Abstract
Objective
To investigate health information needs and their association with health-related quality of life (HRQOL) in a diverse, population-based sample of long-term cancer survivors.
Methods
We analyzed health information needs from 1197 cancer survivors 4–14 years post-diagnosis drawn from two cancer registries in California. Multivariable regression models were used to identify factors associated with endorsement of total number and different categories of needs. The relationship between number of needs and HRQOL and effect modification by confidence for obtaining information was examined.
Results
Survivors reported a high prevalence of unmet information needs in the following categories: side effects & symptoms: 75.8%; tests & treatment: 71.5%; health promotion: 64.5%; interpersonal & emotional: 60.2%; insurance: 39.0%; and sexual functioning & fertility: 34.6%. Survivors who were younger, non-White, and did not receive but wanted a written treatment summary reported a higher number of needs. Number of information needs was inversely related to mental well-being, particularly for those with low confidence for obtaining information (P < 0.05).
Conclusion
These patterns suggest disparities in access to important health information in long-term survivors and that affect HRQOL.
Practice Implications
Findings suggest a need for tailored interventions to equip survivors with comprehensive health information and to bolster skills for obtaining information.
Keywords: Cancer survivorship, Information needs, Self-efficacy, Quality of care, Health-related quality of life
1. Introduction
Cancer survivors encompass the population with a history of cancer from the point of diagnosis through the balance of life [1]. Meeting information needs among post-treatment cancer survivors is a vital component of quality survivorship care [2–4], yet most assessments of cancer survivors’ information needs have focused on the treatment phase [5]. When assessed, survivors have reported higher unmet supportive care needs post-treatment than at completion of treatment [6]. While unmet information needs have been reported across different cancers and populations, disparities in information-seeking experiences have also been found, with low-income and African–American individuals indicating more problems in obtaining health information [7]. Survivors who are Non-Hispanic White and highly educated are more likely to seek information and utilize more sources [8]. Furthermore, low tangible social support has been linked to low health literacy among older adults [9], although whether this relationship holds for cancer survivors is unclear.
Unmet information needs not only reflect the level of need itself but also potentially reduced capacity to obtain information. Bandura’s theory of self-efficacy suggests that expectations of effective performance (e.g., successful procurement of information) are likely to increase a behavior (e.g., information-seeking) to meet needs [10,11]. In addition, having more information about health has been linked to increases in healthy behaviors [12]. Survivors 2–5 years post-diagnosis with high levels of unmet needs have been shown to have poorer health-related quality of life (HRQOL)[13] and increased incidence of anxiety and depression [14]. Thus facilitating the confidence to obtain information may help survivors feel able to meet their own information needs, thereby reducing unmet needs and improving HRQOL.
The U.S. cancer survivor population is growing, and over half of survivors alive today are five or more years past their initial diagnosis [15]. Although many survivors reduce their use of regular follow-up care as time after completion of cancer treatment increases, attention to the late effects from cancer treatment among long-term survivors is rising [16]. Thus, it is important to document health information needs among these long-term survivors to inform them and their healthcare providers across the survivorship continuum. To extend previous work on health information needs in cancer survivors with more recent diagnoses [13], we analyzed information needs experienced by cancer survivors more than 4 years post-diagnosis drawn from a diverse population-based sample. Specifically, we (1) examined the relationship of survivors’ information needs with their socio-demographics and key follow-up care related characteristics; (2) described the type unmet information needs in long-term cancer survivors (3) analyzed associations between information needs and survivors’ HRQOL, investigating confidence to obtain information as a potential moderator of this relationship.
2. Methods
We analyzed data from the FOllow-up Care Use among Survivors (FOCUS) study, a population-based investigation of the follow-up care experiences among long-term cancer survivors (http://cancercontrol.cancer.gov/ocs/focus.html). We investigated health information needs among survivors of breast, prostate, colorectal, and gynecologic (endometrial and ovarian) cancer who were between 4 and 14 years past their initial diagnosis. We included a diverse racial/ethnic population from two Surveillance Epidemiology and End Results (SEER) cancer registries in California: the Los Angeles County Cancer Surveillance Program administered by University of Southern California (USC) and the Greater Bay Area Cancer Registry at the Cancer Prevention Institute of California (CPIC). The study was approved by the institutional review boards at CPIC and USC.
2.1. Patients
We collected data via self-reported questionnaires that were mailed to survivors between March 2005 and July 2006. A total of 6391 cases were sampled by the registries, and of those sampled 4981 were considered eligible for participation. Eligibility criteria included: age at diagnosis 21 and older, ability to read English, no physician refusal of permission to contact, and completion of active treatment. Of the eligible cases, 2977 were located; of those, 1666 completed and returned the survey. The overall participation rate based on eligible, located cases was 56%. Among eligible cases, non-respondents were more likely to be 65 and older, non-White, and with a longer time since diagnosis (P < 0.05). Breast cancer survivors were also more likely to respond than all other cancer survivors (P < 0.05). From the 1666 original respondents, a total of 332 had missing information with respect to health information needs and an additional 137 had missing information on study covariates of interest; these individuals were excluded from analyses, leaving a total of 1197 survivors in the analytic sample.
2.2. Measures
2.2.1. Health information needs
We asked survivors whether they currently needed information on several health-related topics. Twelve specific information needs were assessed in 6 needs categories: tests & treatment, side effects & symptoms, health promotion, interpersonal & emotional, insurance, and sexual functioning & fertility (see Supplementary material for exact wording of all items). Items were adapted from two previous studies conducted with cancer survivors [13,17]. The question wording was as follows: “Little is known about the information needs of long-term cancer survivors. At this time, would you like more information about any of the following health-related topics?” For each item, respondents were offered three response options: “yes,” “no,” and “not sure.” Only a “yes” response was considered an endorsement of a specific need, and if respondents answered “yes” to any of the items in a given information needs category, the category was considered endorsed.
2.2.2. Confidence for obtaining information related to cancer
We measured confidence for obtaining health information with a single item adapted from the Health Information National Trends Survey (HINTS) that asked, “How confident are you at that you could get advice or information related to cancer if you needed it at this time?”. Survivors’ level of confidence was assessed on a 5-point response scale (not at all, a little, somewhat, very, and completely confident) [18]. For simplicity, we refer to this item as informational attainment confidence (IAC).
2.2.3. Sociodemographic characteristics
We collected data on several sociodemographic characteristics: age at survey (divided into four groups: <50, 50–64, 65–79, and >80), gender, race/ethnicity (determined by self-report or SEER registry data if left blank), annual household income, adequacy of financial resources (AFR) to meet daily family needs during the past 4 weeks, and health insurance status (private, private and public, public only, none/unknown). We chose AFR over annual household income in our models for the following reasons: (1) approximately 10.2% versus 2.5% of respondents had missing data on income and AFR respectively; (2) these variables were moderately correlated (ρ = 0.29); and (3) perceived financial stress may be more predictive of psychosocial needs than income [19].
2.2.4. Clinical characteristics
We included data on the following clinical variables: self-reported disease status (active or uncertain disease status, cancer free but experienced recurrence or subsequent cancers, cancer free with no recurrence or subsequent cancer), and number of comorbidities (based on the following 14 conditions: congestive heart failure, cardiomyopathy, heart attack, angina, hypertension, pericarditis, blood clots in the legs or lungs, stroke, chronic lung disease, lung fibrosis, liver disease, diabetes, arthritis, and depression/anxiety) [20].
2.2.5. Social support
We measured social support using a modified 12-item version of the Medical Outcomes Study Social Support Scale, which measures perceived instrumental, informational, and emotional support [21]. Survivors who endorsed >75% of the items were given an average social support score which was transformed to a 0–100 format to facilitate interpretation. A higher score indicated greater social support.
2.2.6. Follow-up care
We asked survivors several questions about the type, frequency, and quality of follow-up care they received over the past 2 years. They were asked who they considered to be their main follow-up care provider: oncologist, primary care physician, gynecologist, urologist, or other provider, and they were asked to rate the quality of the care they had received on a 5-point scale (‘poor’ to ‘excellent’). We also asked survivors if they had received a written summary of their cancer treatment, and if not, would they have wanted one.
2.2.7. Health-related quality of life
We used the Short-Form (SF)-12® health status survey to measure physical and mental health over the past 4 weeks [22]. Responses to the items on the SF-12 were combined to generate two overall scores: a physical component summary score (PCS) and a mental component summary score (MCS). These scores were then normed to the 1999 U.S. general population by transforming them on a T-score metric such that a score of 50 represents the average score in the U.S. general population with a standard deviation of 10 [23]. Higher PCS and MCS scores indicated better physical and mental health respectively.
2.3. Statistical analysis
Analyses were conducted using the Statistical Analysis Software (SAS) callable version of SUDAAN 10.0 (RTI International, Research Triangle Park, NC). The prevalence of information needs across categories was first described for the whole population and across cancer types. We used multivariable modeling (linear and logistic regression) to test factors associated with (1) total number of health information needs (continuous), and (2) endorsement of each need category (dichotomous). Covariates associated with P < 0.20 in the bivariate analyses were included in final multivariable models of number of needs and categories of need.
For the models that examined whether number of health information needs was associated with mental and physical HRQOL, multivariable linear regression models were analyzed, with and without the presence of information attainment confidence (IAC) as an effect modifier. Both number of needs and IAC were centered on their grand mean, and an interaction term of the two centered variables was created. Potential confounders (age, sex, race/ethnicity, marital status, education, current health insurance status, adequate financial resources, cancer site, time since diagnosis, disease status, comorbidity burden, type of main follow-up care physician, receipt of a treatment summary, and self-rated quality of care) were modeled using backward elimination, with a 10–15% change in the coefficient for health information needs indicating confounding [24,25]. Final associations were deemed significant at P < 0.05. Spearman correlations of the independent variables were all low (< |0.25|).
3. Results
3.1. Sample description
Of the 1197 participants, there were 292 breast, 289 prostate, 305 colorectal, and 311 gynecologic cancer survivors. Table 1 describes the sample and presents bivariate associations between sample characteristics and mean number information needs. By design, approximately half (46%) of survivors were diagnosed at least 10 years prior to survey. Forty-two percent were Non-Hispanic White (NHW), 13% were Hispanic White (HW), 20.6% were African-American (AA), 22.3% were Asian-American/Pacific Islander (AAPI), and 1.6% were American Indian/Alaskan Native (AIAN). We suppressed output from AIAN survivors in our analytical models due to low numbers (N = 19).
Table 1.
Sample characteristics and bivariate associations with number of information needs.
| N | Sample % or mean (SD) | Mean number of information needs endorsed (SD) or bivariate correlation | P* | ||
|---|---|---|---|---|---|
| Age at survey | 80 and over | 213 | 17.8 | 3.9 (3.6) | <0.0001 |
| 65–79 | 550 | 45.9 | 5.0 (4.3) | ||
| 50–64 | 354 | 29.6 | 6.2 (4.0) | ||
| Under 50 | 80 | 6.7 | 7.2 (4.2) | ||
| Age continuous | 68.0 (11.4) | −0.32 | <0.0001 | ||
| Years since diagnosis | ≥10 | 551 | 46 | 4.6 (4.2) | 0.03 |
| <10 | 646 | 54 | 5.9 (4.1) | ||
| Time continuous | 8.8 (3.2) | −0.07 | 0.02 | ||
| Gender | Male | 454 | 37.9 | 5.1 (4.3) | 0.16 |
| Female | 743 | 62.1 | 5.5 (4.1) | ||
| Race/ethnicity | Non-Hispanic White | 508 | 42.4 | 4.1 (4) | <0.0001 |
| Hispanic White | 156 | 13 | 5.1 (4.2) | ||
| African-American | 247 | 20.6 | 6.6 (4.3) | ||
| Asian-American/Pacific Islander | 267 | 22.3 | 6.2 (3.8) | ||
| American Indian/Alaska Native | 19 | 1.6 | 8.8 (0.5) | ||
| Education | High school or less | 288 | 24.1 | 5.1 (4.4) | 0.02 |
| Some college | 441 | 36.8 | 5.7 (4.2) | ||
| College graduate or higher | 468 | 39.1 | 5.0 (3.9) | ||
| Adequate financial resources | Yes | 1065 | 89 | 5.1 (4.1) | <0.0001 |
| No | 132 | 11 | 6.8 (4.4) | ||
| Marital status | Married or living as married | 746 | 62.3 | 5.2 (4.2) | 0.39 |
| Never married | 80 | 6.7 | 5.5 (4.2) | ||
| Divorced, separated, or widowed | 371 | 31 | 5.6 (4.1) | ||
| Health insurance status | Private or public/private | 765 | 63.9 | 5.4 (4.1) | <0.0001 |
| Only public-sponsored | 275 | 23 | 4.7 (4.3) | ||
| None/unknown | 157 | 13.1 | 6.0 (4.3) | ||
| Type of cancer | Breast | 292 | 24.4 | 5.5 (4.1) | 0.05 |
| Prostate | 289 | 24.1 | 5.9 (4.4) | ||
| Colon or Rectum | 305 | 25.5 | 4.7 (4.2) | ||
| Gynecologic | 311 | 26 | 5.4 (4.0) | ||
| Disease status | Cancer free, no recurrence/second cancer | 907 | 75.8 | 4.9 (4.1) | 0.003 |
| Currently cancer free, but reported | 145 | 12.1 | 5.9 (4.2) | ||
| recurrence/second cancer | |||||
| Active or uncertain disease status | 145 | 12.1 | 7.4 (4.0) | ||
| Number of comorbidities | 0 | 224 | 18.7 | 5.4 (4.0) | 0.39 |
| 1 | 307 | 25.6 | 5.2 (4.3) | ||
| 2 | 284 | 23.7 | 5.1 (4.2) | ||
| 3+ | 382 | 31.9 | 5.6 (4.1) | ||
| Number of comorbid conditions | 1.7 (1.1) | −0.01 | 0.85 | ||
| Quality of Care | Excellent | 390 | 32.6 | 4.9 (4.1) | <0.0001 |
| Less than excellent care | 462 | 38.6 | 6.6 (4.2) | ||
| No care in the past two years/unknown | 345 | 28.8 | 4.2 (3.9) | ||
| Type of main follow-up doctor | None/unknown | 285 | 23.8 | 4.4 (4.1) | 0.047 |
| PCP | 148 | 12.4 | 5.5 (4.0) | ||
| Oncologist | 491 | 41 | 5.7 (4.2) | ||
| Other specialist | 273 | 22.8 | 5.5 (4.2) | ||
| Receipt of treatment summary | Received | 211 | 17.6 | 5.2 (4.3) | <0.0001 |
| Wanted but did not knowingly receive | 749 | 62.6 | 6.0 (4.1) | ||
| Did not want and did not knowingly receive | 237 | 19.8 | 3.4 (3.6) | ||
| Total Social Support Scale Score | Mean (SD) | 75.6 (21.5) | 0.0001 |
Unadjusted P-values given. P < 0.20 included in subsequent models.
3.2. Number of health information needs
The mean number of health information needs was 5.3 (95% CI: 5.0, 5.5). As shown in Table 2, after adjustment for all covariates in the model, number of needs varied by age at survey (P < 0.001), with younger survivors endorsing a significantly higher number of needs. Number of needs was also associated with cancer type (P < 0.05), with colorectal cancer survivors reporting lower number of needs than breast cancer survivors. Needs also varied significantly by race/ethnicity: NHWs had fewer information needs than all other racial/ethnic groups (P < 0.001). Of the followup care variables analyzed, number of needs differed only by reported receipt of a written treatment summary (P < 0.05); those who wanted but did not receive a treatment summary had a significantly higher number of information needs, and those who neither wanted nor received one had significantly lower number of needs.
Table 2.
Multivariable regression model of number of information needs.a
| Total number of information needs (model adjusted r2 = 0.23) B (SE) (95% CI) | ||
|---|---|---|
| Age at survey | 80 and over | Ref |
| 65–79 | 0.2 (−0.5, 0.9) | |
| 50–64 | 1.6 (0.7, 2.4)*** | |
| Under 50 | 2.4 (1.2, 3.5)*** | |
| Years since diagnosis | ≥10 | |
| <10 | 0.5 (0, 1.1) | |
| Gender | Male | |
| Female | −0.5 (−1.5, 0.6) | |
| Race/ethnicity | Non-Hispanic White | Ref |
| Hispanic White | 1 (0.1, 1.8)* | |
| African-American | 1.4 (0.6, 2.2)*** | |
| Asian-American/Pacific Islander | 1.2 (0.5, 1.9)** | |
| Education | High school or less | Ref |
| Some college | 0.1 (−0.6, 0.8) | |
| College graduate or higher | 0.1 (−0.7, 0.8) | |
| Adequate financial resources | Yes | Ref |
| No | 0.9 (0, 1.9) | |
| Health insurance status | Private or public/private | Ref |
| Only public-sponsored | −0.6 (−1.3, 0.1) | |
| None/unknown | −0.7 (−1.6, 0.2) | |
| Type of cancer | Breast | Ref |
| Prostate | 0.4 (−0.8, 1.7) | |
| Colon or Rectum | −1.1 (−1.9, 0.2)* | |
| Gynecologic | −0.3 (−1, 0.4) | |
| Disease status | Cancer free, no recurrence/second cancer | Ref |
| Currently cancer free, but reported recurrence/second cancer | 0.7 (−0.1, 1.6) | |
| Active or uncertain disease status | 0.8 (0, 1.6) | |
| Quality of care | Excellent | Ref |
| Less than excellent care | 0.5 (−0.2, 1.1) | |
| No care in the past two years/unknown | −0.2 (−1.1, 0.7) | |
| Type of main follow up doctor | None/unknown | −0.8 (−1.8, 0.2) |
| PCP | Ref | |
| Oncologist | −0.1 (−1, 0.8) | |
| Other specialist | −0.5 (−1.6, 0.5) | |
| Receipt of treatment summary | Received | Ref |
| Wanted but did not knowingly receive | 0.8 (0, 1.6)* | |
| Did not want and did not knowingly receive | −1 (−1.8, 0.1)* | |
| Social support | Scale Score | −0.01 (−0.03, 0.00) |
Dependent variable ranged from 0 to 12, total number of needs.
P < 0.05.
P < 0.01.
P < 0.001.
3.3. Type of health information needs
Of the six information needs categories, more than half of survivors expressed a desire for more information about side effects & symptoms (75.8%), tests & treatment (71.5%), health promotion (64.5%), and interpersonal/emotional needs (60.2%) (Table 3). Needs were similar by cancer site, with the following exceptions: colorectal cancer survivors were less likely to report information needs related to side effects & symptoms, health promotion, and interpersonal and emotional needs. Prostate cancer survivors were more likely to report needs related to sexual functioning & fertility.
Table 3.
Information need category endorsements by cancer site
| Total (n = 1197) % (95% CI) |
Breast (n = 292) % (95% CI) |
Prostate (n = 289) % (95% CI) |
Colorectal (n = 305) % (95% CI) |
Gynecologic (n = 311) % (95% CI) |
|
|---|---|---|---|---|---|
| Side effects and symptoms | 75.8 (72.1, 79.1) | 77.2 (69.5, 83.4) | 83.1 (76.8, 88.0) | 61.9 (53.6, 69.6)* | 76.1 (67.7, 82.8) |
| Tests and treatments | 71.5 (67.5, 75.2) | 71.7 (63.6, 78.5) | 78.0 (70.2, 84.1) | 60.8 (52.5, 68.5) | 71.3 (62.7, 78.6) |
| Health promotion | 64.5 (60.3, 68.4) | 66.4 (58.3, 73.6) | 72.4 (64.5, 79.1) | 50.9 (43.0, 58.9)* | 62.3 (53.1, 70.6) |
| Interpersonal and emotional | 60.2 (56.0, 64.2) | 63.6 (55.7, 70.9) | 64.7 (56.5, 72.1) | 46.0 (38.5, 53.8)* | 64.2 (55.6, 72.0) |
| Insurance | 39.0 (35.0, 43.1) | 40.1 (33.0, 47.6) | 44.0 (35.8, 52.5) | 30.3 (23.9, 37.5) | 38.0 (30.9, 45.6) |
| Sexual functioning and fertility | 34.5 (30.6, 38.7) | 22.1 (16.5, 29.0)* | 54.7 (46.1, 63.0)* | 22.5 (16.6, 29.8)* | 23.7 (18.3, 30.2)* |
Significantly different from total (P < 0.05).
Multiple logistic variable regression models examined specific factors associated with each type of health information need category (models not shown). Predictors of endorsement of specific information needs varied greatly by category. African Americans [OR = 2.0 (1.1, 3.7)] and Asian American/Pacific Islanders [OR = 1.7 (1.1, 2.9)] as compared to non Hispanic Whites, those who reported less than excellent [OR = 1.9 (1.2, 3.1)] as compared to excellent follow-up care in the past 2 years, and those who wanted but did not receive a treatment summary [OR = 2.5 (1.5, 4.4)] as compared to those who did were more likely to report side effects & symptoms related information needs. Survivors diagnosed less than 10 years ago [OR = 1.8 (1.2, 2.8)] and Asian American/Pacific Islanders [OR = 1.7 (1.0–2.8)], were more likely to report tests & treatment information needs. In addition, those with active or uncertain disease status [OR = 3.6 (1.8, 7.0)] as compared to those who were cancer free, and those who wanted but did not receive a treatment summary [OR = 1.8 (1.0, 3.2)] were also more likely to report tests & treatment. Survivors who were under 50 at diagnosis [OR = 2.5 (1.0, 6.4)] as compared to 80 and over, those who were non-White [Hispanic OR = 2.1 (1.2, 3.8), African American OR = 1.9 (1.1, 3.4), Asian American/Pacific Islander OR = 2.0 (1.2, 3.3)], reporting inadequate financial resources [OR = 2.8 (1.4, 5.6)], and with lower social support [OR = 0.99 (0.98, 1.00)] were more likely to report health promotion information needs. Younger survivors [under 50 OR = 5.6 (2.2, 14.6), 50–64 OR = 2.3 (1.2, 4.3)] and those less than 10 years since diagnosis [OR = 1.5, (1.0, 2.2)], were more likely to report interpersonal & emotional needs. In addition, non-White survivors [Hispanic OR = 3.0 (1.7, 5.3), African American OR = 2.2 (1.3, 3.8)] and those who wanted but did not receive a written treatment summary [OR = 1.6 (1.0, 2.7)] were more likely to report these needs. Younger survivors [under 50 OR = 6.8 (2.7, 16.8), 50–64 OR = 3.2 (1.7, 6.3)], African American [OR = 2.2 (1.3, 3.8)] and Asian American/Pacific Islanders [OR = 1.8 (1.1, 2.9)], those reporting inadequate financial resources [OR = 2.1 (1.1, 3.8)], and lower social support [OR = 0.99 (0.98, 1.00)] were more likely to report insurance related needs. Finally, younger survivors [under 50 OR = 10.1 (4.0, 25.6), 50–64 OR = 4.3 (2.0, 9.2), 65–79 OR = 2.1 (1.1, 4.4)] and those reporting inadequate financial resources [OR = 2.1 (1.1, 3.8)] were more likely to report sexual functioning & fertility information needs.
3.4. Number of information needs, HRQOL and information attainment confidence
Table 4 shows the adjusted regression parameter estimates of both the mental (MCS) and physical (PCS) component scales of the SF12, without (Model 1) and with (Model 2) an interaction term of confidence for obtaining information by number of needs. Both number of needs [B = −0.4 (−0.6, −0.2), P < 0.001] and IAC [B = 1.2 (0.4, 2.0), P < 0.01] were inversely associated with MCS. The interaction term was also significant [B = 0.2 (0.03, 0.5), P < 0.02], indicating that the relationship between number of health information needs and MCS differed by level of confidence. Fig. 1 shows the relationship between MCS and number of information needs by three categories of confidence: moderate (mean level), low, and high (±1 standard deviation). The negative slope steepens at lower levels of confidence, indicating that the inverse association between information needs and mental HRQOL is strongest among those with low confidence for obtaining information. Neither number of needs, confidence, nor the interaction term was associated with PCS.
Table 4.
SF-12 Parameter estimates of multivariable regression of health-related quality of life (mental and physical component scores) and information attainment confidence on number of health information needs (range 0–12).
| Outcome | Model 1 B (95% CI) |
Model 2 B (95% CI) |
|---|---|---|
| Mental Component Score (range: 0–100)a | ||
| Number of needs | −0.4 (−0.6, −0.2) | −0.4 −0.6, −0.2)*** |
| Information attainment confidence | 1.2 (0.4, 2.0) | 1.1 (0.4, 1.9)** |
| Interaction term | – | 0.2 (0.03, 0.5)* |
| r2 | 0.12 | 0.13 |
| Physical Component Score (range: 0–100)b | ||
| Number of needs | −0.03 (−0.27, 0.21) | −0.03 (−0.27, 0.21) |
| Information attainment confidence | 0.6 (−0.2, 1.4) | 0.6 (−0.2, 1.4) |
| Interaction term | – | −0.03 (−0.23, 0.17) |
| r2 | 0.33 | 0.33 |
Models adjusted for adequate financial resource.
Models adjusted for time since diagnosis, age at diagnosis, race/ethnicity, educational attainment, marital status, adequate financial resources, cancer site, cancer status, number of comorbidities, and social support.
P < 0.05.
P < 0.01.
P < 0.001.
Fig. 1.
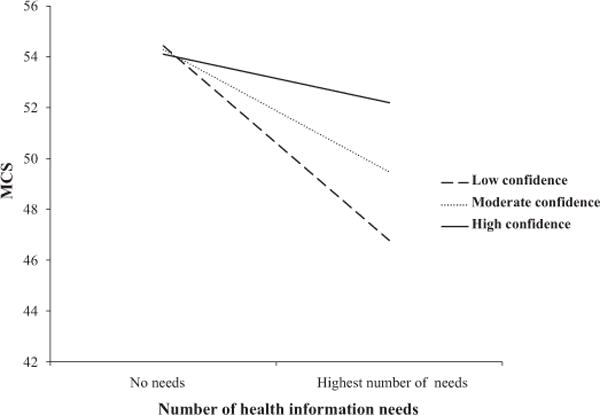
The association between mental health-related quality of life [the mental component scale (MCS) of the SF 12] and number of health information needs at low, moderate, and high levels of confidence to obtain information. Interaction significant at P < 0.02.
4. Discussion and conclusion
4.1. Discussion
Many long-term survivors reported a diverse array of health information needs. Younger survivors, non-Whites, and those who did not receive but wanted a written summary of their cancer treatment reported a higher number of needs. Conversely, colorectal cancer survivors and those who did not want a treatment summary reported a lower number of needs. Information needs by category were highly endorsed in the areas of tests & treatment, health promotion, side effects & symptoms, and interpersonal & emotional issues.
As found in a previous study of survivors surveyed 2–5 years after diagnosis [13], non-White survivors reported a higher number of needs and significantly more health promotion, interpersonal & emotional, side effects & symptoms, and insurance needs. In addition, survivors reporting inadequate access to financial resources endorsed more health promotion and insurance, and sexual functioning & fertility information needs. Previous research has indicated that survivors of lower socioeconomic backgrounds report may need assistance and training in how to gain access to information and may benefit the most from training [7]. One recent patient/physician survey study found that income largely explained racial differences in patient/provider communication. The authors found that for African Americans reduced access to information such as cancer expert referrals persisted despite control for other factors, a finding that suggests the need for careful attention to systemic factors that inhibit information provision for non-White cancer survivors [26].
The main follow-up care variable associated with information needs in terms of both number and type was receipt of a treatment summary. Although 17.6% of our study sample indicated that they had received a one, providing treatment summaries to survivors as they transition off treatment was not common practice at the time when the participants of this study were finishing treatment [27]. A written summary may serve two purposes: as an information reference and as a reassurance that should questions arise in the future, there are resources to turn to. Although previous research in cancer survivors has shown that satisfaction with medical care predicts lower levels of unmet information needs [6], in this study less than excellent self-rated quality of care was only associated with higher likelihood of side effects & symptom information needs.
Our findings that number of information needs was inversely associated with perceived mental but not physical health replicates past work [13]. Our novel finding that confidence for obtaining information acts as a moderator of this relationship suggests a few possibilities. The inverse relationship between health information needs and MCS was strongest among those with low confidence for obtaining information. Thus, opportunities for improving HRQOL in the context of information needs are greatest among those with low confidence. These findings suggest a need to assess and meet current health information needs among survivors, as well as a need to equip survivors with skills, resources, and confidence to obtain information in the future.
In terms of how cancer survivors obtain health information, one study showed that most survivors consider their healthcare providers to be reputable sources of information but often turn instead to the Internet to answer questions [28]. This may reflect a lack of continuity of care, limited access to healthcare providers over time, or limited capacities of some providers to provide this type of comprehensive cancer care [29]. Although the Internet provides survivors with a multitude of information and support opportunities and has been shown to increase self-efficacy to obtain information [10], survivors are often wary of the quality or confused by the content of the information they find [5,7,30].
Confidence to obtain information may stem from both good patient-provider relationships and health literacy. A cross-sectional study of German cancer survivors found that a good patient-provider relationship was the strongest predictor of reporting fulfilled information needs [31]. Given high general population prevalence of low health literacy [32], and findings that low health literacy is associated with lower HRQOL in cancer survivors [33], efforts to improve health literacy may aid in both meeting information needs and improving mental well-being particularly among the less health literate. The open communication that often underlies good doctor-patient relationships may provide the ancillary benefit of equipping survivors with the tools and confidence to obtain information related to maintaining good health over the long term. Our findings suggest two opportunities for providers to meet information needs: at completion of treatment via a treatment summary, and throughout follow-up care by engaging in dialogue with survivors about emerging needs.
A strength of the current study is that it represents one of the few population-based surveys of survivors more than 5 years from diagnosis. However, it is limited by its cross-sectional design. Furthermore, while we oversampled racial/ethnic minorities to represent the diverse population of survivors, there are some limitations on generalizability. The two registries from which survivors were drawn cover predominantly urban/suburban regions of California with both comprehensive cancer centers and several community-based clinics. Non-responders were more likely to be older, diagnosed over 10 years ago, and diagnosed with colorectal cancer. Our findings may also have limited generalizability to survivors living in rural or geographically isolated areas, non-English speakers, or those for whom low levels of literacy hindered participation. Although the cancer sites included in the current analysis represent the most prevalent cancers among survivors in the US [34], results may differ by other cancer types, particularly those with shorter survival or higher treatment burden.
Only survivors who completed the entire health information needs section were included in analysis in order to avoid confusing non-response with a lack of endorsement of a particular need. However, a sensitivity analysis conducted on participants with at least 75% of the health information needs assessment completed yielded similar results to the final analysis. With regard to endorsement of health information needs, an alternative interpretation is that responding “yes” to an individual health information item may indicate interest in, rather than need for, health information in that category. However, this interpretation is less convincing for the following reasons: a prompt before the actual question stating that little is known about information needs of long-term cancer survivors was given; previous research which used similar items found very similar patterns in health information needs [13]; and the significant associations between higher endorsed health information needs and lower HRQOL. The use of a single-item measure of confidence to obtain information from the Health Information National Trends Survey [18], while informative, limits the depth of understanding of this construct. Future studies should expand upon this construct to examine its meaning and impact health outcomes. Finally, these data were collected in 2005–2006, and since that time increasing attention to the need for survivorship care planning in the US has occurred, including the American College of Surgeons Commission on Cancer decision to require evidence of systematic survivorship care planning as part of hospital certification, to take effect in 2015. Thus it is possible that we overestimate current health information needs among long-term cancer survivors. However, given the limited basis on which these documents are provided to date [35], we see no indications that that health information needs or associations between needs and HRQOL would have meaningfully changed.
4.2. Conclusions
Our findings suggest that that despite being years from diagnosis, many survivors have lingering questions about cancer survivorship. The pattern of needs endorsed by survivors suggests persistent health disparities in access to important health information. Findings also suggest the need for treatment summaries and possibly survivorship care plans to equip survivors with comprehensive health information, resources and instructions for obtaining info in the future, as well as tailored approaches for diverse survivor populations. Future research linking information needs, confidence for obtaining information from a variety of sources, and information seeking-behavior in the context of different models of cancer follow-up care and in diverse populations is needed to help improve cancer communication.
4.3. Practice implications
Since the publication of the Institute of Medicine’s report From Cancer Patient to Cancer Survivor: Lost in Transition [36], there has been much discussion over the information that should be included in both a treatment summary and survivorship care plan, as well as who should be responsible for their development and delivery. Research on patient preferences regarding these documents indicates that survivors want a concise and easy-to-read plan, that contains diagnosis information, details of treatments received, clinical trial information, potential late effects of treatment, and a follow-up care plan [37]. Some of the barriers to providing survivorship care plans have included costs and fears about increasing patient anxiety (although recent evidence suggests the contrary [38,39]). Our finding that greater information needs were associated with poorer mental well-being also suggest that interventions such as care plans that address survivors’ information needs are likely to reduce, not increase, patient anxiety.
In the US, although several models are currently being tested [40] there has not yet been comparative effectiveness research to generate an evidence base for the efficacy of survivorship care plans [41] despite the Commission on Cancer’s upcoming requirement for implementation of survivorship care plans as part of their accreditation process [42]. As this research progresses, observing the experiences of implementing survivorship care planning in other countries like Canada [43] and the United Kingdom [44] as well as paying careful attention to the choice of evaluation outcome measures [45] will be critical for the US. If future intervention research confirms our findings, then educating survivors by providing them a SCP from diagnosis may serve to improve QOL, particularly for survivors who lack the confidence to seek out information themselves.
The current study supports the need for research to evaluate ways to provide survivors with comprehensive health information that (1) tailors to individual survivors with respect to information readiness, self-efficacy, and cultural sensitivity and (2) addresses numerous psychosocial needs that survivors express interest in, for example, information on nutrition and physical activity after cancer, managing recurrence anxiety, and understanding familial cancer risks. The majority of cancer patients in the US are 65 and older, survive 5 or more years after diagnosis and face potential increased physical and psychological sequelae [15]. Understanding the information needs of these survivors will better prepare the research and practice community to provide the tools survivors require to maintain good long-term health.
Supplementary Material
Acknowledgments
This study was supported under NCI SEER contract numbers: N01-PC-35136, N01-PC-35139 (http://cancercontrol.cancer.gov/ocs/focus.html). The authors would like to acknowledge Ms. Gretchen Keel (Information Management Services, Inc., Rockville, MD) for her assistance with data analysis on this project. This study has been presented in part at a poster at the 2011 American Association of Cancer Research Science of Cancer Health Disparities Meeting, on September 19, 2011, in Washington, DC.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.pec.2012.08.014.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Cancer Institute or the National Institutes of Health. We confirm all patient/personal identifiers have been removed or disguised so the patient/person(s) described are not identifiable and cannot be identified through the details of the story.
This manuscript was written in the course of employment by the United States Government and it is not subject to copyright in the United States.
References
- 1.Rowland JH, Hewitt M, Ganz PA. Cancer survivorship: a new challenge in delivering quality cancer care. J Clin Oncol. 2006;24:5101–4. doi: 10.1200/JCO.2006.09.2700. [DOI] [PubMed] [Google Scholar]
- 2.Corner J. Addressing the needs of cancer survivors: issues and challenges. Expert Rev Pharmacoecon Outcomes Res. 2008;8:443–51. doi: 10.1586/14737167.8.5.443. [DOI] [PubMed] [Google Scholar]
- 3.ASCO-ESMO. Consensus statement on quality cancer care. J Clin Oncol. 2006;24:3498–9. doi: 10.1200/JCO.2006.07.4021. [DOI] [PubMed] [Google Scholar]
- 4.Epstein R, Street R. Patient-centred communication in cancer care promoting healing and reducing suffering. Bethesda, MD: National Cancer Institute; 2007. [Google Scholar]
- 5.Rutten LJ, Arora NK, Bakos AD, Aziz N, Rowland J. Information needs and sources of information among cancer patients: a systematic review of research (1980–2003) Patient Educ Couns. 2005;57:250–61. doi: 10.1016/j.pec.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 6.McDowell ME, Occhipinti S, Ferguson M, Dunn JSK. Chambers predictors of change in unmet supportive care needs in cancer. Psychooncology. 2010;19:508–16. doi: 10.1002/pon.1604. [DOI] [PubMed] [Google Scholar]
- 7.McInnes DK, Cleary PD, Stein KD, Ding L, Mehta CC, Ayanian JZ. Perceptions of cancer-related information among cancer survivors: a report from the American Cancer Society’s Studies of Cancer Survivors. Cancer. 2008;113:1471–9. doi: 10.1002/cncr.23713. [DOI] [PubMed] [Google Scholar]
- 8.Walsh MC, Trentham-Dietz A, Schroepfer TA, Reding DJ, Campbell B, Foote ML, et al. Cancer information sources used by patients to inform and influence treatment decisions. J Health Commun. 2010;15:445–63. doi: 10.1080/10810731003753109. [DOI] [PubMed] [Google Scholar]
- 9.Lee SD, Gazmararian JA, Arozulla AM. Health literacy and social support among elderly medicare enrollees in a managed care plan. Journal of Applied Geronotology. 2006;25:324–37. [Google Scholar]
- 10.Bass SB, Ruzek SB, Gordon TF, Fleisher L, McKeown-Conn N, Moore D. Relationship of Internet health information use with patient behavior and self-efficacy: experiences of newly diagnosed cancer patients who contact the National Cancer Institute’s Cancer Information Service. J Health Commun. 2006;11:219–36. doi: 10.1080/10810730500526794. [DOI] [PubMed] [Google Scholar]
- 11.Dey A. Consumer health informatics: an overview of patient perspectives on health information needs. HIMJ. 2004;33:121–6. doi: 10.1177/183335830403300404. [DOI] [PubMed] [Google Scholar]
- 12.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 13.Beckjord EB, Arora NK, McLaughlin W, Oakley-Girvan I, Hamilton AS, Hesse BW. Health-related information needs in a large and diverse sample of adult cancer survivors: implications for cancer care. J Cancer Surviv. 2008;2:179–89. doi: 10.1007/s11764-008-0055-0. [DOI] [PubMed] [Google Scholar]
- 14.Husson O, Mols F, van de Poll-Franse LV. The relation between information provision and health-related quality of life, anxiety and depression among cancer survivors: a systematic review. Ann Oncol. 2010;22:761–72. doi: 10.1093/annonc/mdq413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH. Cancer survivors: a booming population. Cancer Epidemiol Biomarkers Prev. 2011;20:1996–2005. doi: 10.1158/1055-9965.EPI-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganz PA. Survivorship: adult cancer survivors. Prim Care. 2009;36:721–41. doi: 10.1016/j.pop.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Mallinger JB, Griggs JJ, Shields CG. Patient-centered care and breast cancer survivors’ satisfaction with information. Patient Educ Couns. 2005;57:342–9. doi: 10.1016/j.pec.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Nelson DE, Kreps GL, Hesse BW, Croyle RT, Willis G, Arora NK, et al. The health information national trends survey (HINTS): development, design, and dissemination. J Health Commun. 2004;9:443–60. doi: 10.1080/10810730490504233. [discussion 81–4] [DOI] [PubMed] [Google Scholar]
- 19.Gage E. Examining the most relevant conceptualization of the socioeconomic status construct for cancer research. Cancer Nurs. 2010;33:E1–9. doi: 10.1097/NCC.0b013e3181c29583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potosky AL, Harlan LC, Stanford JL, Gilliland FD, Hamilton AS, Albertsen PC, et al. Prostate cancer practice patterns and quality of life: the prostate cancer outcomes study. J Natl Cancer Inst. 1999;91:1719–24. doi: 10.1093/jnci/91.20.1719. [DOI] [PubMed] [Google Scholar]
- 21.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–14. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 22.Ware J, Jr, Kosinski M, Keller SDA. 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Ware JE, Jr, Kosinski M, Turner-Bowker DM, Gandek B, Lincoln RI. How to score version 2 of the sf-12® health survey (with a supplement documenting version 1) Health Assessment Lab; 2002. [Google Scholar]
- 24.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–37. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 25.Thompson WD. Statistical analysis of case-control studies. Epidemiol Rev. 1994;16:33–50. doi: 10.1093/oxfordjournals.epirev.a036143. [DOI] [PubMed] [Google Scholar]
- 26.Manfredi C, Kaiser K, Matthews AK, Johnson TP. Are racial differences in patient-physician cancer communication and information explained by background, predisposing, and enabling factors? J Health Commun. 2010;15:272–92. doi: 10.1080/10810731003686598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Institute of Medicine. From cancer patient to cancer survivor: lost in transition. Washington DC: National Academies Press; 2006. [Google Scholar]
- 28.Hesse BW, Arora NK, Burke Beckjord E, Finney Rutten LJ. Information support for cancer survivors. Cancer. 2008;112:2529–40. doi: 10.1002/cncr.23445. [DOI] [PubMed] [Google Scholar]
- 29.Adler NE, Page AEK, editors. Institute of Medicine. Cancer care for the whole patient: meeting psychosocial health needs. Washington, DC: The National Academies Press; 2008. [PubMed] [Google Scholar]
- 30.LaCoursiere SP, Knobf MT, McCorkle R. Cancer patients’ self-reported attitudes about the Internet. J Med Internet Res. 2005;7:e22. doi: 10.2196/jmir.7.3.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neumann M, Wirtz M, Ernstmann N, Ommen O, Langler A, Edelhauser F, et al. Identifying and predicting subgroups of information needs among cancer patients: an initial study using latent class analysis. Support Care Cancer. 2010;19:1197–209. doi: 10.1007/s00520-010-0939-1. [DOI] [PubMed] [Google Scholar]
- 32.Paasche-Orlow MK, Parker RM, Gazmararian JA, Nielsen-Bohlman LT, Rudd RR. The prevalence of limited health literacy. J Gen Intern Med. 2005;20:175–84. doi: 10.1111/j.1525-1497.2005.40245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song L, Mishel M, Bensen JT, Chen RC, Knafl GJ, Blackard B, et al. How does health literacy affect quality of life among men with newly diagnosed clinically localized prostate cancer?: findings from the North Carolina-Louisiana Prostate Cancer Project (PCaP) Cancer. 2011;118:3842–51. doi: 10.1002/cncr.26713. [DOI] [PubMed] [Google Scholar]
- 34.Mariotto AB, Rowland JH, Ries LA, Scoppa S, Feuer EJ. Multiple cancer prevalence: a growing challenge in long-term survivorship. Cancer Epidemiol Biomarkers Prev. 2007;16:566–71. doi: 10.1158/1055-9965.EPI-06-0782. [DOI] [PubMed] [Google Scholar]
- 35.Stricker CT, Jacobs LA, Risendal B, Jones A, Panzer S, Ganz PA, et al. Survivorship care planning after the institute of medicine recommendations: how are we faring? J Cancer Surviv. 2011;5:358–70. doi: 10.1007/s11764-011-0196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Institute of Medicine. From cancer patient to cancer survivor: lost in transition. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 37.Marbach TJ, Griffie J. Patient preferences concerning treatment plans, survivorship care plans, education, and support services. Oncol Nurs Forum. 2011;38:335–42. doi: 10.1188/11.ONF.335-342. [DOI] [PubMed] [Google Scholar]
- 38.Gravis G, Protiere C, Eisinger F, Boher JM, Tarpin C, Coso D, et al. Full access to medical records does not modify anxiety in cancer patients: results of a randomized study. Cancer. 2011;117:4796–804. doi: 10.1002/cncr.26083. [DOI] [PubMed] [Google Scholar]
- 39.Wiljer D, Leonard KJ, Urowitz S, Apatu E, Massey C, Quartey NK, et al. The anxious wait: assessing the impact of patient accessible EHRs for breast cancer patients. BMC Med Inform Decis Mak. 2010;10:46. doi: 10.1186/1472-6947-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCabe MS, Jacobs L. Survivorship care: models and programs. Semin Oncol Nurs. 2008;24:202–7. doi: 10.1016/j.soncn.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 41.Earle CC. Long term care planning for cancer survivors: a health services research agenda. J Cancer Surviv. 2007;1:64–74. doi: 10.1007/s11764-006-0003-9. [DOI] [PubMed] [Google Scholar]
- 42.Commission on Cancer. Cancer program standards 2012 ensuring patient-centered care. Vol. 1. Chicago, IL: American College of Surgeons; 2012. [Google Scholar]
- 43.Grunfeld E, Julian JA, Pond G, Maunsell E, Coyle D, Folkes A, et al. Evaluating survivorship care plans: results of a randomized, clinical trial of patients with breast cancer. J Clin Oncol. 2011;29:4755–62. doi: 10.1200/JCO.2011.36.8373. [DOI] [PubMed] [Google Scholar]
- 44.Richards M, Corner J, Maher J. The National Cancer Survivorship Initiative: new and emerging evidence on the ongoing needs of cancer survivors. Br J Cancer. 2011;105(Suppl. 1):S1–4. doi: 10.1038/bjc.2011.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith TJ, Snyder C. Is it time for (survivorship care) plan B? J Clin Oncol. 2011;29:4740–2. doi: 10.1200/JCO.2011.38.8397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


