Abstract
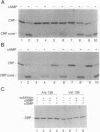
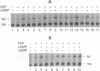
The cAMP receptor protein (CRP) of Escherichia coli needs cAMP for an allosteric change to regulate gene expression by binding to specific DNA sites. The hinge region connecting the DNA-binding domain to the cAMP-binding domain has been proposed to participate in the cAMP-induced allosteric change necessary to adjust C and D alpha-helices for movement of the DNA-binding F alpha-helix away from the protein surface. The role of the hinge region for a conformation change in CRP was tested by studying the effects of single amino acid substitutions at residue 138 located within the hinge. Physiological studies of wild-type and mutant cells and biochemical analysis of purified wild-type and mutant CRP revealed at least three groups of altered CRPs: (i) CRP that behaves like wild type (CRP+); (ii) CRP that binds cAMP but does not complete the structural changes required for specific DNA binding, proteolytic cleavage, and transcription activation (CRPallo); and (iii) CRP that shows some or all of these conformational changes without cAMP (CRP*). These results show a pivotal role of position 138 from which change emanates and provide further evidence that a hinge reorientation involving residue 138 is involved in the interhelical adjustments.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Krakow J. S. Isolation and characterization of the amino and carboxyl proximal fragments of the adenosine cyclic 3' ,5'-phosphate receptor protein of Escherichia coli. Biochemistry. 1981 Aug 4;20(16):4774–4780. doi: 10.1021/bi00519a038. [DOI] [PubMed] [Google Scholar]
- Aiba H., Nakamura T., Mitani H., Mori H. Mutations that alter the allosteric nature of cAMP receptor protein of Escherichia coli. EMBO J. 1985 Dec 1;4(12):3329–3332. doi: 10.1002/j.1460-2075.1985.tb04084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botsford J. L., Harman J. G. Cyclic AMP in prokaryotes. Microbiol Rev. 1992 Mar;56(1):100–122. doi: 10.1128/mr.56.1.100-122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGrazia H., Harman J. G., Tan G. S., Wartell R. M. Investigation of the cAMP receptor protein secondary structure by Raman spectroscopy. Biochemistry. 1990 Apr 10;29(14):3557–3562. doi: 10.1021/bi00466a019. [DOI] [PubMed] [Google Scholar]
- Ebright R. H., Ebright Y. W., Gunasekera A. Consensus DNA site for the Escherichia coli catabolite gene activator protein (CAP): CAP exhibits a 450-fold higher affinity for the consensus DNA site than for the E. coli lac DNA site. Nucleic Acids Res. 1989 Dec 25;17(24):10295–10305. doi: 10.1093/nar/17.24.10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebright R. H., Le Grice S. F., Miller J. P., Krakow J. S. Analogs of cyclic AMP that elicit the biochemically defined conformational change in catabolite gene activator protein (CAP) but do not stimulate binding to DNA. J Mol Biol. 1985 Mar 5;182(1):91–107. doi: 10.1016/0022-2836(85)90030-0. [DOI] [PubMed] [Google Scholar]
- Eilen E., Krakow J. S. Cyclic AMP-mediated intersubunit disulfide crosslinking of the cyclic AMP receptor protein of Escherichia coli. J Mol Biol. 1977 Jul;114(1):47–60. doi: 10.1016/0022-2836(77)90282-0. [DOI] [PubMed] [Google Scholar]
- Eilen E., Pampeno C., Krakow J. S. Production and properties of the alpha core derived from the cyclic adenosine monophosphate receptor protein of Escherichia coli. Biochemistry. 1978 Jun 27;17(13):2469–2473. doi: 10.1021/bi00606a001. [DOI] [PubMed] [Google Scholar]
- Garges S., Adhya S. Cyclic AMP-induced conformational change of cyclic AMP receptor protein (CRP): intragenic suppressors of cyclic AMP-independent CRP mutations. J Bacteriol. 1988 Apr;170(4):1417–1422. doi: 10.1128/jb.170.4.1417-1422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garges S., Adhya S. Sites of allosteric shift in the structure of the cyclic AMP receptor protein. Cell. 1985 Jul;41(3):745–751. doi: 10.1016/s0092-8674(85)80055-6. [DOI] [PubMed] [Google Scholar]
- Harman J. G., McKenney K., Peterkofsky A. Structure-function analysis of three cAMP-independent forms of the cAMP receptor protein. J Biol Chem. 1986 Dec 15;261(35):16332–16339. [PubMed] [Google Scholar]
- Heyduk T., Lee J. C. Escherichia coli cAMP receptor protein: evidence for three protein conformational states with different promoter binding affinities. Biochemistry. 1989 Aug 22;28(17):6914–6924. doi: 10.1021/bi00443a021. [DOI] [PubMed] [Google Scholar]
- Kim J., Adhya S., Garges S. Allosteric changes in the cAMP receptor protein of Escherichia coli: hinge reorientation. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9700–9704. doi: 10.1073/pnas.89.20.9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S. A., Murthy N. S., Krakow J. S. Ligand-induced change in the radius of gyration of cAMP receptor protein from Escherichia coli. FEBS Lett. 1980 Jan 1;109(1):121–124. doi: 10.1016/0014-5793(80)81324-x. [DOI] [PubMed] [Google Scholar]
- McKay D. B., Weber I. T., Steitz T. A. Structure of catabolite gene activator protein at 2.9-A resolution. Incorporation of amino acid sequence and interactions with cyclic AMP. J Biol Chem. 1982 Aug 25;257(16):9518–9524. [PubMed] [Google Scholar]
- Melton T., Snow L. L., Freitag C. S., Dobrogosz W. J. Isolation and characterization of cAMP suppressor mutants of Escherichia coli K12. Mol Gen Genet. 1981;182(3):480–489. doi: 10.1007/BF00293939. [DOI] [PubMed] [Google Scholar]
- Reznikoff W. S. Catabolite gene activator protein activation of lac transcription. J Bacteriol. 1992 Feb;174(3):655–658. doi: 10.1128/jb.174.3.655-658.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. C., Shields G. C., Steitz T. A. Crystal structure of a CAP-DNA complex: the DNA is bent by 90 degrees. Science. 1991 Aug 30;253(5023):1001–1007. doi: 10.1126/science.1653449. [DOI] [PubMed] [Google Scholar]
- Tan G. S., Kelly P., Kim J., Wartell R. M. Comparison of cAMP receptor protein (CRP) and a cAMP-independent form of CRP by Raman spectroscopy and DNA binding. Biochemistry. 1991 May 21;30(20):5076–5080. doi: 10.1021/bi00234a034. [DOI] [PubMed] [Google Scholar]
- Weber I. T., Steitz T. A. Structure of a complex of catabolite gene activator protein and cyclic AMP refined at 2.5 A resolution. J Mol Biol. 1987 Nov 20;198(2):311–326. doi: 10.1016/0022-2836(87)90315-9. [DOI] [PubMed] [Google Scholar]
- Wu C. W., Wu F. Y. Conformational transitions of cyclic adenosine monophosphate receptor protein of Escherichia coli. A temperature-jump study. Biochemistry. 1974 Jun 4;13(12):2573–2578. doi: 10.1021/bi00709a016. [DOI] [PubMed] [Google Scholar]
- Wu F. Y., Nath K., Wu C. W. Conformational transitions of cyclic adenosine monophosphate receptor protein of Escherichia coli. A fluorescent probe study. Biochemistry. 1974 Jun 4;13(12):2567–2572. doi: 10.1021/bi00709a015. [DOI] [PubMed] [Google Scholar]
- Zubay G. The isolation and properties of CAP, the catabolite gene activator. Methods Enzymol. 1980;65(1):856–877. doi: 10.1016/s0076-6879(80)65079-4. [DOI] [PubMed] [Google Scholar]
- de Crombrugghe B., Busby S., Buc H. Cyclic AMP receptor protein: role in transcription activation. Science. 1984 May 25;224(4651):831–838. doi: 10.1126/science.6372090. [DOI] [PubMed] [Google Scholar]