ABSTRACT
Growth of Escherichia coli on glucose in batch culture is accompanied by the excretion of acetate, which is consumed by the cells when glucose is exhausted. This glucose-acetate transition is classically described as a diauxie (two successive growth stages). Here, we investigated the physiological and metabolic properties of cells after glucose exhaustion through the analysis of growth parameters and gene expression. We found that E. coli cells grown on glucose in batch culture produce acetate and consume it after glucose exhaustion but do not grow on acetate. Acetate is catabolized, but key anabolic genes—such as the genes encoding enzymes of the glyoxylate shunt—are not upregulated, hence preventing growth. Both the induction of the latter anabolic genes and growth were observed only after prolonged exposure to low concentrations of acetate and could be accelerated by high acetate concentrations. We postulate that such decoupling between acetate catabolism and acetate anabolism might be an advantage for the survival of E. coli in the ever-changing environment of the intestine.
IMPORTANCE The glucose-acetate transition is a valuable experimental model for comprehensive investigations of metabolic adaptation and a current paradigm for developing modeling approaches in systems microbiology. Yet, the work reported in our paper demonstrates that the metabolic behavior of Escherichia coli during the glucose-acetate transition is much more complex than what has been reported so far. A decoupling between acetate catabolism and acetate anabolism was observed after glucose exhaustion, which has not been reported previously. This phenomenon could represent a strategy for optimal utilization of carbon resources during colonization and persistence of E. coli in the gut and is also of significant interest for biotechnological applications.
INTRODUCTION
The glucose-acetate transition has become a paradigm for the understanding and modeling of metabolic adaptation in Escherichia coli. This Gram-negative bacterium produces acetate during aerobic growth on glucose under batch conditions (1). Once glucose is exhausted, the cells consume the acetate that they have produced (2). This is classically described as a diauxic phenomenon, in which growth on glucose is followed by growth on acetate (2–4). The adaptation mechanisms underlying this behavior have been investigated by means of switch experiments in which cells are grown on glucose and then transferred to fresh medium containing acetate as a sole carbon source (5, 6). While this approach could differ from a physiological diauxie, it provides a valuable experimental framework to detail the metabolic changes and regulatory processes that must occur in glucose-growing cells to achieve growth on acetate. Basically, the switch from glucose medium to acetate medium requires the transition from glycolytic to gluconeogenic metabolism (3, 7, 8). Accordingly, glycolytic enzymes are expressed during growth on glucose and downregulated during growth on acetate. The gluconeogenic processes behave in the opposite way, i.e., they are downregulated on glucose and upregulated on acetate. Significant changes also occur in the tricarboxylic acid (TCA) cycle with the downregulation of isocitrate dehydrogenase on acetate while the enzymes enabling acetate utilization and that of the glyoxylate shunt are upregulated upon growth on acetate (9, 10). The glyoxylate shunt enables the conversion of acetate into C4 compounds that feed the gluconeogenic pathways and ultimately enable all biosynthetic pathways to produce biomass (11). Hence, this shunt is instrumental for growth on acetate, and its regulation, as well as acetate production and consumption processes, is still the subject of intense studies (12, 13).
Apart from switch experiments, very few studies have investigated the metabolic changes occurring throughout the progressive transition that occurs during standard growth on glucose. Interestingly, a close examination of some studies incidentally using the glucose-acetate transitions showed that acetate is consumed after glucose exhaustion but that there is no obvious growth during the same period of time (2, 14–17). This raises questions regarding not only the occurrence of true diauxic behavior during the glucose-acetate transition but also the metabolic adaptations that may occur in this situation.
Here, we report on the detailed investigation of the metabolic changes that occur during the glucose-acetate transition when E. coli is cultured on glucose in batch culture, showing that diauxic behavior does not occur under such conditions. We report a decoupling between acetate catabolism and acetate anabolism after glucose exhaustion. While catabolism is induced immediately after glucose exhaustion, the induction of key anabolic processes is delayed. The lag time before induction of the anabolic processes depends on acetate exposure: the higher the acetate concentration, the shorter the delay. In this paper, we discuss the function and consequences of this phenomenon.
MATERIALS AND METHODS
Strains, media, and growth conditions.
E. coli K-12 MG1655 cultures were carried out in M9 mineral medium complemented with 2.7 g · liter−1 (15 mM) d-glucose (18). Shake-flask cultures were performed in volumes of 50 ml or 200 ml with an M9 volume of a maximal 15% of the culture vessel. Bioreactor cultures were performed as described in reference 2. Briefly, pH was set at 7, temperature was set at 37°C, and dissolved oxygen tension was always maintained at over 20% to avoid micro-oxic conditions. In the acetate stabilization experiments, 0.5 M glacial acetic solution (pH 3) was used to maintain the pH at 7 until growth started again. The percentages of CO2 and O2 in the gas output were monitored using a Dycor ProLine process mass spectrometer (Ametek, DE). Cultures in bioreactors and shake flasks were inoculated with a washed-cell sample obtained from an overnight preculture on M9 glucose (the preculture is centrifuged for 3 min at 8,000 rpm, the supernatant is discarded, and the pellet is resuspended in the culture medium). Optical densities at 600 nm (OD600) were measured using a spectrophotometer (Genesys6 from Thermo Scientific, MA). Apparent growth rates at the periods described were calculated using the formula μ = ln(OD2/OD1)/(t2 − t1). All cultures were performed independently in duplicate or triplicate.
Exometabolome.
Extracellular metabolites were identified and quantified by nuclear magnetic resonance (NMR) using an Avance 500-MHz NMR spectrometer (Bruker, Rheinstatten, Germany) as described in reference 2.
RT-qPCR analysis.
Gene expression was monitored by reverse transcriptase quantitative PCR (RT-qPCR) as described in reference 2. The primers used in this work are listed in Table 1.
TABLE 1.
RT-qPCR primers
| Name | Sequence (5′ to 3′) |
|---|---|
| Q-16S-3′ | ATCCGGACTACGACGCACTT |
| Q-16S-5′ | ACGACCAGGGCTACACACG |
| Q-acs-3′ | GGATCTTCGGCGTTCATCTC |
| Q-acs-5′ | GGGAAAATTGACTGGCAGGA |
| Q-fbp3′ | GTAGAGATAAATACCGCCTTTCAGCA |
| Q-fbp5′ | ATAAATCCACCAACCGCCCTTA |
| Q-Icd-3′ | TTCGTCACCGATGTTTGCAC |
| Q-Icd-5′ | CGCCTGTATGAACCTGAACG |
| Q-icl-3′ | AACCAGCAGGGTTGGAACG |
| Q-icl-5′ | ACATGGGCGGCAAAGTTTTA |
| Q-ihfB-3′ | CAAAGAGAAACTGCCGAAACC |
| Q-ihfB-5′ | GCCAAGACGGTTGAAGATGC |
| Q-mls-3′ | TCAGGCCATAAATCGGCACA |
| Q-mls-5′ | GGTGAACGCACCGAAGAAGG |
| Q-pck3′ | GTGTCTACGCCCGGCAGTTC |
| Q-pck5′ | GACGCCATCCTCAACGGTTC |
| Q-pfkA3′ | CACCCATGTAGGAACCGTCA |
| Q-pfkA5′ | AATTCCGCGACGAGAACATC |
| Q-ppc3′ | CAGGCGAGAACGCAGGTTTT |
| Q-ppc5′ | ATGGTTGAAGCGACCCCTGA |
| Q-pps3′ | CTGGCTCGTAACGCTCACCA |
| Q-pps5′ | GTGCCGCGTTTTATCCGAAG |
| Q-pykF3′ | GCAACCATGATGCCGTCAGA |
| Q-pykF5′ | CGGCGAAAACATCCACATCA |
Switch experiments.
To investigate the timing of phenotypic adaptation throughout the glucose-acetate transition, cell samples were collected from a “mother” culture at 10-min intervals, starting 60 min before glucose exhaustion (GE) to 90 min after. The cell samples were rapidly filtered (Minisart 0.45-μm filter from Sartorius, Göttingen, Germany) and rinsed on the filter with fresh medium containing either glucose or acetate, depending on the substrate used in the following culture. The washed cells were used to inoculate 250-ml Erlenmeyer flasks filled with 30 ml of acetate medium or glucose medium, to reach an initial OD600 of 0.2. To minimize stress for the cells, all the equipment and solutions were prewarmed at 37°C and all the switches were performed within less than 2 min. Growth of the “daughter” cultures was monitored at times 0, 60, and 90 min after inoculation by spectrophotometry at 600 nm. We made sure that the cells from the daughter culture reached the maximal growth rate (μmax) by measuring the growth rate between 60 and 90 min. The μmax values were determined as 0.23 h−1 on M9-acetate and 0.57 h−1 on M9-glucose. To calculate the lag time before this maximal growth, we used the following reasoning: if the growth rate is maximal from t0 (i.e., no delay), tm is the theoretical time value needed to increase the biomass from X0 to X1 (as illustrated in Fig. 1). If there is a delay, X1 will be obtained at t1 (i.e., later than tm). So, the lag time is equal to t1 − tm. By definition,
| (1) |
FIG 1.
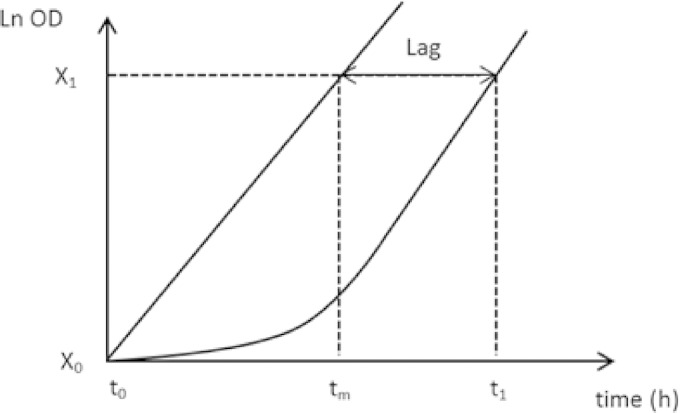
Theoretical growth profiles for the calculation of the lag in the switch experiments. tm is the theoretical time value needed to increase the biomass from X0 to X1 if the growth rate is maximal (μmax) from t0. If there is a delay, X1 will be obtained at t1 (i.e., later than tm). From these elements, the lag can be determined as demonstrated in Materials and Methods.
from which
| (2) |
since
| (3) |
From equations 3 and 2,
| (4) |
We used equation 4 to calculate the lag time in the daughter culture with t1 = 90 min and the biomass values at time zero and 90 min after the inoculation as X0 and X1, respectively.
RESULTS
Growth on acetate was not observed after glucose exhaustion.
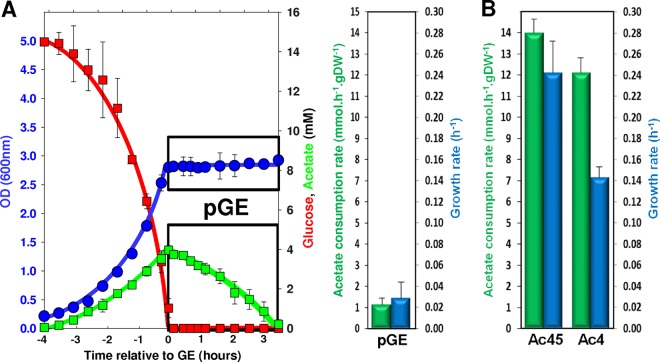
Figure 2A shows a typical cultivation profile of E. coli cells grown under aerobic batch conditions on 15 mM glucose in minimal medium. Exponential growth was observed until glucose was exhausted (growth referring to the population behavior in this report). At the time of glucose exhaustion (here referred to as GE), about 4 mM acetate was produced by the cells. Subsequently, acetate was consumed at the rate of 1.14 ± 0.31 mmol · h−1 · g (dry weight [DW])−1, but no significant growth was observed (apparent μ = 0.03 ± 0.02 h−1 [Fig. 2A]). To check if, for any reason, the culture conditions used in this work could prevent growth on acetate, we performed cultures under the same conditions as described above but with 45 mM acetate instead of 15 mM glucose (i.e., the same carbon molar content in the two cases) as the sole carbon source. Growth on acetate was observed at a rate of 0.24 ± 0.03 h−1, and the measured acetate consumption rate was 13.9 ± 0.5 mmol · h−1 · g (DW)−1. These values are in agreement with values reported in the literature (14, 19) and showed that the culture conditions did not prevent growth on acetate. Since the Monod constant for acetate in E. coli was established at a Ks of 8.5 mM (20), a possible explanation for the lack of post-GE growth upon culture on 15 mM glucose could be that the amount of acetate produced (4 mM) is too small to enable growth. When E. coli cells were grown on minimal medium with 4 mM acetate as the sole carbon source, growth at a rate of 0.13 ± 0.02 h−1 could be measured and the rate of acetate consumption was 12.1 ± 0.9 mmol · h−1 · g (DW)−1, i.e., well above that observed after growth on glucose (Fig. 2B). These data demonstrate that upon growth on 15 mM glucose, there is no growth on acetate after GE, although the level of acetate is sufficient to allow such growth.
FIG 2.
Growth parameters after the glucose exhaustion in a glucose-acetate transition and during growth on acetate as sole carbon source. (A) Glucose, acetate, and biomass (OD) profiles of a culture performed in minimal M9 medium plus 15 mM glucose are shown on the left, with the period following glucose exhaustion boxed (pGE, post-glucose exhaustion). On the right, acetate consumption rates (millimoles of acetate consumed per hour and per gram of biomass [dry weight]) and the growth rate during the post-GE period are presented. (B) Acetate consumption rates and growth rates during growth on 45 mM acetate (Ac45) or 4 mM acetate (Ac4).
Acetate anabolism is not fully induced after GE.
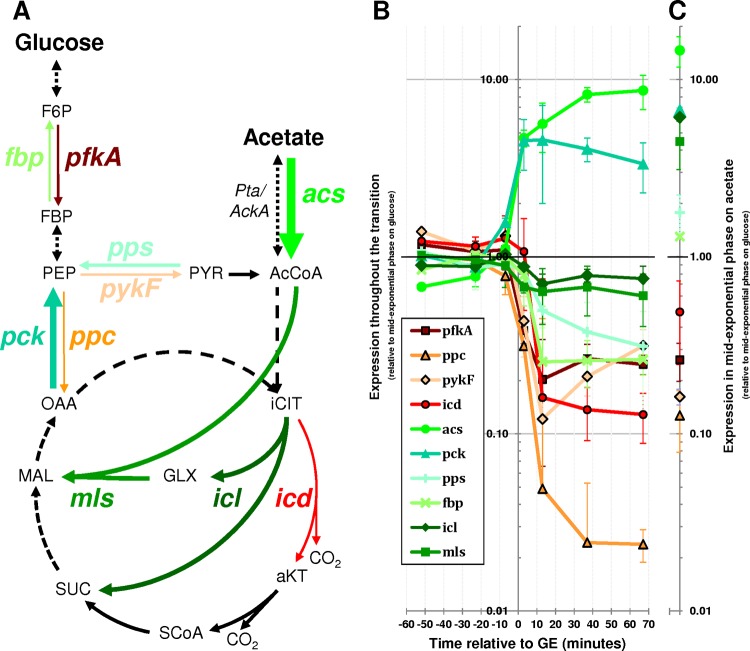
To investigate the metabolic fate of acetate after GE, the expression of key metabolic genes (Fig. 3A) was measured during the entire period of glucose-acetate transition (Fig. 3B) and during exponential growth on acetate (Fig. 3C).
FIG 3.
Expression of key metabolic genes throughout the glucose-acetate transition. (A) Position of investigated genes in E. coli metabolism. Dashed lines represent several reaction steps. The thickness of the arrows increases or decreases with higher or lower gene expression 70 min after glucose exhaustion as found in panel B. (B) Cells were cultured in M9 minimal medium with 15 mM glucose, and RT-qPCR was used to quantify gene expression from 60 min before glucose exhaustion (GE) to 70 min after GE. The expression levels are relative to the levels measured at mid-exponential growth on glucose (115 min before GE). (C) The expression levels of the same set of genes were also measured during exponential growth on 45 mM acetate as the sole carbon source. The expression levels are relative to the levels measured at mid-exponential growth on glucose (as in panel B). pfkA, phosphofructokinase (EG10699); ppc, phosphoenolpyruvate carboxylase (EG10756); pykF, pyruvate kinase (EG10804); icd, isocitrate dehydrogenase (EG10489); acs, acetyl coenzyme A synthetase (EG11448); pck, phosphoenolpyruvate carboxykinase (EG10688); pps, phosphoenolpyruvate synthetase (EG10759); fbp, fructose-1,6-biphosphatase (EG10283); icl, isocitrate lyase (EG10022); mls, malate synthase (EG10023); F6P, fructose-6-phosphate; FBP, fructose-1,6-biphosphate; PEP, phosphoenolpyruvate; OAA, oxaloacetate; PYR, pyruvate; AcCoA, acetyl coenzyme A; iCIT, isocitrate; aKT, 2-oxoglutarate; SCoA, succinyl coenzyme A; SUC, succinate; MAL, malate.
First, the levels of gene expression in cells exponentially growing in fresh medium with acetate as the sole carbon source were compared to those of cells exponentially growing on glucose (Fig. 3C). Compared to cells grown on glucose, cells grown on acetate poorly expressed genes encoding key glycolytic enzymes but expressed more genes associated with acetate consumption (acs) and anabolism (pps, fbp, and pck and the glyoxylate shunt genes icl and mls), as expected from the literature (19, 21). These data are in complete agreement with expectations and more specifically with the role of specific anabolic processes—e.g., glyoxylate shunt—in the ability of E. coli to grow on acetate.
Then, the change of gene expression during the glucose-acetate transition was analyzed (Fig. 3B). The gene expression levels were quite stable until glucose was exhausted. After GE, the glycolytic genes pfkA and pykF, the anaplerotic gene ppc, and the TCA cycle gene icd were downregulated. Both the acs and pck genes were upregulated. These data pointed to a metabolic change in response to the disappearance of glucose, consistent with the observed consumption of acetate. In contrast, some acetate anabolic genes (pps and fbp), as well as the glyoxylate shunt genes icl and mls, were not upregulated after GE (Fig. 3B). The two glyoxylate shunt genes showed somewhat lower levels of expression after GE. The pattern of gene expression established a few minutes after GE remained stable until acetate was exhausted (Fig. 3B). This means that genes essential for acetate anabolism are not upregulated after GE, although acetate is consumed. The pattern of gene expression in cells utilizing acetate after growth on glucose (Fig. 3B) is therefore different from that of cells growing on acetate as the sole carbon source (Fig. 3C). In particular, because the glyoxylate shunt is essential for the production of biomass from acetate, the lack of icl and mls upregulation is consistent with the absence of growth on acetate after GE.
Post-GE cells can rapidly resume growth on glucose but are not adapted to growth on acetate.
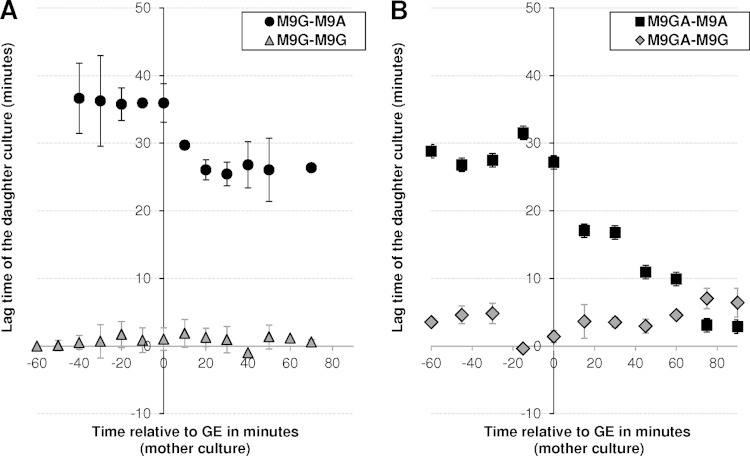
To strengthen the observation that the metabolic behavior of cells using acetate after GE is different from that of cells growing on fresh acetate medium, we analyzed the capacity of the former cells to adapt to growth on acetate or glucose as the sole carbon source. This was performed using switch experiments (Fig. 4) in which cells were initially grown in M9 minimal medium containing 15 mM glucose as the sole carbon source (mother cultures). Culture samples from this mother culture were then collected at 10-min intervals, starting from 60 min before GE to 70 min after GE. The collected cells were rapidly transferred to fresh medium (daughter cultures) containing either 15 mM glucose (M9G-M9G) or 45 mM acetate as sole carbon source (M9G-M9A).
FIG 4.
Impact of the cultivation time on the delay in achieving maximal growth in fresh medium on acetate or glucose. Cells were grown in M9 minimal medium containing either 15 mM glucose (A) or 15 mM glucose plus 32 mM acetate as the substrate (B). From 60 min before to 90 min after glucose exhaustion, cell samples were collected at 10-min intervals and used to inoculate fresh medium containing either 15 mM glucose or 32 mM acetate as the sole carbon source. In total, four conditions were investigated: M9 glucose switched to M9 acetate (M9G-M9A) and M9 glucose switched to M9 glucose (M9G-M9G) (A) and M9 glucose plus acetate switched to M9 acetate (M9GA-M9A) and M9 glucose plus acetate switched to M9 glucose (M9GA-M9G) (B). For each switch experiment, the time needed before adaptation was defined as the time needed for the cells collected from the mother culture to reach maximal growth rate after being switched to the daughter culture medium.
Whatever the collection time, cells collected from the mother culture and transferred to fresh glucose medium were immediately able to achieve maximal growth (i.e., lag times close to 0 [M9G-M9G in Fig. 4A]). This was expected for cells collected before GE but was more surprising for cells collected after GE, since the analysis of gene expression levels has evidenced metabolic adaptations in these cells. These results indicate that the metabolic adaptations occurring after GE do not modify the cells' efficiency in growing on glucose. Cells collected before GE in the mother culture and transferred to fresh acetate medium reached maximal growth on acetate after 36 ± 2 min (M9G-M9A in Fig. 4A). This delay decreased for cells collected after GE, reaching 25 ± 1 min when cells were collected 20 min after GE, but did not decrease further for cells collected later. This means that after growth on glucose, post-GE cells are not fully able to grow on acetate, although they consume this compound, but remain able to grow on glucose.
To investigate if priming the cells with a high concentration of acetate could reduce the delay in reaching maximal growth on acetate, novel switch experiments were performed in which mother cultures consisted of cells grown on 15 mM glucose plus 32 mM acetate (Fig. 4B). Cells grown on glucose plus acetate and collected before GE required slightly less time to reach maximal growth rate on fresh acetate medium than cells grown on glucose (29 ± 2 versus 36 ± 2 min, respectively [M9GA-M9A in Fig. 4B]). After GE, the delay to reach maximal acetate growth decreased with the time of cell collection. No significant delay was observed for cells collected 75 min after GE or later, indicating that cells progressively adapted to acetate growth during the post-GE period. Interestingly, cells grown on glucose plus acetate and collected after GE showed a slight decrease in their ability to resume growth on glucose, which became clear in cells collected 70 min after GE (M9GA-M9G in Fig. 4B). These data show that cells grown on glucose did not fully adapt to growth on acetate after GE unless they were exposed to significant amounts of acetate.
Growth after GE at high acetate concentrations.
We then investigated in more detail the impact of high acetate concentrations on the ability of E. coli to grow on acetate after GE. This was tested by growing cells on mixtures of 15 mM glucose and acetate concentrations ranging from 2 to 128 mM and by analyzing the impact of acetate on the post-GE phase. No growth was detected after GE for cells grown in mixtures containing 2 to 8 mM acetate (Fig. 5A). In contrast, growth was observed after GE when cells were grown with mixtures in which the acetate concentration was at least 16 mM, i.e., four times the acetate concentration produced from 15 mM glucose (Fig. 5A). In parallel, significant increases in acetate consumption rates were observed (Fig. 5B). Thus, both the growth and acetate consumption rates observed after GE increased with acetate concentration (Fig. 5A and B). After GE, the growth rate of cells cultured on glucose plus 128 mM acetate became close to that of cells growing on 45 mM acetate as the sole carbon source (0.17 ± 0.01 versus 0.24 ± 0.03 h−1, respectively). The rates of acetate utilization also became closer (9.2 ± 1.2 versus 13.9 ± 0.5 mmol · h−1 · g [DW]−1). These data show that growth on acetate after GE can be achieved when acetate is available at high concentrations. To support these observations, E. coli cells were cultured on glucose-acetate mixtures and the expression of acetate anabolic genes (pps, fbp, icl, and mls) was monitored by RT-qPCR before (at mid-exponential phase) and after (90 min after GE) glucose exhaustion (Fig. 5C). Before GE, the expression levels in cells grown on glucose-acetate mixtures were similar for all four genes to those observed in cells grown on glucose (data not shown), showing that the expression of these genes was not influenced by the concentrations of acetate in the medium. Expression of fbp and pps decreased after GE, as observed with cells grown on 15 mM glucose as the sole carbon source. This phenomenon tended to progressively disappear when acetate was added at high concentrations. At concentrations of up to 8 mM acetate, the glyoxylate shunt genes icl and mls were not upregulated after GE. At higher concentrations, the two genes were clearly upregulated 90 min after GE. The expression of these genes is consistent with the growth observed under the same conditions. Taken together, these data showed that the addition of high concentrations of acetate (16 mM or above) allowed complete upregulation of the anabolic machinery required for growth on acetate. These data further support the fact that post-GE cells that have grown on glucose as the sole carbon source do not express the complete anabolic machinery to grow on acetate, at least for 90 min after GE.
FIG 5.
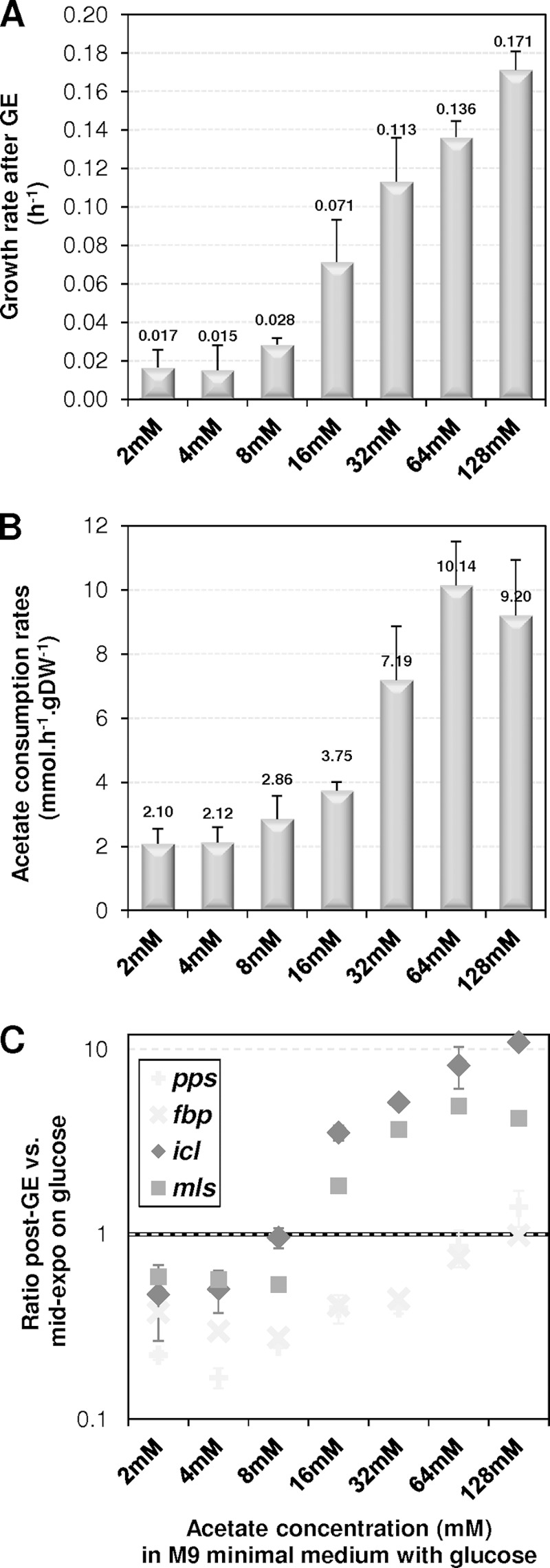
Impact of acetate concentration on acetate metabolism after glucose exhaustion. Cells were grown in M9 minimal medium containing glucose-acetate mixtures (15 mM glucose plus 0 to 128 mM acetate) as the substrate. Growth and expression of key acetate anabolism genes were measured after glucose exhaustion (GE). (A) Maximal growth rates after GE. (B) Maximal acetate consumption rates after GE. (C) Expression of acetate anabolic genes 90 min after glucose exhaustion, relative to levels at mid-exponential growth on glucose.
Acetate exposure determines the onset of anabolism after GE.
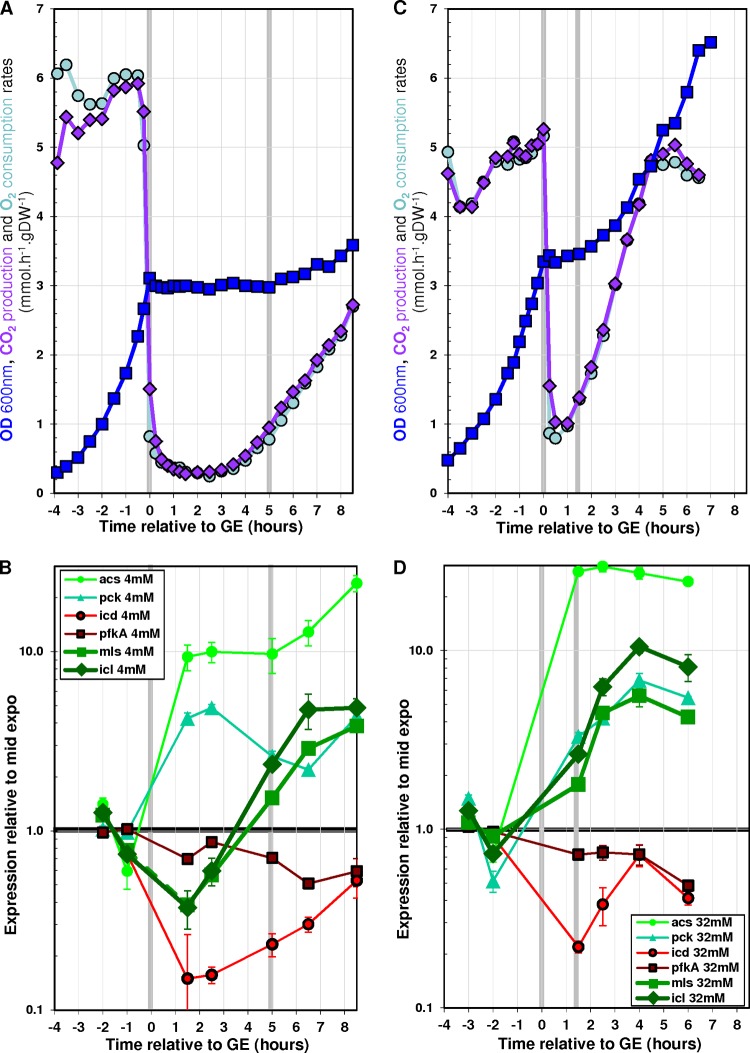
Two scenarios could explain the above results. In scenario 1, the induction of acetate anabolic genes after GE requires a threshold in acetate concentration which would be between 8 and 16 mM; in scenario 2, the time needed for effective induction of acetate anabolic genes after GE depends on acetate concentration and is higher than 90 min when acetate concentration is equal to or below 8 mM. To investigate these hypotheses, we performed an experiment in which cells were cultured in minimal medium containing 15 mM glucose as the sole initial carbon source but in which the acetate concentration was maintained at 4 mM after GE. This concentration is under the putative threshold from scenario 1 and therefore should not enable growth on acetate if this scenario is correct. According to scenario 2, maintaining a low concentration of acetate over a long time should enable growth on acetate. The maintenance of 4 mM acetate after GE was achieved by using a strategy in which acetate was continuously fed just after GE to keep the acetate concentration constant (see Materials and Methods for details). Figure 6A shows the growth profiles obtained during these cultures. A drop in both CO2 production and O2 consumption was observed during the first 3 h following GE, while the biomass concentration remained constant over the same period. Three hours post-GE, a dramatic increase in both CO2 production and O2 consumption was observed. Furthermore, a significant increase in biomass concentration was observed 5 h after GE. These data show that growth on 4 mM acetate after growth on glucose can be achieved provided that the concentration of acetate is maintained for 5 h or more.
FIG 6.
Effect of artificial stabilization of acetate concentration after glucose exhaustion on acetate metabolism. Cells were grown in batch reactors, and glacial acetic acid was added after glucose exhaustion to maintain both pH and acetate concentration. The cultures were performed in M9 minimal medium supplemented with either 15 mM glucose (A and B) (resulting in 4 mM acetate after GE) or 15 mM glucose plus 32 mM acetate (C and D). Time zero is defined as the time of glucose exhaustion. (A and C) ODs, CO2 production rates, and O2 consumption rates. (B and D) Gene expression profiles throughout the transition relative to the mid-exponential phase of growth on glucose.
The levels of gene expression during these cultures were monitored by RT-qPCR. Consistent with the above results, the genes acs and pck were upregulated soon after GE, while icd and pfkA were downregulated (Fig. 6B). The expression of these genes remained almost stable for the next 8 h of the experiment. The glyoxylate shunt genes icl and mls were clearly upregulated 5 h after GE, in agreement with the observed increase in biomass. These results show that post-GE growth on 4 mM acetate is concomitant with the induction of the glyoxylate shunt genes.
Taken together, all the above results indicate that post-GE growth on acetate can occur (i) after a significant delay if low concentrations of acetate are maintained for a long period of time (Fig. 6A) or (ii) more rapidly if high concentrations of acetate are added (Fig. 5A). These observations suggest that the concentration of acetate per se does not determine the capability of cells to induce the machinery enabling growth on acetate but determines the time needed to achieve complete induction of this machinery. To confirm this hypothesis, an experiment was performed in which cells were grown on 15 mM glucose plus 32 mM acetate and the post-GE concentration of acetate was maintained at 32 mM (Fig. 6C). Under such conditions, growth on acetate was observed within 90 min after GE, i.e., much sooner than when the concentration was maintained at 4 mM acetate (Fig. 6A). The earlier onset of growth was correlated with the earlier upregulation of icl and mls expression (Fig. 6D). We therefore conclude that the concentration of acetate reduces the duration of the adaptation to acetate growth by accelerating the upregulation of the glyoxylate shunt genes. Thus, the induction of acetate anabolism appears to depend on the total exposure to acetate. It can be achieved rapidly at high acetate concentrations but requires at least 5 h at 4 mM acetate. Finally, this means that growth does not occur under standard laboratory conditions (i.e., growth on 15 mM glucose as the sole carbon source) because the 4 mM acetate initially produced was consumed within 3.5 h after GE (Fig. 2), before the 5 h needed to induce the growth capability.
DISCUSSION
The data reported here indicate that the behavior of E. coli during the glucose-acetate transition is far more complex than a simple switch from growth on glucose to growth on acetate, as both the time scale of adaptation and the sequence of gene induction appear to differ. In a switch from glucose medium to acetate medium, all the genes involved in acetate consumption and anabolism are induced at the same time, thereby enabling growth. In contrast, cells grown on glucose and producing acetate do not immediately upregulate all the genes required for acetate anabolism once glucose is exhausted, whereas they readily upregulate genes allowing more efficient consumption of acetate (e.g., acs and pck). This leads to decoupling between the induction of acetate catabolism and that of acetate anabolism. The upregulation of the key anabolic processes (e.g., glyoxylate shunt) requires significant exposure to acetate. It is fast at a high concentration of acetate (1.5 h after GE at 32 mM acetate) but slow at a low concentration of acetate (5 h after GE at 4 mM acetate). Under the standard laboratory conditions considered in this work, acetate was exhausted before anabolism was established. This explains the lack of growth after GE and hence the absence of a diauxic behavior. This is reported “in passing” in the literature (2, 14–17). This clarification about the sequence of adaptation events is of importance given the current mathematical efforts to model the kinetics of the glucose-acetate transition (4, 22). Such models are often calibrated using data from experiments in which the cells do grow on acetate as the sole carbon source, which does not appear to be representative of the physiological transition. For instance, our attempts to predict the lack of growth on acetate after GE using the model developed by Kotte et al. failed since the model was calibrated from chemostat cultures with either glucose or acetate as the sole carbon source (4).
It was recently suggested that metabolic adaptation could be achieved only by a subset of the cell population (20, 23, 24). Interestingly, Kotte et al. also reported that the size of the E. coli subpopulation able to adapt to acetate—after being switched from glucose medium to acetate medium—increased with an increase in the concentration of acetate (20). Hence, in our experiments, the faster induction of key anabolic genes at high concentrations of acetate could result from a stronger impact on the entire population or, alternatively, on the existence of a larger subpopulation able to adapt to acetate growth, or even both.
The data reported here raise the question of the role of the decoupling between acetate anabolism and acetate catabolism observed after glucose exhaustion. Acetate is a poor growth substrate for E. coli compared to other substrates used by the bacterium, such as sugars and sugar derivatives (19, 25–27). The delay in inducing anabolism at low acetate concentrations might be a mechanism by which the preferential utilization of other carbon sources is ensured. But the gut, which is the main natural reservoir of E. coli, contains high levels of acetate, since concentrations of up to 30 to 100 mM have been reported (28–30). The fact that the rapid induction of acetate anabolism—enabling growth on acetate—requires elevated acetate concentrations is likely the result of an adaptation to the environmental conditions found in the gut. Because there are permanent changes in substrate availability in the gut, one can imagine that, at low acetate concentrations, the pure catabolic use of this organic acid allows cells to satisfy their energy requirements and maintain cellular integrity. If alternative substrates become available in the meantime, then the cells can rapidly resume growth on these compounds. If no alternative substrate becomes available, complete induction of acetate anabolism is achieved and cells can grow on acetate, although at a low rate. It was recently reported that nongrowing stationary-phase bacteria can maintain constant protein production for prolonged periods of time (31), enabling them to rapidly resume growth when appropriate substrates become available. The observation in our experiments that post-GE cells (not fully adapted to acetate growth on acetate) are able to resume rapid growth on glucose is consistent with this idea. This is also consistent with a previous observation made during glucose-to-acetate switch experiments, whereby cells not able to adapt to acetate consumption are more able to resume growth when glycolytic conditions return (20). Since the gut is an ever-changing and highly competitive environment (26, 32–34), the ability to optimize substrate use is a valuable tradeoff between optimality and minimal adjustment to alternative conditions (35) and is likely to be an advantage for the life of E. coli in its main reservoir.
ACKNOWLEDGMENTS
B.E. was supported by the INRA (Institut National de la Recherche Agronomique) and the INSA (Institut National des Sciences Appliquées) (Program Chaire d'Excellence).
We are grateful to the members of the MetaSys team (LISBP, Toulouse, France) for fruitful discussions. The support of Lindsay Peyriga and Edern Cahoreau from the METATOUL platform (LISBP, Toulouse, France), of Maëlis Pinault, and of Christian Treitz and Andreas Tholey from Kiel University (Germany) is gratefully acknowledged.
REFERENCES
- 1.Luli GW, Strohl WR. 1990. Comparison of growth, acetate production, and acetate inhibition of Escherichia coli strains in batch and fed-batch fermentations. Appl Environ Microbiol 56:1004–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enjalbert B, Letisse F, Portais JC. 2013. Physiological and molecular timing of the glucose to acetate transition in Escherichia coli. Metabolites 3:820–837. doi: 10.3390/metabo3030820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfe AJ. 2005. The acetate switch. Microbiol Mol Biol Rev 69:12–50. doi: 10.1128/MMBR.69.1.12-50.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotte O, Zaugg JB, Heinemann M. 2010. Bacterial adaptation through distributed sensing of metabolic fluxes. Mol Syst Biol 6:355. doi: 10.1038/msb.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh MK, Rohlin L, Kao KC, Liao JC. 2002. Global expression profiling of acetate-grown Escherichia coli. J Biol Chem 277:13175–13183. doi: 10.1074/jbc.M110809200. [DOI] [PubMed] [Google Scholar]
- 6.Kao KC, Tran LM, Liao JC. 2005. A global regulatory role of gluconeogenic genes in Escherichia coli revealed by transcriptome network analysis. J Biol Chem 280:36079–36087. doi: 10.1074/jbc.M508202200. [DOI] [PubMed] [Google Scholar]
- 7.El-Mansi M, Cozzone AJ, Shiloach J, Eikmanns BJ. 2006. Control of carbon flux through enzymes of central and intermediary metabolism during growth of Escherichia coli on acetate. Curr Opin Microbiol 9:173–179. doi: 10.1016/j.mib.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Monod J. 1949. The growth of bacterial cultures. Annu Rev Microbiol 3:371–394. doi: 10.1146/annurev.mi.03.100149.002103. [DOI] [Google Scholar]
- 9.Peng L, Shimizu K. 2003. Global metabolic regulation analysis for Escherichia coli K-12 based on protein expression by 2-dimensional electrophoresis and enzyme activity measurement. Appl Microbiol Biotechnol 61:163–178. doi: 10.1007/s00253-002-1202-6. [DOI] [PubMed] [Google Scholar]
- 10.Prasad Maharjan R, Yu PL, Seeto S, Ferenci T. 2005. The role of isocitrate lyase and the glyoxylate cycle in Escherichia coli growing under glucose limitation. Res Microbiol 156:178–183. doi: 10.1016/j.resmic.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Walsh K, Koshland DE. 1984. Determination of flux through the branch point of two metabolic cycles. J Biol Chem 259:9646–9654. [PubMed] [Google Scholar]
- 12.Peebo K, Valgepea K, Nahku R, Riis G, Oun M, Adamberg K, Vilu R. 2014. Coordinated activation of PTA-ACS and TCA cycles strongly reduces overflow metabolism of acetate in Escherichia coli. Appl Microbiol Biotechnol 98:5131–5143. doi: 10.1007/s00253-014-5613-y. [DOI] [PubMed] [Google Scholar]
- 13.Castano-Cerezo S, Bernal V, Post H, Fuhrer T, Cappadona S, Sanchez-Diaz NC, Sauer U, Heck AJ, Altelaar AM, Canovas M. 2014. Protein acetylation affects acetate metabolism, motility and acid stress response in Escherichia coli. Mol Syst Biol 10:762. doi: 10.15252/msb.20145227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen KB, von Meyenburg K. 1980. Are growth rates of Escherichia coli in batch culture limited by respiration? J Bacteriol 144:114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varma A, Palsson BO. 1994. Stoichiometric flux balance models quantitatively predict growth and metabolic by-product secretion in wild-type Escherichia coli W3110. Appl Environ Microbiol 60:3724–3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu B, Jahic M, Enfors SO. 1999. Modeling of overflow metabolism in batch and fed-batch cultures of Escherichia coli. Biotechnol Prog 15:81–90. doi: 10.1021/bp9801087. [DOI] [PubMed] [Google Scholar]
- 17.O'Beirne D, Hamer G. 2000. The utilisation of glucose/acetate mixtures by Escherichia coli W3110 under aerobic growth conditions. Bioprocess Eng 23:375–380. doi: 10.1007/s004499900176. [DOI] [Google Scholar]
- 18.Nicolas C, Kiefer P, Letisse F, Kromer J, Massou S, Soucaille P, Wittmann C, Lindley ND, Portais JC. 2007. Response of the central metabolism of Escherichia coli to modified expression of the gene encoding the glucose-6-phosphate dehydrogenase. FEBS Lett 581:3771–3776. doi: 10.1016/j.febslet.2007.06.066. [DOI] [PubMed] [Google Scholar]
- 19.Liu M, Durfee T, Cabrera JE, Zhao K, Jin DJ, Blattner FR. 2005. Global transcriptional programs reveal a carbon source foraging strategy by Escherichia coli. J Biol Chem 280:15921–15927. doi: 10.1074/jbc.M414050200. [DOI] [PubMed] [Google Scholar]
- 20.Kotte O, Volkmer B, Radzikowski JL, Heinemann M. 2014. Phenotypic bistability in Escherichia coli's central carbon metabolism. Mol Syst Biol 10:736. doi: 10.15252/msb.20135022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao YP, Patnaik R, Roof WD, Young RF, Liao JC. 1993. Control of gluconeogenic growth by pps and pck in Escherichia coli. J Bacteriol 175:6939–6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baldazzi V, Ropers D, Geiselmann J, Kahn D, de Jong H. 2012. Importance of metabolic coupling for the dynamics of gene expression following a diauxic shift in Escherichia coli. J Theor Biol 295:100–115. doi: 10.1016/j.jtbi.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 23.van Heerden JH, Wortel MT, Bruggeman FJ, Heijnen JJ, Bollen YJ, Planque R, Hulshof J, O'Toole TG, Wahl SA, Teusink B. 2014. Lost in transition: start-up of glycolysis yields subpopulations of nongrowing cells. Science 343:1245114. doi: 10.1126/science.1245114. [DOI] [PubMed] [Google Scholar]
- 24.Solopova A, van Gestel J, Weissing FJ, Bachmann H, Teusink B, Kok J, Kuipers OP. 2014. Bet-hedging during bacterial diauxic shift. Proc Natl Acad Sci U S A 111:7427–7432. doi: 10.1073/pnas.1320063111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beg QK, Vazquez A, Ernst J, de Menezes MA, Bar-Joseph Z, Barabasi AL, Oltvai ZN. 2007. Intracellular crowding defines the mode and sequence of substrate uptake by Escherichia coli and constrains its metabolic activity. Proc Natl Acad Sci U S A 104:12663–12668. doi: 10.1073/pnas.0609845104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fabich AJ, Jones SA, Chowdhury FZ, Cernosek A, Anderson A, Smalley D, McHargue JW, Hightower GA, Smith JT, Autieri SM, Leatham MP, Lins JJ, Allen RL, Laux DC, Cohen PS, Conway T. 2008. Comparison of carbon nutrition for pathogenic and commensal Escherichia coli strains in the mouse intestine. Infect Immun 76:1143–1152. doi: 10.1128/IAI.01386-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Y, Vazquez A, Wise A, Warita T, Warita K, Bar-Joseph Z, Oltvai ZN. 2013. Carbon catabolite repression correlates with the maintenance of near invariant molecular crowding in proliferating E. coli cells. BMC Syst Biol 7:138. doi: 10.1186/1752-0509-7-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Argenzio RA, Southworth M, Stevens CE. 1974. Sites of organic acid production and absorption in the equine gastrointestinal tract. Am J Physiol 226:1043–1050. [DOI] [PubMed] [Google Scholar]
- 29.Cummings JH, Englyst HN. 1987. Fermentation in the human large intestine and the available substrates. Am J Clin Nutr 45:1243–1255. [DOI] [PubMed] [Google Scholar]
- 30.Macfarlane GT, Gibson GR, Cummings JH. 1992. Comparison of fermentation reactions in different regions of the human colon. J Appl Bacteriol 72:57–64. doi: 10.1111/j.1365-2672.1992.tb04882.x. [DOI] [PubMed] [Google Scholar]
- 31.Gefen O, Fridman O, Ronin I, Balaban NQ. 2014. Direct observation of single stationary-phase bacteria reveals a surprisingly long period of constant protein production activity. Proc Natl Acad Sci U S A 111:556–561. doi: 10.1073/pnas.1314114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koch AL. 1971. The adaptive responses of Escherichia coli to a feast and famine existence. Adv Microb Physiol 6:147–217. doi: 10.1016/S0065-2911(08)60069-7. [DOI] [PubMed] [Google Scholar]
- 33.Chang DE, Smalley DJ, Tucker DL, Leatham MP, Norris WE, Stevenson SJ, Anderson AB, Grissom JE, Laux DC, Cohen PS, Conway T. 2004. Carbon nutrition of Escherichia coli in the mouse intestine. Proc Natl Acad Sci U S A 101:7427–7432. doi: 10.1073/pnas.0307888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miranda RL, Conway T, Leatham MP, Chang DE, Norris WE, Allen JH, Stevenson SJ, Laux DC, Cohen PS. 2004. Glycolytic and gluconeogenic growth of Escherichia coli O157:H7 (EDL933) and E. coli K-12 (MG1655) in the mouse intestine. Infect Immun 72:1666–1676. doi: 10.1128/IAI.72.3.1666-1676.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuetz R, Kuepfer L, Sauer U. 2007. Systematic evaluation of objective functions for predicting intracellular fluxes in Escherichia coli. Mol Syst Biol 3:119. [DOI] [PMC free article] [PubMed] [Google Scholar]