Abstract
The effect of postural orientation on the motor corticospinal excitability (MCE) of proximal and distal upper extremity (UE) muscles was investigated. In a crossover design, recruitment curves (RCs), short interval cortical inhibition (SICI) and intracortical facilitation (ICF) of resting anterior deltoid (AD) and first dorsal interosseus (FDI) was assessed in two postures: sitting and standing. Six healthy adults without contraindications to transcranial magnetic stimulation (TMS) participated in the study. TMS was applied over the motor cortical representation of FDI and AD at intensities ranging from 90% to 200% of resting motor threshold (RMT) in increments of 10%. SICI and ICF were assessed for each muscle using a conditioning stimulus (80% RMT) preceding a test stimulus (120% RMT) with an interstimulus interval of 2 ms and 15 ms, respectively. For AD, but not FDI, there was a significant and consistent increase in RC slope during standing compared to sitting. For FDI, there was no difference in ICF and SICI between sitting and standing. However, for AD, while there was no difference in ICF between the two postures, there was a clear trend for SICI to decrease (p = 0.06) in standing compared to sitting. These results indicate that postural change from sitting to standing, affects the MCE of proximal but not distal muscles. While this indicates the role of proximal UE muscles in postural control, it also implies that rehabilitation protocols for enhancing proximal arm motor function may be advantaged if administered in a standing posture.
Keywords: Motor corticospinal excitability, Posture, Arm muscles
Graphical Abstract
1. Introduction
Changes in posture are known to influence motor function of lower extremity, upper extremity (UE), and speech [3–5,22]. Change in posture from sitting to standing increases the complexity of arm reaching dynamics due to additional challenges for postural control in standing [1]. Coordination between center of mass movement and arm movement during a pointing task is different in seated compared to standing position [17]. Such differences indicate that UE control strategies are dependent on postural orientation. Despite these reported posture-related changes in UE function, little is known about how postural changes impact the corticomotor system projecting to the UE muscles.
Transcranial magnetic stimulation (TMS) non-invasively and trans-synaptically activates pyramidal tract neurons to evoke electromyographic motor evoked potentials (MEPs) that provide quantitative information about excitability of the corticospinal system (MCE) [7]. While previous studies have investigated the effects of postural changes on MCE of the lower extremity muscles [14,24], evidence regarding the effect of change in posture from sitting to standing on MCE of UE muscles could not be identified. Obata et al. observed an increase in the plateau value and maximum slope of the RC of soleus and tibialis anterior muscle in standing compared to sitting [14]. However, it is not known if MCE of the UE is modulated by changes in postural orientation (sitting compared to standing).
Proximal and distal muscles of the UE have distinct roles in arm and hand function. Proximal muscles provide the ability to reach to a target while distal muscles are engaged in object manipulation. Larger proximal muscles are more likely to have a greater influence in the control of center of mass kinematics while distal muscles likely have little influence on COM control [13,16]. Moreover, more proximal limb and axial muscles may be differentially controlled by ventromedial motor pathways than distal limb muscles involving dorsolateral pathways [11]. As a result, postural orientation may influence MCE differentially in proximal compared to distal muscles. The purpose of this study was to compare the effect of posture (sitting vs. standing) on MCE of proximal and distal UE muscles. We hypothesized that change in posture from sitting to standing will enhance the MCE of the proximal arm muscle but not distal arm muscle.
2. Materials and methods
Six healthy right-handed adults (mean age 51.8 ± 8.1 years) without any history of neurological or orthopedic impairment participated in this study approved by the institutional review board. None of the subjects showed any contraindication to TMS [9,19,25]. Informed consent was obtained from all participants.
We employed a within-subject design in which participants were tested for changes in MCE, inhibition and facilitation for first dorsal interosseus (FDI) and anterior deltoid (AD) during sitting compared to standing. We obtained RCs, intracortical inhibition (ICI) and intracortical facilitation (ICF) for FDI and AD in sitting and standing to understand the posture-related changes in MCE, inhibition and facilitation.
Each participant was tested on four different sessions separated by at least 1 day. All participants were tested first for AD and then for FDI. For each muscle, recruitment curves (RCs) and intracortical facilitation–inhibition (ICI and ICF) measures were obtained on separate sessions. At each session, data were collected in two postures: sitting and standing. Surface EMG was recorded from the right FDI or AD muscle with adhesive electrodes placed in a tendon-belly arrangement over the bulk of the muscle. The EMG signal was filtered with a band pass of 30–1000 Hz, amplified, and digitized at 1000 Hz. The data were graphically displayed and stored for offline analysis.
Single TMS pulses were applied over the left motor cortex with a 70 mm figure of eight coil attached to Magstim Rapid Stimulator (Magstim, Whitland, UK). The coil was held tangentially to the scalp with the handle pointing posteriorly away from the midline at an angle of 45°. Current induced from this position is directed approximately perpendicular to the central sulcus [2,12]. A “hot-spot” for AD and FDI was determined as the site at which the largest MEP was obtained from the respective muscle at lowest TMS intensity. Then intensity was systematically reduced to determine the resting motor threshold (RMT) which is the minimum TMS intensity required to invoke an MEP amplitude of at least 50 μV in 5 out of 10 consecutive trials [20]. RMT was assessed in each posture.
2.1. Recruitment curve (RC) determination
For each muscle, we determined the TMS recruitment curve in sitting and standing after finding RMT in both positions. Participants were randomized into either a sit- or stand-first group. If randomized to the stand-first group, data were obtained in standing first and seated position second and vice versa. RC was collected with 10 MEPs collected at each intensity from 90% to 200% RMT (or 100% of stimulator output whichever is reached first) with 10% increments. In each posture, the test arm was relaxed at the participants’ side and rested in neutral position, confirmed by no activity in EMG. In each posture, the non-test forearm was supported with shoulder in neutral, elbow 90° flexion and wrist and hand relaxed. This supported arm should minimize sway and asymmetrical stance of the subject during testing. Rest breaks were provided as necessary throughout the testing. Total testing time for this session is approximately 2 h.
2.2. Intracortical inhibition/facilitation determination
In the subsequent session paired-pulse TMS was used to assess the inhibition and facilitation to the AD and FDI. For ICI and ICF, participants were tested in seated position first and then standing position. In sitting, a test stimulus (TS) with an intensity of 120% of RMT was preceded by a conditioning stimulus (CS) 80% of RMT with an interstimulus interval (ISI) of 2 ms for inhibition (10 MEPs) and 15 ms for facilitation (10 MEPs) [10]. In the standing position, TMS intensity necessary to achieve the same average MEP amplitude elicited with the TS in sitting was obtained. This was identified as the standing-TS for paired-pulse testing in standing. Then, we adjusted the CS based on an algorithm that assured it was 20% below RMT (given that the TS value was 20% above RMT). Ten MEPs were obtained each with TS alone; CS preceding the TS with ISI of 2 ms (ICI) and 15 ms (ICF). The effect of the preceding CS on the TS was investigated for each muscle for both ISIs.
2.3. MEP analysis
Peak to peak MEP amplitude was measured for each potential. Averages were calculated for each of the different intensity levels for RC analysis. RCs were plotted for each muscle in every participant.
For the paired-pulse data, average conditioned peak-to-peak MEP amplitude was measured and expressed as a percentage of the average unconditioned (or test) MEP (MEP ratio). MEP ratio of 100 would indicate no effect of the CS. MEP ratio less than 100 indicates inhibition while MEP ratio of more than 100 indicates facilitation.
2.4. Statistical analysis
We used mixed effects regression models (also known as “hierarchical models”) to assess the association between position and RCs, accounting for the repeated measures on the same subjects. Initially, we fit a model that made no assumptions about the relationship between mean MEP amplitude and TMS intensity and included a random intercept. Subsequently, because the RCs appeared reasonably linear, we fit a model that included a random intercept and random slopes at each position. The models were fit using SAS Proc Mixed using the REML approach.
For the paired-pulse TMS experiments, a paired t-test was used to compare the MEP ratio in sitting to that in standing at each ISI (i.e. for ICI and ICF).
3. Results
Table 1 summarizes demographic data, testing order and RMT for both muscles for all the participants. Statistical analyses indicated that RMT did not significantly differ across postures for the two muscles. Fig. 1 shows an ensemble average of 10 MEPs of AD and FDI muscle of participant 5 in sitting and standing. Fig. 2 illustrates RCs for each of the 6 participants across the two postures for AD and FDI, respectively. For AD (top row), there was a consistent effect of posture on the RC slope. In all participants, the RC slope was steeper during standing compared to sitting posture. Based on a model that made no functional assumptions about the relationship between mean MEP amplitude and TMS intensity, there was a significant effect of position (Fig. 3, p < 0.0001). Based on a model which postulated an effect of position on average slope, we found a significantly steeper average slope in the standing position (p = 0.030). In contrast, for FDI, based on the unstructured model, there was no significant effect of position (Fig. 3, p = 0.96).
Table 1.
Participant data for age, handedness, order of posture during testing of anterior deltoid (AD) and first dorsal interosseus (FDI), and resting motor thresholds (RMT, % of maximum stimulator output, MSO) of AD and FDI in sitting (SIT) and standing (STA).
| SUB | Age | Handedness | Order (AD) | RMT (AD) (%MSO) |
Order (FDI) | RMT (FDI) (%MSO) |
||
|---|---|---|---|---|---|---|---|---|
| SIT | STA | SIT | STA | |||||
| 1 | 52 | RT | Sit–sta | 60 | 60 | Sit–sta | 48 | 48 |
| 2 | 59 | RT | Sit–sta | 36 | 36 | Sta–sit | 51 | 50 |
| 3 | 39 | RT | Sta–sit | 36 | 34 | Sit–sta | 39 | 41 |
| 4 | 63 | RT | Sta–sit | 53 | 53 | Sit–sta | 63 | 63 |
| 5 | 56 | RT | Sit–sta | 55 | 50 | Sta–sit | 38 | 33 |
| 6 | 49 | RT | Sta–sit | 34 | 34 | Sta–sit | 46 | 33 |
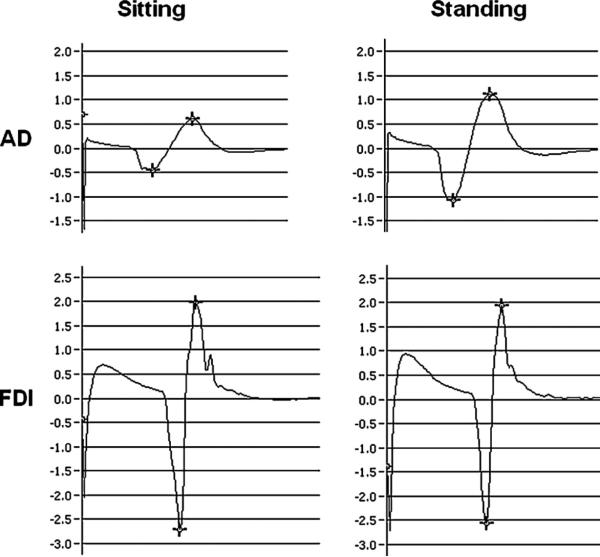
Fig. 1.
Ensemble average MEPs from anterior deltoid (AD, top row) and first dorsal interosseus (FDI, bottom row) muscle of representative participant (S5) evoked in sitting and standing evoked at TMS intensity of 150% RMT.
Fig. 2.
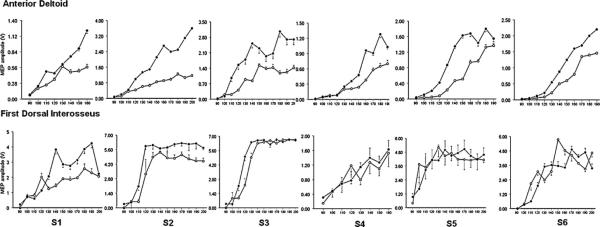
Individual participant recruitment curves for AD (upper row) and FDI (lower row) in sitting (open circles) compared to standing (bold circles).
Fig. 3.
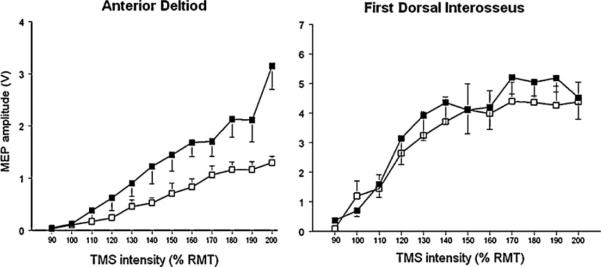
Group recruitment curve for AD and FDI in sitting and standing. While there was no significant effect of posture on the recruitment curve slope in the FDI muscle (p = 0.96), for the AD muscle, the recruitment curve was significantly steeper in standing than sitting (p < 0.001).
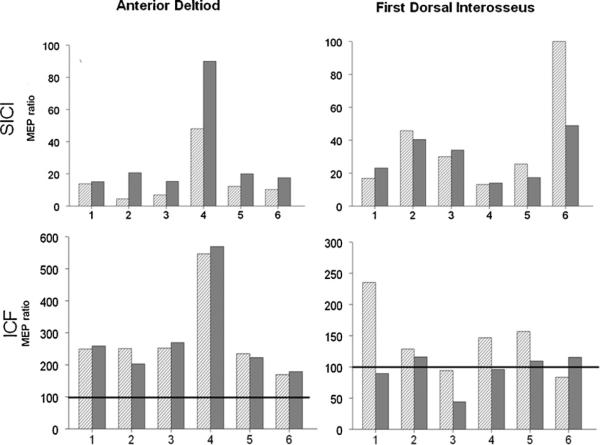
Fig. 4 illustrates the MEP ratios for AD and FDI, respectively. For AD, there was reduced intracortical inhibition during standing (higher MEP ratios) compared to sitting. Although the effect was not statistically significant, there is a clear trend with a uniform effect on all participants (p = 0.06; medium effect size: Cohen's d = 0.6). There was no significant effect of posture on ICF in AD and on ICI and ICF in FDI.
Fig. 4.
SICI and ICF: MEP ratio data for participants 1–6 in sitting (striped bar) and standing (solid gray bars). MEP ratio of 100 (dark horizontal line in the lower row) would indicate no effect of the conditioning stimulus. MEP ratio less than 100 indicates inhibition while MEP ratio of more than 100 indicates facilitation. For the AD muscle, the MEP ratio is consistently higher (indicating less inhibition) in standing compared to sitting (p = 0.06; effect size = 0.6).
4. Discussion
The present report investigates the effect of posture on MCE, intracortical inhibition and facilitation in a proximal and distal upper extremity muscle. We observed that the effect of posture on MCE was different for the proximal and distal UE muscles. While MCE of the proximal UE muscle (AD) was significantly enhanced in standing compared to sitting, there was no effect of posture on the MCE of the distal UE muscle (FDI). The increased MCE of AD in standing is likely to be mediated by a decrease in the ICI during standing compared to sitting. Thus, the present results demonstrate that a posture-related modulation in the MCE for the proximal arm muscle but not the distal one.
Change in MCE with posture may be mediated by multiple factors such as a generalized increased in alertness in standing or may reflect the possible role of proximal arm muscles in postural control during standing. Enhancement of MCE in AD but not FDI during standing suggests that it was not a generalized effect. Instead, our data indicate dissociation between the proximal and distal UE muscle response to postural change.
Proximal muscles are relatively bulky, have attachments on the trunk, and control movement of the whole UE segment; therefore making them biomechanically more susceptible to influence COM kinematics. In contrast, smaller distal hand muscles have little influence on the COM. Proximal arm muscles are known to influence postural control. Arm movements after perturbations like tripping over an obstacle have been suggested to serve a protective function and contribute to balance recovery [15,18]. Conversely, changes in postural control are also known to influence proximal UE control. Training focused on improving postural control improved arm control in patients who had ataxia secondary to a brain stem stroke [23]. This suggests an influence of posture on arm control. In the current study, we provide further physiological evidence for this effect of posture on proximal arm control. One potential limitation of the present study is that postural sway in standing may also affect the role of the proximal UE in postural control. Although this was not directly assessed in the current study, the postural sway was minimized by having the participants support their non-test forearm in standing.
The present findings also bring to light the role of primary motor cortex as a neural substrate that may mediate synergistic relationship between posture and proximal UE movements. Increased challenges to the postural-control system in standing may enhance the MCE of the proximal UE muscles such as the AD possibly via mechanisms such as subliminal fringe. Subliminal fringe refers to a zone of subthreshold excitability of neurons that often project to synergistic muscles and receive excitatory post-synaptic potentials from the depolarized neuronal group [6]. These subliminal fringe neurons have a higher threshold for activation either because they are less excitable, have a lower synaptic innervation density, or because they are spatially further from the center of TMS activation (hotspot). Progressive growth of MEP size with increase in TMS intensities (suprathreshold) is mediated by activation of neurons besides those in the core region activated at threshold [7]. Support for subliminal fringe mechanisms for this effect is evident in the RCs. A close inspection of the RCs for AD reveals that for all participants, differences between sitting and standing are more evident at higher stimulation intensities. This suggests that in standing posture the neurons of the AD subliminal system are likely more excitable compared to sitting posture. This may also reflect the modulatory influences of descending vestibular and reticular brainstem pathways associated with postural control. Postural orientation may affect ventromedial motor pathways involving proximal limb and axial muscles differently than distal hand muscles engaged in grasp and fine motor skills. Another likely mechanism responsible for increased excitability may relate to reduced GABA-A related activity in the motor cortex. There was a trend toward decrease in GABA-A-mediated ICI in standing compared to sitting posture for AD; possibly indicating a lower GABA-A interneuronal activity in the motor cortex of AD in standing. While we assessed ICI with one ISI (2 ms), assessment of ICI with multiple ISIs would have possibly yielded more robust results.
MEP amplitude is influenced by cortical excitability as well as the excitability of spinal motor neurons in the spinal cord. Here we did not directly measure the excitability of the spinal motor neurons; therefore we cannot ascertain which level in the central nervous system mediated the enhancement of MEP amplitudes in standing. There is evidence that, when the target muscle contraction is low, the contribution of spinal mechanisms to MEP amplitude modulation is negligible [8,21]. In the present study, the test muscles were at rest and electrically silent. Therefore, it is likely that the modulation of RCs in standing was likely mediated by changes at the cortical level. Further, a trend for reduction in GABA-A mediated ICI for proximal arm muscle in standing indicates that at least part of posture-related excitability changes are mediated at the cortical level. However, future studies are needed to definitively identify the source of modulation of MEPs.
5. Conclusion
To our knowledge, this is the first study to investigate posture-related changes in MCE in UE muscles. We found that MCE of proximal but not distal UE muscles is enhanced in standing compared to sitting. From a theoretical perspective, this implies a differential role of proximal UE muscles in postural control compared to distal UE muscles. Further, they suggest that primary motor cortex may be engaged in mediating the synergistic relationship between body posture and proximal UE movements. From a practical standpoint, these findings have implications for UE rehabilitation in patients with neurological disorders such as in hemiparesis after stroke. It is conceivable that rehabilitation protocols for enhancing proximal arm movement function may be more effective if administered in a standing posture. While future studies are needed to investigate the effects of posture on proximal UE training protocols, the current findings provide a neurophysiological basis for the effects of posture on MCE of UE muscles.
HIGHLIGHTS.
▶ Effect of whole body posture on motor cortical excitability (MCE) of upper extremity is not known.
▶ MCE of proximal and distal upper extremity muscles was tested in sitting and standing.
▶ There was an increased MCE in standing compared to sitting for proximal muscle (anterior deltoid).
▶ For distal muscle (first dorsal interosseus), there was no significant effect of posture on MCE.
References
- 1.Berrigan F, Simoneau M, Martin O, Teasdale N. Coordination between posture and movement: interaction between postural and accuracy constraints. Experimental Brain Research. 2006;170:255–264. doi: 10.1007/s00221-005-0210-z. [DOI] [PubMed] [Google Scholar]
- 2.Brasil-Neto JP, McShane LM, Fuhr P, Hallett M, Cohen LG. Topographic mapping of the human motor cortex with magnetic stimulation: factors affecting accuracy and reproducibility. Electroencephalography and Clinical Neuro-physiology. 1992;85:9–16. doi: 10.1016/0168-5597(92)90095-s. [DOI] [PubMed] [Google Scholar]
- 3.Chan AH, Ng AW. Lateral foot-movement times in sitting and standing postures. Perceptual and Motor Skills. 2008;106:215–224. doi: 10.2466/pms.106.1.215-224. [DOI] [PubMed] [Google Scholar]
- 4.Cuisinier R, Olivier I, Nougier V. Effects of foreperiod duration on anticipatory postural adjustments: determination of an optimal preparation in standing and sitting for a raising arm movement. Brain Research Bulletin. 2005;66:163–170. doi: 10.1016/j.brainresbull.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Cuisinier R, Olivier I, Nougier V. The increased foreperiod duration to attain the neutral optimal preparation from sitting to standing. Experimental Brain Research. 2007;180:321–331. doi: 10.1007/s00221-007-0862-y. [DOI] [PubMed] [Google Scholar]
- 6.Denny-Brown DE, Sherrington CS. Subliminal fringe in spinal flexion. Journal of Physiology. 1928;66:175–180. doi: 10.1113/jphysiol.1928.sp002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devanne H, Lavoie BA, Capaday C. Input–output properties and gain changes in the human corticospinal pathway. Experimental Brain Research. 1997;114:329–338. doi: 10.1007/pl00005641. [DOI] [PubMed] [Google Scholar]
- 8.Fujiwara K, Tomita H, Kunita K. Increase in corticospinal excitability of limb and trunk muscles according to maintenance of neck flexion. Neuroscience Letters. 2009;461:235–239. doi: 10.1016/j.neulet.2009.06.047. [DOI] [PubMed] [Google Scholar]
- 9.Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, Cramer SC. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nature Neuroscience. 2006;9:735–737. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- 10.Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. Journal of Physiology. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemon RN. Descending pathways in motor control. Annual Review of Neuro-science. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- 12.Mills KR, Boniface SJ, Schubert M. Magnetic brain stimulation with a double coil: the importance of coil orientation. Electroencephalography and Clinical Neurophysiology. 1992;85:17–21. doi: 10.1016/0168-5597(92)90096-t. [DOI] [PubMed] [Google Scholar]
- 13.Milosevic M, McConville KM, Masani K. Arm movement improves performance in clinical balance and mobility tests. Gait and Posture. 2011;33:507–509. doi: 10.1016/j.gaitpost.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Obata H, Sekiguchi H, Nakazawa K, Ohtsuki T. Enhanced excitability of the corticospinal pathway of the ankle extensor and flexor muscles during standing in humans. Experimental Brain Research. 2009;197:207–213. doi: 10.1007/s00221-009-1874-6. [DOI] [PubMed] [Google Scholar]
- 15.Pijnappels M, Kingma I, Wezenberg D, Reurink G, van Dieen JH. Armed against falls: the contribution of arm movements to balance recovery after tripping. Experimental Brain Research. 2010;201:689–699. doi: 10.1007/s00221-009-2088-7. [DOI] [PubMed] [Google Scholar]
- 16.Pozzo T, Ouamer M, Gentil C. Simulating mechanical consequences of voluntary movement upon whole-body equilibrium: the arm-raising paradigm revisited. Biological Cybernetics. 2001;85:39–49. doi: 10.1007/PL00007995. [DOI] [PubMed] [Google Scholar]
- 17.Pozzo T, Stapley PJ, Papaxanthis C. Coordination between equilibrium and hand trajectories during whole body pointing movements. Experimental Brain Research. 2002;144:343–350. doi: 10.1007/s00221-002-1052-6. [DOI] [PubMed] [Google Scholar]
- 18.Roos PE, McGuigan MP, Kerwin DG, Trewartha G. The role of arm movement in early trip recovery in younger and older adults. Gait and Posture. 2008;27:352–356. doi: 10.1016/j.gaitpost.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalography and Clinical Neurophysiology. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 21.Stedman A, Davey NJ, Ellaway PH. Facilitation of human first dorsal interosseous muscle responses to transcranial magnetic stimulation during voluntary contraction of the contralateral homonymous muscle. Muscle and Nerve. 1998;21:1033–1039. doi: 10.1002/(sici)1097-4598(199808)21:8<1033::aid-mus7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 22.Stone M, Stock G, Bunin K, Kumar K, Epstein M, Kambhamettu C, Li M, Parthasarathy V, Prince J. Comparison of speech production in upright and supine position. Journal of the Acoustical Society of America. 2007;122:532–541. doi: 10.1121/1.2715659. [DOI] [PubMed] [Google Scholar]
- 23.Stoykov ME, Stojakovich M, Stevens JA. Beneficial effects of postural intervention on prehensile action for an individual with ataxia resulting from brainstem stroke. NeuroRehabilitation. 2005;20:85–89. [PubMed] [Google Scholar]
- 24.Tokuno CD, Taube W, Cresswell AG. An enhanced level of motor cortical excitability during the control of human standing. Acta Physiologica (Oxford) 2009;195:385–395. doi: 10.1111/j.1748-1716.2008.01898.x. [DOI] [PubMed] [Google Scholar]
- 25.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalography and Clinical Neurophysiology. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]