB in two cohorts at different ages, 6 weeks (35 g) and 10 weeks (50 g), to determine the impact of pre-surgery body weight in the surgery effect. In the first cohort, a transient weight loss was observed in the 6-week group with 4 g weight reduction in the first week post-surgery (Figure 1b). Interestingly, a persistent weight gain was observed in the mice thereafter. The body weight became 20% above the pre-surgical levels 8 weeks post-surgery (Figures 1b–d). Compared with sham-operated mice, RYGB mice exhibited less weight regain after surgery. In the second cohort, a similar pattern of weight regain was observed in 10-week-old ob/ob mice although the pre-surgery body weight was much higher (Figure 1c). Percentage weight loss was compared between WT and ob/ob mice at 1, 3 and 8 weeks post surgery. Persistent weight loss beyond pre-surgery levels was only observed in WT mice, but not in ob/ob mice after RYGB (Figure 1d). ob/ob mice exhibited some weight loss after 10 weeks of RYGB in the second cohort, which might be a consequence of stress responses from the tests, such as insulin tolerance test and metabolic chamber study. Even though, the body weight remained above the presurgery levels (Figure 1c). Such a weight reduction was not observed in the sham ob/ob mice, suggesting that RYGB may increase ob/ob mouse sensitivity to stressful challenges. These data suggest that in contract to that in WT mice, RYGB could not prevent weight regain in ob/ob mice.
Body fat reduction accounts for the surgery-induced weight loss. Fat mass was measured using nuclear magnetic resonance in WT and ob/ob mice. DIO mice exhibited 40% reduction in fat mass 8 weeks after RYGB (Figure 1d). A portion of the reduction was a result of switching from high-to-medium-fat diet after surgery, as about 9% weight reduction was also observed in sham-operated WT mice. HFD was replaced by the medium-fat diet (breeder chow) after surgery to reflect diet preference in RYGB patients and to maintain the adiposity. ob/ob mice did not exhibit a reduction in fat mass after RYGB as indicated by data in the first cohort (Figure 1f). Instead, fat mass was increased over the pre-surgery levels in both sham and RYGB ob/ob mice. The data suggest that RYGB fails to reduce fat mass below pre-surgery levels persistently in ob/ob mice.
NRYGB increased energy expenditure in WT, but not ob/ob mice
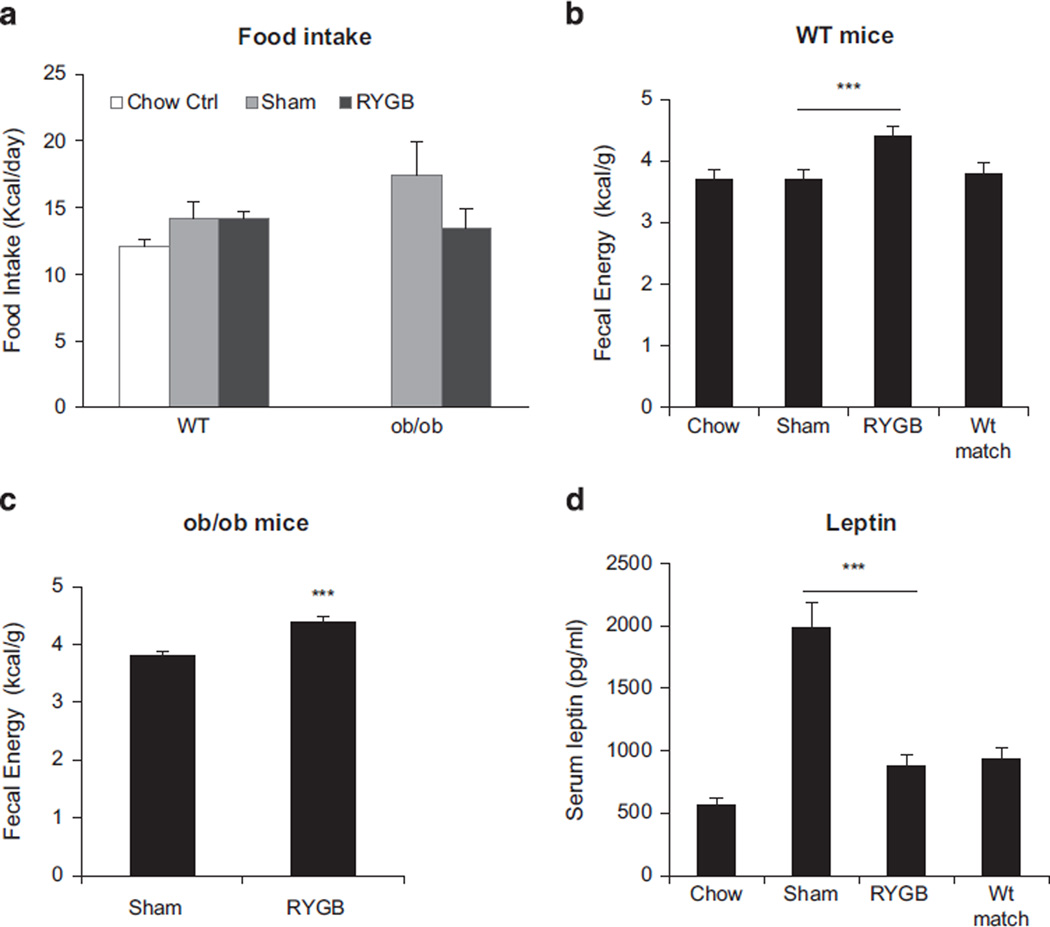
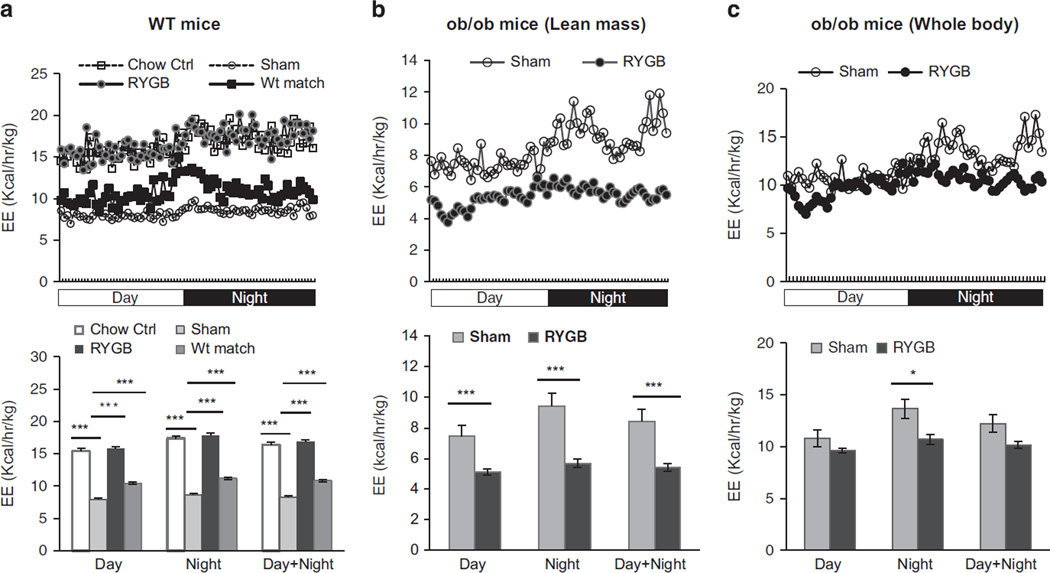
Energy intake, fecal energy loss and energy expenditure were examined at 5–8 weeks after RYGB when the body weight was stabilized at the lower level in DIO mice. Daily food intake per mouse was not significantly reduced by RYGB in DIO mice (Figure 2a). A modest reduction was observed in ob/ob mice after RYGB (Figure 2a), but the change is not significant. Fecal calorie content was significantly increased by RYGB in both WT and ob/ob mice (Figures 2b and c). Serum leptin was significantly decreased in WT mice by RYGB or calorie restriction in weight-matched group (Figure 2d). Energy expenditure per lean body mass was significantly increased by RYGB in WT, but not in ob/ob mice (Figures 3a and b). To the contrary, energy expenditure was decreased by RYGB in ob/ob mice (Figure 3b). The decrease was observed when the data were normalized with either lean body mass (Figure 3b) or whole-body weight (Figure 3c). The decrease was significant at day and night time when normalized with lean mass, but only night time when normalized with whole-body mass. The difference between WT and ob/ob mice suggests that RYGB was not able to induce energy expenditure in ob/ob mice.
Figure 2.
Energy intake and fecal calorie content after RYGB. (a) Energy intake of WT and ob/ob mice. The mice were single housed and food intake was measured for 3 days after 4 day adaptation. The food intake was conducted at 6 wks post surgery and expressed in kcal per day per mouse (N = 5–6). (b) Fecal calorie content in WT mice (N = 5–6). The test was done at 39 wks post surgery with dry feces. (c) Fecal calorie content in ob/ob mice of the 9-wk-old group (n = 5). The test was done at 23 wks post surgery. (d) Serum leptin. The test was conducted in fasting blood at 6 wks after RYGB. The data are expressed as mean ± s.e. ***P < 0.001 vs sham control by t-test.
Figure 3.
Energy expenditure after RYGB. (a) Energy expenditure of WT mice. The mice were single housed and energy expenditure was normalized with lean body mass (n = 7–8). (b) Energy expenditure in ob/ob mice of the first cohort (n = 5). The test was conducted at 8 wks post surgery and the result was normalized with lean body mass. (c) Energy expenditure of ob/ob mice normalized with whole-body weight. The data are expressed as mean ± s.e. *P < 0.05. ***P < 0.001 vs sham by t-test.
RYGB improved insulin sensitivity in WT, but not ob/ob mice
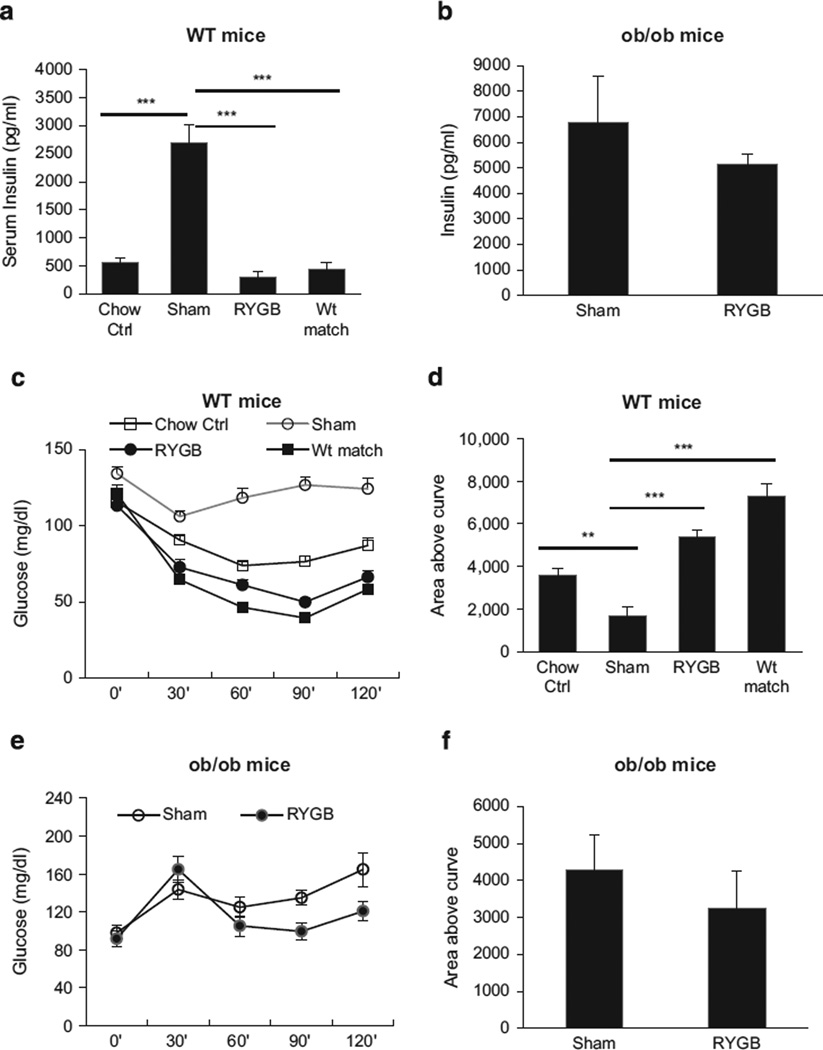
Insulin sensitivity was examined with fasting insulin and insulin tolerance in this study. In WT mice, serum insulin was increased by obesity five- to sixfold (Figure 4a, sham). The insulin was restored to the level of lean control mice by RYGB at 6 weeks post surgery (Figure 4a, RYGB). The same reduction was observed in weight-matched DIO mice (Figure 4a, Wt-matched). However, the RYGB effect was not observed in ob/ob mice (Figure 4b). In insulin tolerance test, a similar improvement was observed in WT mice after RYGB or calorie restriction (Figures 4c and d). A significant improvement was not observed ob/ob mice after RYGB (Figures 4e and f). The data suggest that RYGB improves insulin sensitivity in WT, but not in ob/ob mice.
Figure 4.
Insulin sensitivity. (a) Fasting insulin of WT mice. The fasting blood insulin was examined at 6 wks after RYGB. (b) Fasting insulin in ob/ob mice of the 6-wk-old group. (c) Insulin tolerance test (ITT) in WT mice. Insulin was administrated at 0.7 U kg−1 by i.p. injection. (d) Area above the curve for ITT in WT mice. (e) ITT in ob/ob mice (10-wk group). ITT was conducted at 11 wks post surgery. (f) Area above the curve (AAC) of ob/ob mice. The data are expressed as mean ± s.e. **P < 0.01, ***P < 0.001 vs sham by t-test. In this study, mouse number is n = 7 (WT), and n = 6 (ob/ob).
To test the weight-dependent effect of RYGB on insulin sensitivity, weight-matched mice were compared with RYGB mice in insulin sensitivity, which was conducted in WT mice (Figure 1). After calorie restriction, the weight-matched group exhibited identical improvement in insulin sensitivity to RYGB group in terms of changes in fasting insulin and insulin tolerance (Figures 4a–c), suggesting that weight loss from RYGB is critical in the maintenance of insulin sensitivity.
Leptin restored RYGB effects in ob/ob mice
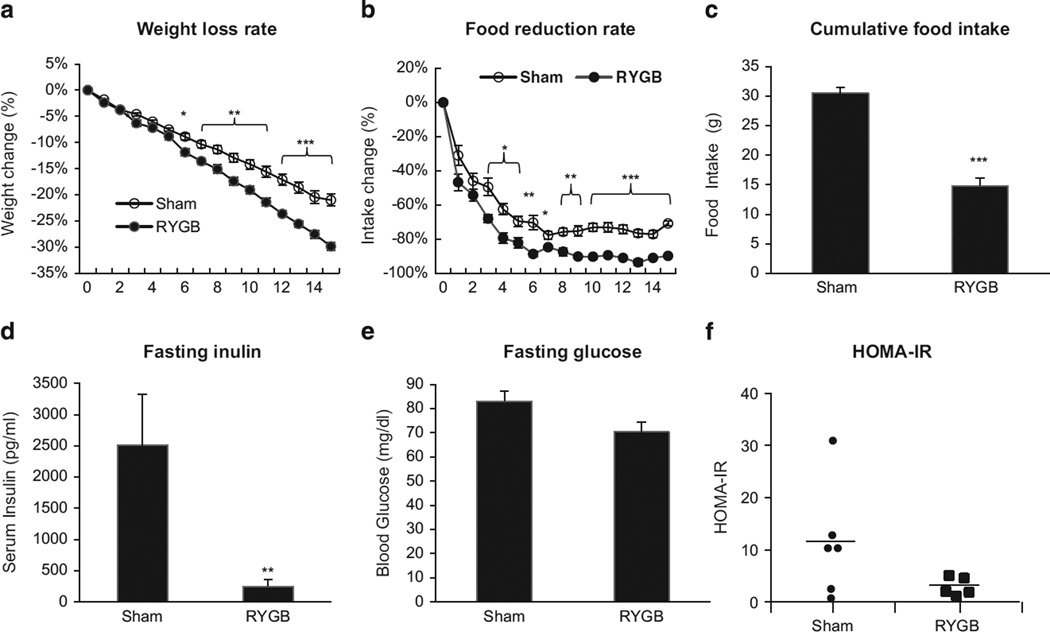
To test leptin in the effects of RYGB, we administrated leptin in ob/ob mice (1 mg kg−1 per day, i.p.) in a 2-week study. Body weight, food intake and insulin sensitivity were monitored in the study. Reduction in body weight and food intake was observed in both sham and RYGB mice following leptin treatment (Figures 5a and b). The weight reduction was identical in mass (g) in the two groups. However, percentage weight loss was significantly larger in the RYGB group because of lower body weight. The reduction in food intake was significantly more (51%) in the RYGB group compared with the sham group (Figure 5c). Insulin sensitivity was measured by fasting insulin and homeostatic model assessment-insulin resistance. More Improvement was observed in RYGB mice after the leptin treatment (Figures 5d–f). Fasting blood glucose was not significantly different in the two groups of mice after the leptin treatment (Figure 5e). These data suggest that leptin injection significantly improves ob/ob mouse response to RYGB surgery.
Figure 5.
Leptin effect in ob/ob mice. Leptin was administrated in ob/ob mice (9-wk group) at 25 wks after RYGB. (a) Weight loss rate. The reduction in body weight was calculated in percentage relative to the pre-treatment level. (b) Reduction rate in daily food intake. The reduction was in percentage relative to the pretreatment level. (c) Cumulative food intake. The number represents a sum of daily food intake over the 2 wks of leptin treatment. (d) Fasting blood insulin after the leptin treatment. (e) Fasting blood glucose after the leptin treatment. (f) MOHA-IR in ob/ob mice after the leptin treatment. The data are expressed as mean ± s.e. (n = 5–6). *P < 0.05, *P < 0.01, ***P < 0.001 vs sham by t-test.
DISCUSSION
Our mouse model of RYGB allowed us to compare ob/ob mice and DIO mice in RYGB surgery. The model is characterized by the close similarity with RYGB surgery in humans, including the ~35% sustained weight reduction and insulin sensitization for up to 39 (10 months) weeks (equivalent to ~20 years in humans) and low mortality. These effects are coupled with a negative energy balance in DIO mice after RYGB.
ob/ob mice failed to exhibit substantial weight loss after RYGB surgery. The mice exhibited a transient weight loss of ~10% in the first week post surgery, but the loss disappeared in 2 weeks after the surgery, which was associated with a sustained weight regain. Although the body weight exceeded pre-surgery level after 3 weeks in RYGB group, the weight regain was much less than that of sham group, suggesting that RYGB delayed weight gain in ob/ob mice. The reduction in nutrient absorption may contribute to the delay in RYGB mice. However, the nutrient reduction was not sufficient to keep the body weight below the pre-surgery level in ob/ob mice. In clinical practice, weight reduction beyond the pre-surgery level is used to determine the therapeutic effects of bariatric bypass surgeries.1 A systematic review and meta-analysis of large sets of published data suggest that RYGB therapy fails to improve glucose metabolism in 15–20% obese patients.1 In light of this criterion, our data suggest that the leptin signal may be required for prevention of weight regain in RYGB.
RYGB fails to enhance energy expenditure in ob/ob mice. Leptin, a hormone produced by adipose tissue, inhibits food intake and stimulates energy expenditure. Lack of leptin makes ob/ob mouse an excellent model to test leptin in RYGB. Although the hypothalamic circuitry is altered in ob/ob mice,23 the neuron response to leptin is not influenced by the alteration24 and leptin is able to restore the metabolic disorders in ob/ob mice.25 Energy expenditure was increased by RYGB in DIO mice, but not in ob/ob mice, suggesting that lack of leptin is responsible for the low energy expenditure in ob/ob mice. Leptin sensitivity was decreased by obesity, and improved by RYGB as suggested by serum leptin alteration in DIO mice in this study. The improvement is translated into sustained weight loss in DIO mice, but not in ob/ob mice. Leptin is required by RYGB to enhance energy expenditure and decrease body weight.
ob/ob mice failed to show persistent improvement in insulin sensitivity after RYGB. In ob/ob mice, insulin sensitivity was tested with insulin tolerance test and homeostatic model assessment-insulin resistance. Blood glucose was increased instead of decreasing after insulin injection in insulin tolerance test (Figure 4e). The increase is likely a result of stress response under severe insulin resistance in ob/ob mice, which prevent insulin action in the reduction of glucose. Our data suggest that RYGB was unable to improve insulin sensitivity in ob/ob mice. However, the improvement was observed after the 14 day leptin-replacement therapy, suggesting that leptin may be required for the improved insulin sensitivity after RYGB. In DIO model, insulin sensitivity was improved to the same level in the RYGB and WT-matched groups with identical weight loss, suggesting that the weight loss is critical for the sustained insulin sensitization after RYGB. Although the weight-matched strategy was not used in the study of ob/ob mice, the weight loss-dependent effect may apply to ob/ob mice as well. In leptin therapy, the more improvement in insulin sensitivity was coupled with a larger reduction in food intake in RYGB ob/ob mice, supporting the role of negative energy balance in the control of insulin sensitization. Several recent reports suggest that the negative energy balance from calorie restriction has an equivalent effect to RYGB on improving insulin sensitivity in patients.6–28 Future experiments with different doses of leptin and clamp techniques will be necessary to shed light on the mechanisms of insulin sensitization in ob/ob mice by RYGB.
This study suggests that the intact function of leptin circuitry may be required for RYGB effects. The leptin signaling pathway includes multiple proteins such as leptin receptor, melanocortin 4 receptor (MC4R), agouti-related protein and peptide tyrosine tyrosine. In the pathway, leptin binds to the receptor in proopiomelanocortin neurons to induce the secretion of melanocyte-stimulating hormone (α-MSH) and β-MSH. α-MSH activates MC4R to increase cAMP in the neurons for sensation of satiety. Leptin also inhibits expression of neuropeptide Y and agouti-related protein, which induce food intake. Agouti-related protein stimulates appetite through the inhibition of MC4R.29 Mutation in any of the signaling proteins may lead to functional deficiency of the leptin signaling pathway. Our study suggests that a defect in the leptin signaling pathway may decrease the efficacy of RYGB surgery. Although leptin mutation is rare in humans, mutations in the downstream signaling proteins are often found in severe obese patients.30 MC4R mutation is associated with severe obesity31 and loss of MC4R function attenuates the effects of RYGB surgery.32 The MC4R activity has been verified in MC4R knockout mice, which failed to respond to RYGB surgery.33 In two previous studies of Zucker obese rats (leptin receptor deficient), RYGB decreased body weight below pre-surgery level.19,20 It is not clear which of the many differences (species, surgery, diet, and timeline) between this and other studies accounts for the discrepant outcome in body weight. While, 4 weeks may have been too short to detect weight regain in Zucker rats after RYGB, particularly with a small (about 14%) weight loss at 4 weeks in one study.20 Leptin may not be the only factor in the prevention of weight regain. There are other factors contributing to the weight control by RYGB, such as bile acid that signals via the farnesoid × receptor,34 re-programming of gut glucose utilization35 and increased levels of circulating peptide tyrosine tyrosine.8 As ob/ob mouse is fragile and easily stressed, it was quite a challenge to keep mortality low after RYGB. Carefully maintaining body temperature and hydration levels during surgery and immediate post surgery was crucial.
In conclusion, our data demonstrate the difference of ob/ob and DIO mice in response to RYGB surgery. In DIO mice, RYGB generated a persistent weight loss and insulin sensitization like what have reported in 80–85% obese patients in the clinical studies. In ob/ob mice, although RYGB attenuated weight gain compared with sham operation, it failed to keep the body weight below the pre-surgery level. The weight regain resembles what has been reported in obese patients with MCR4 mutation after RYGB. Our observations suggest that leptin sensitivity is improved by RYGB. The improvement is translated into sustained weight loss in DIO mice, but not in ob/ob mice. Leptin appears to be one of the endocrine factors required for the therapeutic effects of RYGB surgery. Lack of leptin or dysfunction of leptin signaling circuitry may contribute to the weight regain and diabetes recurrence in 15–20% obese patients after RYGB surgery. This study re-enforces that weight loss contributes substantially to insulin sensitization in RYGB. These conclusions may help to predict efficacy of RYGB surgery before the surgery, and explain the weight regain after surgery.
ACKNOWLEDGEMENTS
This study is supported by the National Institutes of Health research projects DK085495 and DK068036 (ZH and JY), DK047348 (HRB), DK072476 (HM), F32-DK097896 (KRZ), COBRE (NIH P20-RR021945) and CNRU (NIH 1P30-DK072476) center grants (Cell Biology and Bio-imaging Core facilities).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
ZH, KR-Z, CD, RAM and HL, performed the experiments. HM, MK, H-RB and JY designed the study and wrote the manuscript. JY is fully responsible for this article. All authors read and approved the final manuscript.
REFERENCE
- 1.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 2.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, et al. Bariatric surgery versus intensive medical therapy for diabetes - 3-year outcomes. N Engl J Med. 2014;370:2002–2013. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coll AP, Farooqi IS, O'Rahilly S. The hormonal control of food Intake. Cell. 2007;129:251–262. doi: 10.1016/j.cell.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stefater MA, Wilson-Perez HE, Chambers AP, Sandoval DA, Seeley RJ. All bariatric surgeries are not created equal: insights from mechanistic comparisons. Endocr Rev. 2012;33:595–622. doi: 10.1210/er.2011-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–242. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laferrere B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–1716. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korner J, Inabnet W, Conwell IM, Taveras C, Daud A, Olivero-Rivera L, et al. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity (Silver Spring) 2006;14:1553–1561. doi: 10.1038/oby.2006.179. [DOI] [PubMed] [Google Scholar]
- 8.Chandarana K, Gelegen C, Karra E, Choudhury AI, Drew ME, Fauveau V, et al. Diet and gastrointestinal bypass-induced weight loss: the roles of ghrelin and peptide YY. Diabetes. 2011;60:810–818. doi: 10.2337/db10-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab. 2004;89:2608–2615. doi: 10.1210/jc.2004-0433. [DOI] [PubMed] [Google Scholar]
- 10.Ye J, Hao Z, Mumphrey MB, Townsend RL, Patterson LM, Stylopoulos N, et al. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. Am J Physiol Regul Integr Comp Physiol. 2014;306:R352–R362. doi: 10.1152/ajpregu.00491.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mokadem M, Zechner JF, Margolskee RF, Drucker DJ, Aguirre V. Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol Metab. 2013;3:191–201. doi: 10.1016/j.molmet.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chambers AP, Jessen L, Ryan KK, Sisley S, Wilson-Perez HE, Stefater MA, et al. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology. 2011;141:950–958. doi: 10.1053/j.gastro.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abegg K, Schiesser M, Lutz TA, Bueter M. Acute peripheral GLP-1 receptor agonism or antagonism does not alter energy expenditure in rats after Roux-en-Y gastric bypass. Physiol Behav. 2013;121:70–78. doi: 10.1016/j.physbeh.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson-Perez HE, Chambers AP, Sandoval DA, Stefater MA, Woods SC, Benoit SC, et al. The effect of vertical sleeve gastrectomy on food choice in rats. Int J Obes (Lond) 2013;37:288–295. doi: 10.1038/ijo.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stefater MA, Perez-Tilve D, Chambers AP, Wilson-Perez HE, Sandoval DA, Berger J, et al. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology. 2010;138:2426–2436. doi: 10.1053/j.gastro.2010.02.059. 2436 e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grayson BE, Schneider KM, Woods SC, Seeley RJ. Improved rodent maternal metabolism but reduced intrauterine growth after vertical sleeve gastrectomy. Science Transl Med. 2013;5:199ra112. doi: 10.1126/scitranslmed.3006505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 18.Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology. 2010;151:1588–1597. doi: 10.1210/en.2009-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Y, Ohinata K, Meguid MM, Marx W, Tada T, Chen C, et al. Gastric bypass model in the obese rat to study metabolic mechanisms of weight loss. J Surg Res. 2002;107:56–63. doi: 10.1006/jsre.2002.6508. [DOI] [PubMed] [Google Scholar]
- 20.Meirelles K, Ahmed T, Culnan DM, Lynch CJ, Lang CH, Cooney RN. Mechanisms of glucose homeostasis after Roux-en-Y gastric bypass surgery in the obese, insulin-resistant Zucker rat. Ann Surg. 2009;249:277–285. doi: 10.1097/SLA.0b013e3181904af0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hao Z, Zhao Z, Berthoud H-R, Ye J. Development and erification of a mouse model for Roux-en-Y gastric bypass surgery with a small gastric pouch. PLoS One. 2013;8:e52922. doi: 10.1371/journal.pone.0052922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang T, Zhang J, Yin J, Staszkiewicz J, Gawronska-Kozak B, Mynatt R, et al. Uncoupling of inflammation and insulin resistance by NF-kB in transgenic mice through induction of energy expenditure. J Biol Chem. 2010;285:4637–4644. doi: 10.1074/jbc.M109.068007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 24.Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet. 1996;14:95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 25.Harris RB, Zhou J, Redmann SM, Jr, Smagin GN, Smith SR, Rodgers E, et al. A leptin dose-response study in obese (ob/ob) and lean (+/?) mice. Endocrinology. 1998;139:8–19. doi: 10.1210/endo.139.1.5675. [DOI] [PubMed] [Google Scholar]
- 26.Isbell JM, Tamboli RA, Hansen EN, Saliba J, Dunn JP, Phillips SE, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. 2010;33:1438–1442. doi: 10.2337/dc09-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackness C, Karmally W, Febres G, Conwell IM, Ahmed L, Bessler M, et al. Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and beta-cell function in type 2 diabetic patients. Diabetes. 2013;62:3027–3032. doi: 10.2337/db12-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lingvay I, Guth E, Islam A, Livingston E. Rapid improvement of diabetes after gastric bypass surgery: is it the diet or surgery? Diabetes Care. 2013;36:2741–2747. doi: 10.2337/dc12-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buch TR, Heling D, Damm E, Gudermann T, Breit A. Pertussis toxin-sensitive signaling of melanocortin-4 receptors in hypothalamic GT1–7 cells defines agouti-related protein as a biased agonist. J Biol Chem. 2009;284:26411–26420. doi: 10.1074/jbc.M109.039339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farooqi S, O'Rahilly S. Genetics of obesity in humans. Endocr Rev. 2006;27:710–718. doi: 10.1210/er.2006-0040. [DOI] [PubMed] [Google Scholar]
- 31.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 32.Hatoum IJ, Stylopoulos N, Vanhoose AM, Boyd KL, Yin DP, Ellacott KL, et al. Melanocortin-4 receptor signaling Is required for weight loss after gastric bypass surgery. J Clin Endocrinol Metab. 2012;97:E1023–E1031. doi: 10.1210/jc.2011-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zechner JF, Mirshahi UL, Satapati S, Berglund ED, Rossi J, Scott MM, et al. Weight-independent effects of roux-en-Y gastric bypass on glucose homeostasis via melanocortin-4 receptors in mice and humans. Gastroenterology. 2013;144:580–590. e7. doi: 10.1053/j.gastro.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saeidi N, Meoli L, Nestoridi E, Gupta NK, Kvas S, Kucharczyk J, et al. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science. 2013;341:406–410. doi: 10.1126/science.1235103. [DOI] [PMC free article] [PubMed] [Google Scholar]