Abstract
Angiogenesis, the formation of new blood vessels from preexisting vessels, is a highly complex process. It is regulated in a finely-tuned manner by numerous molecules including not only soluble growth factors such as vascular endothelial growth factor and several other growth factors, but also a diverse set of insoluble molecules, particularly collagenous and non-collagenous matrix constituents. In this review we have focused on the role and potential mechanisms of a multifunctional small leucine-rich proteoglycan decorin in angiogenesis. Depending on the cellular and molecular microenvironment where angiogenesis occurs, decorin can exhibit either a proangiogenic or an antiangiogenic activity. Nevertheless, in tumorigenesis-associated angiogenesis and in various inflammatory processes, particularly foreign body reactions and scarring, decorin exhibits an antiangiogenic activity, thus providing a potential basis for the development of decorin-based therapies in these pathological situations.
Keywords: Angiogenesis, extracellular matrix, proteoglycan, decorin, mechanism, therapy
Introduction
Angiogenesis, the formation of new blood vessels from preexisting vessels through sprouting or intussusception, is a fundamental process in mammalian reproduction, development, and wound repair [1–3]. Angiogenesis also plays a critical role in a variety of pathological situations including malignant, inflammatory, and ischemic disorders [4]. Furthermore, there is an association between angiogenesis, scarring, and fibrosis [5].
For some time, we have understood that in addition to soluble molecules, particularly growth factors such as vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β), and several other growth factors, insoluble extracellular matrix (ECM) macromolecules are of great importance in the angiogenic process [6–8]. Indeed, today we know that the structure of the ECM in itself has a great impact on angiogenesis via directly or indirectly regulating endothelial cell (EC) behavior [8–11]. Angiogenesis requires the generation of “activated migratory” ECs (tip cells) which guide the developing vascular sprout [12–15]. Remodeling of the ECM by ECs as angiogenesis proceeds enables initiation, formation, and finally, the stabilization of new blood vessels.
ECM and Angiogenesis
A number of individual ECM macromolecules participate in angiogenesis, either promoting or restricting events involved in this process [6,8,9]. Different collagens such as types I, III, IV, and VI collagen [16–19], a variety of glycoproteins, particularly fibronectin [20,21], vitronectin [22], laminins [23] and matricellular proteins such as thrombospondin [24] and SPARC (Secreted Protein Acidic and Rich in Cysteine) [25] have been shown to contribute to angiogenesis. Furthermore, specific proteoglycans (PGs) and glycosaminoglycans (GAGs) including the heparan sulfate PGs perlecan [26] and syndecans [27,28], the dermatan sulfate PGs decorin [29,30] and biglycan [14,31], the chondroitin sulfate PG versican [14,32,33], the keratan sulfate PGs fibromodulin [34] and lumican [35], and, finally, hyaluronan (HA) [7,36,37] are involved in angiogenesis as well. In addition, several proteolytically cleaved fragments of the matrix macromolecules, called matrikines and matricryptins, are active in modulating angiogenesis [8,14,33,38–41]. One of the most well-known examples of these cleavage products is the carboxyl terminal fragment of type XVIII collagen, called endostatin, which is a potent angiogenesis inhibitor [42]. Other similar cleavage products with antiangiogenic activity are canstatin and tumstatin, both derived from type IV collagen [43,44], endorepellin, the C-terminus of the heparan sulfate PG perlecan [45], and hyaluronan fragments [7,46]. Matrix macromolecules and/or their cleavage products can participate in angiogenesis at all different stages beginning with vascular sprouting and eventually ending in vessel stabilization [47].
Almost 30 years ago, we made the observation that ECs in confluent monolayer culture synthesized primarily biglycan, but not the highly homologous SLRP (small leucine-rich proteoglycan) family member decorin [48,49]. However, ECs switched to synthesis of decorin when they were stimulated to sprout and form tubes in vitro [29]. Subsequently, it was demonstrated that when ECs were co-cultured with fibroblasts in a collagen gel, they formed cord-like structures which was accompanied by a 100-fold increase in the synthesis of decorin [30].
In this review, we have focused on highlighting the multifunctionality of decorin in angiogenesis, as has become apparent over the last several years. We describe its role in regulating ECM stiffness and rigidity, in modulating angiogenic growth factor activation/deactivation, in binding to several cell surface receptors involved in angiogenesis and exciting new studies that highlight its role in autophagy as possible mechanism(s) by which this PG contributes to angiogenesis.
Decorin
Decorin, in earlier literature also called PG-II, PG-40 and PG-S2 [50–52], is the prototype molecule of the SLRP gene family that encompasses 18 members [53,54]. The name decorin originates from its ability to decorate collagen type I fibrils. Decorin has been shown to bind to the d and e bands of type I collagen via its core protein, “decoron,” thereby controlling fibril formation [55–57] and regulating mechanical properties of these fibrils [58]. The effects of decorin on fibrillogenesis are also true in vivo [59]. In addition, decorin has been suggested to play a regulatory role in several other biological and physiological processes such as myogenesis [60] and fetal membrane development [61] as well as tissue repair [62]. Notably, the importance of decorin in various pathological conditions e.g. cancer, is also established [63,64]. Decorin is mainly expressed by various mesenchymal cells, such as fibroblasts, chondrocytes, and smooth muscle cells [49,65], but in specific situations also by ECs as will be described below.
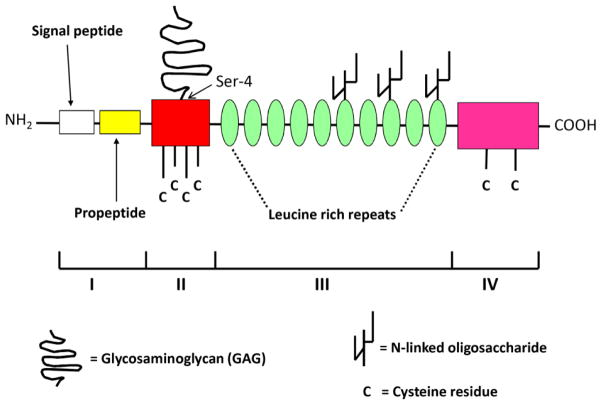
Decorin is usually composed of a core glycoprotein with the relative molecular weight of about 40 kDa and one either chondroitin or dermatan sulfate GAG side chain which is attached to the serine residue 4 [66,67] (Fig. 1). In the core protein of decorin, four distinct domains can be identified [68]. The first domain consists of a 14-amino acid signal peptide and a 16-amino acid propeptide, both of which are cleaved before decorin is secreted. The second domain that is rich in cysteine is the GAG side chain-carrying domain. The third domain is the leucine-rich repeat region consisting of 10 repeats of 24 amino acids rich in leucine. This domain results in the three-dimensional structure of decorin resembling an arch [69], a typical architecture of all proteins with leucine-rich repeat motifs [70]. The fourth domain of the decorin core protein is the carboxyl terminal domain which contains two cysteine residues and a conserved disulfide loop. These structural features of decorin enable it to bind and interact with numerous other ECM macromolecules as well as with different growth factors and cytokines [63,68]. Furthermore, when in a soluble form, decorin can interact with certain cell surface receptors and thereby it can have a direct influence on intracellular signaling [54,71]. Both the core protein and the GAG chain are variously responsible for the different effects of decorin on cellular functions [72–74]. For example, the core protein of decorin can act as an inhibitor of tumor growth in different xenograft models such as breast and prostate cancers via downregulating the members of the ErbB receptor tyrosine kinase family [75,76]. The GAG chain, on the other hand, is able to influence migration of cells such as smooth muscle cells and melanoma cells via mechanisms including intracellular acidification [77,78]. In addition, the length of the decorin GAG chain affects matrix assembly by determining the distance between separate collagen fibrils [79], affecting angiogenesis [80]. Thus, as with most PGs, the bioactivity of decorin as a molecule must be considered as a sum of its parts [74].
Fig. 1.
Schematic drawing of the molecular structure of decorin. All four domains I–IV of decorin core protein are indicated (for details see the text). The GAG side chain attached to serine residue 4 of the second domain is also shown.
Decorin in Angiogenesis
Immunostaining for decorin is present in microvessels in human atherosclerotic plaques [81], in ECs in human granulomatous tissue [30], and in newly formed microvessels within the thickened intima of human arterial wall in giant cell arteritis [82], whereas decorin immunostaining is absent from the endothelium of resting capillaries [83]. Furthermore, decorin positive microvessels have been detected at the base of pseudoaneurysms in the temporal artery of human patients [84]. Decorin has also been found to be expressed in significant amounts around neovessels after varicose vein surgery in patients [85]. Alternatively, although decorin null mice do not exhibit any abnormalities in their vasculature [59], decorin deficiency causes impaired angiogenesis in the injured cornea of these animals [86]. Similarly, reduced decorin expression in oral squamous cell carcinomas and in human microvascular ECs leads to decreased angiogenesis [87,88].
While the studies described above suggest that decorin has a stimulatory role in the angiogenic response, there are several studies supporting the opposite view. Decorin inhibits endothelial tube formation in vitro, which is potentiated with the addition of thrombospondin [89]. Decorin suppresses angiogenesis in tumors [90] and is differentially expressed in human benign versus malignant vascular tumors [91]. Specifically, in Kaposi’s sarcoma and angiosarcoma, both of which represent malignant vascular neoplasms, decorin expression is completely lacking, whereas in benign vascular tumors, namely in hemangiomas, where capillary growth has ceased, decorin is expressed in readily detectable amounts. In addition, there is an increase in vascular invasion in polyvinyl alcohol sponges implanted in decorin-deficient mice compared to vascular invasion in sponges implanted in wild-type control mice [92]. Studies have also demonstrated that even fragments of decorin can exhibit antiangiogenic activity, partially through the ability of these fragments to depress VEGF-induced focal adhesion kinase phosphorylation and assembly of focal adhesions [93]. In addition, overexpression of decorin retards corneal neovascularization via downregulation of proangiogenic molecules including VEGF [94]. Thus, growing evidence since the 1990’s indicates a critical role for decorin in the angiogenic response, particularly angiogenesis associated with inflammatory processes and tumor growth. However, whether decorin’s activity will be pro- or antiangiogenic appears to depend on the physiological or pathological condition of the tissue.
Potential Mechanism(s) for Decorin in Angiogenesis
There are a number of ways by which decorin can influence angiogenesis in either positive or negative ways. It may interact with various ECM macromolecules promoting assembly of a complex ECM and preventing turnover, enabling the formation of an ECM conducive for angiogenesis [59,95–99]. For example, decorin is known to control collagen fibril formation of, e.g., type I collagen [57] and type I collagen fibrils, in turn, provide a template for vascular tube formation when in contact with the apical side of the endothelium, thus promoting angiogenesis. The interaction of decorin with collagen fibrils also makes decorin resistant to proteolytic attack, resulting in a more stabilized fibrillar network [100]. Binding of decorin to the matrix proteins not only leads to the stabilization of the fibrillar network, but concomitantly causes alterations in the biomechanical properties of the ECM, particularly in the tensile strength and rigidity of the matrix [58,97,101]. Stiffness and rigidity are two central properties of the ECM that are known to influence angiogenesis [12,102].
While decorin can promote the formation and maintenance of the highly-ordered structures of fibrillar proteins, it may also have a role in either preserving or destroying these fiber systems. Indeed, the core protein of decorin is capable of stimulating the expression of matrix metalloproteinase-1 (MMP-1) [103,104], a collagenase that is highly active during angiogenesis. This protease promotes the expression of vascular endothelial growth factor receptor-2 (VEGFR2) through stimulation of protease activated receptor-1 (PAR-1) and activation of nuclear factor-κB (NF-κB) [105]. Decorin also stimulates the synthesis of another collagenase, namely MMP-2 [106], that degrades type IV collagen, the major structural component of basement membranes. Similarly to MMP-1, MMP-2 has been reported to enhance vascular proliferation. On the other hand, decorin is able to stimulate synthesis of tissue inhibitors of matrix metalloproteinases (TIMPs), particularly TIMP-2 [106] and TIMP-3 [107], which both decrease angiogenesis [108–110].
Decorin can also influence cell-ECM interactions by affecting the integrin adhesion receptors. For example, decorin modulates the activity of α2β1 integrin [99]. More specifically, decorin can allosterically modulate α2β1 integrin’s collagen binding activity by interacting with the α2 subunit of this integrin via its GAG moiety and/or core protein [111]. Furthermore, decorin can influence the expression of integrins. DCN−/− fibroblasts treated with decorin have reduced expression of α2β1 integrin [112]. In addition, in human airway smooth muscle cells, decorin increases the synthesis of integrin αv subunit [113] that together with β3 subunit is abundantly expressed on angiogenic ECs, but not on normal, quiescent ECs [114]. However, although αvβ3 integrin has been suggested to play a key role in angiogenesis [115], there is currently no published data to demonstrate that decorin influences the expression or function of this integrin in ECs. Nevertheless, study of decorin’s role in the regulation of integrin activity and function provides an intriguing field of angiogenic research.
Decorin may also impact angiogenesis by binding directly to other cell surface receptors or signaling molecules involved in angiogenesis. Currently decorin is known to be a ligand for several tyrosine kinases including the epidermal growth factor receptor (EGFR) [116,117], Met, which is the receptor for the hepatocyte growth factor (HGF) [118], insulin-like growth factor receptor-I (IGF-IR) [119–121] and VEGFR2 [122]. Furthermore, decorin has been suggested to bind to platelet derived growth factor receptor (PDGFR), but further studies are still required to confirm this [123]. Engagement of decorin with cell surface receptors can either activate or inhibit the function of the receptor [99,120], depending on the physiological state of the tissue. In disease, decorin is more likely to have an antagonizing effect on the aforementioned receptors [107,121,124].
Decorin also influences the expression and bioavailability of several angiogenic growth factors and cytokines. For example, decorin binds to VEGF and may impact the availability and activity of this angiogenic factor. Evidence is available that decorin-expressing sarcoma cells produce reduced amounts of VEGF, leading to suppressed tumor-cell mediated angiogenesis [90]. Similarly, virus-mediated decorin gene delivery decreases angiogenesis in the cornea of rabbits via downregulating VEGF expression, in addition to downregulating the expression of two other proangiogenic molecules, namely monocyte chemoattractant protein-1 (MCP-1) and angiopoietin [94]. However, others have found decorin to have the opposite effect on VEGF expression. In dysplastic and malignant oral epithelial cells aberrantly expressing nuclear localized decorin, knockdown of decorin expression attenuates angiogenesis via simultaneously silencing angiogenic mediators including VEGF [125]. Consistent with this finding, in fetal growth restriction, where decorin expression is decreased, VEGF-A expression as well as angiogenesis are decreased [88]. Additionally, mouse cerebral ECs treated with decorin stimulate VEGF expression via activation of specific transcription factors resulting in increased angiogenesis [126]. It still remains to be verified whether these observed effects of decorin on angiogenesis are truly VEGF-dependent. Thus, more in depth studies are needed to decipher the molecular mechanism(s) involved in decorin’s role in either stimulating or inhibiting angiogenesis through VEGF pathways.
Another growth factor vital not only in fibrosis [127] but also in angiogenesis is TGF-β [128–133]. Decorin can bind TGF-β and neutralize its activity [134–137]. Hence, the bioavailability of TGF-β is markedly under the control of decorin. Indeed, degradation of decorin by different proteases (e.g., MMP-2, -3 and -7 and granzyme B) releases sequestered TGF-β and restores its bioavailability [138,139]. Furthermore, overexpression of decorin inhibits TGF-β expression [140,141]. However, it still has to be clarified whether there is a causal relationship between decorin and TGF-β in the regulation of angiogenesis.
In addition to VEGF and TGF-β, decorin interacts with several other angiogenic growth factors, including platelet-derived growth factor (PDGF) [123,142,143], fibroblast growth factor (FGF) [93,144], insulin-like growth factor (IGF) [120,121,145], connective tissue growth factor (CTGF) [146–148], and HGF [118,149]. Furthermore, decorin influences the availability of the proangiogenic factor angiopoietin, as well [94]. A summary of studies addressing the involvement of decorin in regulating the activity and availability of angiogenic growth factors is presented as Table I and diagrammatically as Fig. 2.
Table I.
Angiogenic growth factors regulated by decorin.
| Molecule | Abbreviation | Relationship with decorin | Reference |
|---|---|---|---|
| Angiopoietin | ANG | Inhibition by decorin | [90] |
| Connective tissue growth factor | CTGF | Induces decorin synthesis/decorin regulates CTGF activity in fibrotic conditions | [142–144] |
| Fibroblast growth factor | FGF | Decorin promotes activity after injury/inhibition by decorin | [140/89] |
| Hepatocyte growth factor | HGF | Inhibition by decorin | [114,145] |
| Insulin-like growth factor | IGF-I | In normal cells, DCN activates IGF-I/in transformed cells, decorin inhibits IGF-I activation | [116,117,141] |
| Platelet derived growth factor | PDGF | Inhibition by decorin | [119,138,139] |
| Transforming growth factor beta | TGF-β | Inhibition by decorin | [131,133,136] |
| Vascular endothelial growth factor | VEGF | Inhibition by decorin | [86,89,90] |
Fig. 2.
Interaction of decorin with various ECM macromolecules and growth factors in the modulation of angiogenesis. (1.) Decorin is able to bind to other ECM macromolecules, especially types I and VI collagen, and fibronectin, and to modulate rigidity and stiffness of the ECM. (2.) By sequestering growth factors to the ECM, decorin can inhibit their angiogenic activity. (3.) Decorin can regulate the expression of specific MMPs and TIMPSs thereby influencing the structure and mechanochemical properties of the ECM. MMPs degrade the ECM structure and provide room for vascular sprouting while TIMPs inhibit the activity of MMPs. (4.) MMPs can free decorin-bound growth factors thus restoring their angiogenic activity. (5.) Decorin is also able to bind to growth factor receptors thus blocking their interaction with their natural ligands and their subsequent activation.
Apart from different angiogenic growth factors, decorin has also been shown to markedly contribute to the regulation of angiogenic cytokine expression [150]. Cytokines form a group of small proteins (5–20 kDa) including chemokines, interferons, and interleukins that are vitally important for the immune system and the inflammatory process, and as such, they also play a crucial role in a variety of pathologies and associated phenomena, such as angiogenesis [151,152]. The finding that decorin is capable of downregulating the expression of chemokines, particularly MCP-1 [94], suggests that decorin potentially attenuates inflammation-associated angiogenesis [153]. In line with this, decorin could also decrease inflammation-associated angiogenesis by potentiating the activity of interferons, particularly interferon-γ, a well-known antiangiogenic molecule [154,155]. However, being an endogenous ligand of toll-like receptors 2 and 4 in macrophages, decorin stimulates the expression of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), and simultaneously reduces the expression of anti-inflammatory interleukin-10 (IL-10) [150]. This suggests that decorin plays a dual role in inflammation and consequently also a double role in inflammation-associated angiogenesis [82,92].
A completely new mechanism whereby decorin may be linked with the regulation of angiogenesis is its role in autophagy [156]. Autophagy is the major intracellular catabolic mechanism whereby unnecessary or dysfunctional cytosolic components, proteins, and organelles are degraded by lysosomes leading to cellular renovation and homeostasis [157]. Interestingly, angiogenesis inhibitors are known to activate autophagy in ECs [158]. Regarding decorin, its soluble form has been shown to cause autophagy in both microvascular and macrovascular ECs leading to decreased angiogenesis [159]. Mechanistically, this effect of decorin on EC autophagy has been shown to be mediated via direct interaction with VEGFR2 which causes activation of adenosine monophosphate (AMP) kinase signaling and inactivation of mTOR (mammalian target of rapamycin) [156,160]. AMP kinase phosphorylation leads to modulation of paternally-expressed gene 3 (Peg3), a key player in autophagy that then goes on to control the expression of beclin 1 and microtubule-associated protein 1A/1B-light chain 3 (LC3) [159–161].
Decorin may also modulate angiogenesis via influencing apoptosis of ECs. Originally, decorin has been suggested to have an antiapoptotic effect on ECs during angiogenesis [30]. However, it was later shown that the peptides derived from the decorin leucine-rich repeat cause induction of EC apoptosis concomitantly with the inhibition of EC tube formation [93]. The apoptosis-promoting activity of decorin has also been described for other cells, particularly for malignant cells such as breast cancer, cholangiocarcinoma, and hepatocellular carcinoma cells [162–164]. Thus, the action of decorin on EC apoptosis may be context-dependent [165].
Therapeutic Potential of Decorin as an Angiogenic Modulator
As we have discussed above, decorin can impact angiogenesis in multiple ways. Although decorin has variously been shown to either promote or inhibit angiogenesis, its effect on tumorigenesis-associated angiogenesis has been shown to be an inhibitory one [90,91,166]. Because tumor growth and metastasis are crucially dependent on angiogenesis [167], the development of new decorin-based adjuvant therapies in malignancies is rational despite the fact that antiangiogenic drugs and therapies have not yet produced widespread or enduring clinical benefits [168]. In addition to inhibiting angiogenesis in tumors, decorin has been shown to inhibit angiogenesis associated with foreign body reactions [92]. This provides a mechanistic basis for why decorin would be a very promising biological agent to prevent scarring [5,169]. The multifunctional nature of decorin also enables it to be a potential therapeutic agent for a variety of other pathologies, even for those which are not angiogenesis-dependent. These pathologies include glomerulonephritis [140] and peritoneal fibrosis [170], both of which are highly dependent on TGF-β. On the other hand, therapeutic use of decorin as an angiogenesis-promoting molecule has also been indicated. For example, after partial hepatectomy in fibrotic mice, decorin has been found to accelerate liver generation [171].
Conclusion
Angiogenesis is the result of a dynamic interplay between numerous molecules in the ECM and cellular milieu. In this review, we have focused on the role and potential mechanisms of the multifunctional SLRP decorin in angiogenesis. We have aimed to convince the reader that decorin is not only associated with angiogenesis, but more importantly, it plays a causal role in this process. Furthermore, depending on the molecular microenvironment where angiogenesis is induced, decorin can either promote or inhibit angiogenesis. This regulation occurs via mechanisms involving decorin’s ability to interact with and modulate the actions of other ECM macromolecules, a variety of growth factors and cytokines as well as certain cell surface receptors. Thus, it is clear that decorin impacts the life and death of endothelial cells and may have therapeutic potential to regulate the angiogenic response.
Highlights.
Different ECM macromolecules contribute to the process of angiogenesis
Decorin is a multifunctional small leucine-rich proteoglycan
Decorin can exhibit either a proangiogenic or an antiangiogenic activity
In pathological conditions, decorin’s role in angiogenesis is mainly inhibitory
Decorin-based therapies show great potential in the future
Acknowledgments
This study has been supported by the Medical Research Fund of Turku University Hospital, Cancer Foundation of South-Western Finland, Turku University Foundation, Fund of Turku University, Finnish Foundation for Cardiovascular Research (HJ), and National Institutes of Health grants R01 HL064387 and EB 012558 (TNW). We thank Dr. Virginia M. Green for her careful reading and editing of the manuscript.
Abbreviations used
- VEGF
vascular endothelial growth factor
- TGF-β
transforming growth factor-β
- ECM
extracellular matrix
- EC
endothelial cell
- SPARC
secreted protein acidic and rich in cysteine
- PG
proteoglycan
- GAG
glycosaminoglycan
- SLRP
small leucine-rich proteoglycan
- MMP-1
matrix metalloproteinase-1
- VEGFR2
vascular endothelial growth factor receptor-2
- PAR-1
protease activated receptor-1
- NF-κB
nuclear factor-κB
- TIMPs
tissue inhibitors of matrix metalloproteinases
- EGFR
epidermal growth factor receptor
- HGF
hepatocyte growth factor
- IGF-IR
insulin-like growth factor receptor-I
- PDGFR
platelet derived growth factor receptor
- MCP-1
monocyte chemoattractant protein-1
- PDGF
platelet-derived growth factor
- FGF
fibroblast growth factor
- IGF
insulin-like growth factor
- CTGF
connective tissue growth factor
- TNF-α
tumor necrosis factor-α
- IL
interleukin
- AMP
adenosine monophosphate
- mTOR
mammalian target of rapamycin
- Peg3
paternally-expressed gene 3
- LC3
light chain 3
Footnotes
This article is dedicated to the late Elke Schönherr, MD, PhD, who, together with Dr. Järveläinen, did her postdoctoral work in 1988–1991 in the laboratory of Thomas N. Wight at the University of Washington in Seattle. Elke passed away far too early in her career (August 7, 2005), but her work has stimulated all of us to think about proteoglycans, such as decorin, as effectors of cell behavior and to recognize that single components of the ECM can regulate specific events in homeostasis and in disease. Her contributions have laid the foundation for much of the outstanding work on this molecule that has followed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–4. [PubMed] [Google Scholar]
- 2.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–4. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–95. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–60. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 5.van der Veer WM, Niessen FB, Ferreira JA, Zwiers PJ, de Jong EH, Middelkoop E, et al. Time course of the angiogenic response during normotrophic and hypertrophic scar formation in humans. Wound Repair Regen. 2011;19:292–301. doi: 10.1111/j.1524-475X.2011.00692.x. [DOI] [PubMed] [Google Scholar]
- 6.Ingber DE, Folkman J. How does extracellular matrix control capillary morphogenesis? Cell. 1989;58:803–5. doi: 10.1016/0092-8674(89)90928-8. [DOI] [PubMed] [Google Scholar]
- 7.Slevin M, Krupinski J, Gaffney J, Matou S, West D, Delisser H, et al. Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol. 2007;26:58–68. doi: 10.1016/j.matbio.2006.08.261. [DOI] [PubMed] [Google Scholar]
- 8.Neve A, Cantatore FP, Maruotti N, Corrado A, Ribatti D. Extracellular Matrix Modulates Angiogenesis in physiological and pathological conditions. Biomed Res Int. 2014;2014:756078. doi: 10.1155/2014/756078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belotti D, Foglieni C, Resovi A, Giavazzi R, Taraboletti G. Targeting angiogenesis with compounds from the extracellular matrix. Int J Biochem Cell Biol. 2011;43:1674–85. doi: 10.1016/j.biocel.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park KP, Du P, Subbiah R, Park JH. Vascular morphogenesis of human umbilical vein endothelial cells on cell-derived macromolecular matrix microenvironment. Tissue Eng Part A. 2014;20:2365–77. doi: 10.1089/ten.tea.2013.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vernon RB, Sage EH. Between molecules and morphology. Extracellular matrix and creation of vascular form. Am J Pathol. 1995;147:873–83. [PMC free article] [PubMed] [Google Scholar]
- 13.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–77. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obika M, Vernon RB, Gooden MD, Braun KR, Chan CK, Wight TN. ADAMTS-4 and biglycan are expressed at high levels and co-localize to podosomes during endothelial cell tubulogenesis in vitro. J Histochem Cytochem. 2014;62:34–49. doi: 10.1369/0022155413507727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seano G, Chiaverina G, Gagliardi PA, di Blasio L, Puliafito A, Bouvard C, et al. Endothelial podosome rosettes regulate vascular branching in tumour angiogenesis. Nat Cell Biol. 2014;16:931–41. doi: 10.1038/ncb3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sweeney SM, DiLullo G, Slater SJ, Martinez J, Iozzo RV, Lauer-Fields JL, et al. Angiogenesis in collagen I requires alpha2beta1 ligation of a GFP*GER sequence and possibly p38 MAPK activation and focal adhesion disassembly. J Biol Chem. 2003;278:30516–24. doi: 10.1074/jbc.M304237200. [DOI] [PubMed] [Google Scholar]
- 17.Twardowski T, Fertala A, Orgel JP, San Antonio JD. Type I collagen and collagen mimetics as angiogenesis promoting superpolymers. Curr Pharm Des. 2007;13:3608–21. doi: 10.2174/138161207782794176. [DOI] [PubMed] [Google Scholar]
- 18.Bahramsoltani M, Harms T, Drewes B, Plendl J. Searching for markers to identify angiogenic endothelial cells: a proteomic approach. Clin Hemorheol Microcirc. 2013;55:255–69. doi: 10.3233/CH-2012-1631. [DOI] [PubMed] [Google Scholar]
- 19.Mammoto T, Jiang A, Jiang E, Panigrahy D, Kieran MW, Mammoto A. Role of collagen matrix in tumor angiogenesis and glioblastoma multiforme progression. Am J Pathol. 2013;183:1293–305. doi: 10.1016/j.ajpath.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicosia RF, Bonanno E, Smith M. Fibronectin promotes the elongation of microvessels during angiogenesis in vitro. J Cell Physiol. 1993;154:654–61. doi: 10.1002/jcp.1041540325. [DOI] [PubMed] [Google Scholar]
- 21.Yi M, Ruoslahti E. A fibronectin fragment inhibits tumor growth, angiogenesis, and metastasis. Proc Natl Acad Sci U S A. 2001;98:620–4. doi: 10.1073/pnas.98.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li R, Luo M, Ren M, Chen N, Xia J, Deng X, et al. Vitronectin regulation of vascular endothelial growth factor-mediated angiogenesis. J Vasc Res. 2014;51:110–7. doi: 10.1159/000360085. [DOI] [PubMed] [Google Scholar]
- 23.Simon-Assmann P, Orend G, Mammadova-Bach E, Spenle C, Lefebvre O. Role of laminins in physiological and pathological angiogenesis. Int J Dev Biol. 2011;55:455–65. doi: 10.1387/ijdb.103223ps. [DOI] [PubMed] [Google Scholar]
- 24.Lawler PR, Lawler J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb Perspect Med. 2012;2:a006627. doi: 10.1101/cshperspect.a006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jendraschak E, Sage EH. Regulation of angiogenesis by SPARC and angiostatin: implications for tumor cell biology. Semin Cancer Biol. 1996;7:139–46. doi: 10.1006/scbi.1996.0019. [DOI] [PubMed] [Google Scholar]
- 26.Aviezer D, Hecht D, Safran M, Eisinger M, David G, Yayon A. Perlecan, basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell. 1994;79:1005–13. doi: 10.1016/0092-8674(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 27.Echtermeyer F, Streit M, Wilcox-Adelman S, Saoncella S, Denhez F, Detmar M, et al. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J Clin Invest. 2001;107:R9–R14. doi: 10.1172/JCI10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Rossi G, Whiteford JR. Syndecans in angiogenesis and endothelial cell biology. Biochem Soc Trans. 2014;42:1643–6. doi: 10.1042/BST20140232. [DOI] [PubMed] [Google Scholar]
- 29.Järveläinen HT, Iruela-Arispe ML, Kinsella MG, Sandell LJ, Sage EH, Wight TN. Expression of decorin by sprouting bovine aortic endothelial cells exhibiting angiogenesis in vitro. Exp Cell Res. 1992;203:395–401. doi: 10.1016/0014-4827(92)90013-x. [DOI] [PubMed] [Google Scholar]
- 30.Schönherr E, O’Connell BC, Schittny J, Robenek H, Fastermann D, Fisher LW, et al. Paracrine or virus-mediated induction of decorin expression by endothelial cells contributes to tube formation and prevention of apoptosis in collagen lattices. Eur J Cell Biol. 1999;78:44–55. doi: 10.1016/S0171-9335(99)80006-5. [DOI] [PubMed] [Google Scholar]
- 31.Berendsen AD, Pinnow EL, Maeda A, Brown AC, McCartney-Francis N, Kram V, et al. Biglycan modulates angiogenesis and bone formation during fracture healing. Matrix Biol. 2014;35:223–31. doi: 10.1016/j.matbio.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng PS, Wen J, Ang LC, Sheng W, Viloria-Petit A, Wang Y, et al. Versican/PG-M G3 domain promotes tumor growth and angiogenesis. FASEB J. 2004;18:754–6. doi: 10.1096/fj.03-0545fje. [DOI] [PubMed] [Google Scholar]
- 33.Fu Y, Nagy JA, Brown LF, Shih SC, Johnson PY, Chan CK, et al. Proteolytic cleavage of versican and involvement of ADAMTS-1 in VEGF-A/VPF-induced pathological angiogenesis. J Histochem Cytochem. 2011;59:463–73. doi: 10.1369/0022155411401748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jian J, Zheng Z, Zhang K, Rackohn TM, Hsu C, Levin A, et al. Fibromodulin promoted in vitro and in vivo angiogenesis. Biochem Biophys Res Commun. 2013;436:530–5. doi: 10.1016/j.bbrc.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albig AR, Roy TG, Becenti DJ, Schiemann WP. Transcriptome analysis of endothelial cell gene expression induced by growth on matrigel matrices: identification and characterization of MAGP-2 and lumican as novel regulators of angiogenesis. Angiogenesis. 2007;10:197–216. doi: 10.1007/s10456-007-9075-z. [DOI] [PubMed] [Google Scholar]
- 36.West DC, Kumar S. Hyaluronan and angiogenesis. Ciba Found Symp. 1989;143:187–201. doi: 10.1002/9780470513774.ch12. [DOI] [PubMed] [Google Scholar]
- 37.Savani RC, Cao G, Pooler PM, Zaman A, Zhou Z, DeLisser HM. Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J Biol Chem. 2001;276:36770–8. doi: 10.1074/jbc.M102273200. [DOI] [PubMed] [Google Scholar]
- 38.Ingber D. Extracellular matrix and cell shape: potential control points for inhibition of angiogenesis. J Cell Biochem. 1991;47:236–41. doi: 10.1002/jcb.240470309. [DOI] [PubMed] [Google Scholar]
- 39.Maquart FX, Simeon A, Pasco S, Monboisse JC. Regulation of cell activity by the extracellular matrix: the concept of matrikines. J Soc Biol. 1999;193:423–8. [PubMed] [Google Scholar]
- 40.Davis GE, Bayless KJ, Davis MJ, Meininger GA. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am J Pathol. 2000;156:1489–98. doi: 10.1016/S0002-9440(10)65020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ricard-Blum S, Salza R. Matricryptins and matrikines: biologically active fragments of the extracellular matrix. Exp Dermatol. 2014 doi: 10.1111/exd.12435. [DOI] [PubMed] [Google Scholar]
- 42.Marneros AG, Olsen BR. Physiological role of collagen XVIII and endostatin. FASEB J. 2005;19:716–28. doi: 10.1096/fj.04-2134rev. [DOI] [PubMed] [Google Scholar]
- 43.Kamphaus GD, Colorado PC, Panka DJ, Hopfer H, Ramchandran R, Torre A, et al. Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. J Biol Chem. 2000;275:1209–15. doi: 10.1074/jbc.275.2.1209. [DOI] [PubMed] [Google Scholar]
- 44.Maeshima Y, Sudhakar A, Lively JC, Ueki K, Kharbanda S, Kahn CR, et al. Tumstatin, an endothelial cell-specific inhibitor of protein synthesis. Science. 2002;295:140–3. doi: 10.1126/science.1065298. [DOI] [PubMed] [Google Scholar]
- 45.Mongiat M, Sweeney SM, San Antonio JD, Fu J, Iozzo RV. Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of perlecan. J Biol Chem. 2003;278:4238–49. doi: 10.1074/jbc.M210445200. [DOI] [PubMed] [Google Scholar]
- 46.West DC, Hampson IN, Arnold F, Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985;228:1324–6. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]
- 47.Senger DR, Davis GE. Angiogenesis. Cold Spring Harb Perspect Biol. 2011;3:a005090. doi: 10.1101/cshperspect.a005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kinsella MG, Wight TN. Modulation of sulfated proteoglycan synthesis by bovine aortic endothelial cells during migration. J Cell Biol. 1986;102:679–87. doi: 10.1083/jcb.102.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Järveläinen HT, Kinsella MG, Wight TN, Sandell LJ. Differential expression of small chondroitin/dermatan sulfate proteoglycans, PG-I/biglycan and PG-II/decorin, by vascular smooth muscle and endothelial cells in culture. J Biol Chem. 1991;266:23274–81. [PubMed] [Google Scholar]
- 50.Salisbury BG, Wagner WD. Isolation and preliminary characterization of proteoglycans dissociatively extracted from human aorta. J Biol Chem. 1981;256:8050–7. [PubMed] [Google Scholar]
- 51.Oldberg Å, Antonsson P, Lindblom K, Heinegard D. A collagen-binding 59-kd protein (fibromodulin) is structurally related to the small interstitial proteoglycans PG-S1 and PG-S2 (decorin) EMBO J. 1989;8:2601–4. doi: 10.1002/j.1460-2075.1989.tb08399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adany R, Heimer R, Caterson B, Sorrell JM, Iozzo RV. Altered expression of chondroitin sulfate proteoglycan in the stroma of human colon carcinoma. Hypomethylation of PG-40 gene correlates with increased PG-40 content and mRNA levels. J Biol Chem. 1990;265:11389–96. [PubMed] [Google Scholar]
- 53.Iozzo RV, Murdoch AD. Proteoglycans of the extracellular environment: clues from the gene and protein side offer novel perspectives in molecular diversity and function. FASEB J. 1996;10:598–614. [PubMed] [Google Scholar]
- 54.Nastase MV, Iozzo RV, Schaefer L. Key roles for the small leucine-rich proteoglycans in renal and pulmonary pathophysiology. Biochim Biophys Acta. 2014;1840:2460–70. doi: 10.1016/j.bbagen.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pogany G, Vogel KG. The interaction of decorin core protein fragments with type I collagen. Biochem Biophys Res Commun. 1992;189:165–72. doi: 10.1016/0006-291x(92)91539-3. [DOI] [PubMed] [Google Scholar]
- 56.Orgel JP, Eid A, Antipova O, Bella J, Scott JE. Decorin core protein (decoron) shape complements collagen fibril surface structure and mediates its binding. PLoS One. 2009;4:e7028. doi: 10.1371/journal.pone.0007028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalamajski S, Oldberg Å. The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol. 2010;29:248–53. doi: 10.1016/j.matbio.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 58.Reese SP, Underwood CJ, Weiss JA. Effects of decorin proteoglycan on fibrillogenesis, ultrastructure, and mechanics of type I collagen gels. Matrix Biol. 2013;32:414–23. doi: 10.1016/j.matbio.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–43. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brandan E, Gutierrez J. Role of skeletal muscle proteoglycans during myogenesis. Matrix Biol. 2013;32:289–97. doi: 10.1016/j.matbio.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 61.Wu Z, Horgan CE, Carr O, Owens RT, Iozzo RV, Lechner BE. Biglycan and decorin differentially regulate signaling in the fetal membranes. Matrix Biol. 2014;35:266–75. doi: 10.1016/j.matbio.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dunkman AA, Buckley MR, Mienaltowski MJ, Adams SM, Thomas SJ, Kumar A, et al. The injury response of aged tendons in the absence of biglycan and decorin. Matrix Biol. 2014;35:232–8. doi: 10.1016/j.matbio.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sofeu Feugaing DD, Gotte M, Viola M. More than matrix: the multifaceted role of decorin in cancer. Eur J Cell Biol. 2013;92:1–11. doi: 10.1016/j.ejcb.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 64.Horvath Z, Kovalszky I, Fullar A, Kiss K, Schaff Z, Iozzo RV, et al. Decorin deficiency promotes hepatic carcinogenesis. Matrix Biol. 2014;35:194–205. doi: 10.1016/j.matbio.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bianco P, Fisher LW, Young MF, Termine JD, Robey PG. Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non-skeletal tissues. J Histochem Cytochem. 1990;38:1549–63. doi: 10.1177/38.11.2212616. [DOI] [PubMed] [Google Scholar]
- 66.Krusius T, Ruoslahti E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci U S A. 1986;83:7683–7. doi: 10.1073/pnas.83.20.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mann DM, Yamaguchi Y, Bourdon MA, Ruoslahti E. Analysis of glycosaminoglycan substitution in decorin by site-directed mutagenesis. J Biol Chem. 1990;265:5317–23. [PubMed] [Google Scholar]
- 68.Iozzo RV. The family of the small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol. 1997;32:141–74. doi: 10.3109/10409239709108551. [DOI] [PubMed] [Google Scholar]
- 69.Weber IT, Harrison RW, Iozzo RV. Model structure of decorin and implications for collagen fibrillogenesis. J Biol Chem. 1996;271:31767–70. doi: 10.1074/jbc.271.50.31767. [DOI] [PubMed] [Google Scholar]
- 70.Bella J, Hindle KL, McEwan PA, Lovell SC. The leucine-rich repeat structure. Cell Mol Life Sci. 2008;65:2307–33. doi: 10.1007/s00018-008-8019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goldoni S, Owens RT, McQuillan DJ, Shriver Z, Sasisekharan R, Birk DE, et al. Biologically active decorin is a monomer in solution. J Biol Chem. 2004;279:6606–12. doi: 10.1074/jbc.M310342200. [DOI] [PubMed] [Google Scholar]
- 72.Seidler DG, Goldoni S, Agnew C, Cardi C, Thakur ML, Owens RT, et al. Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase-3 activation. J Biol Chem. 2006;281:26408–18. doi: 10.1074/jbc.M602853200. [DOI] [PubMed] [Google Scholar]
- 73.Seidler DG, Dreier R. Decorin and its galactosaminoglycan chain: extracellular regulator of cellular function? IUBMB Life. 2008;60:729–33. doi: 10.1002/iub.115. [DOI] [PubMed] [Google Scholar]
- 74.Seidler DG. The galactosaminoglycan-containing decorin and its impact on diseases. Curr Opin Struct Biol. 2012;22:578–82. doi: 10.1016/j.sbi.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 75.Goldoni S, Seidler DG, Heath J, Fassan M, Baffa R, Thakur ML, et al. An antimetastatic role for decorin in breast cancer. Am J Pathol. 2008;173:844–55. doi: 10.2353/ajpath.2008.080275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu Y, Sun H, Owens RT, Wu J, Chen YQ, Berquin IM, et al. Decorin suppresses prostate tumor growth through inhibition of epidermal growth factor and androgen receptor pathways. Neoplasia. 2009;11:1042–53. doi: 10.1593/neo.09760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bartolini B, Thelin MA, Svensson L, Ghiselli G, van Kuppevelt TH, Malmström A, et al. Iduronic acid in chondroitin/dermatan sulfate affects directional migration of aortic smooth muscle cells. PLoS One. 2013;8:e66704. doi: 10.1371/journal.pone.0066704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stock C, Jungmann O, Seidler DG. Decorin and chondroitin-6 sulfate inhibit B16V melanoma cell migration and invasion by cellular acidification. J Cell Physiol. 2011;226:2641–50. doi: 10.1002/jcp.22612. [DOI] [PubMed] [Google Scholar]
- 79.Nomura Y. Structural change in decorin with skin aging. Connect Tissue Res. 2006;47:249–55. doi: 10.1080/03008200600846606. [DOI] [PubMed] [Google Scholar]
- 80.Kirkpatrick ND, Andreou S, Hoying JB, Utzinger U. Live imaging of collagen remodeling during angiogenesis. Am J Physiol Heart Circ Physiol. 2007;292:H3198–206. doi: 10.1152/ajpheart.01234.2006. [DOI] [PubMed] [Google Scholar]
- 81.Gutierrez P, O’Brien KD, Ferguson M, Nikkari ST, Alpers CE, Wight TN. Differences in the distribution of versican, decorin, and biglycan in atherosclerotic human coronary arteries. Cardiovascular Pathology. 1997;6:271–8. doi: 10.1016/S1054-8807(97)00001-X. [DOI] [PubMed] [Google Scholar]
- 82.Nelimarkka L, Salminen H, Kuopio T, Nikkari S, Ekfors T, Laine J, et al. Decorin is produced by capillary endothelial cells in inflammation-associated angiogenesis. Am J Pathol. 2001;158:345–53. doi: 10.1016/S0002-9440(10)63975-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bosse A, Schwarz K, Vollmer E, Kresse H. Divergent and co-localization of the two small proteoglycans decorin and proteoglycan-100 in human skeletal tissues and tumors. J Histochem Cytochem. 1993;41:13–9. doi: 10.1177/41.1.8417108. [DOI] [PubMed] [Google Scholar]
- 84.Burke AP, Järveläinen H, Kolodgie FD, Goel A, Wight TN, Virmani R. Superficial pseudoaneurysms: clinicopathologic aspects and involvement of extracellular matrix proteoglycans. Mod Pathol. 2004;17:482–8. doi: 10.1038/modpathol.3800060. [DOI] [PubMed] [Google Scholar]
- 85.Reich-Schupke S, Mumme A, Altmeyer P, Stuecker M. Decorin expression with stump recurrence and neovascularization after varicose vein surgery--a pilot study. Dermatol Surg. 2011;37:480–5. doi: 10.1111/j.1524-4725.2011.01912.x. [DOI] [PubMed] [Google Scholar]
- 86.Schönherr E, Sunderkötter C, Schaefer L, Thanos S, Grassel S, Oldberg Å, et al. Decorin deficiency leads to impaired angiogenesis in injured mouse cornea. J Vasc Res. 2004;41:499–508. doi: 10.1159/000081806. [DOI] [PubMed] [Google Scholar]
- 87.Nayak S, Goel MM, Bhatia V, Chandra S, Makker A, Kumar S, et al. Molecular and phenotypic expression of decorin as modulator of angiogenesis in human potentially malignant oral lesions and oral squamous cell carcinomas. Indian J Pathol Microbiol. 2013;56:204–10. doi: 10.4103/0377-4929.120366. [DOI] [PubMed] [Google Scholar]
- 88.Chui A, Murthi P, Gunatillake T, Brennecke SP, Ignjatovic V, Monagle PT, et al. Altered decorin leads to disrupted endothelial cell function: A possible mechanism in the pathogenesis of fetal growth restriction? Placenta. 2014;35:596–605. doi: 10.1016/j.placenta.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 89.de Davies CL, Melder RJ, Munn LL, Mouta-Carreira C, Jain RK, Boucher Y. Decorin inhibits endothelial migration and tube-like structure formation: role of thrombospondin-1. Microvasc Res. 2001;62:26–42. doi: 10.1006/mvre.2001.2311. [DOI] [PubMed] [Google Scholar]
- 90.Grant DS, Yenisey C, Rose RW, Tootell M, Santra M, Iozzo RV. Decorin suppresses tumor cell-mediated angiogenesis. Oncogene. 2002;21:4765–77. doi: 10.1038/sj.onc.1205595. [DOI] [PubMed] [Google Scholar]
- 91.Salomäki HH, Sainio AO, Söderström M, Pakkanen S, Laine J, Jarvelainen HT. Differential expression of decorin by human malignant and benign vascular tumors. J Histochem Cytochem. 2008;56:639–46. doi: 10.1369/jhc.2008.950287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Järveläinen H, Puolakkainen P, Pakkanen S, Brown EL, Hook M, Iozzo RV, et al. A role for decorin in cutaneous wound healing and angiogenesis. Wound Repair Regen. 2006;14:443–52. doi: 10.1111/j.1743-6109.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- 93.Sulochana KN, Fan H, Jois S, Subramanian V, Sun F, Kini RM, et al. Peptides derived from human decorin leucine-rich repeat 5 inhibit angiogenesis. J Biol Chem. 2005;280:27935–48. doi: 10.1074/jbc.M414320200. [DOI] [PubMed] [Google Scholar]
- 94.Mohan RR, Tovey JC, Sharma A, Schultz GS, Cowden JW, Tandon A. Targeted decorin gene therapy delivered with adeno-associated virus effectively retards corneal neovascularization in vivo. PLoS One. 2011;6:e26432. doi: 10.1371/journal.pone.0026432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rada JA, Cornuet PK, Hassell JR. Regulation of corneal collagen fibrillogenesis in vitro by corneal proteoglycan (lumican and decorin) core proteins. Exp Eye Res. 1993;56:635–48. doi: 10.1006/exer.1993.1081. [DOI] [PubMed] [Google Scholar]
- 96.Kinsella MG, Fischer JW, Mason DP, Wight TN. Retrovirally mediated expression of decorin by macrovascular endothelial cells. Effects on cellular migration and fibronectin fibrillogenesis in vitro. J Biol Chem. 2000;275:13924–32. doi: 10.1074/jbc.275.18.13924. [DOI] [PubMed] [Google Scholar]
- 97.Järveläinen H, Vernon RB, Gooden MD, Francki A, Lara S, Johnson PY, et al. Overexpression of decorin by rat arterial smooth muscle cells enhances contraction of type I collagen in vitro. Arterioscler Thromb Vasc Biol. 2004;24:67–72. doi: 10.1161/01.ATV.0000107026.98626.3b. [DOI] [PubMed] [Google Scholar]
- 98.Rühland C, Schönherr E, Robenek H, Hansen U, Iozzo RV, Bruckner P, et al. The glycosaminoglycan chain of decorin plays an important role in collagen fibril formation at the early stages of fibrillogenesis. FEBS J. 2007;274:4246–55. doi: 10.1111/j.1742-4658.2007.05951.x. [DOI] [PubMed] [Google Scholar]
- 99.Fiedler LR, Schönherr E, Waddington R, Niland S, Seidler DG, Aeschlimann D, et al. Decorin regulates endothelial cell motility on collagen I through activation of insulin-like growth factor I receptor and modulation of alpha2beta1 integrin activity. J Biol Chem. 2008;283:17406–15. doi: 10.1074/jbc.M710025200. [DOI] [PubMed] [Google Scholar]
- 100.Sztrolovics R, White RJ, Poole AR, Mort JS, Roughley PJ. Resistance of small leucine-rich repeat proteoglycans to proteolytic degradation during interleukin-1-stimulated cartilage catabolism. Biochem J. 1999;339(Pt 3):571–7. [PMC free article] [PubMed] [Google Scholar]
- 101.Pins GD, Christiansen DL, Patel R, Silver FH. Self-assembly of collagen fibers. Influence of fibrillar alignment and decorin on mechanical properties. Biophys J. 1997;73:2164–72. doi: 10.1016/S0006-3495(97)78247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Krishnan L, Hoying JB, Nguyen H, Song H, Weiss JA. Interaction of angiogenic microvessels with the extracellular matrix. Am J Physiol Heart Circ Physiol. 2007;293:H3650–8. doi: 10.1152/ajpheart.00772.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huttenlocher A, Werb Z, Tremble P, Huhtala P, Rosenberg L, Damsky CH. Decorin regulates collagenase gene expression in fibroblasts adhering to vitronectin. Matrix Biol. 1996;15:239–50. doi: 10.1016/s0945-053x(96)90115-8. [DOI] [PubMed] [Google Scholar]
- 104.Schönherr E, Schaefer L, O’Connell BC, Kresse H. Matrix metalloproteinase expression by endothelial cells in collagen lattices changes during co-culture with fibroblasts and upon induction of decorin expression. J Cell Physiol. 2001;187:37–47. doi: 10.1002/1097-4652(2001)9999:9999<::AID-JCP1048>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 105.Mazor R, Alsaigh T, Shaked H, Altshuler AE, Pocock ES, Kistler EB, et al. Matrix metalloproteinase-1-mediated up-regulation of vascular endothelial growth factor-2 in endothelial cells. J Biol Chem. 2013;288:598–607. doi: 10.1074/jbc.M112.417451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Al Haj Zen A, Lafont A, Durand E, Brasselet C, Lemarchand P, Godeau G, et al. Effect of adenovirus-mediated overexpression of decorin on metalloproteinases, tissue inhibitors of metalloproteinases and cytokines secretion by human gingival fibroblasts. Matrix Biol. 2003;22:251–8. doi: 10.1016/s0945-053x(03)00018-0. [DOI] [PubMed] [Google Scholar]
- 107.Neill T, Painter H, Buraschi S, Owens RT, Lisanti MP, Schaefer L, et al. Decorin antagonizes the angiogenic network: concurrent inhibition of Met, hypoxia inducible factor 1alpha, vascular endothelial growth factor A, and induction of thrombospondin-1 and TIMP3. J Biol Chem. 2012;287:5492–506. doi: 10.1074/jbc.M111.283499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Valente P, Fassina G, Melchiori A, Masiello L, Cilli M, Vacca A, et al. TIMP-2 over-expression reduces invasion and angiogenesis and protects B16F10 melanoma cells from apoptosis. Int J Cancer. 1998;75:246–53. doi: 10.1002/(sici)1097-0215(19980119)75:2<246::aid-ijc13>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 109.Qi JH, Ebrahem Q, Moore N, Murphy G, Claesson-Welsh L, Bond M, et al. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med. 2003;9:407–15. doi: 10.1038/nm846. [DOI] [PubMed] [Google Scholar]
- 110.Rojiani MV, Alidina J, Esposito N, Rojiani AM. Expression of MMP-2 correlates with increased angiogenesis in CNS metastasis of lung carcinoma. Int J Clin Exp Pathol. 2010;3:775–81. [PMC free article] [PubMed] [Google Scholar]
- 111.Fiedler LR, Eble JA. Decorin regulates endothelial cell-matrix interactions during angiogenesis. Cell Adh Migr. 2009;3:3–6. doi: 10.4161/cam.3.1.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jungmann O, Nikolovska K, Stock C, Schulz JN, Eckes B, Riethmuller C, et al. The dermatan sulfate proteoglycan decorin modulates alpha2beta1 integrin and the vimentin intermediate filament system during collagen synthesis. PLoS One. 2012;7:e50809. doi: 10.1371/journal.pone.0050809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.D’Antoni ML, Risse PA, Ferraro P, Martin JG, Ludwig MS. Effects of decorin and biglycan on human airway smooth muscle cell adhesion. Matrix Biol. 2012;31:101–12. doi: 10.1016/j.matbio.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 114.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–71. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 115.Weis SM, Cheresh DA. alphaV integrins in angiogenesis and cancer. Cold Spring Harb Perspect Med. 2011;1:a006478. doi: 10.1101/cshperspect.a006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moscatello DK, Santra M, Mann DM, McQuillan DJ, Wong AJ, Iozzo RV. Decorin suppresses tumor cell growth by activating the epidermal growth factor receptor. J Clin Invest. 1998;101:406–12. doi: 10.1172/JCI846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Iozzo RV, Moscatello DK, McQuillan DJ, Eichstetter I. Decorin is a biological ligand for the epidermal growth factor receptor. J Biol Chem. 1999;274:4489–92. doi: 10.1074/jbc.274.8.4489. [DOI] [PubMed] [Google Scholar]
- 118.Goldoni S, Humphries A, Nyström A, Sattar S, Owens RT, McQuillan DJ, et al. Decorin is a novel antagonistic ligand of the Met receptor. J Cell Biol. 2009;185:743–54. doi: 10.1083/jcb.200901129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schönherr E, Sunderkötter C, Iozzo RV, Schaefer L. Decorin, a novel player in the insulin-like growth factor system. J Biol Chem. 2005;280:15767–72. doi: 10.1074/jbc.M500451200. [DOI] [PubMed] [Google Scholar]
- 120.Iozzo RV, Buraschi S, Genua M, Xu SQ, Solomides CC, Peiper SC, et al. Decorin antagonizes IGF receptor I (IGF-IR) function by interfering with IGF-IR activity and attenuating downstream signaling. J Biol Chem. 2011;286:34712–21. doi: 10.1074/jbc.M111.262766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Morrione A, Neill T, Iozzo RV. Dichotomy of decorin activity on the insulin-like growth factor-I system. FEBS J. 2013;280:2138–49. doi: 10.1111/febs.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Khan GA, Girish GV, Lala N, Di Guglielmo GM, Lala PK. Decorin is a novel VEGFR-2-binding antagonist for the human extravillous trophoblast. Mol Endocrinol. 2011;25:1431–43. doi: 10.1210/me.2010-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baghy K, Horvath Z, Regos E, Kiss K, Schaff Z, Iozzo RV, et al. Decorin interferes with platelet-derived growth factor receptor signaling in experimental hepatocarcinogenesis. FEBS J. 2013;280:2150–64. doi: 10.1111/febs.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reed CC, Gauldie J, Iozzo RV. Suppression of tumorigenicity by adenovirus-mediated gene transfer of decorin. Oncogene. 2002;21:3688–95. doi: 10.1038/sj.onc.1205470. [DOI] [PubMed] [Google Scholar]
- 125.Dil N, Banerjee AG. Knockdown of aberrantly expressed nuclear localized decorin attenuates tumour angiogenesis related mediators in oral cancer progression model in vitro. Head Neck Oncol. 2012;4:11,3284-4-11. doi: 10.1186/1758-3284-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Santra M, Santra S, Zhang J, Chopp M. Ectopic decorin expression up-regulates VEGF expression in mouse cerebral endothelial cells via activation of the transcription factors Sp1, HIF1alpha, and Stat3. J Neurochem. 2008;105:324–37. doi: 10.1111/j.1471-4159.2007.05134.x. [DOI] [PubMed] [Google Scholar]
- 127.Rosenbloom J, Castro SV, Jimenez SA. Narrative review: fibrotic diseases: cellular and molecular mechanisms and novel therapies. Ann Intern Med. 2010;152:159–66. doi: 10.7326/0003-4819-152-3-201002020-00007. [DOI] [PubMed] [Google Scholar]
- 128.Kuzuya M, Kinsella JL. Induction of endothelial cell differentiation in vitro by fibroblast-derived soluble factors. Exp Cell Res. 1994;215:310–8. doi: 10.1006/excr.1994.1347. [DOI] [PubMed] [Google Scholar]
- 129.Kuzuya M, Kinsella JL. Reorganization of endothelial cord-like structures on basement membrane complex (Matrigel): involvement of transforming growth factor beta 1. J Cell Physiol. 1994;161:267–76. doi: 10.1002/jcp.1041610211. [DOI] [PubMed] [Google Scholar]
- 130.Mandriota SJ, Menoud PA, Pepper MS. Transforming growth factor beta 1 down-regulates vascular endothelial growth factor receptor 2/flk-1 expression in vascular endothelial cells. J Biol Chem. 1996;271:11500–5. doi: 10.1074/jbc.271.19.11500. [DOI] [PubMed] [Google Scholar]
- 131.Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002;21:1743–53. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ferrari G, Cook BD, Terushkin V, Pintucci G, Mignatti P. Transforming growth factor-beta 1 (TGF-beta1) induces angiogenesis through vascular endothelial growth factor (VEGF)-mediated apoptosis. J Cell Physiol. 2009;219:449–58. doi: 10.1002/jcp.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mahmoud M, Upton PD, Arthur HM. Angiogenesis regulation by TGFbeta signalling: clues from an inherited vascular disease. Biochem Soc Trans. 2011;39:1659–66. doi: 10.1042/BST20110664. [DOI] [PubMed] [Google Scholar]
- 134.Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346:281–4. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- 135.Border WA, Ruoslahti E. Transforming growth factor-beta in disease: the dark side of tissue repair. J Clin Invest. 1992;90:1–7. doi: 10.1172/JCI115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hildebrand A, Romaris M, Rasmussen LM, Heinegård D, Twardzik DR, Border WA, et al. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994;302(Pt 2):527–34. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schönherr E, Broszat M, Brandan E, Bruckner P, Kresse H. Decorin core protein fragment Leu155-Val260 interacts with TGF-beta but does not compete for decorin binding to type I collagen. Arch Biochem Biophys. 1998;355:241–8. doi: 10.1006/abbi.1998.0720. [DOI] [PubMed] [Google Scholar]
- 138.Imai K, Hiramatsu A, Fukushima D, Pierschbacher MD, Okada Y. Degradation of decorin by matrix metalloproteinases: identification of the cleavage sites, kinetic analyses and transforming growth factor-beta1 release. Biochem J. 1997;322:809–14. doi: 10.1042/bj3220809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Boivin WA, Shackleford M, Vanden Hoek A, Zhao H, Hackett TL, Knight DA, et al. Granzyme B cleaves decorin, biglycan and soluble betaglycan, releasing active transforming growth factor-beta1. PLoS One. 2012;7:e33163. doi: 10.1371/journal.pone.0033163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Isaka Y, Brees DK, Ikegaya K, Kaneda Y, Imai E, Noble NA, et al. Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nat Med. 1996;2:418–23. doi: 10.1038/nm0496-418. [DOI] [PubMed] [Google Scholar]
- 141.Stander M, Naumann U, Dumitrescu L, Heneka M, Loschmann P, Gulbins E, et al. Decorin gene transfer-mediated suppression of TGF-beta synthesis abrogates experimental malignant glioma growth in vivo. Gene Ther. 1998;5:1187–94. doi: 10.1038/sj.gt.3300709. [DOI] [PubMed] [Google Scholar]
- 142.Nili N, Cheema AN, Giordano FJ, Barolet AW, Babaei S, Hickey R, et al. Decorin inhibition of PDGF-stimulated vascular smooth muscle cell function: potential mechanism for inhibition of intimal hyperplasia after balloon angioplasty. Am J Pathol. 2003;163:869–78. doi: 10.1016/S0002-9440(10)63447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Scott RA, Panitch A. Decorin mimic regulates platelet-derived growth factor and interferon-gamma stimulation of vascular smooth muscle cells. Biomacromolecules. 2014;15:2090–103. doi: 10.1021/bm500224f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Penc SF, Pomahac B, Winkler T, Dorschner RA, Eriksson E, Herndon M, et al. Dermatan sulfate released after injury is a potent promoter of fibroblast growth factor-2 function. J Biol Chem. 1998;273:28116–21. doi: 10.1074/jbc.273.43.28116. [DOI] [PubMed] [Google Scholar]
- 145.Morcavallo A, Buraschi S, Xu SQ, Belfiore A, Schaefer L, Iozzo RV, et al. Decorin differentially modulates the activity of insulin receptor isoform A ligands. Matrix Biol. 2014;35:82–90. doi: 10.1016/j.matbio.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kaji T, Yamamoto C, Oh-i M, Nishida T, Takigawa M. Differential regulation of biglycan and decorin synthesis by connective tissue growth factor in cultured vascular endothelial cells. Biochem Biophys Res Commun. 2004;322:22–8. doi: 10.1016/j.bbrc.2004.07.078. [DOI] [PubMed] [Google Scholar]
- 147.Ward WK, Li AG, Siddiqui Y, Federiuk IF, Wang XJ. Increased expression of Interleukin-13 and connective tissue growth factor, and their potential roles during foreign body encapsulation of subcutaneous implants. J Biomater Sci Polym Ed. 2008;19:1065–72. doi: 10.1163/156856208784909408. [DOI] [PubMed] [Google Scholar]
- 148.Vial C, Gutierrez J, Santander C, Cabrera D, Brandan E. Decorin interacts with connective tissue growth factor (CTGF)/CCN2 by LRR12 inhibiting its biological activity. J Biol Chem. 2011;286:24242–52. doi: 10.1074/jbc.M110.189365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kristensen IB, Pedersen L, Ro TB, Christensen JH, Lyng MB, Rasmussen LM, et al. Decorin is down-regulated in multiple myeloma and MGUS bone marrow plasma and inhibits HGF-induced myeloma plasma cell viability and migration. Eur J Haematol. 2013;91:196–200. doi: 10.1111/ejh.12125. [DOI] [PubMed] [Google Scholar]
- 150.Moreth K, Iozzo RV, Schaefer L. Small leucine-rich proteoglycans orchestrate receptor crosstalk during inflammation. Cell Cycle. 2012;11:2084–91. doi: 10.4161/cc.20316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bruno A, Pagani A, Pulze L, Albini A, Dallaglio K, Noonan DM, et al. Orchestration of angiogenesis by immune cells. Front Oncol. 2014;4:131. doi: 10.3389/fonc.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Niu J, Azfer A, Zhelyabovska O, Fatma S, Kolattukudy PE. Monocyte chemotactic protein (MCP)-1 promotes angiogenesis via a novel transcription factor, MCP-1-induced protein (MCPIP) J Biol Chem. 2008;283:14542–51. doi: 10.1074/jbc.M802139200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lindner DJ. Interferons as antiangiogenic agents. Curr Oncol Rep. 2002;4:510–4. doi: 10.1007/s11912-002-0065-4. [DOI] [PubMed] [Google Scholar]
- 155.Bocian C, Urbanowitz AK, Owens RT, Iozzo RV, Gotte M, Seidler DG. Decorin potentiates interferon-gamma activity in a model of allergic inflammation. J Biol Chem. 2013;288:12699–711. doi: 10.1074/jbc.M112.419366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Neill T, Schaefer L, Iozzo RV. Instructive roles of extracellular matrix on autophagy. Am J Pathol. 2014;184:2146–53. doi: 10.1016/j.ajpath.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–41. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 158.Ramakrishnan S, Nguyen TM, Subramanian IV, Kelekar A. Autophagy and angiogenesis inhibition. Autophagy. 2007;3:512–5. doi: 10.4161/auto.4734. [DOI] [PubMed] [Google Scholar]
- 159.Buraschi S, Neill T, Goyal A, Poluzzi C, Smythies J, Owens RT, et al. Decorin causes autophagy in endothelial cells via Peg3. Proc Natl Acad Sci U S A. 2013;110:E2582–91. doi: 10.1073/pnas.1305732110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Goyal A, Neill T, Owens RT, Schaefer L, Iozzo RV. Reprint of: Decorin activates AMPK, an energy sensor kinase, to induce autophagy in endothelial cells. Matrix Biol. 2014;35:42–50. doi: 10.1016/j.matbio.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Neill T, Torres A, Buraschi S, Iozzo RV. Decorin has an appetite for endothelial cell autophagy. Autophagy. 2013;9:1626–8. doi: 10.4161/auto.25881. [DOI] [PubMed] [Google Scholar]
- 162.Boström P, Sainio A, Kakko T, Savontaus M, Söderström M, Järveläinen H. Localization of decorin gene expression in normal human breast tissue and in benign and malignant tumors of the human breast. Histochem Cell Biol. 2013;139:161–71. doi: 10.1007/s00418-012-1026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hamid AS, Li J, Wang Y, Wu X, Ali HA, Du Z, et al. Recombinant human decorin upregulates p57KIP(2) expression in HepG2 hepatoma cell lines. Mol Med Rep. 2013;8:511–6. doi: 10.3892/mmr.2013.1510. [DOI] [PubMed] [Google Scholar]
- 164.Yu X, Zou Y, Li Q, Mao Y, Zhu H, Huang G, et al. Decorin-mediated inhibition of cholangiocarcinoma cell growth and migration and promotion of apoptosis are associated with E-cadherin in vitro. Tumour Biol. 2014;35:3103–12. doi: 10.1007/s13277-013-1402-y. [DOI] [PubMed] [Google Scholar]
- 165.Dimmeler S, Zeiher AM. Endothelial cell apoptosis in angiogenesis and vessel regression. Circ Res. 2000;87:434–9. doi: 10.1161/01.res.87.6.434. [DOI] [PubMed] [Google Scholar]
- 166.Neill T, Schaefer L, Iozzo RV. Decorin: a guardian from the matrix. Am J Pathol. 2012;181:380–7. doi: 10.1016/j.ajpath.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 168.Moserle L, Jimenez-Valerio G, Casanovas O. Antiangiogenic therapies: going beyond their limits. Cancer Discov. 2014;4:31–41. doi: 10.1158/2159-8290.CD-13-0199. [DOI] [PubMed] [Google Scholar]
- 169.Järvinen TA, Ruoslahti E. Targeted antiscarring therapy for tissue injuries. Adv Wound Care (New Rochelle) 2013;2:50–4. doi: 10.1089/wound.2011.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chaudhary K, Moore H, Tandon A, Gupta S, Khanna R, Mohan RR. Nanotechnology and Adeno-associated virus based decorin gene therapy ameliorates Peritoneal Fibrosis. Am J Physiol Renal Physiol. 2014 doi: 10.1152/ajprenal.00653.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ma R, Chen J, Li Z, Tang J, Wang Y, Cai X. Decorin accelerates the liver regeneration after partial hepatectomy in fibrotic mice. Chin Med J (Engl) 2014;127:2679–85. [PubMed] [Google Scholar]