Abstract
Aims
The prevalence of highly insulin resistant diabetes is increasing and treatment requires the use of very high doses of insulin. This study was performed to analyze efficacy and patient satisfaction with use of U-500 concentrated insulin.
Methods
The medical records of 40 patients using U-500 insulin for at least 3 months were reviewed. A quality of life questionnaire was administered 6 or more months after U-500 was initiated. Effects of U-500 use on HbA1c, weight, total daily insulin use, hypoglycemia, and patient satisfaction were measured.
Results
Patients had uncontrolled diabetes for 3 years prior to U-500 initiation despite insulin titration. Subjects required continued insulin titration to attain glycemic control even after U-500 initiation, but HbA1c decreased by 1.5% within three months. Subjects gained weight with insulin titration. Hypoglycemic symptoms increased early after transition to U-500 insulin, but patients reported fewer hypoglycemic episodes on the quality of life questionnaire. Patient satisfaction with diabetes care and control was significantly improved following transition to U-500 insulin.
Conclusions
Use of U-500 insulin assists with attaining glycemic control in highly insulin resistant subjects, but at the cost of weight gain and increased insulin doses. However, patient satisfaction is improved with U-500 insulin use.
Keywords: insulin resistance, HbA1c, weight gain, hypoglycemia
Introduction
The prevalence of diagnosed and undiagnosed diabetes in the United States is 23.6 million individuals (7.8% of the population). Diabetes mellitus is accompanied by long-term microvascular, neurologic, and macrovascular complications including retinopathy, nephropathy, neuropathy, and cardiovascular disease. The Diabetes Control and Complications Trial (DCCT) and the United Kingdom Prospective Diabetes Study (UKPDS) demonstrated that improvement of glycemic control reduces diabetes-related microvascular complications[1, 2]. However, due to a high prevalence of insulin resistance in Type 2 Diabetes attaining good glycemic control often requires large doses of insulin. Administration of large volumes of insulin can lead to leakage and poor absorption. Additionally, large volume injections can cause discomfort and lead to poor compliance with treatment[3].
U-500 is fivefold more concentrated (500 units/ml) than U-100 regular insulin (100 units/ml). Due to its concentration, administration of 100 units of insulin requires an injection volume of only 0.20 ml of U-500 insulin (compared to 1.0 ml for U-100 insulin). Therefore, there is less potential for leakage and a greater potential for absorption albeit with delayed time to peak[4, 5]. There are a number of small reports that have noted the effects of U-500 insulin on changes in HbA1c, weight, and total daily insulin dosage[3, 6-11]. All studies have shown that U-500 insulin leads to a reduction in HbA1c level but have not been consistent on changes in body weight or total daily insulin dosage. The purpose of this study was to determine the effect of U-500 insulin on glycemic control, changes in body weight, total daily insulin dosage, incidence of hypoglycemia, or effect on lipid levels and blood pressure as well as patient satisfaction and quality of life.
Subjects, Materials and Methods
All patients attending diabetes clinics at the University of Kentucky or the Lexington, KY Department of Veterans Affairs medical centers who were using U-500 insulin for at least 3 months were invited to participate. All subjects provided written informed consent as approved by the University of Kentucky Institutional Review Board and the VA Research and Development committee. There were no exclusion criteria. The medical records of patients who had been using U-500 insulin for a minimum of 3 months were reviewed up to 5 years prior to the use of U-500 insulin. Data included body weight and height, blood pressure, levels of HbA1c, total daily insulin dose, accounts of hypoglycemia, fasting lipid levels, urinary albumin excretion, and concurrent medications and diagnoses. This study was an observational study including retrospective chart review. At the time of data analysis only 7 subjects had been using U-500 for 2 years, so data presented includes 3, 6 and 12 months post U-500 initiation (data for 40, 36 and 24 subjects respectively). The baseline visit is defined as the time of U-500 initiation.
A quality of life questionnaire was developed by the authors specifically for this study based on a previously published and validated quality of life questionnaire[12], and was given to each subject 6 months after U-500 initiation. Subjects were asked to recall their experiences prior to U-500 use, compared to their current experience with U-500 insulin (Table 1). If an eligible subject had already been using U-500 insulin for > 6 months, the questionnaire was administered upon entrance into our study. The answers were rated numerically according to the scales as indicated on Table 1.
Table 1.
Quality of life questionnaire
| Questions | Before U-500 | After U-500 | P value |
|---|---|---|---|
| Part 1: Rated on a scale of: Very satisfied (2); Moderately satisfied (1); neither (0); Moderately dissatisfied (−1); Very dissatisfied (−2) | |||
| How satisfied are you with your diabetes treatment? | −0.26±0.30 | 1.77±0.08; (74%) | P<0.001 |
| How satisfied are you with the amount of time it takes to manage your diabetes? | −0.13±0.28 | 1.71±0.08; (77%) | P<0.001 |
| How satisfied are you with your knowledge about your diabetes? | 0.71±0.23 | 1.77±0.08; (61%) | P<0.001 |
| How satisfied are you with your ability to determine your blood sugar level? | 0.48±0.26 | 1.64±0.14; (55%) | P<0.001 |
| How satisfied are you with your overall diabetes control? | −0.74±0.26 | 1.48±0.15; (81%) | P<0.001 |
| Part 2: Rated on a scale of: Never (0); Very Seldom (1); Sometimes (2); Often (3); Always (4) | |||
| How often did you have a bad night's sleep because of diabetes? | 2.23±0.21 | 1.57±0.17; (60%) | P=0.02 |
| How often did you feel diabetes limited your career? | 2.37±0.23 | 1.47±0.23; (60%) | P=0.008 |
| How often did you have pain because of your diabetes treatment? | 2.70±0.26 | 1.20±0.15; (53%) | P=0.002 |
| How often did you feel physically ill? | 2.03±0.19 | 1.20±0.13; (63%) | P<0.001 |
| How often did you worry about whether you would pass out? | 1.60±0.21 | 0.70±0.11; (63%) | P<0.001 |
| How often did you worry about whether you would miss work? | 1.73±0.32 | 0.68±0.23 (45%) | P=0.01 |
| Part 3: Numerical responses | |||
| How many times in an average week would you feel the symptoms of a low blood sugar? | 1.60±0.38 | 0.98±0.16; (31%) | NS |
| How many times in an average week did you find a blood sugar level < 60 mg/dl? | 0.84±0.20 | 0.52±0.13; (14%) | NS |
| How many times in an average week would you require the assistance of someone else because you had a severely low blood sugar? | 0.74±0.25 | 0.19±0.08 (3%) | P=0.04 |
| How many times in an average week would you notice leakage of insulin following an injection? | 3.55±0.90 | 0.72±0.22 (14%) | P=0.005 |
Data is presented as mean±SEM based on numeric scores. For parts 1 and 2 the percent reporting any improvement after U-500 initiation (based on change in numeric responses to paired questions) is shown in parentheses; for part 3 the percent reporting any increase in symptoms after U-500 initiation (based on change in numeric responses to paired questions) is shown in parentheses.
Statistical analyses: Data are presented as mean±SEM, and were analyzed by one way repeated measures ANOVA. The quality of life questionnaire contained paired questions and data is analyzed by t-test. P values < 0.05 are considered statistically significant.
Results
The patient demographic consisted of 40 Caucasian patients (33 males and 7 females). The majority of the subjects were recruited from the Lexington KY Veterans Affairs medical center. Two subjects discontinued U-500 use prior to 3 months of use and were not included in the analysis; all other subjects continue on U-500 insulin at the time of this analysis. The mean age of the patients at the time of U-500 initiation was 57±2 years. The mean BMI at the time of U-500 initiation was 40.5±1.2 kg/m2, and had been gradually rising over the prior 3 years for most individuals (Fig. 1A). All subjects had been using insulin prior to U-500 use: 23 of 40 on premix NPH/regular; 2 using premix analog insulin; 14 using basal/bolus (6 with analog rapid acting, 8 using regular insulin), and one subject on basal insulin only. Most subjects were on multiple medications including 25 of 40 using metformin and an additional 4 subjects using a thiazolidinedione. Most subjects were at or near targets for blood pressure and total cholesterol and LDL cholesterol levels, but had elevated triglycerides and low HDL cholesterol levels (Table 2). Urinary albumin excretion was available for 24 subjects, and was modestly elevated (Table 2). Treatment with U-500 insulin led to no significant changes in blood pressure or lipid values or number of required blood pressure or lipid lowering medications (Table 2, showing baseline and 6 months post U-500 use values).
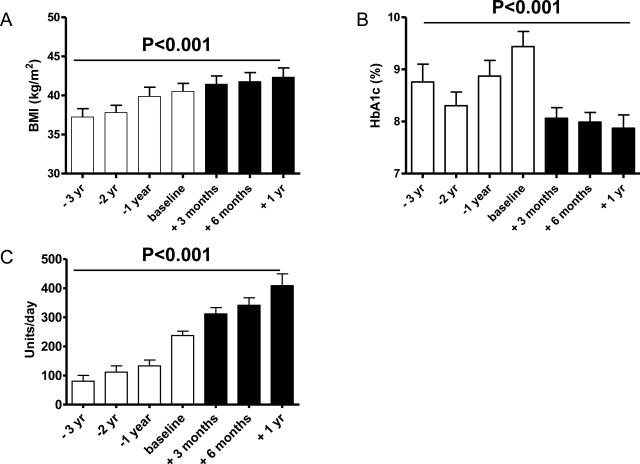
Figure 1. Effects of U-500 use on BMI, glycemic control and insulin use.
Body mass index (BMI, kg/m2; panel A), HbA1c (panel B), and total daily insulin doses (panel C) were recorded from chart review at the indicated times prior to and following initiation with U-500 insulin. Baseline is defined as the time of U-500 initiation. Open bars represent time points prior to U-500 use, solid bars represent time points after U-500 initiation. Data were analyzed by one way repeated measures ANOVA.
Table 2.
Metabolic features and medication use.
| Parameter | Baseline visit | 6 months after U-500 |
|---|---|---|
| Systolic BP (mmHg) | 134±2.5 | 128±3.4 |
| Diastolic BP (mmHg) | 73±1.4 | 72±1.6 |
| Number of BP medications | 2.2±0.1 | 2.3±0.2 |
| Total cholesterol (mg/dl) | 181.6±20.7 | 181.8±13.9 |
| LDL cholesterol (mg/dl) | 70.3±5.3 | 72.6±6.6 |
| HDL cholesterol (mg/dl) | 31.8±1.6 | 32.6±2.5 |
| Triglycerides (mg/dl) | 738±302 | 591±256 |
| Number of Lipid medications | 1.4±0.2 | 1.4±0.2 |
| Urine albumin excretion (mg/g creatinine) | 34.9±11.1 | NA |
BP: blood pressure; NA: not available; baseline visit is defined as the time of U-500 insulin initiation. Data is presented as mean±SEM.
The mean HbA1c at the time of U-500 initiation was 9.4±0.3% and had gradually risen over the 3 year period prior to U-500 initiation for most subjects (Fig. 1B). Within 3 months of U-500 use, the average HbA1c significantly decreased to 8.0±0.2% and remained at this level up through 1 year of follow up (P<0.001; Fig 1B). At the time of U-500 initiation only 7.5% (3/40) subjects had HbA1c ≤7.5%; by 3, 6 and 12 months of use this was achieved by 30% (9/30), 38% (13/34) and 38% (9/24) respectively. The mean BMI for patients 1 year prior to U-500 initiation was 39.9±1.2 kg/m2 and at U-500 initiation was 40.5±1.0 kg/m2, reflecting an average weight gain of 9.2±2.7 lbs the year prior to U-500 initiation. Following initiation of U-500 there was an average weight gain of 12.6± 2.9 lbs over one year; however, 4 subjects actually lost weight following initiation of U-500 insulin. The mean total daily insulin dose at the time of U-500 insulin initiation was 239.6±15.0 units/day, or 1.75±0.14 units/kg/day. Within 6 months of U-500 use, the average total daily insulin dose significantly increased by 70% (P<0.001; average increase of 97 units/day; range −128 to +522 units/day; Fig. 1C) giving a mean total daily insulin use of 338.5±26.4 units/day, or 2.21±0.18 units/kg/day.
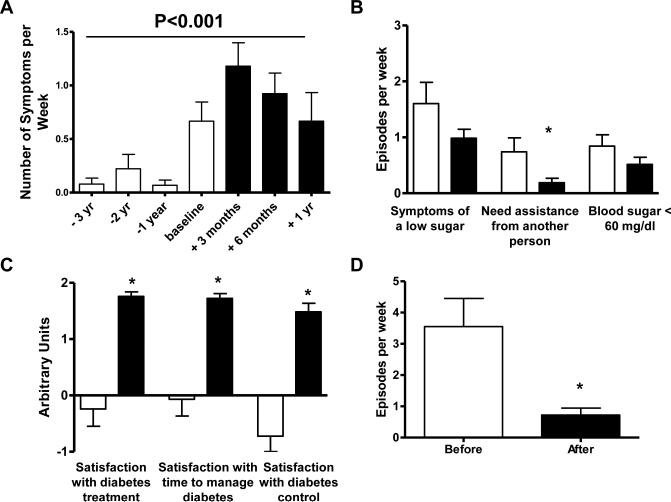
Patients were given a quality of life questionnaire at a minimum of 6 months after U-500 insulin initiation (Table 1). Not all subjects completed the quality of life questionnaires, and data is available for 31 of the 36 subjects that had been using U-500 for at least 6 months. There was no difference in HbA1c after 6 months of U-500 use between responders and non-responders (8.1±0.2 vs 7.9±0.7 respectively, P=NS). Frequency of hypoglycemia was evaluated both from self-reported hypoglycemia documented in clinical charts, and from self-reported hypoglycemia in the quality of life questionnaire. As the mean total daily insulin dose increased, there was a significant increase in frequency of self-reported hypoglycemia documented in clinical charts, which peaked at 3 months after U-500 initiation, then declined (P<0.001; Fig 2A). However, it is important to note that most of these hypoglycemia episodes were not documented, and for those that were many patients reported symptoms of hypoglycemia even when the blood glucose levels were above 70 mg/dl, and often above 100 mg/dl. Conversely, evaluation of hypoglycemia obtained from the quality of life questionnaire demonstrated a non-significant tendency towards fewer symptoms of low sugar, fewer reports of needing assistance from another for a low sugar (P=0.04), and fewer self-reported blood sugar values < 60 mg/dl after use of U-500 insulin (Fig. 2B).
Figure 2. Effect of U-500 use on hypoglycemia and patient satisfaction.
A. Hypoglycemia symptoms (episodes/week) were recorded from chart review at the indicated times prior to and following initiation with U-500 insulin. Baseline is defined as the time of U-500 initiation. Data were analyzed by one way repeated measures ANOVA. B. Hypoglycemia (episodes/week) as reported on the quality of life questionnaire administered 6 or more months after initiation of U-500 insulin. C. Patient satisfaction as reported on the quality of life questionnaire administered 6 or more months after initiation of U-500 insulin. D. Frequency of leakage of injected insulin (episodes/week) as reported on the quality of life questionnaire. Open bars represent time points prior to U-500 use, solid bars represent time points after U-500 initiation. * represents P<0.05.
Patient satisfaction with their diabetes was improved following use of U-500 insulin (Table 1). Specifically, patients had increased satisfaction with their diabetes treatment, time to manage their diabetes, and diabetes control (all P<0.001; Fig 2C). In addition, patients reported significantly fewer problems with leakage of insulin from injections following use of U-500 (Fig. 2D).
Discussion
In summary, in this observational study of subjects using U-500 insulin, we found that within 6 months of U-500 insulin use, the subjects had significantly improved glycemic control: mean HbA1c significantly decreased from 9.4±0.3% to 8.0±0.2%. This was accomplished by increased insulin doses (mean insulin dose increased from 1.75 to 2.21 units/kg/d). The subjects had weight gain that paralleled the increased insulin dose with an average gain of 10-12 lbs over the 6-12 months following initiation of U-500. However, the year prior to initiation of U-500 insulin the subjects had a similar increase in total daily insulin dose (75%), a similar increase in weight (9 lbs), yet an increase in their HbA1c. Thus, although there was a significant increase in total daily insulin dose and weight gain following transition to U-500 insulin, the subjects did have a significant improvement in glycemic control.
As seen in the DCCT and UKPDS trials, better glycemic control leads to decreased incidence of diabetic-related microvascular complications including neuropathy, retinopathy, nephropathy, and cardiovascular disease[1, 2]. Furthermore, improved glycemic control appears to lead to reduced macrovascular complications[13, 14], the leading cause of death in diabetes. The groups treated with intensive therapy in both the DCCT and the UKPDS trials had increased weight gain compared to the control groups[2, 15]; however, despite this increased weight gain, both studies demonstrated fewer complications in the intensively treated groups. Thus, although weight gain is frustrating and undesirable for patients, it is offset by the benefits of improved glycemic control. Furthermore, our findings support the need for continued insulin titration: despite a weight gain with insulin titration prior to initiation of U-500 insulin there were no improvements in HbA1c, with continued insulin titration using U-500 insulin we were able to attain improved glycemic control, albeit with further weight gain. Given the improvement in glycemic control attained after U-500 insulin usage, compared to the lack of improvement in glycemic control prior to U-500 insulin use, the relative weight gain for the glycemic control was less on U-500. However, 4 subjects actually lost weight following U-500 transition (range 5-30 pounds), suggesting that perhaps with improved satisfaction with their diabetes treatments subjects had more motivation to improve their lifestyle.
Patients in this study were started on U-500 insulin by their diabetes provider following guidelines in published algorithms[9, 16]. As seen in Fig. 1C most subjects had been undergoing insulin titration over the 3 years prior to U-500 insulin initiation, although without any improvement in glycemic control. The lack of improvement in HbA1c could be attributed to lack of efficacy of the U-100 insulin formulations patients had been using due to leakage of injected insulin (reported by most subjects), poor absorption from the large volumes injected[17], or poor adherence with treatment prescriptions. By changing patients to U-500 insulin, the injection volumes are reduced by 80%, which allowed for effective insulin titration likely contributing to our ability to lower HbA1c. Nevertheless, it is important to note that patients continued to need significant insulin titration following transition to U-500 insulin in order to attain improved glycemic control, reflecting the considerable insulin requirements of these subjects. However, as all patients were started on U-500 shortly after transfer of care to a new diabetes provider (LRT) we cannot exclude the possibility of an effect of a new clinic or new provider on the ability to attain improved glycemic control.
Although improved glycemic control was noted within 3 months of initiation of U-500 insulin, most subjects did not attain glycemic targets as defined by the American Diabetes Association. Furthermore, it is disappointing to note that there did not appear to be any ongoing improvement in HbA1c with continued use of U-500 insulin, despite continued insulin titration. By 6 and 12 months of U-500 use, 13 of 34, and 9 of 24 subjects respectively had achieved HbA1c < 7.5%, but only 5 of 34, and 6 of 24 subjects respectively had achieved HbA1c < 7.0%. All subjects were seen by the same provider (LRT) at one of two medical centers (the Lexington Veterans Affairs Medical Center or the University of Kentucky medical center), and received the same diabetes education and instructions, thus the difference between patients attaining or not attaining glycemic goals cannot be attributed to their medical care. The relative lack of success in obtaining glycemic goals is more likely a reflection of the degree of insulin resistance of these subjects (20 of 36 subjects were using > 300 units/day, with only 6 of 35 subjects using < 200 units/day 6 months after U-500 initiation) and the general difficulty of attaining glycemic goals in clinical practice.
An interesting observation in this study is the discrepancy between hypoglycemia reports obtained from chart review, with hypoglycemia reports obtained from the quality of life questionnaire. It is well known that hypoglycemia symptoms can occur even with blood sugar levels in the normal or elevated range, particularly in subjects who have maintained chronically poor glycemic control. The medical charts contain only a statement as to whether the patient reports symptoms of hypoglycemia, and approximate number of episodes per week. No documentation of blood sugar levels is recorded. Given that the subjects had chronically poor glycemic control prior to U-500 initiation, and fairly rapid and considerable insulin titration, it is certainly reasonable that some of these reported hypoglycemic episodes occurred in the absence of blood sugar levels < 70 mg/dl. The medical chart reported hypoglycemia events peaked at 3 months after U-500 initiation, at which time the subjects had already had an average 1.5% decrease in HbA1c, suggesting that the hypoglycemia frequency may have been due to the relatively rapid lowering of HbA1c. There was a trend towards fewer hypoglycemia events at 6 and 12 months after U-500 initiation, implying that subjects did not continue to have increased hypoglycemia episodes despite maintaining improved glycemic control. Furthermore, many hypoglycemia episodes are due to a relative mismatch between insulin administration and food intake; the first 3 months after U-500 initiation may reflect the learning period as subjects became accustomed to the time of action of U-500. The decrease in hypoglycemia episodes after the first 3 months supports this hypothesis. The quality of life questionnaire was administered 6 or more months after U-500 initiation. By this time, most subjects would have gained familiarity with U-500 action, and had maintained improved glycemic control for 6 or more months. Furthermore, this questionnaire included questions aimed to define the hypoglycemia: in addition to asking about symptoms, subjects were asked to document the number of blood glucose levels < 60 mg/dl a week, and the number of times they needed assistance from another person for hypoglycemia symptoms. Thus, the trend towards decreased frequency of hypoglycemia with U-500 use is encouraging. The quality of life questionnaire supports that users of U-500 were generally pleased with this insulin compared to their previous treatments.
There have been several previous reports on U-500 insulin use, including reports of use of U-500 in insulin pumps[7-9]. Of our 40 subjects, only 2 are using U-500 insulin in a pump, with the rest administering it subcutaneously in 2-4 divided doses per day. The previous reports have been varied in their reports of HbA1c reduction, weight gain, hypoglycemia and total daily insulin dose. A recent article summarized these publications, and reports an average improvement in HbA1c of 1.6%, a weight gain of 4.2 kg, and no significant changes in total daily insulin dose[5]. Previous reports did not explore the effect treatment with U-500 insulin may have had on lipid levels, blood pressure, or other metabolic changes that often correlate with improved glycemic control such as albuminuria levels. Additionally, previous research had not evaluated the effect U-500 on patient satisfaction. A major focus of this study was to evaluate patient satisfaction with U-500 insulin use, based on the presumption that improved patient satisfaction leads to improved adherence to treatment recommendations, and improved outcomes. Furthermore, our study expands on previous observations in that we include data on a relatively large number of subjects (40) and long duration of U-500 use (up to 12 months). Overall, subjects were significantly more satisfied with U-500 treatment in comparison to prior treatment and care of their Type 2 diabetes. This improvement in patient satisfaction can be attributed to a number of factors including the improvement in glycemic control after years of lack of improvement despite insulin titration for most subjects, to the smaller injection volumes with less insulin leakage, burning or pain, and to fewer numbers of injections for most subjects. The lack of patient perceived increase in hypoglycemia likely reflects the increased sense of satisfaction with their overall diabetes care reflected by the subjects in this study. U-500 insulin use did not significantly change blood pressure or lipid values or number of required blood pressure or lipid lowering medications. As mentioned previously, all patients were started on U-500 shortly after initiating care with a new diabetes provider (LRT); thus, we cannot exclude the possibility of an effect of a new clinic or new provider on the quality of life survey.
In conclusion, U-500 insulin is an effective method of treatment for highly insulin-resistant Type 2 diabetes. Treatment with U-500 insulin leads to decreased HbA1c and increased total daily insulin while improving glycemic control. Although there was an increase in body weight previous studies have suggested the benefits from improved glycemic control outweigh the disadvantages of increased weight. U-500 insulin did not show significant changes in blood pressure, lipid values, or number of required blood pressure or lipid lowering medications. U-500 insulin results in markedly improved patient satisfaction with diabetes, with no perceived increase in hypoglycemia.
Acknowledgements
The authors thank the participants of the study, and thank clinic staff Elizabeth Holden, RN; Sheri Setser, RD, and Rebecca Cole, ARNP for their support with the management of the participants. This work was supported in part by Clinical Center for Translational Science Professional Student Mentored Research Program and the Medical Student Federal Work Study Program (both to AD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Presented in part at the 91st Annual Meeting of the Endocrine Society, Washington, DC, June 2009. There are no financial disclosures or conflicting interests for any author.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N. Engl. J. Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 3.Garg R, Johnston V, McNally PG, et al. U-500 insulin: why, when and how to use in clinical practice. Diabetes Metab Res Rev. 2007;23:265–268. doi: 10.1002/dmrr.709. [DOI] [PubMed] [Google Scholar]
- 4.Galloway JA, Spradlin CT, Nelson RL, et al. Factors influencing the absorption, serum insulin concentration, and blood glucose responses after injections of regular insulin and various insulin mixtures. Diabetes Care. 1981;4:366–376. doi: 10.2337/diacare.4.3.366. [DOI] [PubMed] [Google Scholar]
- 5.Lane WS, Cochran EK, Jackson JA, et al. High-dose insulin therapy: is it time for U-500 insulin? Endocr Pract. 2009;15:71–79. doi: 10.4158/EP.15.1.71. [DOI] [PubMed] [Google Scholar]
- 6.Ballani P, Tran MT, Navar MD, et al. Clinical experience with U-500 regular insulin in obese, markedly insulin-resistant type 2 diabetic patients. Diabetes Care. 2006;29:2504–2505. doi: 10.2337/dc06-1478. [DOI] [PubMed] [Google Scholar]
- 7.Bulchandani DG, Konrady T, Hamburg MS. Clinical efficacy and patient satisfaction with U-500 insulin pump therapy in patients with type 2 diabetes. Endocr Pract. 2007;13:721–725. doi: 10.4158/EP.13.7.721. [DOI] [PubMed] [Google Scholar]
- 8.Knee TS, Seidensticker DF, Walton JL, et al. A novel use of U-500 insulin for continuous subcutaneous insulin infusion in patients with insulin resistance: a case series. Endocr Pract. 2003;9:181–186. doi: 10.4158/EP.9.3.181. [DOI] [PubMed] [Google Scholar]
- 9.Lane WS. Use of U-500 regular insulin by continuous subcutaneous insulin infusion in patients with type 2 diabetes and severe insulin resistance. Endocr Pract. 2006;12:251–256. doi: 10.4158/EP.12.3.251. [DOI] [PubMed] [Google Scholar]
- 10.Neal JM. Analysis of effectiveness of human U-500 insulin in patients unresponsive to conventional insulin therapy. Endocr Pract. 2005;11:305–307. doi: 10.4158/EP.11.5.305. [DOI] [PubMed] [Google Scholar]
- 11.Wafa WS, Khan MI. Use of U-500 regular insulin in type 2 diabetes. Diabetes Care. 2006;29:2175–2176. doi: 10.2337/dc06-1148. [DOI] [PubMed] [Google Scholar]
- 12.Burroughs TE, Desikan R, Waterman BM, et al. Development and Validation of the Diabetes Quality of Life Brief Clinical Inventory. 2004;17:41–49. [Google Scholar]
- 13.Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am. J. Cardiol. 1995;75:894–903. doi: 10.1016/s0002-9149(99)80683-3. [DOI] [PubMed] [Google Scholar]
- 14.Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 15.Weight gain associated with intensive therapy in the diabetes control and complications trial. The DCCT Research Group. Diabetes Care. 1988;11:567–573. doi: 10.2337/diacare.11.7.567. [DOI] [PubMed] [Google Scholar]
- 16.Crasto W, Jarvis J, Hackett E, et al. Insulin U-500 in severe insulin resistance in type 2 diabetes mellitus. Postgrad Med J. 2009;85:219–222. doi: 10.1136/pgmj.2008.073379. [DOI] [PubMed] [Google Scholar]
- 17.Binder C. Absorption of injected insulin. A clinical-pharmacological study. Acta Pharmacol Toxicol (Copenh) 1969;27(Suppl 2):1–84. doi: 10.1111/j.1600-0773.1969.tb03069.x. [DOI] [PubMed] [Google Scholar]