Abstract
Vincristine (VCR) is a potent anti-cancer drug but its clinical efficacy is dose-limited by severe neurotoxicity. Prophylactic and palliative treatment of VCR-induced toxicity have been explored without significant findings. The field of drug delivery may provide an opportunity to increase the therapeutic index of VCR by delivering the drug specifically to tumor sites while sparing normal tissue. We have recently developed a telodendrimer (PEG5k-Cys4-L8-CA8) capable of forming disulfide cross-linked micelles which can encapsulate a variety of chemotherapeutics. In the present study, we encapsulate VCR into these micelles (DCM-VCR) and use them to treat lymphoma bearing mice. DCM-VCR particles have a size of 16 nm which is ideal for tumor accumulation via the enhanced permeability and retention (EPR) effect. Compared to our first-generation non-cross-linked micelles, DCM-VCR demonstrated greater stability and slower drug release in physiological conditions. In addition, DCM-VCR exhibited an MTD of 3.5 mg/kg while the MTD for conventional VCR was only 1.5 mg/kg. Using a NIRF dye, we showed that DCM-VCR accumulated at the tumor site starting 1 h after injection and persisted up to 72 h in lymphoma bearing mice. In an in vivo efficacy study, high dose (2.5 mg/kg) DCM-VCR exhibited the greatest reduction in tumor volume. High dose DCM-VCR was well tolerated and did not cause additional toxicity as measured by body weight, complete blood count, serum chemistry and histological analyses of the sciatic nerve. Mice treated with equivalent concentrations (1 mg/kg) of conventional VCR and DCM-VCR controlled tumor growth equally, however, in combination with on-demand addition of the reducing agent, N-acetylcysteine, DCM-VCR exhibited a superior anti-tumor effect compared to conventional VCR.
Keywords: drug delivery, lymphoma, micelle, disulfide crosslink, vincristine
1. Introduction
Vincristine (VCR) is a vinca alkaloid with broad anti-tumor activity and is effective for the treatment of a variety of cancers including non-Hodgkin’s lymphoma (NHL). In 2011, ~75,000 new cases of NHL will occur and the overall 1and 5year relative survival is 80% and 67%, respectively [1]. VCR acts as a mitotic inhibitor by binding to tubulin and preventing its polymerization into microtubules, thus arresting cell division in metaphase. The clinical efficacy of VCR is limited however, due to its neurotoxic effect which manifests as sensorimotor peripheral neuropathy. Drugs to prevent and treat VCR-induced neurotoxicity have been extensively studied but further research is warranted [2–5]. One approach to reduce the toxicity of VCR while simultaneously enhancing its efficacy is to selectively deliver the drug to tumor tissue while decreasing its exposure to normal tissues.
The advent of nanomedicine has led to the development of a variety of drug carriers capable of encapsulating chemotherapeutics. The rationale for using such drug carriers is that they can prolong the circulation time of the drug, decrease the exposure to normal tissues and increase drug accumulation at tumor sites. Several groups have developed a liposomal formulation of VCR and showed that it exhibits a greater anti-tumor effect in xenograft models of cancer compared to conventional VCR [6–9]. In addition, Marqibo is the first liposomal formulation of VCR to reach clinical trials and is currently being tested in several types of cancer. Preliminary results from these trials showed that liposomal VCR was tolerated at twice the dose intensity of conventional VCR with the dose- limiting toxicity being peripheral neuropathy [10]. Although promising, liposomal VCR is not without limitations and there remains a need to develop a superior VCR nanocarrier. Current liposomal formulations of VCR lack polyethylene glycol (PEG) which aids in controlled drug release and masks liposomes from premature uptake by the reticuloendothelial system, both being crucial for effective drug delivery. In addition, the relatively large size (~150 nm) of liposomes may hinder passive tumor accumulation via the enhanced permeability and retention (EPR) effect as well as deep tumor penetration once at the tumor site [11].
Polymeric micelles are a large family of nano-sized particles with unique core–shell structure which may offer some therapeutic advantages over liposomes. These particles are 10–100 nm and as drug carriers they are the optimal size for passive tumor targeting via the EPR effect [12, 13]. We have previously reported several micellar nanocarriers based on linear PEG and dendritic cholic acid block copolymers (called telodendrimers) capable of efficient anticancer drug delivery [14–16]. When loaded with paclitaxel (PTX), our first-generation micelles exhibited a greater anti-tumor effect than equivalent doses of the FDA-approved free drug (TAXOL®) and the albumin-bound formulation (Abraxane®). Furthermore, we have recently completed a phase I clinical trial in companion dogs with lymphoma and demonstrated these micelles to be well tolerated and exhibit anti-cancer efficacy.
We have recently reported on our second-generation telodendrimer which contains thiol groups capable of forming intra-micellar disulfide bonds for the purpose of enhanced particle stability [17]. Using PTX as a model anti-cancer drug, we showed these micelles to be more resistant to premature drug release, circulate in the blood longer and exhibit a greater anti-tumor effect compared to their non-crosslinked counterparts. Upon reaching the tumor site, intra-micellar disulfide bonds are cleaved by the highly reductive intracellular environment or by on-demand addition of a reducing agent and the drug payload is released. In the present study, we loaded our second-generation micelles with VCR and characterized their stability and cytotoxicity in vitro. We then loaded these micelles with VCR (DCM-VCR) and assessed their ability to alter the efficacy and toxicity of the free drug.
The morphology, particle size distribution, stability and drug release profile of VCR-loaded micelles were characterized by transmission electron microscopy (TEM), dynamic light scattering (DLS) and the in vitro dialysis method, respectively. The in vitro cytotoxicity of conventional VCR, non-cross-linked micelle-VCR (NCM-VCR) and DCM-VCR were assessed in the NHL cell line Raji. Next, we used healthy balb/c mice to investigate whether the maximum tolerated dose of conventional VCR could be increased by using our disulfide cross-linked micelles. Finally, we compared the in vivo efficacy and toxicity of conventional VCR and DCM-VCR in a xenograft model of NHL.
2. Experimental Section
2.1. Materials
Monomethyl-terminated poly(ethylene glycol) monoamine (MeO-PEG-NH2, Mw: 5000 Da) was purchased from Rapp Polymere (Germany). Vincristine sulfate was purchased from AvaChem Scientific (San Antonio, TX). The conventional (clinical) formulation of vincristine sulfate was obtained from the Cancer Center of University of California, Davis. (Fmoc)Lys(Boc)-OH, (Fmoc)Lys(Dde)-OH, (Fmoc)Lys(Fmoc)-OH, (Fmoc)Cys(Trt)-OH and (Fmoc)Ebes-OH were obtained from AnaSpec Inc. (San Jose, CA). 1,10-dioctadecyl-3,3,30,30 tetramethylindodicarbocyanine perchlorate (DiD) was purchased from Invitrogen. Tetrazolium compound [3-(4, 5-dimethylthiazol-2-yl)-5-(3 carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, MTS] and phenazine methosulfate (PMS) were purchased from Promega (Madison, Wisconsin). Cholic acid, Ellman’s reagent [DTNB, 5,59 dithiobis(2-nitrobenzoic acid)], triethylamine (TEA) and all other chemicals were purchased from Sigma Aldrich (St. Louis).
2.2. Synthesis of telodendrimers
The thiolated telodendrimer (named as PEG5k-Cys4-L8-CA8) was synthesized via solution-phase condensation reactions from MeO-PEG-NH2 utilizing stepwise peptide chemistry as described previously [17]. In addition, the PEG5k-CA8 thiol-free telodendrimer was synthesized for the preparation of non-cross-linked micelles [15, 16].
2.3. Preparation and characterization of VCR-loaded micelles
DCM-VCR was prepared by the solvent evaporation method as described in previous studies [14–18]. 1 mg of vincristine sulfate powder was dissolved with 3 molar equivalents of triethylamine (TEA) in chloroform and mixed with 20 mg PEG5k-Cys4-L8-CA8 telodendrimers in a 10 mL round bottom flask. Chloroform was evaporated under vacuum to form a thin film followed by hydration of the film with 1 mL PBS buffer and 30 min of sonication. The VCR-loaded micelles were then cross-linked via O2-mediated oxidation as described previously [17]. The level of free thiol groups was monitored by Ellman’s test over time. The micelle solution was used for further characterizations without dialysis after the level of free thiol groups remained at a constant low value. Drug loading was analyzed by spectrophotometric detection of VCR at 299 nm after releasing the drug from the micelles by adding 9 times of DMSO and 10 min sonication. Drug loading was determined using a standard curve generated with a series of VCR/DMSO standard solutions (Fig. S1). NCM-VCR was prepared using PEG5k-CA8 thiol-free telodendrimer as reported previously [15]. The hydrophobic NIRF dye, DiD (0.5 mg/mL) was loaded into the disulfide cross-linked micelles using the method described above. Finally, the micelle solutions were sterilized by passage through a 0.22 µm filter.
The morphology and particle size distribution of DCM-VCR were characterized by Philips CM-120 transmission electron microscopy (TEM) and dynamic light scattering (DLS) instruments (Microtrac), respectively. The micelle concentrations were kept at 1 mg/mL for DLS measurements. All measurements were performed at 25°C and data were analyzed by Microtrac FLEX Software 10.5.3. For TEM, the aqueous micelle solution (1 mg/mL) was deposited onto copper grids, stained with phosphotungstic acid and measured at room temperature.
2.4. Stability of micelles in SDS and human plasma
The stabilities of NCM-VCR and DCM-VCR were assessed under micelle disrupting conditions by measuring particle size. First, NCM-VCR and DCM-VCR were incubated with sodium dodecyl sulfate (SDS) which was reported to be able to efficiently break down polymeric micelles [19]. The final SDS concentration was 2.5 mg/mL and the micelle concentration was fixed at 1 mg/mL. Next, we measured the size of NCM-VCR and DCM-VCR following incubation in 50% (v/v) plasma from healthy human volunteers at physiological body temperature (37 °C). DCM-VCR was additionally incubated with SDS and 20 mM of the reducing agent N-acetylcysteine (NAC). The size and size distribution of the micelle solutions were measured at predetermined time intervals.
2.5. Drug release study
The in vitro drug release profiles for the different formulations of VCR were measured using the dialysis method [15]. NCMs and DCMs were prepared with 20 mg/mL telodendrimer and loaded with 1 mg/mL VCR. Aliquots of the conventional formulation of VCR (1 mg/mL), NCM-VCR and DCM-VCR were injected into dialysis cartridges with a MWCO of 3.5 kDa (Thermo Scientific, Rockford, IL). Cartridges were dialyzed against 1 L PBS at 37°C with shaking at 100 rpm in the presence of 10 g/L activated charcoal to create a sink condition. The concentration of VCR remaining in the dialysis cartridge at various time points was measured by spectrophotometry after releasing the drug from the micelles by adding 9 times of DMSO and 10 min sonication. Values were reported as the means for each triplicate sample.
2.6. Cell culture and xenograft model
The Burkitt’s B-cell lymphoma cell line, Raji, was purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). Cells were cultured in ATCC formulated RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin G and 100 µg/mL streptomycin at 37 °C using a humidified 5% CO2 incubator.
6–8 week old female athymic nude mice were obtained from Harlan Sprague–Dawley (Indianapolis, IN) and maintained in micro-isolation cages under pathogen free conditions in the UC Davis animal facility. All procedures were conducted under an approved protocol (protocol No.) according to guidelines specified by the National Institute of Health Guide for Animal Use and Care. Mice were allowed to acclimatize for at least 4 days prior to the start of any experiment. 3 days before tumor cell implantation, mice received 400 rads of whole body radiation. To establish tumors, 5×106 Raji cells resuspended in PBS were subcutaneously implanted on to the flank of each animal.
2.7. in vitro cytotoxicity assay
Raji cells were seeded in 96-well plates at a density of 10,000 cells/well. The cells were treated with the conventional formulation of VCR, NCM-VCR and DCM-VCR continuously for 72 h. In a separate experiment, Raji cells were incubated with the VCR formulations for 2 h, washed 3 times and then resuspended in media and incubated for an additional 70 h. After 72 h, cell viability was assessed using the CellTiter 96 AQueous One Solution Cell Proliferation Assay according to the manufacturer's instructions. 20 uL MTS solution was added to each well and cell viability assessed after a 1 h incubation. Cell viability as a percent of the untreated control for triplicate wells was calculated as follows: [(OD490 treated − OD490 background) / (OD490 control − OD490 background)*100].
2.8. Maximum tolerated dose
The maximum tolerated dose of the conventional formulation of VCR and DCM-VCR was investigated in healthy female balb/c mice. Mice (n=4) were treated with the conventional formulation of VCR or DCM-VCR at doses of 1.5, 2.5, 3.5 and 4.5 mg VCR/kg. VCR was administered via the tail vein every 10 days for a total of two treatments. Body weight and other symptoms of toxicity (unkempt fur, ataxia, piloerection, hind limb paralysis) were observed daily for 20 days. Mice were sacrificed upon losing 20% of their initial weight. Please define what is MDT.
2.9. NIRF optical imaging
Nude mice with subcutaneous Raji tumors of an approximate 8–10 mm diameter were subjected to in vivo NIRF optical imaging. At different time points post-injection of free DiD or DiD/VCR co-loaded disulfide cross-linked micelles (DiD and VCR both 0.5 mg/mL), mice were scanned using a Kodak multimodal imaging system IS2000MM with an excitation bandpass filter at 625 nm and an emission at 700 nm. The mice were anaesthetized by intraperitoneal injection of pentobarbital (60 mg/kg) before each imaging. 72 h after the initial injection, animals were euthanized by CO2 overdose and tumors and major organs were excised and imaged with the Kodak imaging station.
2.10. in vivo therapeutic study
NHL Raji tumors were allowed to grow until they reached a range of 100–200 mm3 and this was designated as day 0. Mice were then randomly separated into 5 treatment groups (n=6–8 per group). The first treatment group consisted of PBS as a control. The second and third groups consisted of the conventional formulation of VCR and DCM-VCR, both at a dose of 1 mg/kg. The fourth group consisted of DCM-VCR (1 mg/kg) plus N-Acetylcysteine (NAC) at 100 mg/kg. NAC is a reducing agent and has been approved by FDA for mucolytic therapy (Mucomyst®) and the treatment of acetaminophen overdose. NAC was given 24 h after the administration of DCM-VCR. The fifth group consisted of DCM-VCR alone at a dose of 2.5 mg/kg. Treatments were administered on days 0 and 9 and were injected via the tail vein. Body weight and tumor volume were checked twice per week with tumor volume being measured by digital calipers and the equation: (LxW2)/2. Mice were sacrificed when tumor volume exceeded 1500 mm3 or 20 mm in either dimension.
2.11. Toxicity
Mice from the above mentioned therapeutic study were used to investigate VCR toxicity. Seven days after the final treatment, the blood of mice (n=3) from each group was collected for determination of complete blood count as well as analysis of serum chemistry including alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, blood urea nitrogen (BUN) and creatinine. To compare the neurotoxic effects of the vincristine formulations, mice (n=3) were sacrificed seven days after the final treatment and a portion of the sciatic nerve was dissected. The nerves were processed into 1 um epoxy resin sections and stained with toluidine blue for light microscopy.
2.12. Statistical analysis
Statistical analysis was performed by Student’s t-test for two groups, and one way ANOVA for multiple groups. All results were expressed as the mean ± standard error (SEM) unless otherwise noted. A value of p < 0.05 was considered statistically significant.
3. Results and discussion
3.1. Preparation and characterization of VCR-loaded micelles
Disulfide cross-linked micelles were formed by self-assembly of the PEG5k-Cys4-L8-CA8 telodendrimer followed by oxidation as previously described [17]. The first-generation thiol-free telodendrimer, PEG5k-CA8, which forms non-cross-linked micelles was also synthesized for comparison and its synthesis was described previously [16]. The chemical structures of both telodendrimers were determined by 1H-NMR spectrometry and their molecular weights were analyzed using MALDI-TOF mass spectrometry [15, 17].
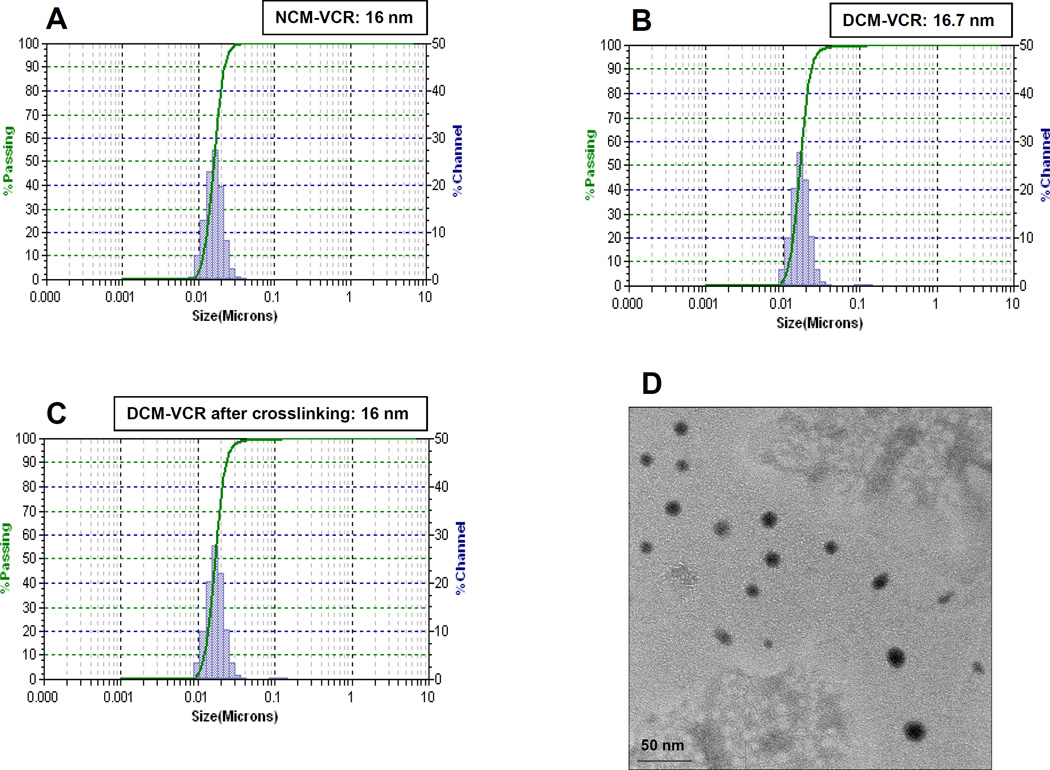
To load VCR into the micelles, 1 mg of vincristine sulfate powder was first dissolved in 3 molar equivalents of TEA in chloroform. TEA is used to neutralize the vincristine salt, allowing the drug to exist in its free form which enables superior drug loading. 20 mg of PEG5k-Cys4-L8-CA8 or PEG5k-CA8 telodendrimer was added to this solution, vortexed and then a vacuum was applied to evaporate the chloroform yielding a thin film. Subsequent hydration with PBS and sonication yielded NCM-VCR and DCM-VCR with a size of approximately 16 nm as determined by dynamic light scattering (Fig. 1A,1B). Due to the well-defined structure of the telodendrimers, the micelles exhibited a narrow distribution in size. At the ratio of telodendrimer to VCR used (20:1 w/w), VCR loading into NCM-VCR and DCM-VCR approached 100% as determined by the spectrophotometric method. To crosslink the thiol groups of DCM-VCR, oxygen was bubbled in for 48 h at which point all of the thiol groups were oxidized to disulfide bonds [17]. After O2-mediated oxidation, the size and size distribution of DCM-VCR remained the same (Fig. 1C). Transmission electron microscopy (TEM) was used to further examine the particle size and morphology of DCM-VCR after crosslinking. TEM revealed DCM-VCR to have a size similar to that measured by DLS and to be roughly spherical in shape (Fig. 1D).
Figure 1.
3.2. Micelle stability
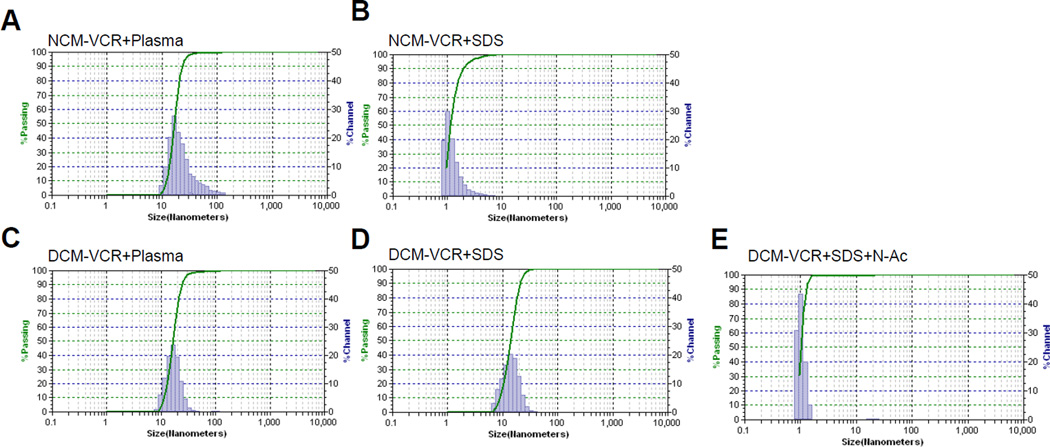
In an aqueous environment, micelles exist in a thermodynamic equilibrium between unimers and micelles. When injected intravenously, unimers are susceptible to interaction with serum proteins and lipoproteins which can shift the equilibrium toward the unimer form and cause premature drug release [20]. In addition, once in the blood stream, micelles are susceptible to dilution below their critical micelle concentration (CMC) which can result in their dissociation to unimers and subsequent drug release. The PEG5k-Cys4-L8-CA8 telodendrimer contains thiol groups capable of forming intra-micellar disulfide bonds, thus stabilizing the micelle and preventing premature drug release. To this end, we compared the stability of NCM-VCR and DCM-VCR in physiological and severe micelle disrupting conditions. Both types of micelles were incubated with 50% human plasma at 37°C and their size was monitored over time using DLS. After 24 h, the size of NCM-VCR increased slightly from 16 to 31 nm and the particle size distribution was broadened with some particles as large as 100 nm (Fig. 2A). In comparison, the size of DCM-VCR was unchanged during the 24 h period (Fig. 2C). To further examine micelle stability, we monitored particle size after incubating NCM-VCR and DCM-VCR with 2.5 mg/mL SDS which is known to efficiently break down polymeric micelles [19]. Immediately upon addition of SDS to NCM-VCR, micelle size was reduced from 15 to 1 nm, indicating complete micelle disruption (Fig. 2B). DCM-VCR was able to resist micelle disruption and maintain particle size (Fig. 2D). The intra-micellar disulfide bonds which stabilize DCM-VCR are reversible which allows for drug release when inside the highly reducing environment of the target cell or upon external addition of a reducing agent such as N-acetylcysteine (NAC). In the presence of SDS and NAC (20 mM), the integrity of DCM-VCR was compromised and full disruption of the particle was observed after 1 h (Fig. 2E). This redox sensitive disintegration of the micellesis important because it allows on-demand release of the VCR once DCM-VCR has accumulated at the tumor site by using NAC.
Figure 2.
3.3. Drug release
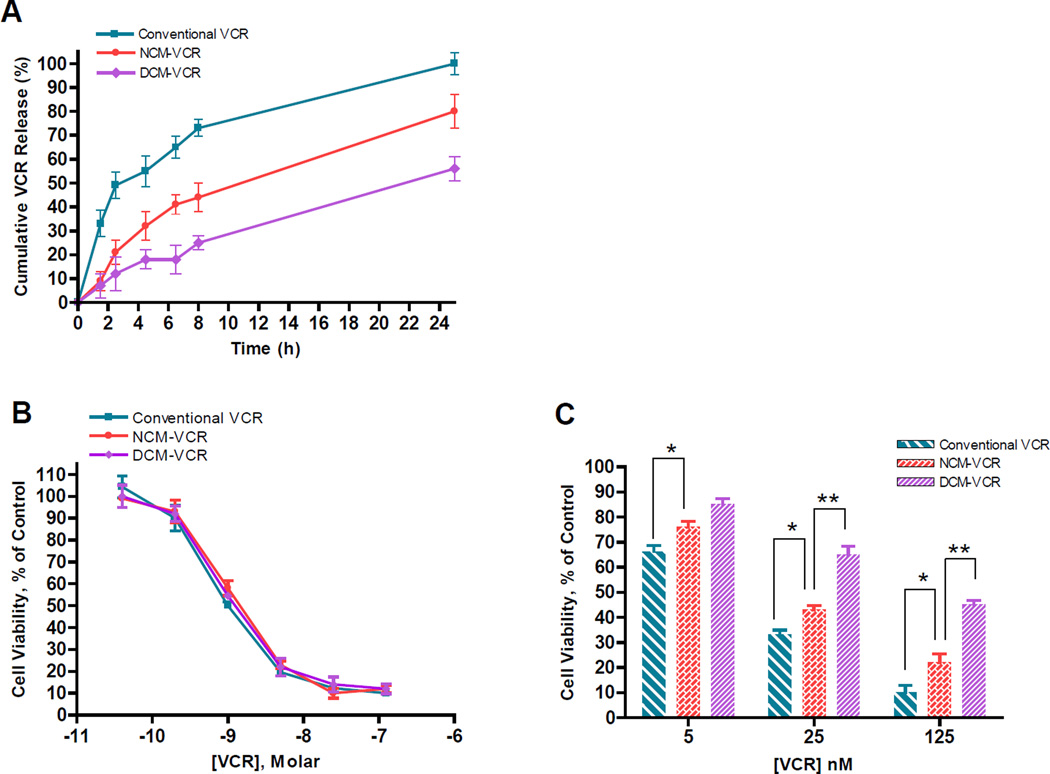
To deliver the highest concentration of drug to tumor sites and to prevent unwanted interaction with healthy tissues, a nanocarrier with sustained drug release is essential. Premature drug release can lead to off target toxicity as well as a reduction in the anti-tumor efficacy of the nanocarrier due to much of the toxic payload being released prior to reaching the tumor. Understanding the kinetics of drug release is especially important for VCR drug delivery systems because VCR is a cell-cycle sensitive drug which is most effective when tumor cells are continuously exposed for a prolonged duration [21]. In fact, a continuous intravenous infusion of the drug has the greatest anti-tumor effect but is hindered by severe toxicity [22]. We used the dialysis method to study the release profile of DCM-VCR and included conventional VCR and NCM-VCR for comparison (Fig. 3A). The three VCR formulations were loaded into dialysis cassettes with a MWCO of 3.5 kDa and dialyzed at 37°C against 1L PBS with 10 g/L charcoal to maintain an ideal sink condition. At predetermined time points, a sample was removed from the cassettes for determination of VCR concentration and calculation of VCR remaining. After 8 h, the conventional VCR sample had released 73% VCR compared to 44% and 24% for NCM-VCR and DCM-VCR, respectively. By 24 h, almost 100% of the conventional VCR had been released while NCM-VCR and DCM-VCR retained 20% and 43% of their original VCR content. We have previously demonstrated that when a reducing agent such as NAC or glutathione (GSH) is added to the dialysate, drug release can be rapidly facilitated [17].
Figure 3.
3.4. in vitro cytotoxicity
The in vitro cytotoxicity of DCM-VCR was investigated in the NHL cell line, Raji, and compared with conventional VCR and NCM-VCR in a 72 h continuous and a 2 h washout assay. In the 72 h continuous treatment assay, the 3 VCR formulations exhibited almost identical IC50 values of ~1 nM (Fig. 3B). This result was not surprising since NCM-VCR and DCM-VCR were expected to release most of their drug contents over the 72 h period, thus exhibiting a cytotoxic effect equal to that of conventional VCR. In the washout assay, Raji cells were incubated with the 3 VCR formulations for 2 h, washed and then incubated for an additional 70 h followed by assessment of cell viability. At the tested VCR concentrations (5, 25, 125 nM), conventional VCR exhibited a greater cytotoxic effect than NCM-VCR and DCM-VCR (Fig. 3C). Between the two VCR micelle formulations, NCM-VCR was more cytotoxic than DCM-VCR. Conventional VCR at a concentration of 25 nM killed 68% of the cells while the same concentration of NCM-VCR and DCM-VCR killed 58% and 36% of cells, respectively. The reduction in cytotoxicity when NCM-VCR and DCM-VCR are used is attributed to the controlled release of VCR from these particles. During the 2 h incubation period, NCM-VCR and DCM-VCR sequester the drug, thereby preventing it from diffusing into the cells. The greater cytotoxic effect of NCM-VCR compared to DCM-VCR can be attributed to its faster drug release and instability in the presence of serum proteins (Figs. 2A, 3A). The sustained release of VCR from DCM-VCR is critical because VCR is a cell-cycle dependent drug which exerts its greatest effect when cells are exposed for increased durations of time. Rapid and premature drug release from NCM-VCR and washout of conventional VCR may prohibit them from exerting their maximum cytotoxic effect in vivo. However, micelles with exceptional drug retention and stability suffer from releasing low concentrations of drug which are not therapeutically relevant. The on-demand drug release feature of DCM-VCR can overcome this problem and we have previously showed that in vitro and in vivo addition of the reducing agent, NAC, facilitated the release of PTX from DCM-PTX [17].
3.5. Maximum tolerated dose
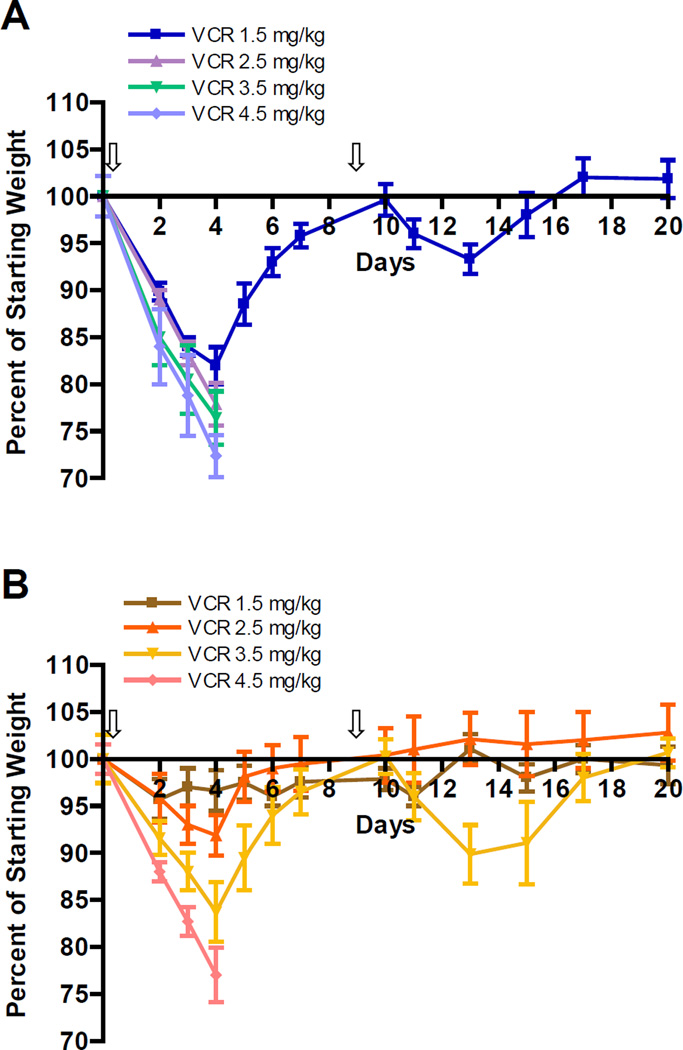
Groups of female balb/c mice (n=4) were treated with 1.5, 2.5, 3.5 or 4.5 mg VCR/kg in the form of conventional VCR or DCM-VCR to investigate the maximum tolerated dose. Mice were injected intravenously on days 0 and 10 with either formula and their body weight and general signs of toxicity were monitored for 20 days. The mice treated with 1.5 mg/kg of conventional VCR exhibited significant weight loss (18%) and unkempt fur, with the nadir being on day 4 (Fig. 4A). After day 4, these mice started to regain weight and reached their initial weight on day 10. The mice treated with doses higher than 1.5 mg/kg of conventional VCR lost >20% of their initial weight and were sacrificed. The MTD of conventional VCR determined in this study was similar to the MTD observed previously by other groups [23–25]. Mice treated with the same doses of VCR in the form of DCM-VCR exhibited significantly less weight loss at all doses (Fig. 4B). Only the group of mice receiving the highest dose of 4.5 mg/kg DCM-VCR lost >20% of their initial weight and had to be sacrificed. Thus, DCM-VCR was able to increase the MTD of VCR from 1.5 to 3.5 mg/kg (>2-fold). Dose intensification of VCR in a clinical setting is significant as it may allow patients to receive a full dose of chemotherapy without the limiting toxicities.
Figure 4.
3.6. NIRF optical imaging
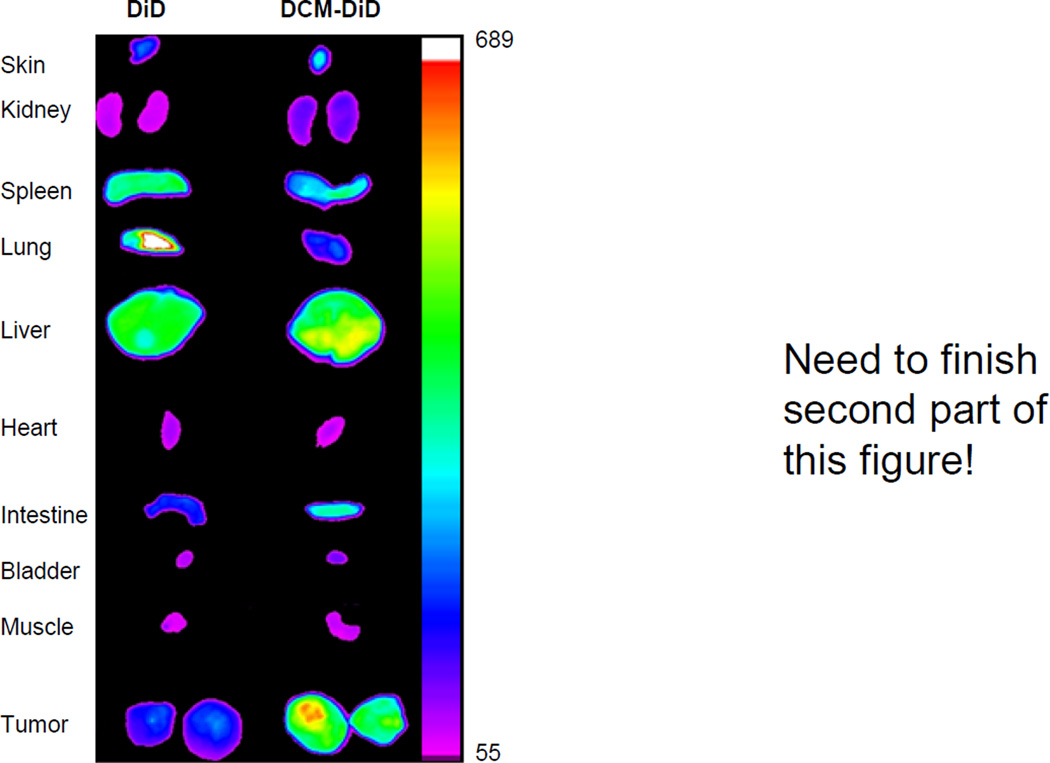
To follow the in vivo fate of DCM-VCR, a NIRF optical imaging approach was used. DCM-VCR was co-loaded with the fluorescent dye, DiD, and intravenously injected in to Raji tumor bearing mice. As a control, a separate group of mice received free DiD. We have previously demonstrated that our first- and second-generation micelles are capable of accumulating at tumor sites via the EPR effect [15, 17]. In the present study, DCM-VCR/DiD was able to accumulate at the site of the tumor beginning 1 h after the injection and up to 72 h later (Fig. 5). Free DiD distributed throughout the body without obvious uptake by a particular organ. After 72 h, ex vivo analysis revealed that DCM-VCR/DiD exhibited highest uptake in the tumor followed by the liver. High liver uptake was not surprising as this is a normal phenomenon with nanocarriers due to the presence of macrophages which engulf these particles. For free DiD, ex vivo analysis revealed the distribution to be markedly different, with high uptake by the lung and little tumor uptake.
Figure 5.
3.7. Therapeutic efficacy and toxicity
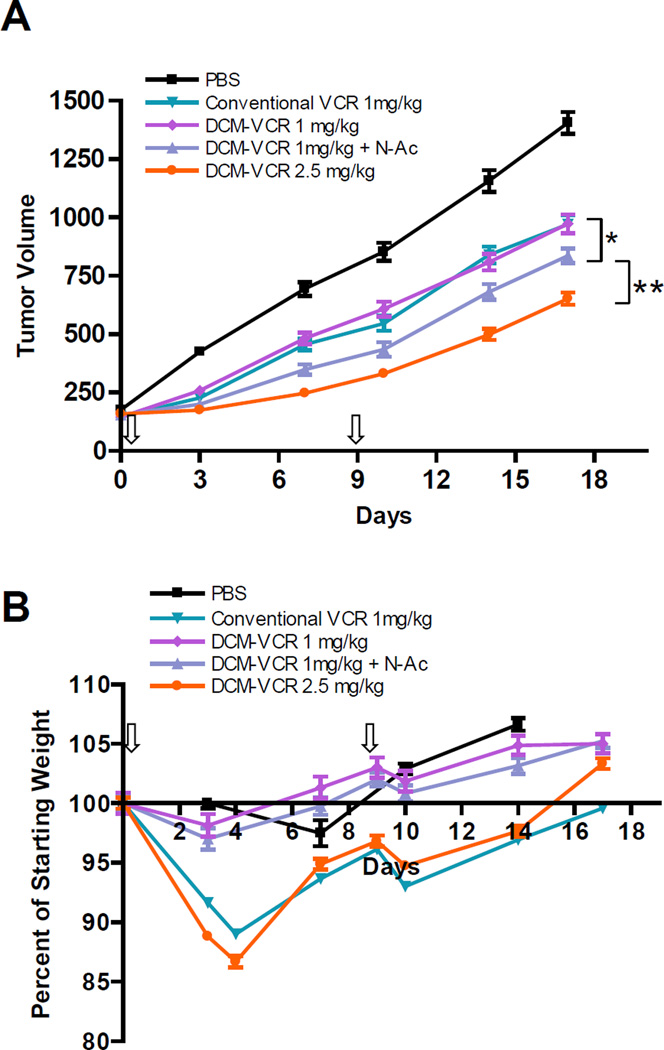
The anti-tumor effect of DCM-VCR was investigated in a xenograft model of NHL and compared to conventional VCR. Raji tumor bearing mice were treated with PBS, conventional VCR (1 mg/kg), DCM-VCR (1 mg/kg) plus or minus 100 mg/kg NAC or high dose DCM-VCR (2.5 mg/kg). All VCR treatment groups caused a significant (p<0.05) reduction in tumor growth as compared to the PBS control (Fig. 6A). Interestingly, mice receiving 1 mg/kg DCM-VCR did not exhibit a superior anti-tumor effect compared with conventional VCR at the same dose. However, mice receiving 1 mg/kg DCM-VCR followed by 100 mg/kg NAC 24 h later, did exhibit a greater reduction in tumor volume compared with conventional VCR at the same dose (p<0.05). We attribute the greater efficacy to the on-demand drug release from DCM-VCR once it accumulated at the tumor site. Although 1 mg/kg conventional VCR and DCM-VCR exhibited equivalent efficacy, the group of mice receiving DCM-VCR (with and without NAC) lost significantly less weight than the group of mice receiving conventional VCR (Fig. 6B). In terms of a clinical benefit, DCM-VCR may offer a less toxic treatment option that doesn’t sacrifice efficacy. The greatest reduction in tumor volume was observed in the group receiving 2.5 mg/kg DCM-VCR which exhibited a significantly greater tumor reduction than all treatment groups (p<0.005). It should be noted that the 2.5 mg/kg DCM-VCR treatment group lost an almost equivalent amount of weight as the 1 mg/kg conventional VCR group (Fig. 6B).
Figure 6.
Having showed that 2.5 mg/kg DCM-VCR was tolerable and the most effective treatment, we sought to evaluate the toxicity of the different VCR formulations. Seven days after the final treatment from the above study, the blood of mice (n=3) from each group was collected for determination of complete blood count (Table S1). The counts (RBC, WBC, hemoglobin, platelets) from all the treatment groups were within the normal range and revealed no significant differences between groups. In addition, the blood of mice from the PBS, conventional VCR (1 mg/kg) and DCM-VCR (2.5 mg/kg) groups was used to assess serum chemistry including alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, blood urea nitrogen (BUN) and creatinine (Table S2). As with the CBC, serum chemistry analysis revealed values within the normal range and no significant differences between groups.
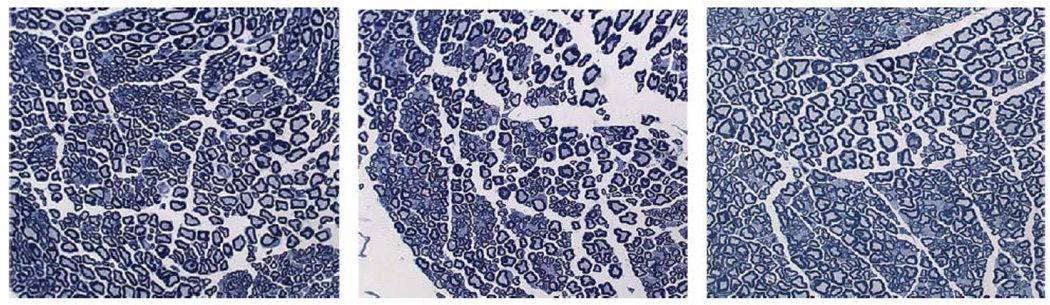
The dose limiting toxicity of VCR, peripheral neuropathy, can be assessed in animal models using histological, electrophysiological and behavioral methods [2, 26]. However, VCR-induced changes generally require prolonged exposure to a low dose of the drug [27]. There is no VCR-induced model of neuropathy which follows the dosage regimen used in our in vivo efficacy study (two treatments, 9 days apart). Seven days after the final treatment, we dissected the sciatic nerve from the PBS, conventional VCR (1 mg/kg) and DCM-VCR (2.5 mg/kg) groups for histological analysis. VCR is known to cause axonal degeneration and demyelination of nerve fibers which can be observed with either light or electron microscopy. Histological analysis revealed no obvious damage to the nerve fibers from the groups treated with conventional VCR (1 mg/kg) and DCM-VCR (2.5 mg/kg) and no differences compared to the PBS control group (Fig. 7).
Figure 7.
4. Conclusion
We used our second-generation disulfide crosslinked micelles to ecapsulate VCR for specific delivery to NHL xenograft tumors. We demonstrated DCM-VCR to be more stable in physiological conditions and to release drug more slowly than the non-cross-linked counterpart in in vitro tests. Further, we showed that the MTD of conventional VCR could be increased >2-fold by using DCM-VCR. In a xenograft model of NHL, high dose DCM-VCR was tolerable and proved to be more efficacious than conventional VCR. Finally, we showed that high dose DCM-VCR was not myelosuppressive, did not alter kidney or liver function and did not induce neurotoxicity. DCM-VCR represents an alternative treatment option to conventional VCR with the advantage of tolerable dose intensification which may provide a superior clinical effect.
Supplementary Material
Footnotes
Appendix A. Supplementary data
References
- 1.Society AC. Cancer Facts and Figures 2011. American Cancer Society; Atlants: 2011. [Google Scholar]
- 2.Contreras PC, Vaught JL, Gruner JA, Brosnan C, Steffler C, Arezzo JC, Lewis ME, Kessler JA, Apfel SC. Insulin-like growth factor-I prevents development of a vincristine neuropathy in mice. Brain research. 1997;774:20–26. doi: 10.1016/s0006-8993(97)81682-4. [DOI] [PubMed] [Google Scholar]
- 3.Moore A, Pinkerton R. Vincristine: Can its therapeutic index be enhanced? Pediatric blood & cancer. 2009;53:1180–1187. doi: 10.1002/pbc.22161. [DOI] [PubMed] [Google Scholar]
- 4.Callizot N, Andriambeloson E, Glass J, Revel M, Ferro P, Cirillo R, Vitte PA, Dreano M. Interleukin-6 protects against paclitaxel, cisplatin and vincristine-induced neuropathies without impairing chemotherapeutic activity. Cancer chemotherapy and pharmacology. 2008;62:995–1007. doi: 10.1007/s00280-008-0689-7. [DOI] [PubMed] [Google Scholar]
- 5.Apfel SC, Lipton RB, Arezzo JC, Kessler JA. Nerve growth factor prevents toxic neuropathy in mice. Annals of neurology. 1991;29:87–90. doi: 10.1002/ana.410290115. [DOI] [PubMed] [Google Scholar]
- 6.Mayer LD, Masin D, Nayar R, Boman NL, Bally MB. Pharmacology of liposomal vincristine in mice bearing L1210 ascitic and B16/BL6 solid tumours. British journal of cancer. 1995;71:482–488. doi: 10.1038/bjc.1995.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Gao H, Chen L, Wu B, Zheng Y, Liao R, Jiang Y, He F. Tumor targeting of vincristine by mBAFF-modified PEG liposomes in B lymphoma cells. Cancer letters. 2008;269:26–36. doi: 10.1016/j.canlet.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 8.Shan S, Flowers C, Peltz CD, Sweet H, Maurer N, Kwon EJ, Krol A, Yuan F, Dewhirst MW. Preferential extravasation and accumulation of liposomal vincristine in tumor comparing to normal tissue enhances antitumor activity. Cancer chemotherapy and pharmacology. 2006;58:245–255. doi: 10.1007/s00280-005-0145-x. [DOI] [PubMed] [Google Scholar]
- 9.Noble CO, Guo Z, Hayes ME, Marks JD, Park JW, Benz CC, Kirpotin DB, Drummond DC. Characterization of highly stable liposomal and immunoliposomal formulations of vincristine and vinblastine. Cancer chemotherapy and pharmacology. 2009;64:741–751. doi: 10.1007/s00280-008-0923-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez MA, Pytlik R, Kozak T, Chhanabhai M, Gascoyne R, Lu B, Deitcher SR, Winter JN. Vincristine sulfate liposomes injection (Marqibo) in heavily pretreated patients with refractory aggressive non-Hodgkin lymphoma: report of the pivotal phase 2 study. Cancer. 2009;115:3475–3482. doi: 10.1002/cncr.24359. [DOI] [PubMed] [Google Scholar]
- 11.Andresen TL, Jensen SS, Jorgensen K. Advanced strategies in liposomal cancer therapy: problems and prospects of active and tumor specific drug release. Progress in lipid research. 2005;44:68–97. doi: 10.1016/j.plipres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Bae Y, Nishiyama N, Fukushima S, Koyama H, Yasuhiro M, Kataoka K. Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconjugate chemistry. 2005;16:122–130. doi: 10.1021/bc0498166. [DOI] [PubMed] [Google Scholar]
- 13.Tsukioka Y, Matsumura Y, Hamaguchi T, Koike H, Moriyasu F, Kakizoe T. Pharmaceutical and biomedical differences between micellar doxorubicin (NK911) and liposomal doxorubicin (Doxil) Japanese journal of cancer research : Gann. 2002;93:1145–1153. doi: 10.1111/j.1349-7006.2002.tb01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Xiao K, Luo J, Lee J, Pan S, Lam KS. A novel size-tunable nanocarrier system for targeted anticancer drug delivery. Journal of controlled release : official journal of the Controlled Release Society. 2010;144:314–323. doi: 10.1016/j.jconrel.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo J, Xiao K, Li Y, Lee JS, Shi L, Tan YH, Xing L, Holland Cheng R, Liu GY, Lam KS. Well-defined, size-tunable, multifunctional micelles for efficient paclitaxel delivery for cancer treatment. Bioconjugate chemistry. 2010;21:1216–1224. doi: 10.1021/bc1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao K, Luo J, Fowler WL, Li Y, Lee JS, Xing L, Cheng RH, Wang L, Lam KS. A self-assembling nanoparticle for paclitaxel delivery in ovarian cancer. Biomaterials. 2009;30:6006–6016. doi: 10.1016/j.biomaterials.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Xiao K, Luo J, Xiao W, Lee JS, Gonik AM, Kato J, Dong TA, Lam KS. Well-defined, reversible disulfide cross-linked micelles for on-demand paclitaxel delivery. Biomaterials. 2011;32:6633–6645. doi: 10.1016/j.biomaterials.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao K, Li Y, Luo J, Lee JS, Xiao W, Gonik AM, Agarwal RG, Lam KS. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials. 2011;32:3435–3446. doi: 10.1016/j.biomaterials.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koo AN, Lee HJ, Kim SE, Chang JH, Park C, Kim C, Park JH, Lee SC. Disulfide-cross-linked PEG-poly(amino acid)s copolymer micelles for glutathione-mediated intracellular drug delivery. Chem Commun (Camb) 2008:6570–6572. doi: 10.1039/b815918a. [DOI] [PubMed] [Google Scholar]
- 20.Rijcken CJ, Snel CJ, Schiffelers RM, van Nostrum CF, Hennink WE. Hydrolysable core-crosslinked thermosensitive polymeric micelles: synthesis, characterisation and in vivo studies. Biomaterials. 2007;28:5581–5593. doi: 10.1016/j.biomaterials.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 21.Pinkerton CR, McDermott B, Philip T, Biron P, Ardiet C, Vandenberg H, Brunat-Mentigny M. Continuous vincristine infusion as part of a high dose chemoradiotherapy regimen: drug kinetics and toxicity. Cancer chemotherapy and pharmacology. 1988;22:271–274. doi: 10.1007/BF00273423. [DOI] [PubMed] [Google Scholar]
- 22.Jackson DV, Jr, Sethi VS, Spurr CL, Willard V, White DR, Richards F, 2nd, Stuart JJ, Muss HB, Cooper MR, Homesley HD, Jobson VW, Castle MC. Intravenous vincristine infusion: phase I trial. Cancer. 1981;48:2559–2564. doi: 10.1002/1097-0142(19811215)48:12<2559::aid-cncr2820481203>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Allen TM, Newman MS, Woodle MC, Mayhew E, Uster PS. Pharmacokinetics and anti-tumor activity of vincristine encapsulated in sterically stabilized liposomes. International journal of cancer. Journal international du cancer. 1995;62:199–204. doi: 10.1002/ijc.2910620215. [DOI] [PubMed] [Google Scholar]
- 24.Leonetti C, Scarsella M, Semple SC, Molinari A, D'Angelo C, Stoppacciaro A, Biroccio A, Zupi G. In vivo administration of liposomal vincristine sensitizes drug-resistant human solid tumors. International journal of cancer. Journal international du cancer. 2004;110:767–774. doi: 10.1002/ijc.20174. [DOI] [PubMed] [Google Scholar]
- 25.Kanter PM, Klaich GM, Bullard GA, King JM, Bally MB, Mayer LD. Liposome encapsulated vincristine: preclinical toxicologic and pharmacologic comparison with free vincristine and empty liposomes in mice, rats and dogs. Anti-cancer drugs. 1994;5:579–590. doi: 10.1097/00001813-199410000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Uceyler N, Kobsar I, Biko L, Ulzheimer J, Levinson SR, Martini R, Sommer C. Heterozygous P0 deficiency protects mice from vincristine-induced polyneuropathy. Journal of neuroscience research. 2006;84:37–46. doi: 10.1002/jnr.20873. [DOI] [PubMed] [Google Scholar]
- 27.Kamei J, Nozaki C, Saitoh A. Effect of mexiletine on vincristine-induced painful neuropathy in mice. European journal of pharmacology. 2006;536:123–127. doi: 10.1016/j.ejphar.2006.02.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.