Supplemental Digital Content is available in the text.
Background:
Virtual surgery planning has proven useful for reconstructing head and neck defects by fibula osteocutaneous free flaps (FOFF). Benefits include improved healing, function, and aesthetics, as well as cost savings. But available virtual surgery planning systems incorporating fibula in craniomaxillofacial reconstruction simulate only bone reconstruction without considering vessels and soft tissue.
Methods:
The Haptics-Assisted Surgery Planning (HASP) system incorporates bone, vessels, and soft tissue of the FOFF in craniomaxillofacial defect reconstruction. Two surgeons tested HASP on 4 cases they had previously operated on: 3 with composite mandibular defects and 1 with a composite cervical spine defect. With the HASP stereographics and haptic feedback, using patient-specific computed tomography angiogram data, the surgeons planned the 4 cases, including bone resection, fibula design, recipient vessels selection, pedicle and perforator location selection, and skin paddle configuration.
Results:
Some problems encountered during the actual surgery could have been avoided as they became evident with HASP. In one case, the fibula reconstruction was incomplete because the fibula had to be reversed and thus did not reach the temporal fossa. In another case, the fibula had to be rotated 180 degrees to correct the plate and screw placement in relation to the perforator. In the spinal case, difficulty in finding the optimal fibula shape and position required extra ischemia time.
Conclusions:
The surgeons found HASP to be an efficient planning tool for FOFF reconstructions. The testing of alternative reconstructions to arrive at an optimal FOFF solution preoperatively potentially improves patient function and aesthetics and reduces operating room time.
Traumatic injuries, congenital defects, and malignant tumors in the head and neck region often cause severe disfigurement and suffering. Aspects that profoundly affect the survival rate and quality of life include competence of the surgeon, time from diagnosis to treatment,1 and quality of the preoperative plan.2–4 For example, the 5-year survival rate of gingival mandibular cancer is only about 50%, highlighting the importance of optimizing the diagnostic and treatment processes.5,6
For reconstruction of long bone defects in the head and neck area, different microvascular free flaps with donor sites such as the iliac, humerus, and radius bone have been explored with various degrees of success.7 However, the fibula osteocutaneous free flap (FOFF) became the more popular donor site when Hidalgo8 introduced it for mandibular reconstruction in 1989. Wei et al9 had already demonstrated the vascular supply of the skin paddle by a septocutaneous perforator. The fibula free flap is widely used all over the world for orofacial reconstruction due to its well-known benefits including a long pedicle, low donor-site morbidity, possibility of a 2-team approach, and reliability of its skin paddle for simultaneous soft-tissue reconstruction. However, this type of complex surgery has a long learning curve, especially in centers with low case load, making the need for extra training modalities obvious.10
Virtual surgery planning (VSP) and preoperative fabrication of implants and cutting guides for osteosynthesis have proven valuable in reconstructive surgery, with benefits including reduced time to shape the bone segments,11 reduced ischemia time,12 and improved accuracy with increased bony contact between fibular segments. This leads to benefits for the patient, such as better healing, function, and aesthetics. Furthermore, the planning session gives surgeons and engineers the opportunity to solve medical and technical patient-specific issues before surgery, with decreased intraoperative time yielding considerable cost savings. In a comparative study between 9 preplanned and 11 freehand reconstructions, Zweifel et al13 report the average time for the freehand reconstructions to be approximately 88 minutes and for the preplanned cases 21 minutes, resulting in an average time gain of approximately 67 minutes.
Examples of current commercial VSP system providers include Planmeca,14 Materialise,15 and Brainlab.16 Dérand et al17 demonstrate the use of generic 3D modeling software for surgery planning. However, the user interfaces of these systems are limited to 2D interaction with 3D data, which can be nonintuitive for 3D tasks such as surgery planning, and often require the assistance of a technician. Our goal is to shorten the preoperative planning and production from days to hours. This requires, in addition to in-house production of plates and cutting guides, a user-friendly planning system that can easily be used by the surgeons themselves without the assistance of technicians and 3D modeling experts.
Three-dimensional interaction through stereographics combined with haptics has the potential to make VSP systems easier to use by providing a visual sense of depth and an additional interaction and information channel, that is, touch. The combination of stereographics and haptics is motivated by the inherently visual and tactile nature of surgery. The use of haptics in craniomaxillofacial (CMF) surgery planning is rare; Parthasarathy18 describes the use of haptics in a planning workflow for cranioplasty and Murray et al19 for the repositioning of zygomatic bones. Schvartzman et al20 and Petersson and Åkerlund21 describe VSP systems with haptics for CMF surgery, but none of these systems support reconstructive planning using an FOFF. In addition, there is a need to incorporate into the surgical planning the soft-tissue component and the vessels along with the bone, which is not possible with current virtual planning systems.22
We describe a user-friendly VSP research system under development, the Haptics-Assisted Surgery Planning (HASP) system, which relies on stereographics and haptics to allow surgeons, with minimal system training and without support of technicians, to plan complex surgical procedures. In a retrospective pilot study, a surgical team interactively and iteratively uses the system to find the optimal fibula flap solution by defining and refining fibula osteotomies, anastomosis sites, and configuration of the skin paddle for 4 reconstructive cases of the head and neck region.
METHODS
HASP System Overview
HASP offers an alternative to traditional 2D monitor/mouse interfaces, giving the surgeon a convenient 3D working environment where he/she can view virtual patient-specific anatomical models in stereo and directly feel and interact with the models, in a manner similar to working with physical anatomical models. The display and stereo glasses (Fig. 1A, a and e) provide a stereoscopic view of a virtual 3D model of the patient-specific anatomy; individual bones or bone fragments are shown in unique colors and vessels in red. The surgeon may translate and rotate the entire 3D model, and reposition the bone fragments, fibula segments, and vessels, with the pen-like handle on a haptic device (3D pen with force feedback, see Fig. 1B, c) as we have described previously.23 During interaction, contact forces are computed in real time with high spatial resolution, which gives an impression similar to that of manipulating real, physical objects. The half-transparent mirror (Fig. 1B, b) allows the haptic and visual workspaces to be colocated. The infrared cameras track the markers on the stereo glasses for user “look-around” (Fig. 1A, d and e), that is, the surgeon can view the 3D model from different viewpoints by moving his/her head. This is essential for detecting anatomical details that are occluded from certain viewpoints and can also be useful for aligning objects.
Fig. 1.
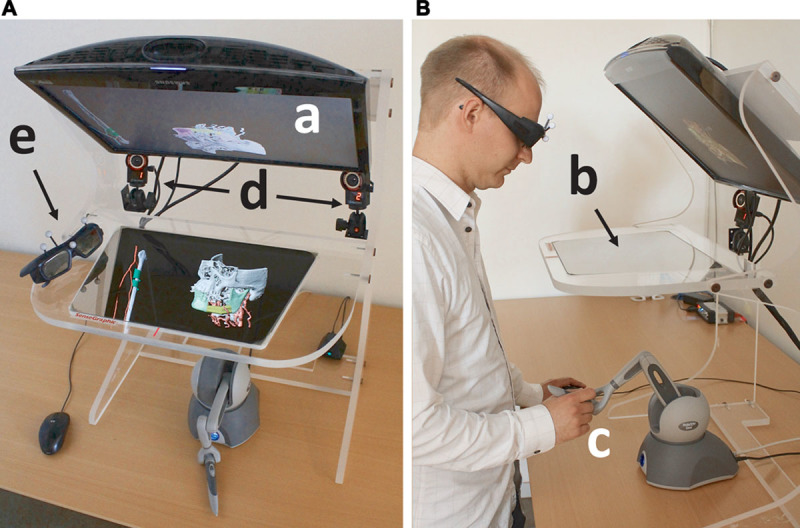
The system hardware as seen from above (A) and from the side (B). The monitor (a) displays the anatomical 3D model, which is reflected on the half-transparent mirror (b). The surgeon manipulates the 3D model with the haptic device (c) under the mirror. The infrared cameras (d) track the markers on the stereo glasses (e) for user look-around.
Application of HASP in the Design of an FOFF
Figure 2 shows the FOFF planning workflow in HASP: preparation, design, and test of the FOFF leading to the resulting plan for the osteotomy positions and angles, anastomosis sites, and skin paddle configuration. The video demonstrates the planning of a mandibular reconstruction using an FOFF (See Video 1, Supplemental Digital Content 1, this video is available in the “Related Videos” section of the full-text articles at http://www.PRSGO.com or available at http://links.lww.com/PRSGO/A120).
Fig. 2.
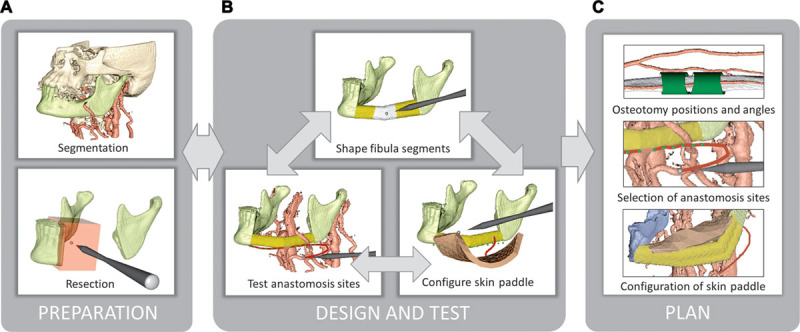
Reconstruction planning workflow. A, Load segmented bone and vessels from computed tomography angiogram data and resect bone to prepare the recipient site for reconstruction. B, Define the positions, orientations, and angulations of fibula segments; test pedicle reach to anastomosis sites on the recipient vessels; and test possible skin paddle configurations. C, Resulting plan. The user iterates within the Design and Test stage to find a suitable configuration for the fibula, vessels, and skin paddle.
Preparation
The surgeon begins by loading presegmented volumetric computed tomography angiogram data from the head and neck and from the lower leg. A standard fibula model with vessels may substitute the leg computed tomography. The recipient site is prepared for the transplant with a virtual resection tool (Fig. 3A) to remove malignant tissue in the bone with sufficient margin or to reshape fracture surfaces for better load-bearing properties. The resection tool provides planar or arbitrary cuts.
Fig. 3.
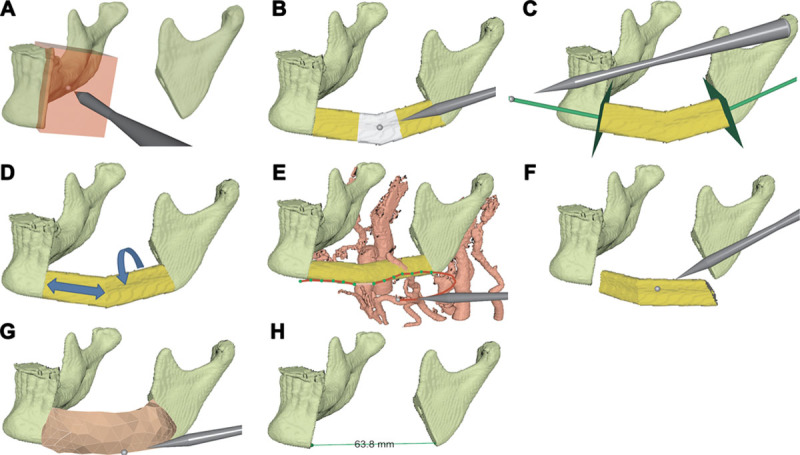
Workflow steps. A, Resect malignant tissue. B, Define angulations. C, Adjust the most proximal and distal osteotomies. D, Select section and rotation of fibula. E, Test anastomosis sites. F, Test fit. G, Configure skin paddle. H, Measure distances.
Design and Test
Control points positioned with the haptic pen define an initial contour of adjacent fibula segments. Two control points define the beginning and the end position of the fibula graft; additional control points may be added to create angulations. Control points may be moved or removed to adjust the contour (Fig. 3B). The orientations of the most proximal and distal fibular osteotomies may be adjusted to match the contact surfaces at the recipient site (Fig. 3C). The surgeon chooses what section of the fibula to use as well as its rotation around its long axis (Fig. 3D). These choices may significantly influence which anastomosis sites are reachable by the pedicle and how the perforator constrains the placement of the skin paddle. The system continually computes the fibula osteotomy positions and angles from the user-defined control points and displays the resulting contour and the osteotomies in relation to the surrounding vessels (Fig. 3E).
With the haptic device, the surgeon may test the fit of the bony part of the FOFF by virtually removing and reinserting it into the recipient site (Fig. 3F). In addition to visual feedback, haptic feedback enables the surgeon to feel whenever the fibula segments make contact with neighboring bone. If the fit is not satisfactory, the reconstruction parameters can be adjusted and tested again.
After designing the bony part of the flap, the surgeon may test the reach of the pedicle. The pedicle is represented by a deformable model that allows moving, cutting, bending, and stretching using the haptic device. Grasping and moving the end of the pedicle tests its reach to potential anastomosis sites on the recipient vessels (Fig. 3E). The pedicle may be split into 1 artery and 2 veins. If the pedicle is stretched, that is, a desired site is beyond reach, the surgeon receives both a visual cue, the pedicle color lightens, and a haptic cue similar to the resistance when stretching a rubber band. If the reach is insufficient, the surgeon may redesign the fibula angulations or use a different section or orientation of the fibula.
HASP allows design of a skin paddle for soft-tissue reconstruction. The skin paddle is a deformable skin model with configurable size, connected to the fibula by a deformable model of a perforator. With the haptic device, the surgeon may move, orient, reshape and suture the skin paddle, and test its reach under the constraint of the perforator (Fig. 3G). If the skin paddle or perforator vessel is stretched, the surgeon receives both a visual cue, that is the skin paddle or perforator color lightens, and a haptic feedback that resists the stretch. If the perforator reach is insufficient, the surgeon may redesign the angulations or use a different section or orientation of the fibula.
Resulting Plan
The plan from HASP includes the orientation of the fibula, positions and angles of the fibula osteotomies which may be used to generate a cutting guide, selection of recipient vessels and anastomosis sites, determination of pedicle length, location of the pedicle (lingual or buccal), location of the skin perforator in relation to the osteotomies, and location and configuration of the skin paddle. A measurement tool (Fig. 3H) may be used at any stage in the planning to measure, for example, the length of the defect, the pedicle, or the fibula segments.
HASP SYSTEM EVALUATION WITH CLINICAL CASES
Two surgeons (a plastic surgeon, A.R.-L., and a maxillofacial Surgeon, A.T.) from the Uppsala University Hospital evaluated HASP, with 4 cases that they had operated on previously. We describe the cases in Table 1 and present the plan derived with HASP in Table 2. The first case served as practice; the surgeons received instructions on how to use the system during the planning.
Table 1.
Case Descriptions
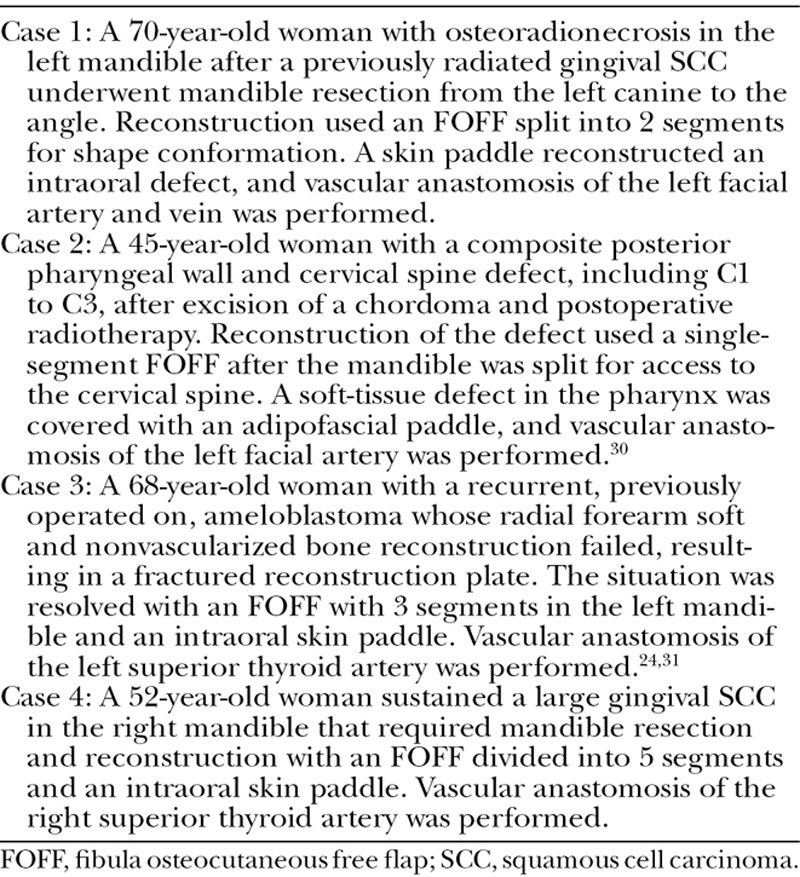
Table 2.
The Resulting Plan from HASP Includes Notes and Measurements of Bone Defect at the Recipient Site, Bone Preparation, Determination of Anastomosis Site, Number of Osteotomies and Segments, Pedicle Length, Number of Angulations, Length of Fibula Segments, Distance between from Perforator to Closest Osteotomy Site(s), Pedicle Location, Skin Paddle Location, and Total Planning Time
RESULTS
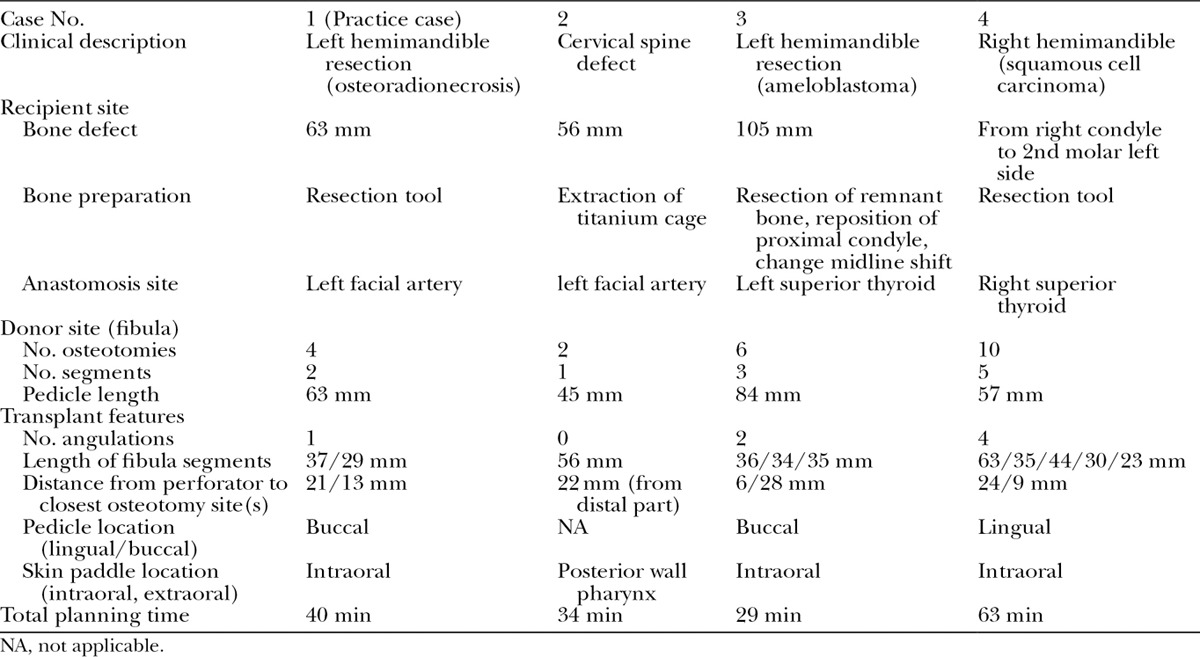
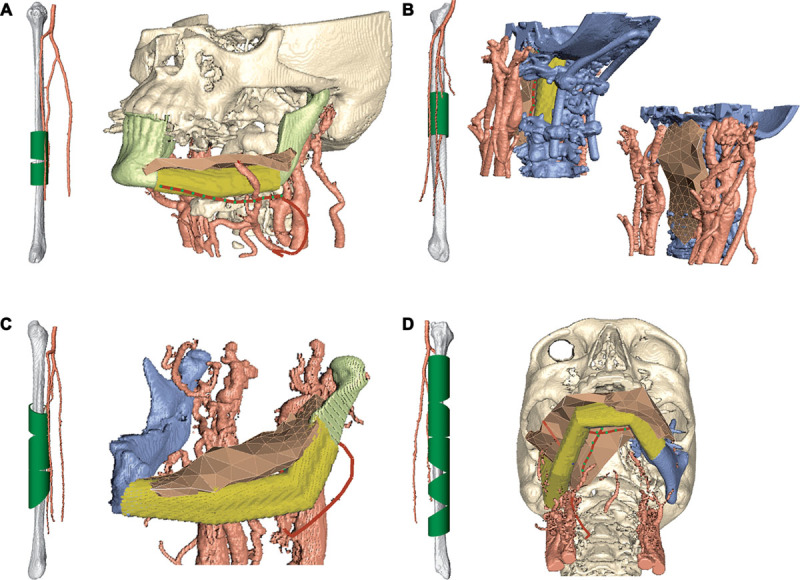
The planning session results shown in Figure 4 were similar in outcome to the actual surgeries, with the following exceptions. In Case 3, the fibula had to be rotated 180 degrees about its length axis intraoperatively to avoid plate and screw interference with the septocutaneous perforator to the skin. In Case 4, the fibula had to be reversed 180 degrees from the surgical plan derived with conventional planning software, as the pedicle did not reach the initially suggested anastomosis site. As a result, the distance between the bone and the reconstruction plate increased, the fit between the fibula segments became suboptimal, and the fibula did not reach the temporal fossa, as can be seen in Figure 5. These issues were evident in the planning with HASP and could most likely have been avoided. In Case 2, it was difficult to find the optimal fibula shape and position, resulting in extra time in the operating room which could have been reduced with prior planning with HASP. The surgeons estimate that the ischemia time could have been reduced up to 25% in this case. The results show that the surgeons could make a detailed plan in a short time, between 29 and 63 minutes per case, after only 40 minutes of training.
Fig. 4.
Final plans for the 4 cases. A, Case 1, osteoradionecrosis. B, Case 2, cervical spine defect. C, Case 3, ameloblastoma. D, Case 4, squamous cell carcinoma. The whole fibula with osteotomy positions and orientations is shown in relation to surrounding vessels to the left in each case.
Fig. 5.
A, Surgery outcome for Case 4. Due to an intraoperative reversal of the fibula, the resulting reconstruction did not reach the temporal fossa. B, During the planning session of Case 4 with HASP, this problem was avoided.
The surgeons found several aspects of HASP useful. In particular, the possibility to iterate between the different components of the FOFF (skin, bone, and vessels) was appreciated during the collaborative planning. From a microvascular standpoint, the bone defect was easily visualized, and the surgeons could adjust where on the fibula to place the osteotomies to optimize the locations of the perforators to the skin paddle and the reach of the vascular pedicle. The vessels can be picked up, measured, and moved to the anastomosis sites, suggesting adjustments such as rotations of the fibula before the placement of plates. The surgeons can anticipate the size of the defect and virtually move around the skin paddle to cover the defect over the fibula bony part intra- or extraorally. The planning of the soft tissue and the ability to anticipate possible “bulkiness” of the flap play an important role when the patient will receive dental implants. Today, during real surgery, the inset of the soft-tissue paddle is done last and is mainly performed by freehand.
Other feedback from the surgeons includes that the ability to work in 3D with “look-around” is very helpful for perception of the anatomy and alignment of objects. Haptic feedback assists the contouring of the reconstruction and the insetting of the FOFF, giving a striking difference to other systems without haptic feedback previously tried by the 2 participating surgeons. The haptic feedback was especially appreciated for the feel of the bone (resistance when hitting the ends of the resection) and when planning the length of the fibula. The foremost complaints concerned system ergonomics, including limited screen size, arm fatigue after extended use of the haptics device, and that only one user at a time can fully benefit from the head tracking.
DISCUSSION
The priorities in CMF surgery are to first save life, secondly to restore function, and thirdly to achieve a cosmetically acceptable result. Major recent advancements include better imaging and the introduction of VSP. There are limitations in the reconstructive options due to medical and anatomical restrictions in donor and recipient sites, but VSP allows for accurate planning of bone reconstruction and production of cutting guides24 and patient-specific fixation plates,17 which optimizes the outcome from a functional and esthetical point of view.2,3,25–28
The drawbacks of commercial VSP systems are due to the reliance on several iterations with technicians at external companies which delay the surgery with a negative impact on the prognosis. The process often needs 8–10 working days, including transport of plates and cutting guides between countries. By contrast, the surgeons in this study planned the cases themselves in 1 hour or less. The planning with HASP illustrates that issues encountered during the actual surgery could have been identified beforehand and ischemia time could have been reduced. Shorter ischemia time leads to a better prognosis for the flap, and in general, shorter time in the operating room leads to a better prognosis for the patient. In addition, and in contrast to available VSP systems, HASP allows not only planning of the bony part22 but lack variables related to soft tissue and vessels, which are crucial for the success of the vascularized bone and soft-tissue transfer.
The surgeons participating in this study had previously operated the cases, which could lead to a bias as they were familiar with them. Although this may be regarded as a weakness, it may also be seen as a strength because the surgeons became very aware during the planning session of which problems could have been prevented with our system, such as fibula orientation and pedicle reach.
No VSP system can anticipate all challenges encountered in the operating room, but the ease of changing parameters in HASP, such as reversing direction of the fibula, or adding and removing osteotomies, allows straightforward preparation of alternative reconstruction plans including alternative anastomosis sites. In addition to the quantitative planning data (Table 2), the surgeon gains a “mental map” of the case during preoperative planning, which may facilitate work during surgery. This also motivates the use of HASP as a teaching tool. Finally, HASP is a vehicle for collaborative planning by teams from diverse disciplines, such as CMF; ear, nose, and throat; and plastic surgery.
FUTURE WORK
The most important next step is a prospective study on a larger population to find support for the observations made by the surgeons in the pilot study described herein. Another important next step is to ensure that the plan derived from HASP can be transferred to the operating room by automatically generated cutting guides for fibula and mandibular resections as well as integration with a surgical navigator. Intraoral scans would give an opportunity to optimize the resulting occlusion by so-called backward virtual planning of dental implants. Magnetic resonance scans would allow a more detailed recipient site model, potentially including additional patient-specific soft tissue. Other possible extensions include support for double barrel and dual-paddle configurations and a more accurate model of a skin paddle. Finally, we will address the ergonomic issues of the system.
CONCLUSIONS
One key concept in HASP that leads to an optimal FOFF is the iterative design of the osteotomies, the location of the anastomosis sites, and the configuration of the skin paddle (Fig. 2). In other words, the 3 components of the FOFF design do not have to be planned in any particular order after the initial osteotomies are defined, and the user gets immediate feedback how the change of one component affects the other components. The other key concept is the underlying interaction paradigm, combining stereo visualization and haptics, which gives a natural interface for an application that is inherently highly visual and highly tactile.
The results clearly show that complex planning with HASP can be managed in-house by the surgeon himself or herself, or in collaboration between surgeons, in an hour or less after a short training session. This expands the possibilities to use virtual planning not only in oncological surgery but also when lead time to surgery is critical such as in trauma reconstruction.
Hidalgo29 stated that “Shaping of mandible grafts is an artistic process that will remain partially intuitive despite attempts to more rigidly define the process scientifically”; the HASP system’s natural interface allows the surgeon to move the intuitive, artistic process to a preoperative stage.
Video Graphic 1.
See video, Supplemental Digital Content 1, which displays a demonstration of reconstruction planning with the HASP system. This video is available in the “Related Videos” section of the full-text articles at http://www.PRSGO.com or available at http://links.lww.com/PRSGO/A120.
ACKNOWLEDGMENT
We thank Johan Nysjö for segmenting the image data used in this study.
PATIENT CONSENT
The person in Figure 1 provided written consent for the use of his image. The Ethical Review Board, Uppsala, approved the use of the patient image data, application 2012/269, with addendum approved on November 8, 2013. We received written consent from patients in 2 cases and from next of kin in 2 cases.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. This work was supported by Department of Information Technology, Uppsala University, Uppsala, Sweden; Department of Surgical Sciences, Uppsala University, Uppsala, Sweden; Department of Oral and Maxillofacial Surgery, Uppsala University Hospital, Uppsala, Sweden; ALF Funds, Medical Faculty, Uppsala University, Uppsala, Sweden; Public Health Service of Uppsala County Council, Sweden; and the Thuréus Foundation. The Article Processing Charge was paid for by the Department of Information Technology, Uppsala University, Uppsala, Sweden.
REFERENCES
- 1.Lyhne NM, Christensen A, Alanin MC, et al. Waiting times for diagnosis and treatment of head and neck cancer in Denmark in 2010 compared to 1992 and 2002. Eur J Cancer. 2013;49:1627–1633. doi: 10.1016/j.ejca.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 2.Roser SM, Ramachandra S, Blair H, et al. The accuracy of virtual surgical planning in free fibula mandibular reconstruction: comparison of planned and final results. J Oral Maxillofac Surg. 2010;68:2824–2832. doi: 10.1016/j.joms.2010.06.177. [DOI] [PubMed] [Google Scholar]
- 3.Leiggener CS, Krol Z, Gawelin P, et al. A computer-based comparative quantitative analysis of surgical outcome of mandibular reconstructions with free fibula microvascular flaps. J Plast Surg Hand Surg. 2015;49:95–101. doi: 10.3109/2000656X.2014.920711. [DOI] [PubMed] [Google Scholar]
- 4.Antony AK, Chen WF, Kolokythas A, et al. Use of virtual surgery and stereolithography-guided osteotomy for mandibular reconstruction with the free fibula. Plast Reconstr Surg. 2011;128:1080–1084. doi: 10.1097/PRS.0b013e31822b6723. [DOI] [PubMed] [Google Scholar]
- 5.Adell R, Svensson B, Bågenholm T. Dental rehabilitation in 101 primarily reconstructed jaws after segmental resections—possibilities and problems. An 18-year study. J Craniomaxillofac Surg. 2008;36:395–402. doi: 10.1016/j.jcms.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Larsen MB, Hansen RP, Hansen DG, et al. Secondary care intervals before and after the introduction of urgent referral guidelines for suspected cancer in Denmark: a comparative before-after study. BMC Health Serv Res. 2013;13:348. doi: 10.1186/1472-6963-13-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Testelin S. [History of microsurgical reconstruction of the mandible]. Ann Chir Plast Esthet. 1992;37:241–245. [PubMed] [Google Scholar]
- 8.Hidalgo DA. Fibula free flap: a new method of mandible reconstruction. Plast Reconstr Surg. 1989;84:71–79. [PubMed] [Google Scholar]
- 9.Wei FC, Chen HC, Chuang CC, et al. Fibular osteoseptocutaneous flap: anatomic study and clinical application. Plast Reconstr Surg. 1986;78:191–200. doi: 10.1097/00006534-198608000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Wei FC, Santamaria E, Chang YM, et al. Mandibular reconstruction with fibular osteoseptocutaneous free flap and simultaneous placement of osseointegrated dental implants. J Craniofac Surg. 1997;8:512–521. doi: 10.1097/00001665-199711000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Modabber A, Legros C, Rana M, et al. Evaluation of computer-assisted jaw reconstruction with free vascularized fibular flap compared to conventional surgery: a clinical pilot study. Int J Med Robot. 2012;8:215–220. doi: 10.1002/rcs.456. [DOI] [PubMed] [Google Scholar]
- 12.Seruya M, Fisher M, Rodriguez ED. Computer-assisted versus conventional free fibula flap technique for craniofacial reconstruction: an outcomes comparison. Plast Reconstr Surg. 2013;132:1219–1228. doi: 10.1097/PRS.0b013e3182a3c0b1. [DOI] [PubMed] [Google Scholar]
- 13.Zweifel DF, Simon C, Hoarau R, et al. Are virtual planning and guided surgery for head and neck reconstruction economically viable? J Oral Maxillofac Surg. 2015;73:170–175. doi: 10.1016/j.joms.2014.07.038. [DOI] [PubMed] [Google Scholar]
- 14. Planmeca. Available at: http://www.planmeca.com/. Accessed April 2, 2015.
- 15. Materialise. Available at: http://www.materialise.com/. Accessed April 2, 2015.
- 16. Brainlab. Available at: http://www.brainlab.com/. Accessed April 2, 2015.
- 17.Dérand P, Rännar LE, Hirsch JM. Imaging, virtual planning, design, and production of patient-specific implants and clinical validation in craniomaxillofacial surgery. Craniomaxillofac Trauma Reconstr. 2012;5:137–144. doi: 10.1055/s-0032-1313357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parthasarathy J. 3D modeling, custom implants and its future perspectives in craniofacial surgery. Ann Maxillofac Surg. 2014;4:9–18. doi: 10.4103/2231-0746.133065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray DJ, Edwards G, Mainprize JG, et al. Optimizing craniofacial osteotomies: applications of haptic and rapid prototyping technology. J Oral Maxillofac Surg. 2008;66:1766–1772. doi: 10.1016/j.joms.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 20.Schvartzman SC, Silva R, Salisbury K, et al. Computer-aided trauma simulation system with haptic feedback is easy and fast for oral-maxillofacial surgeons to learn and use. J Oral Maxillofac Surg. 2014;72:1984–1993. doi: 10.1016/j.joms.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Petersson F, Åkerlund C. Haptic Force Feedback Interaction for Planning in Maxillo-Facial Surgery. Linköping, Sweden: Master Thesis. Department of Science and Technology, Linköping University; 2003. [Google Scholar]
- 22.Hoarau R, Zweifel D, Simon C, et al. The use of 3D planning in facial surgery: preliminary observations. Rev Stomatol Chir Maxillofac Chir Orale. 2014;115:353–360. doi: 10.1016/j.revsto.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Olsson P, Nysjö F, Hirsch JM, et al. A haptics-assisted cranio-maxillofacial surgery planning system for restoring skeletal anatomy in complex trauma cases. Int J Comput Assist Radiol Surg. 2013;8:887–894. doi: 10.1007/s11548-013-0827-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leiggener C, Messo E, Thor A, et al. A selective laser sintering guide for transferring a virtual plan to real time surgery in composite mandibular reconstruction with free fibula osseous flaps. Int J Oral Maxillofac Surg. 2009;38:187–192. doi: 10.1016/j.ijom.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 25.Ayoub N, Ghassemi A, Rana M, et al. Evaluation of computer-assisted mandibular reconstruction with vascularized iliac crest bone graft compared to conventional surgery: a randomized prospective clinical trial. Trials. 2014;15:114. doi: 10.1186/1745-6215-15-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foley BD, Thayer WP, Honeybrook A, et al. Mandibular reconstruction using computer-aided design and computer-aided manufacturing: an analysis of surgical results. J Oral Maxillofac Surg. 2013;71:e111–e119. doi: 10.1016/j.joms.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 27.Bai S, Shang H, Liu Y, et al. Computer-aided design and computer-aided manufacturing locating guides accompanied with prebent titanium plates in orthognathic surgery. J Oral Maxillofac Surg. 2012;70:2419–2426. doi: 10.1016/j.joms.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Hirsch DL, Garfein ES, Christensen AM, et al. Use of computer-aided design and computer-aided manufacturing to produce orthognathically ideal surgical outcomes: a paradigm shift in head and neck reconstruction. J Oral Maxillofac Surg. 2009;67:2115–2122. doi: 10.1016/j.joms.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Hidalgo DA. Modeling a fibula transplant in mandibular reconstructions: evaluation of the effects of a minimal number of osteotomies on the contour of the jaw—discussion. Plast Reconstr Surg. 2001;108:1922–1923. doi: 10.1097/00006534-200112000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez–Lorenzo A, Rydevik MM, Thor A, et al. Fibula osteo–adipofascial flap for reconstruction of a cervical spine and posterior pharyngeal wall defect. Microsurgery. 2014;34:314–318. doi: 10.1002/micr.22217. [DOI] [PubMed] [Google Scholar]
- 31.Klee C, Lindskog S, Hirsch JM, Thor A. Recurrent ameloblastoma of the mandible: Surgical seeding or metastasis of malignant ameloblastoma? Case Reports in Clinical Medicine. 2013;2:154. [Google Scholar]


