ABSTRACT
Propionyl coenzyme A (propionyl-CoA) assimilation by Rhodobacter sphaeroides proceeds via the methylmalonyl-CoA pathway. The activity of the key enzyme of the pathway, propionyl-CoA carboxylase (PCC), was upregulated 20-fold during growth with propionate compared to growth with succinate. Because propionyl-CoA is an intermediate in acetyl-CoA assimilation via the ethylmalonyl-CoA pathway, acetate growth also requires the methylmalonyl-CoA pathway. PCC activities were upregulated 8-fold in extracts of acetate-grown cells compared to extracts of succinate-grown cells. The upregulation of PCC activities during growth with propionate or acetate corresponded to increased expression of the pccB gene, which encodes a subunit of PCC. PccR (RSP_2186) was identified to be a transcriptional regulator required for the upregulation of pccB transcript levels and, consequently, PCC activity: growth substrate-dependent regulation was lost when pccR was inactivated by an in-frame deletion. In the pccR mutant, lacZ expression from a 215-bp plasmid-borne pccB upstream fragment including 27 bp of the pccB coding region was also deregulated. A loss of regulation as a result of mutations in the conserved motifs TTTGCAAA-X4-TTTGCAAA in the presence of PccR allowed the prediction of a possible operator site. PccR, together with homologs from other organisms, formed a distinct clade within the family of short-chain fatty acyl coenzyme A regulators (ScfRs) defined here. Some members from other clades within the ScfR family have previously been shown to be involved in regulating acetyl-CoA assimilation by the glyoxylate bypass (RamB) or propionyl-CoA assimilation by the methylcitrate cycle (MccR).
IMPORTANCE Short-chain acyl-CoAs are intermediates in essential biosynthetic and degradative pathways. The regulation of their accumulation is crucial for appropriate cellular function. This work identifies a regulator (PccR) that prevents the accumulation of propionyl-CoA by controlling expression of the gene encoding propionyl-CoA carboxylase, which is responsible for propionyl-CoA consumption by Rhodobacter sphaeroides. Many other Proteobacteria and Actinomycetales contain one or several PccR homologs that group into distinct clades on the basis of the pathway of acyl-CoA metabolism that they control. Furthermore, an upstream analysis of genes encoding PccR homologs allows the prediction of conserved binding motifs for these regulators. Overall, this study evaluates a single regulator of propionyl-CoA assimilation while expanding the knowledge of the regulation of short-chain acyl-CoAs in many bacterial species.
INTRODUCTION
Rhodobacter sphaeroides belongs to the purple nonsulfur bacteria, a physiologically related group of phototrophic bacteria known for their metabolic versatility (1). Given their natural abundance in anaerobic portions of stagnant water, purple nonsulfur bacteria are adept at using light as a source of energy during anaerobic growth while acquiring carbon from fermentation products, such as propionate. Despite the abundance of propionate in its natural environment, R. sphaeroides was originally classified uniquely among the purple nonsulfur bacteria to be unable to use propionate as a carbon source for growth (1). A recent edition of Bergey's Manual of Systematic Bacteriology, however, recognizes that the type strain, R. sphaeroides 2.4.1, does grow with propionate (2).
As a growth substrate, propionate is directly activated to propionyl coenzyme A (propionyl-CoA). Propionyl-CoA is also an intermediate of pathways responsible for the degradation of endogenously derived or exogenously supplied branched-chain amino acids as well as branched-chain and odd-numbered-chain fatty acids (3–6). In addition, propionyl-CoA is formed during the metabolism of 3-hydroxypropionate and during acetyl-CoA assimilation via the ethylmalonyl-CoA pathway (see Fig. S1 in the supplemental material) (7, 8). The ethylmalonyl-CoA pathway substitutes for the glyoxylate bypass in many bacteria and is required for replenishing intermediates of the citric acid cycle for growth with substrates that enter central carbon metabolism at the level of acetyl-CoA (9).
Propionyl-CoA is an inhibitor of several key metabolic enzymes, and therefore, the metabolism of propionyl-CoA needs to be tightly regulated to prevent its accumulation inside the cell (10–15). In most microorganisms, propionyl-CoA levels are controlled via at least two known metabolic pathways: the methylcitrate cycle and the methylmalonyl-CoA pathway (see Fig. S1 in the supplemental material). The methylcitrate cycle is responsible for oxidizing propionyl-CoA to pyruvate, and the pathway is best characterized for enterobacteria (16, 17). The enzymes of the pathway for Salmonella enterica are encoded by the prp operon (18), and the expression of the operon is controlled by PrpR, a σ54-dependent transcriptional activator that responds to intracellular 2-methylcitrate levels (19). The flow of carbon through the methylcitrate cycle in S. enterica is additionally controlled by reversible covalent propionylation of the propionyl-CoA synthetase active site, modulating the amount of propionate that is activated to propionyl-CoA (20, 21).
Similarly, flux through the methylcitrate cycle in members of the order Actinomycetales, such as Corynebacterium glutamicum and Mycobacterium tuberculosis (22, 23), is controlled by reversible acylation of propionyl-CoA synthetase (24, 25) and by transcriptional control of the genes encoding enzymes of the methylcitrate cycle. The transcriptional regulator has been named LrpG (26), PrpR (27), and Rv1129c (28) in M. tuberculosis and PrpR in C. glutamicum (29). It is important to note that the transcriptional regulator from M. tuberculosis and the so-called PrpR regulator from C. glutamicum are unrelated to PrpRs described for enterobacteria like S. enterica. Instead, these regulators of the methylcitrate cycle from the Actinomycetales represent homologs of RamB, a transcriptional regulator first shown to regulate genes of the glyoxylate bypass for C. glutamicum (30) (see below).
The second known pathway for propionyl-CoA assimilation, the methylmalonyl-CoA pathway, operates in a range of organisms (including humans) and across all three domains of life (31–34). The methylmalonyl-CoA pathway has been thoroughly biochemically characterized in a number of organisms, including R. sphaeroides. The pathway requires propionyl-CoA carboxylase (PCC), an ATP- and biotin-dependent enzyme that catalyzes the carboxylation of propionyl-CoA to form (2S)-methylmalonyl-CoA, which is further converted to succinyl-CoA for assimilation.
Although the reactions of the methylmalonyl-CoA pathway are understood, its regulation in bacteria is unstudied. Some insight into the regulation of the methylmalonyl-CoA pathway for propionyl-CoA assimilation may come from several well-studied strategies for regulating the metabolism of other short-chain acyl-CoAs in a variety of bacteria. In Pseudomonas aeruginosa and P. putida, for example, isobutyryl-CoA metabolism produces propionyl-CoA and CO2 in a pathway that requires methylmalonate semialdehyde dehydrogenase (MmsA) (35) and 3-hydroxyisobutyrate dehydrogenase (MmsB) (see Fig. S1 in the supplemental material) (36). The mmsA and mmsB genes are encoded within a single operon that is transcriptionally activated by MmsR, a member of the AraC-type family of regulators (37). For Escherichia coli, the glyoxylate bypass for acetyl-CoA assimilation (see Fig. S1 in the supplemental material) (38) is transcriptionally and posttranscriptionally controlled. For E. coli, an operon encodes isocitrate lyase (AceA), malate synthase (AceB), and a kinase/phosphatase (AceK); AceK is responsible for modulating the activity of isocitrate dehydrogenase by reversible phosphorylation (39). Expression of the operon is largely controlled by IclR in response to pyruvate and glyoxylate (40, 41). C. glutamicum also uses the glyoxylate bypass for acetyl-CoA assimilation. Regulation of aceA and aceB expression in C. glutamicum also occurs transcriptionally, albeit not via IclR. The expression of both genes is activated by the transcriptional activator RamA (42), a LuxR-type regulator, and is repressed by RamB (30). A homolog of RamB also controls the transcription of genes required for propionyl-CoA assimilation via the methylcitrate cycle for C. glutamicum and M. tuberculosis (see above) (26–29).
The following study reveals that a RamB homolog, RSP_2186, is used by R. sphaeroides to control propionyl-CoA assimilation via the methylmalonyl-CoA pathway. The role of RSP_2186 (herein named PccR) as a transcriptional regulator of pccB was examined. Evidence that activation by PccR is the primary mechanism for adjusting the flow of propionyl-CoA through the methylmalonyl-CoA pathway of R. sphaeroides is presented. In addition, the novel ScfR family of transcriptional regulators is defined on the basis of bioinformatics. The ScfR family includes six clades of regulators whose members are likely responsible for regulating pathways for short-chain acyl-CoA assimilation in a variety of organisms.
MATERIALS AND METHODS
Materials.
Propionyl-CoA was synthesized from propionic anhydride as described previously (43). All primers used in the study were obtained from Sigma-Aldrich (St. Louis, MO) and are listed in Table S1 in the supplemental material.
Bacterial strains and growth conditions.
R. sphaeroides 2.4.1 (DSMZ 158) and its derivative strains were grown aerobically in the dark or anaerobically in the light (3,000 lx) at pH 6.8 and 30°C in minimal medium (44) supplemented with 10 mM succinate, acetate, or propionate; because the carbon in propionate is more reduced than cell carbon, 10 mM bicarbonate was also added in the case of propionate. The medium in the plates included 2.5% agar. For enzyme assays and RNA isolation, cells were harvested in early to mid-exponential phase at an optical density at 578 nm (OD578) of 0.4 to 0.5. For growth studies, cells were pregrown anaerobically in minimal medium containing 10 mM sodium succinate, and about 0.1 ml was transferred to 5 ml minimal medium containing the appropriate carbon source. R. sphaeroides strains carrying plasmids were grown in the presence of spectinomycin (25 μg ml−1). The medium used for isolating single-crossover mutant strains contained kanamycin (20 μg ml−1). E. coli strains DH5α, S17-1, and SM10 were grown in Luria-Bertani (LB) broth at 37°C with ampicillin (100 μg ml−1), spectinomycin (50 μg ml−1), or kanamycin (50 μg ml−1), as necessary.
Isolation of the markerless in-frame pccR deletion strain RsΔpccRMC12 and complementation.
The suicide plasmid pMC70 employed for the markerless in-frame inactivation of pccR (rsp_2186) was constructed by amplifying 1,491 bp of an upstream region (primers D_ramB_upF2 and D_ramB_upR2) and 1,822 bp of a downstream region (primers D_ramB_downF2 and D_ramB_downR2) of pccR by PCR and by cloning the products in tandem into pK18mobsacB. The resulting plasmid, pMC70, contains an in-frame deletion of 1,224 bp of the pccR gene. The remaining open reading frame includes 134 bp of the 3′ portion of the original coding region and 28 bp of the 5′ portion separated by a KpnI site and encodes a 55-amino-acid peptide.
RsΔpccRMC12 was isolated by mating R. sphaeroides 2.4.1 with E. coli S17-1 transformed with pMC70. Single-crossover strains were isolated anaerobically in the light on minimal medium plates containing succinate as the carbon source (minimal medium succinate plates) and kanamycin for selection. To allow the second crossover event, the cells from isolated colonies were grown aerobically in the dark overnight in 100 μl minimal medium succinate lacking kanamycin. Overnight cultures were spread on minimal medium succinate plates supplemented with 10% sucrose and grown anaerobically in the light. Isolated colonies were patched as replicates on minimal medium succinate plates with or without kanamycin. For identification of double-crossover strains, colony PCR was performed on kanamycin-sensitive strains using primers DramB_seqUpR1 and DramB_seqDnR1. The PCR product sizes obtained were used to distinguish the isolates as deletion mutants or wild-type revertants. The genotype of RsΔpccRMC12 was confirmed by sequencing the product obtained by PCR with primers DramB_seqUpF1 and DramB_seqUpR1 and the product obtained by PCR with primers DramB_seqDnR1 and D_ramB_downR4.
For complementation of the RsΔpccRMC12 mutant a 1,584-bp fragment was amplified from R. sphaeroides 2.4.1 genomic DNA with primers 2186promF2 and 2186compR1. The fragment contained the pccR gene, which included a 162-bp region upstream of the translational start site. The fragment was digested with XbaI/EcoRI and ligated into pBBRsm2MCS5 (7) to generate pMC66. The pBBRsm2MCS5 plasmid (empty vector control) and pMC66 were independently conjugated into the wild type or RsΔpccRMC12 by mating with E. coli SM10 transformed with the respective plasmid.
Isolation of pccB-lacZ reporter strains.
To construct a scaffold for promoter-lacZ fusions, the lac operon was inserted into the pBBRsm2MCS5 vector (7) and the intrinsic alpha-subunit lacZ portion of the pBBRsm2MCS5 plasmid was removed. A 4,206-bp fragment was PCR amplified from pBBRsm2MCS5 with primers MC75F3 and MC75R2. The lac operon (5,071 bp) was amplified from pMC1403 (45) with primers lacoperon_for1 and lacoperon_rev2. By way of EcoRI/KpnI digestion of both PCR products and subsequent ligation, plasmid pMC75 was obtained and used as a scaffold for pccB promoter-lacZ fusions. Primers pccBGSF0 and pccBGSR0 and chromosomal R. sphaeroides DNA were used to amplify a 640-bp fragment that included the pccB upstream region. The fragment was cloned into pUC18 using BamHI/XbaI, resulting in the plasmid pMC73. Versions of pMC73 with site-directed mutations (pMC73Δ1, pMC73Δ2, and pMC73Δ12) were constructed by amplification of pMC73 with PfuUltra II polymerase (Agilent) and the respective primers indicated in Table S1 in the supplemental material. Unmutated (methylated) template pMC73 was removed by DpnI restriction before the amplified products were used to transform DH5α cells. After purification of the plasmids from DH5α cells, the mutations were confirmed by DNA sequencing. A 230-bp product amplified from pMC73, pMC73Δ1, pMC73Δ2, and pMC73Δ12 with primers pccBpromF1 and pccBpromR1 was restricted with XbaI/EcoRI and ligated into pMC75 to generate pMC85, pMC85Δ1, pMC85Δ2, and pMC85Δ12. These plasmids therefore contained 188 bp of either the original or the mutated pccB upstream region and 27 bp of the pccB coding region fused to the lacZ gene. The variations in the upstream fragments available on each plasmid are detailed in Fig. 2.
FIG 2.
(A) Genomic context of pccB (encoding the β subunit of propionyl-CoA carboxylase), pccR, and the consensus sequence (open boxes) initially identified upstream of pccR genes within several alphaproteobacteria. Striped arrows, genes annotated to encode a multidrug-resistant transporter; checkered arrows, conserved coding regions of unknown function; open arrows, coding regions with no significant sequence identity to the other genes shown. (B) Illustration of the mutated pccB upstream regions that are present on each plasmid in the pMC85 series. Each plasmid contains a derivative of a 215-bp fragment of the pccB upstream fragment including 27 bp of the pccB coding region fused to lacZ. Regulation of expression from each putative promoter is presented in Table 2.
Propionyl-CoA carboxylase and β-galactosidase enzyme assays.
For the preparation of cell extracts, frozen cell pellets (400 to 600 mg [wet weight]) were suspended in 600 μl of 50 mM Tris-HCl, pH 8.0, 5 mM MgCl2, and 0.1 mg ml−1 DNase I. After addition of ∼1 g glass beads (diameter, 0.1 to 0.25 mm; Retsch), the suspension was beaten at 30 Hz for 9 min with a bead beater (model MM200; Retsch). Insoluble cell material and beads were separated from the cell extract by centrifugation at 15,800 × g for 5 min at 4°C. The protein concentration was measured by the method of Bradford (46) using bovine serum albumin as a standard.
Propionyl-CoA carboxylase activity was measured in 400-μl reaction mixtures containing 100 mM Tris, pH 8.0, 5 mM MgCl2, 10 mM KCl, 2.5 mM dithiothreitol, 5 mM ATP, 0.38 mM propionyl-CoA, 12.5 mM NaH14CO3 (3.7 μCi), and 70 to 520 μg cell extract. Reactions were initiated with the addition of propionyl-CoA. At time points of 0, 2.5, and 5 min, an aliquot of 100 μl was removed from the reaction mixture and the reaction was stopped by addition of 50 μl of 100% propionic acid (final pH, ≈2.5); this was followed by centrifugation in open tubes for 30 min to remove unincorporated CO2. Incorporation of acid-stable 14C was quantified by adding 75 μl of each sample to 3.0 ml of scintillation fluid, and the radioactive decay was measured by a scintillation counter. Background values at time zero were subtracted from the other values.
β-Galactosidase activity was measured as the enzyme-dependent cleavage of ortho-nitrophenyl-β-galactoside (ONPG) at 412 nm (ε412 = 4,500 M−1 cm−1) in a cuvette with a 1-cm path length. The reaction was initiated by the addition of 100 μl of 4 mg ml−1 ONPG in Z buffer (50 mM potassium phosphate buffer, pH 7.0, 40 mM KCl, 1 mM MgSO4, 0.35% β-mercaptoethanol) to a 400-μl Z-buffer mixture containing 2 to 300 μg cell extract.
RNA isolation.
Cells from 200 ml of culture at an OD578 of 0.4 to 0.5 were collected by centrifugation (8,000 × g, 10 min, 4°C) and resuspended in 1.3 ml of TRI Reagent (Sigma). After 30 min at room temperature, 650 μl chloroform was added, and the mixture was incubated for an additional 10 min at room temperature. Following centrifugation (15,800 × g, 10 min, 4°C), the process was repeated with the aqueous phase with 650 μl of TRI Reagent and 300 μl of chloroform through a series of 5-min incubations. The final aqueous phase was added to an equal volume (∼1 ml) of isopropanol. After at least 1 h at −20°C, the precipitated nucleic acids were pelleted by centrifugation (15,800 × g, 10 min, 4°C) and dissolved in 400 μl RNase-free water. DNA was degraded at 37°C for 1 h with 500 U RNase-free DNase I (Roche). The RNA was precipitated and stored at −20°C until use. RNA was collected by centrifugation (15,800 × g, 10 min, 4°C) and dissolved in 200 μl RNase-free water immediately before use.
Quantitative reverse transcription-PCR (qRT-PCR).
Whole-cell cDNA was produced in a 100-μl reaction mixture by reverse transcribing 70 μl of whole-cell RNA with 500 U SuperScript III reverse transcriptase (Invitrogen) and 2 nmol of 10-nucleotide oligomers of randomized sequence. The resulting whole-cell cDNA was diluted 44- to 352-fold in 2-fold steps in 22-μl PCR mixtures that contained 14 μl IQ SYBR SuperMix (Bio-Rad) and 40 pmol of each respective primer (see Table S1 in the supplemental material). Amplification was performed in a Bio-Rad CFX96 thermal cycler for 40 cycles with the following amplification protocol: 97°C for 20 s, 64°C for 20 s, and 72°C for 10 s. To prevent the detection of potential primer dimers, the reaction mixture was heated to 81°C for 10 s before the fluorescence was recorded for each round of amplification. Each reaction was performed in triplicate for each dilution. Threshold cycle (CT) values that demonstrated 90% to 110% amplification efficiency were averaged and compared to the values on standard curves in order to estimate the initial absolute value of the cDNA template concentration. The standard curves were generated from the CT values for reaction mixtures containing known concentrations of 10-fold dilutions of purified PCR product that had been obtained from R. sphaeroides chromosomal DNA with the same primers used in the experimental reactions. Final absolute values were normalized to the average of the values of the rpoZ and recA levels for comparison among biological replicates, strains, and growth conditions.
Identification of the pccB transcriptional start site.
A primer extension method was employed to establish the 5′ end of pccB as described previously (47). Primer MSACEpccBRT1 fluorescently labeled with 6-carboxyfluorescein (FAM) on its 5′ terminus was used to prime reverse transcription with whole-cell RNA. Using an ABI 3770 capillary electrophoresis sequencer, the migration of the reverse transcription product was compared to the migration of the product of the sequencing reactions performed on the corresponding DNA region with the same 5′ FAM-labeled primer. Comigration with a given sequencing product was indicative of the 5′ position of the given transcript.
In silico ScfR identification and DNA binding site recognition.
Homologs of PccR were collected as the top 1,000 BLAST (Basic Local Alignment Search Tool) results after querying the National Center for Biotechnology Information (NCBI) nonredundant protein sequence database with the R. sphaeroides PccR (RSP_2186) sequence. Protein sequences that were not associated with a genome in the database were removed. The resulting 327 protein sequences were aligned using the Muscle program within MEGA (version 5) software (48). Nonconserved extensions were removed manually. The adjusted sequences and accession numbers for the protein sequences that were used are listed in Table S2 in the supplemental material. Neighbor-joining trees were constructed using MEGA (version 5). Any presented bootstrap values were predicted from 500 replicates and are based on the statistical model of Jones et al. (49).
To investigate possible DNA binding sites for each family of ScfR proteins, 200-bp fragments of sequence immediately upstream of coding regions for proteins from each family were compared using the MEME suite of tools (50). The sequences that were used in the comparison are listed in Table S2 in the supplemental material. The parameters were adjusted to identify any number of 6-bp to 15-bp conserved motifs per query sequence. The resulting motifs were used to build the DNA logos presented in Fig. 5.
FIG 5.
Neighbor-joining tree of selected ScfR proteins. Nodes with bootstrap values greater than 50% are labeled. Each entry includes the class name assigned to the given protein on the basis of the genomic context of its respective gene. A question mark indicates that the corresponding genetic context does not contain any genes of the indicated metabolic gene clusters. An asterisk indicates a protein that clusters in the tree with proteins of a different class. Following the protein name is the locus tag for the given protein and the species in which the protein is found. To the right of the tree is an illustration of the general genome neighborhood of the genes that encode proteins from the corresponding clade. See Fig. S2 in the supplemental material for the extended tree. A DNA logo (50) is included if a conserved motif was identified upstream of the genes responsible for the proteins in the corresponding clade. The indicated motifs may have occurred multiple times in the queried sequences. Gene annotations are as follows: pccB, propionyl-CoA carboxylase β subunit; pccA, propionyl-CoA carboxylase α subunit; mcm, methylmalonyl-CoA mutase; prpB, 2-methylcitrate lyase; prpC, 2-methylcitrate synthase; prpD, 2-methylcitrate dehydratase; prpE, propionyl-CoA synthetase; mmsA, methylmalonate semialdehyde dehydrogenase; hibA, acyl-CoA dehydrogenase; hibB, enoyl-CoA hydratase; mmsB, 3-hydroxyisobutyrate dehydrogenase; aceA, isocitrate dehydrogenase; aceB, malate synthase.
RESULTS
Propionyl-CoA carboxylase activity is mainly regulated at the level of the pccB transcript.
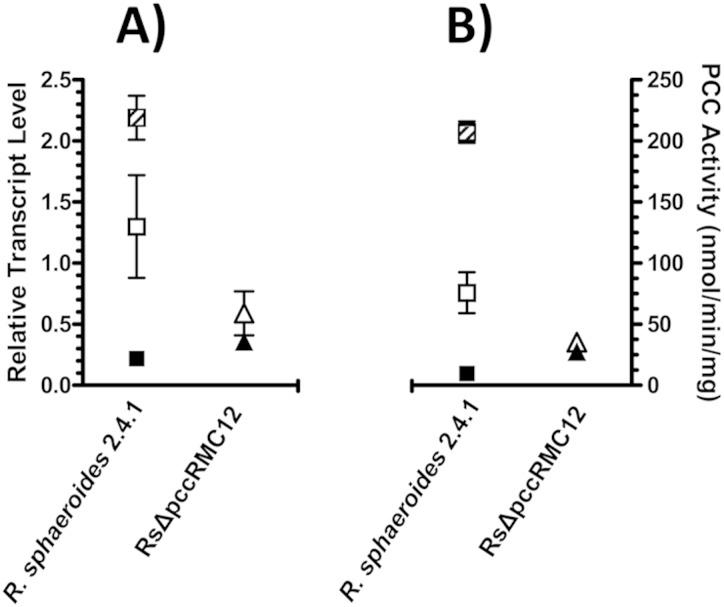
The pccB gene of R. sphaeroides, rsp_2189, encodes the β subunit of propionyl-CoA carboxylase (PCC), an enzyme of the methylmalonyl-CoA pathway required for propionyl-CoA assimilation. The methylmalonyl-CoA pathway is also a component of the ethylmalonyl-CoA pathway for acetyl-CoA assimilation (see Fig. S1 in the supplemental material) (8). PCC activity was measured as the formation of acid-stable 14C-labeled products upon incubation of the cell extract with ATP, propionyl-CoA, and NaH14CO3. PCC activities increased 8- or 20-fold when the activities in the extracts of cells grown with acetate or propionate-HCO3−, respectively, were compared to those in the extracts of cells grown with succinate (Table 1). Similarly, pccB transcript levels, as measured by qRT-PCR, in whole-cell RNA from acetate- and propionate-grown cells were increased 6- and 10-fold, respectively, when they were compared with those in whole-cell RNA from succinate-grown cells (Fig. 1). The correlation of PCC activity and pccB mRNA levels under different growth conditions is consistent with the predominantly transcriptional regulation of pccB expression that is dependent on the carbon source supplied.
TABLE 1.
PCC activity in extracts of photoheterotrophically grown cells
| Strain | PCC activitya (nmol min−1 mg−1) with: |
||
|---|---|---|---|
| Succinate | Acetate | Propionate-HCO3− | |
| Wild type | 10 ± 1 | 76 ± 17 | 207 ± 8 |
| RsΔpccRMC12 | 28 ± 6 | 35 ± 2 | NGb |
| RsΔpccRMC12(pMC66) | 18 | 60 | 191 |
Errors represent the standard deviations from at least three biological replicates.
NG, no growth.
FIG 1.
Comparison of pccB transcript abundance (A) and the PCC activity (B) of R. sphaeroides 2.4.1 and RsΔpccRMC12 cells grown with succinate (filled symbols), acetate (open symbols), and propionate-HCO3− (hatched symbols). Expression levels were relative to the average value of recA and rpoZ transcript abundance. Error bars represent the standard deviations from at least three biological replicates. RsΔpccRMC12 did not grow with propionate-HCO3−.
Identification of a transcriptional regulator candidate.
The genome of R. sphaeroides was analyzed for genes that might encode homologs of known transcriptional regulators involved in acyl-CoA metabolism from other organisms. Homologs of PrpR (STM0367) from S. enterica (18), MmsR (PA3571) from P. aeruginosa (37), IclR (B4018) from E. coli (51), or RamA (Cg2831) from C. glutamicum (42) were not detected. In addition, proteins (STM2651, Rpa3031, and Rv0998) required for acylation of acyl-CoA synthetases in different bacteria (24, 52, 53) are not encoded by the R. sphaeroides genome. The only homolog of known transcriptional regulators of acyl-CoA metabolism encoded by R. sphaeroides is RSP_2186, which shows 38% sequence identity to RamB (Cg0800) from C. glutamicum (30). The rsp_2186 gene, named pccR here, is divergently transcribed from pccB and is separated from pccB by two open reading frames (Fig. 2). Furthermore, both pccR and pccB have two copies of a direct repeat (TTTGCAAA) in their upstream untranslated regions. The same duplicated direct repeats have been shown to be bound by RamB in C. glutamicum (30).
PccR is necessary for transcriptional regulation of pccB.
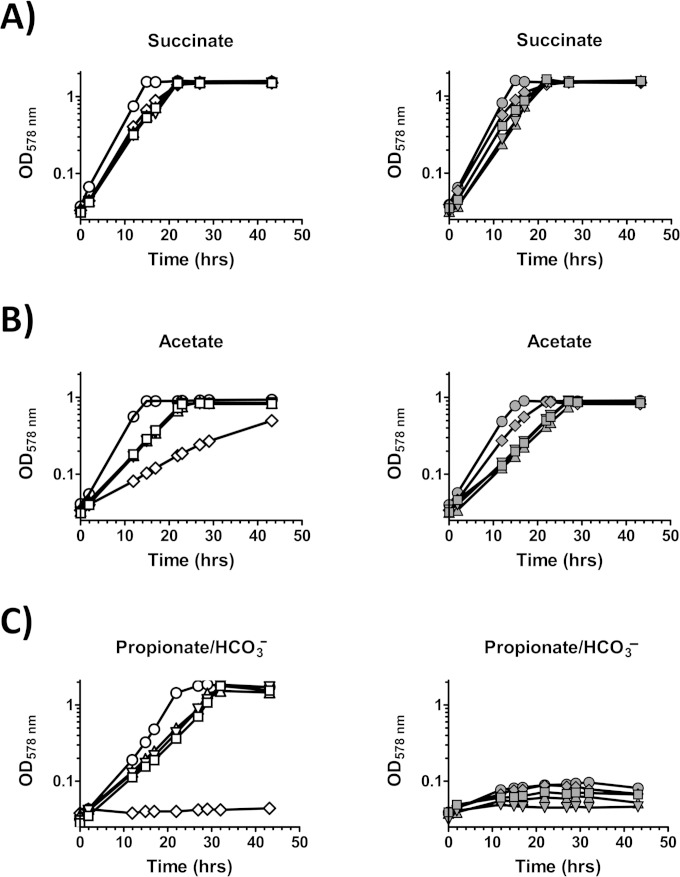
When the pccR coding region was inactivated by an in-frame deletion of 1,224 bp of its original 1,386 bp, the resulting strain, RsΔpccRMC12, was unable to grow with propionate-HCO3− as the carbon source. The mutant RsΔpccRMC12, however, grew normally with succinate or acetate as the carbon source (Fig. 3). When pccR was expressed from its own promoter on plasmid pMC66 in the mutant strain, growth with propionate-HCO3− was restored (Fig. 3), indicating that the growth defect was due to the absence of PccR.
FIG 3.

Photoheterotrophic growth of R. sphaeroides 2.4.1 (open circles), RsΔpccRMC12 (filled circles), RsΔpccRMC12(pBBRsm2MCS5) (crosses), and RsΔpccRMC12(pMC66) (stars) with succinate, acetate, and propionate-HCO3−.
Although the growth of the RsΔpccRMC12 mutant strain with succinate or acetate as the carbon source was not affected, the pccB transcript levels of the mutant grown with either substrate showed significant changes compared to those of the wild type. For the wild type, pccB transcript levels for succinate-grown cells were 6-fold lower than those for acetate-grown cells. In the case of the RsΔpccRMC12 mutant strain, however, pccB transcript levels were the same during growth with succinate or acetate (Fig. 1). The change in the regulation of pccB transcript levels between the wild type and the RsΔpccRMC12 mutant strain was directly reflected in the PCC activity levels (Fig. 1); for the mutant, pccB transcript levels and PCC activities were elevated during growth with succinate and reduced during growth with acetate compared with the results for the wild-type strain (Fig. 1). Based on these results, we concluded that the lower level of PCC activity in the absence of regulation by PccR was sufficient to optimally support growth with acetate yet was insufficient to support growth growth with propionate-HCO3−.
Activation of pccB expression requires the two TTTGCAAA motifs.
The levels of pccB expression were also measured using a translational lacZ reporter fusion on a plasmid; a 157-bp fragment of the pccB upstream region and the first 27 bp of the pccB coding region were fused to the lac operon in pMC85 (Fig. 2). Similar to the chromosomal regulation, the β-galactosidase (LacZ) activity in cell extracts of the wild-type R. sphaeroides strain carrying pMC85 showed the upregulation of expression from the plasmid-borne pccB promoter during growth with acetate compared to the level of regulation during growth with succinate (Table 2). For the RsΔpccRMC12 mutant strain carrying pMC85, LacZ activities were unregulated and lower than those for the wild-type strain carrying pMC85, as expected (Table 2). However, LacZ activities in cell extracts of the succinate-grown RsΔpccRMC12(pMC85) mutant were expected to be higher than those of the succinate-grown wild type strain carrying pMC85, on the basis of the observed elevated pccB transcript levels in the mutant compared with those in the wild-type strain (Fig. 1). Instead, the inverse was true, suggesting that the length of the promoter region used to construct the plasmid might be insufficient to include an additional site required for appropriate negative regulation.
TABLE 2.
LacZ activities in cell extracts of photoheterotrophically grown cells carrying derivatives of the pccB-lacZ translational fusion plasmid pMC85
| Strain and plasmid | LacZ activitya (nmol/min/mg) with: |
||
|---|---|---|---|
| Succinate | Acetate | Propionate-HCO3− | |
| R. sphaeroides 2.4.1 | <5 | <5 | <5 |
| pMC85 | 340 ± 30 | 4,350 ± 1,000 | NGb |
| pMC85Δ1 | 10 ± 0 | 40 ± 0 | 90 ± 10 |
| pMC85Δ2 | 20 ± 0 | 30 ± 10 | 30 ± 20 |
| pMC85Δ12 | 30 ± 10 | 60 ± 20 | 60 ± 20 |
| RsΔpccRMC12 | <5 | <5 | NG |
| pMC85 | 20 ± 10 | 50 ± 20 | NG |
| pMC85Δ1 | 40 ± 20 | 50 ± 10 | NG |
| pMC85Δ2 | 20 ± 10 | 40 ± 10 | NG |
| pMC85Δ12 | 30 ± 10 | 60 ± 10 | NG |
Errors represent the range for at least two biological replicates.
NG, no growth.
The upstream regions of pccB and pccR each contain a proximal duplication of a TTTGCAAA motif, a direct repeat that matches a consensus sequence identified upstream of pccB and pccR in closely related organisms (Fig. 2). In order to contextualize the direct repeats with respect to the pccB promoter, primer extension was used to identify the transcriptional start site of pccB. A major signal was detected for a thymidine 29 bp upstream of the translational start site (see Fig. S2 in the supplemental material). For the functional study of the duplicated direct repeat motifs, each direct repeat in pMC85 was sequentially mutated to GCGTAGCG (Fig. 2). The various plasmids were transferred into wild-type and RsΔpccRMC12 strains, and expression from the pccB promoter and its derivatives was monitored as LacZ activity. When either one or both of the TTTGCAAA sites were mutated, the level of activation of expression from the pccB promoter on the plasmid was lost in the wild type and corresponded to the expression level from the pMC85 plasmid observed in the RsΔpccRMC12 mutant strain (Table 2). The results strongly suggest that activation of expression from the pccB promoter involves an interaction between PccR and both direct repeats.
Because activation of pccB expression from the chromosome was not fully possible for the RsΔpccRMC12 mutant strain, the strain was unable to grow with propionate-HCO3− as the carbon source (Fig. 3). Unexpectedly, the wild type carrying the pMC85 plasmid also failed to grow with propionate-HCO3−, although growth with succinate was unaffected (Fig. 4). In addition, growth of the wild-type strain carrying pMC85 with acetate was impaired (Fig. 4). Mutations of either one or both of the direct repeats on the pMC85 plasmid restored normal growth with propionate-HCO3− and acetate (Fig. 4), suggesting that a possible signal molecule or another transcriptional factor is titrated away through interaction with PccR bound to the plasmid DNA.
FIG 4.
Photoheterotrophic growth of R. sphaeroides 2.4.1 (left) and RsΔpccRMC12 (right) that carry no plasmid (circles), pMC85 (diamonds), pMC85Δ1 (triangles), pMC85Δ2 (inverted triangles), or pMC85Δ12 (squares) with succinate, acetate, or propionate-HCO3−.
PccR is a member of a proposed family of transcriptional regulators controlling the assimilation of short-chain fatty acyl CoAs (ScfRs).
An examination of other genomes for genes that encode homologs of PccR led to the recognition that PccR is a member of a large protein family. The common features of these proteins are an XRE-family helix-turn-helix N-terminal domain for DNA binding and a C terminus that consists of a domain of unknown function (DUF2083). The DUF2083 domain contains five highly conserved cysteines (see Fig. S3 in the supplemental material). Another domain of unknown function (DUF955) is present proximally to DUF2083 and is located between the helix-turn-helix and the DUF2083 domains.
Figure 5, which is an abbreviated version of Fig. S4 in the supplemental material constructed from 327 sequences, shows that the analyzed sequences clustered into six clades. With the exception of 24 out of 327 sequences, trends were observed in the genomic contexts of the genes that encode the proteins of each clade (Fig. 5). The corresponding genes of representatives within a clade generally clustered with genes encoding enzymes of a specific acyl-CoA assimilation pathway. This feature was used to establish the nomenclature of four classes of regulators within this single large protein family. PccR (propionyl-CoA carboxylase regulator)-encoding genes clustered with genes for enzymes of the methylmalonyl-CoA pathway for propionyl-CoA assimilation. Genes encoding enzymes of the methylcitrate cycle for propionyl-CoA assimilation clustered with mccR, so named by us to indicate that it likely encodes a methylcitrate cycle regulator. The RamB (regulator of acetate metabolism) class is named for an aforementioned member that was previously characterized and shown to be required for regulating the glyoxylate bypass for acetyl-CoA assimilation (30). Two RamB clades that contain proteins encoded by genes that colocalize with genes encoding malate synthase (aceB) and/or isocitrate lyase (aceA) of the glyoxylate bypass are present. The two clades are designated RamB subclasses 1 and 2. Proteins of the final clade are notated as IbcRs (isobutyryl-CoA assimilation regulators). Their genes cluster with genes encoding enzymes required for isobutyryl-CoA assimilation via 3-hydroxyisobutyryl-CoA (37). The proteins of all clades are likely responsible for controlling acetyl-CoA, propionyl-CoA, or isobutyryl-CoA metabolism. Therefore, the collection of these protein homologs is denoted here as the ScfR (short-chain fatty acyl-CoA assimilation regulator) family.
In an effort to predict potential operator sites for the regulators within a single clade, we speculated that the regulators regulate the expression of their own genes. A 200-bp portion of genomic sequence was collected from the upstream regions of 37 pccR genes, 24 mccR genes, 27 ramB subclass 1 genes, and 14 ramB subclass 2 genes. Motifs upstream of the genes of each class were identified with MEME (50). The logos in Fig. 5 illustrate the unique motifs that were identified upstream of genes encoding a given class of ScfR. The only exception is the ramB subclass 2 clade, where no consensus motif was identified.
DISCUSSION
PccR from R. sphaeroides, encoded by rsp_2186, was identified to be a transcriptional regulator that is responsible for controlling propionyl-CoA assimilation through the methylmalonyl-CoA pathway by controlling pccB expression and, ultimately, PCC activity. A duplicated direct repeat, TTTGCAAA-Xn-TTTGCAAA, was identified to be a possible operator site located upstream of pccR (n = 12) and of pccB (n = 4); pccB encodes the β subunit of PCC. An examination of the R. sphaeroides 2.4.1 genome (both chromosomes and each of the five plasmids) revealed that only those two loci in the entire genome contain a proximal duplication of this TTTGCAAA site, suggesting that PccR specifically regulates propionyl-CoA assimilation in R. sphaeroides. Because various attempts to purify a soluble PccR protein failed, we were unable to demonstrate direct binding of PccR to the two direct repeats.
The in silico analysis that led to establishment of the classes of the ScfR family also serves to unify some seemingly disconnected reports in the literature. A bioinformatics analysis by Suvorova et al., for example, identified a group of regulators that they named PrpQ* proteins (54). The work recognized that the genes for PrpQ* proteins were conserved in genome neighborhoods that encode pathways for propionyl-CoA assimilation (Suvorova et al. [54] incorrectly refer to the methylmalonyl-CoA pathway as the citramalate cycle) and that the genome neighborhoods contained appropriately located TTTGC(G/A)AA sequences. The analysis presented here replaces the PrpQ* nomenclature with the MccR and PccR classes.
Furthermore, at least three groups, Datta et al. (26), Griffin et al. (28), and Masiewicz et al. (27), independently reported the naming and characterization of the MccR class member Rv1129c from M. tuberculosis. Datta et al. reported that Rv1129c is responsible for activating expression of the prp operon and named the protein LrpG (local regulatory protein G) (26), while Griffin et al. showed that rv1129c is required for propionyl-CoA metabolism during the growth of M. tuberculosis with cholesterol (28). Masiewicz et al. identified that the binding sequence of Rv1129c is a tandem TTTGCAAA and similarly recognized that the protein is involved in activating transcription of the prp operon (27). Given that PrpR was the name given to the σ54-dependent regulator of the prp operon of enterobacteria (18, 19), Masiewicz et al. (27) used the same nomenclature for Rv1129c, despite its dissimilarity in amino acid sequence to PrpR. This name was also later used for describing Cg0800 of C. glutamicum (29, 55). Because PrpR/LrpG (Rv1129c) from M. tuberculosis and PrpR (Cg0800) from C. glutamicum are members of the MccR class of the ScfR family, we recommend adjusting the previously mixed and misleading nomenclature (Fig. 5).
It is worthwhile to note that the TTTGCAAA identified by Masiewicz et al. (27) within the M. tuberculosis MccR binding site is more similar to the motif that we identified for PccR than to the motif represented in the logo for the putative MccR binding site in Fig. 5. However, a closer look at the MccR logo reveals that changes at some of the variable positions could accommodate the half site TTTGCAAA. The resulting conclusion is therefore that conserved ScfR binding sites for any given class are difficult to immediately distinguish across species. However, in organisms such as Rhodobacter capsulatus, whose genome encodes members of three ScfR classes, the binding site of each ScfR protein is likely unique in order to avoid undesired regulatory interference. How specific binding site distinctions permeate throughout the genomes of different organisms that contain only one ScfR protein requires additional investigation.
Given the overall similarity of the ScfRs to each other and the similarity of the substrates of the pathways that the ScfRs are likely to control, it is tempting to speculate that each ScfR senses and binds the acyl-CoA metabolite that is associated with its class. Therefore, we propose the following model for PccR from R. sphaeroides: PccR always interacts with the direct repeats present upstream of pccB. Upon binding of propionyl-CoA and potentially another transcription factor, PccR activates transcription. Because PccR stays bound to the pccB promoter, immediate and tunable activation of transcription is possible. Assuming that each ScfR operates similarly, the model is consistent with the fact that although acetyl-CoA was suspected to be the effector molecule for RamB-mediated regulation in other systems, acetyl-CoA had no direct effect in vitro on binding of RamB to DNA (30, 56).
The regulatory immediacy of the model is proposed in large part because in R. sphaeroides propionyl-CoA inhibits the activity of pyruvate dehydrogenase (12, 57), an enzyme complex which is essential for growth with most substrates. As a result, the cell requires a responsive and specific system for activating the genes whose products are responsible for reducing cellular propionyl-CoA concentrations. In other bacteria, control of propionyl-CoA accumulation is achieved by the rapidly reversible modification of propionyl-CoA synthetase (20, 21, 24, 25, 52, 53, 58, 59). Enzymes known to be necessary for modifying the synthetase, however, are not encoded by the R. sphaeroides genome. Instead, R. sphaeroides involves PccR in modulating cellular propionyl-CoA levels by the tunable control of propionyl-CoA assimilation.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grant MCB0842892 from the National Science Foundation.
We thank Marie Asao for her insightful feedback throughout the work and critical review of the manuscript, F. Robert Tabita for generously providing facilities to perform PCC activity assays, Charles Daniels for continuous support and helpful discussions, and Mike Zianni and the Plant Microbe Genomics Facility at Ohio State University for sharing their expertise regarding qPCR.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00402-15.
REFERENCES
- 1.Van Niel CB. 1944. The culture, general physiology, morphology, and classification of the non-sulfur purple and brown bacteria. Bacteriol Rev 8:1–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imhoff JF. 2005. Genus I. Rhodobacter Imhoff, Trüper and Pfennig 1984, 342VP, p 164 In Brenner DJ, Krieg NR, Staley JT, Garrity GM (ed), Bergey's manual of systematic bacteriology, 2nd ed, vol 2 Springer, New York, NY. [Google Scholar]
- 3.Massey LK, Sokatch JR, Conrad RS. 1976. Branched-chain amino acid catabolism in bacteria. Bacteriol Rev 40:42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham IA, Eastmond PJ. 2002. Pathway of straight and branched chain fatty acid catabolism in higher plants. Prog Lipid Res 41:156–181. doi: 10.1016/S0163-7827(01)00022-4. [DOI] [PubMed] [Google Scholar]
- 5.Zhang YM, Rock CO. 2008. Membrane lipid homeostasis in bacteria. Nat Rev Microbiol 6:222–233. doi: 10.1038/nrmicro1839. [DOI] [PubMed] [Google Scholar]
- 6.Eoh H, Rhee KY. 2014. Methylcitrate cycle defines the bactericidal essentiality of isocitrate lyase for survival of Mycobacterium tuberculosis on fatty acids. Proc Natl Acad Sci U S A 111:4976–4981. doi: 10.1073/pnas.1400390111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider K, Asao M, Carter MS, Alber BE. 2012. Rhodobacter sphaeroides uses a reductive route via propionyl coenzyme A to assimilate 3-hydroxypropionate. J Bacteriol 194:225–232. doi: 10.1128/JB.05959-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erb TJ, Berg IA, Brecht V, Müller M, Fuchs G, Alber BE. 2007. Synthesis of C5-dicarboxylic acids from C2-units involving crotonyl-CoA carboxylase/reductase: the ethylmalonyl-CoA pathway. Proc Natl Acad Sci U S A 104:10631–10636. doi: 10.1073/pnas.0702791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erb TJ, Fuchs G, Alber BE. 2009. (2S)-Methylsuccinyl-CoA dehydrogenase closes the ethylmalonyl-CoA pathway for acetyl-CoA assimilation. Mol Microbiol 73:992–1008. doi: 10.1111/j.1365-2958.2009.06837.x. [DOI] [PubMed] [Google Scholar]
- 10.Gregersen N. 1981. The specific inhibition of the pyruvate dehydrogenase complex from pig kidney by propionyl-CoA and isovaleryl-CoA. Biochem Med 26:20–27. doi: 10.1016/0006-2944(81)90026-0. [DOI] [PubMed] [Google Scholar]
- 11.Shaw L, Engel PC. 1985. The suicide inactivation of ox liver short-chain acyl-CoA dehydrogenase by propionyl-CoA. J Biochem 230:723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maruyama K, Kitamura H. 1985. Mechanisms of growth inhibition by propionate and restoration of growth by sodium bicarbonate or acetate in Rhodopseudomonas sphaeroides S. J Biochem 98:819–824. [DOI] [PubMed] [Google Scholar]
- 13.Man W-J, Li Y, Connor CD, Wilton DC. 1995. The binding of propionyl-CoA and carboxymethyl-CoA to Escherichia coli citrate synthase. Biochim Biophys Acta 1250:69–75. doi: 10.1016/0167-4838(95)00044-U. [DOI] [PubMed] [Google Scholar]
- 14.Brock M, Buckel W. 2004. On the mechanism of action of the antifungal agent propionate. Eur J Biochem 271:3227–3241. doi: 10.1111/j.1432-1033.2004.04255.x. [DOI] [PubMed] [Google Scholar]
- 15.Schwab MA, Sauer SW, Okun JG, Nitjtmans LGJ, Rodenburg RJT, van den Heuvel LP, Dröse S, Brandt U, Hoffmann GF, Ter Laak H, Kölker S, Smeitink JAM. 2006. Secondary mitochondrial dysfunction in propionic aciduria: a pathogenic role of endogenous mitochondrial toxins. Biochem J 398:107–112. doi: 10.1042/BJ20060221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Textor S, Wendisch VF, De Graaf AA, Müller U, Linder MI, Linder D, Buckel W. 1997. Propionate oxidation in Escherichia coli: evidence for the operation of a methylcitrate cycle in bacteria. Arch Microbiol 168:428–436. doi: 10.1007/s002030050518. [DOI] [PubMed] [Google Scholar]
- 17.Horswill AR, Escalante-Semerena JC. 1999. Salmonella typhimurium LT2 catabolizes propionate via the 2-methycitric acid cycle. J Bacteriol 181:5615–5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horswill AR, Escalante-Semerena JC. 1997. Propionate catabolism in Salmonella enterica LT2: two divergently transcribed units comprise the prp locus at 8.5 centrisomes, prpR encodes a member of the sigma-54 family of activators, and the prpBCDE genes constitute an operon. J Bacteriol 179:928–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palacios S, Escalante-Semerena JC. 2004. 2-Methylcitrate-dependent activation of propionate catabolic operon (prpBCDE) of Salmonella enterica by the PrpR protein. Microbiology 150:3877–3887. doi: 10.1099/mic.0.27299-0. [DOI] [PubMed] [Google Scholar]
- 20.Starai VJ, Celic I, Cole RN, Boeke JD, Escalante-Semerena JC. 2002. Sir2-dependent activation of acetyl-CoA synthetase by deacetylation of active lysine. Science 298:2390–2392. doi: 10.1126/science.1077650. [DOI] [PubMed] [Google Scholar]
- 21.Garrity J, Gardner JG, Hawse W, Wolberger C, Escalante-Semerena JC. 2007. N-Lysine propionylation controls the activity of propionyl-CoA synthetase. J Biol Chem 282:30239–30245. doi: 10.1074/jbc.M704409200. [DOI] [PubMed] [Google Scholar]
- 22.Claes WA, Pühler A, Kalinowski J. 2002. Identification of two prpDBC gene clusters in Corynebacterium glutamicum and their involvement in propionate degradation via the 2-methylcitrate cycle. J Bacteriol 184:2728–2739. doi: 10.1128/JB.184.10.2728-2739.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muñoz-Elías EJ, Upton AM, Cherian J, McKinney JD. 2006. Role of the methylcitrate cycle in Mycobacterium tuberculosis metabolism, intracellular growth, and virulence. Mol Microbiol 60:1109–1122. doi: 10.1111/j.1365-2958.2006.05155.x. [DOI] [PubMed] [Google Scholar]
- 24.Nambi S, Gupta K, Bhattacharyya M, Ramakrishnan P, Ravikumar V, Siddiqui N, Thomas AT, Visweswariah SS. 2013. Cyclic AMP-dependent protein lysine acylation in mycobacteria regulates fatty acid and propionate metabolism. J Biol Chem 288:14114–14124. doi: 10.1074/jbc.M113.463992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayden JD, Brown LR, Gunawardena HP, Perkowski EF, Chen X, Braunstein M. 2013. Reversible acetylation regulates acetate and propionate metabolism in Mycobacterium smegmatis. Microbiology 159:1986–1999. doi: 10.1099/mic.0.068585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Datta P, Shi L, Bibi N, Balázsi G, Gennaro ML. 2011. Regulation of central metabolism genes of Mycobacterium tuberculosis by parallel feed-forward loops controlled by sigma factor E (σE). J Bacteriol 193:1154–1160. doi: 10.1128/JB.00459-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masiewicz P, Brzostek A, Wolański M, Dziadek J, Zakrzewska-Czerwińska J. 2012. A novel role of the PrpR as a transcriptional factor involved in the regulation of methylcitrate pathway in Mycobacterium tuberculosis. PLoS One 7:e43651. doi: 10.1371/journal.pone.0043651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffin JE, Pandey AK, Gilmore SA, Mizrahi V, McKinney JD, Bertozzi CR, Sassetti CM. 2012. Cholesterol catabolism by Mycobacterium tuberculosis requires transcriptional and metabolic adaptations. Chem Biol 19:218–227. doi: 10.1016/j.chembiol.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plassmeier JK, Persicke M, Pühler A, Sterthoff C, Rückert C, Kalinowski J. 2012. Molecular characterization of PrpR, the transcriptional activator of propionate catabolism in Corynebacterium glutamicum. J Biotechnol 159:1–11. doi: 10.1016/j.jbiotec.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Gerstmeir R, Cramer A, Dangel P, Schaffer S, Eikmanns BJ. 2004. RamB, a novel transcriptional regulator of genes involved in acetate metabolism of Corynebacterium glutamicum. J Bacteriol 186:2798–2809. doi: 10.1128/JB.186.9.2798-2809.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lardy HA, Peanasky R. 1953. Metabolic functions of biotin. Physiol Rev 33:560–565. [DOI] [PubMed] [Google Scholar]
- 32.Flavin M, Ortiz PJ, Ochoa S. 1955. Metabolism of propionic acid in animal tissues. Nature 176:823–826. doi: 10.1038/176823a0. [DOI] [PubMed] [Google Scholar]
- 33.Mancia F, Smith GA, Evans PR. 1999. Crystal structure of substrate complexes of methylmalonyl-CoA mutase. Biochemistry 38:7999–8005. doi: 10.1021/bi9903852. [DOI] [PubMed] [Google Scholar]
- 34.Huang CS, Sadre-Bazzaz K, Shen Y, Deng B, Zhou ZH, Tong L. 2010. Crystal structure of the α6β6 holoenzyme of propionyl-CoA carboxylase. Nature 466:1001–1005. doi: 10.1038/nature09302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bannerjee D, Sanders LE, Sokatch JR. 1970. Properties of the methylmalonate semialdehyde dehydrogenase of Pseudomonas aeruginosa. J Biol Chem 245:1828–1835. [PubMed] [Google Scholar]
- 36.Marshall VD, Sokatch JR. 1972. Regulation of valine catabolism in Pseudomonas aeruginosa. J Bacteriol 110:1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steele MI, Lorenz D, Hatter K, Park A, Sokatch JR. 1992. Characterization of the mmsAB operon of Pseudomonas aeruginosa PAO encoding methylmalonate-semialdehyde dehydrogenase and 3-hydroxyisobutyrate dehydrogenase. J Biol Chem 267:13585–18592. [PubMed] [Google Scholar]
- 38.Kornberg HL, Krebs HA. 1957. Synthesis of cell constituents from C2-units by a modified tricarboxylic acid cycle. Nature 179:988–991. doi: 10.1038/179988a0. [DOI] [PubMed] [Google Scholar]
- 39.LaPorte DC, Chung T. 1985. A single gene codes for the kinase and phosphatase which regulate isocitrate dehydrogenase. J Biol Chem 260:15291–15297. [PubMed] [Google Scholar]
- 40.Maloy SR, Nunn WD. 1982. Genetic regulation of the glyoxylate shunt in Escherichia coli K-12. J Bacteriol 149:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lorca GL, Ezersky A, Lunin VV, Walker JR, Altamentova S, Evdokimova E, Vedadi M, Bochkarev A, Savchenko A. 2007. Glyoxylate and pyruvate are antagonistic effectors of the Escherichia coli IclR transcriptional regulator. J Biol Chem 282:16476–16491. doi: 10.1074/jbc.M610838200. [DOI] [PubMed] [Google Scholar]
- 42.Cramer A, Gerstmeir R, Schaffer S, Bott M, Eikmanns BJ. 2006. Identification of RamA, a novel LuxR-type regulator of genes involved in acetate metabolism in Corynebacterium glutamicum. J Bacteriol 188:2554–2567. doi: 10.1128/JB.188.7.2554-2567.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simon EJ, Shemin D. 1953. The preparation of S-succinyl coenzyme A. J Am Chem Soc 75:2520. doi: 10.1021/ja01106a522. [DOI] [Google Scholar]
- 44.Alber BE, Spanheimer R, Ebenau-Jehle C, Fuchs G. 2006. Study of an alternate glyoxylate cycle for acetate assimilation by Rhodobacter sphaeroides. Mol Microbiol 61:297–309. doi: 10.1111/j.1365-2958.2006.05238.x. [DOI] [PubMed] [Google Scholar]
- 45.Shapira SK, Chou J, Richaud FV, Casadaban MJ. 1983. New versatile plasmid vectors for expression of hybrid proteins coded by a cloned gene fused to lacA gene sequences encoding an enzymatically active carboxy-terminal portion of β-galactosidase. Gene 11:71–82. [DOI] [PubMed] [Google Scholar]
- 46.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 47.Merighi M, Majerczak DR, Zianni M, Tessanne K, Coplin DL. 2006. Molecular characterization of Pantoea stewartii subsp. stewartii HrpY, a conserved response regulator of the Hrp type II secretion system, and its interaction with the hrpS promoter. J Bacteriol 188:5089–5100. doi: 10.1128/JB.01929-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones DT, Taylor WR, Thornton JM. 1992. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci 8:275–282. [DOI] [PubMed] [Google Scholar]
- 50.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. 2009. MEME suite: tools for motif discovery and searching. Nucleic Acids Res 37:202–208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sunnarborg A, Klumpp D, Chung T, LaPorte DC. 1990. Regulation of the glyoxylate bypass operon: cloning and characterization of iclR. J Bacteriol 172:2642–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Starai VJ, Escalante-Semerena JC. 2004. Identification of the protein acetyltransferase (Pat) enzyme that acetylates acetyl-CoA synthetase in Salmonella enterica. J Mol Biol 340:1005–1012. doi: 10.1016/j.jmb.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 53.Crosby HA, Pelletier DA, Hurst GB, Escalante-Semerena JC. 2012. System-wide studies of N-lysine acetylation in Rhodopseudomonas palustris reveal substrate specificity of protein acetyltransferases. J Biol Chem 287:15590–15601. doi: 10.1074/jbc.M112.352104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suvorova IA, Ravcheev DA, Gelfand MS. 2012. Regulation and evolution of malonate and propionate catabolism in proteobacteria. J Bacteriol 194:3234–3240. doi: 10.1128/JB.00163-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Radmacher E, Eggerling L. 2007. The three tricarboxylate synthase activities of Corynebacterium glutamicum and increase of l-lysine synthesis. Appl Microbiol Biotechnol 76:587–595. doi: 10.1007/s00253-007-1105-7. [DOI] [PubMed] [Google Scholar]
- 56.Wendisch VF, Spies M, Reinscheid DJ, Schnicke S, Sahm H, Eikmanns BJ. 1997. Regulation of acetate metabolism in Corynebacterium glutamicum: transcriptional control of the isocitrate lyase and malate synthase genes. Arch Microbiol 168:262–269. doi: 10.1007/s002030050497. [DOI] [PubMed] [Google Scholar]
- 57.Maruyama K, Kitamura H. 1975. Some effects of propionate on the growth of Rhodopseudomonas sphaeroides S. Agric Biol Chem 39:1521–1526. doi: 10.1271/bbb1961.39.1521. [DOI] [Google Scholar]
- 58.Chan CH, Garrity J, Crosby HA, Escalante-Semerena JC. 2011. In Salmonella enterica, the sirtuin-dependent protein acylation/deacylation system (SDPADS) maintains energy homeostasis during growth on low concentration of acetate. Mol Microbiol 80:168–183. doi: 10.1111/j.1365-2958.2011.07566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Starai VJ, Takahashi H, Boeke JD, Escalante-Semerena JC. 2004. A link between transcription and intermediary metabolism: a role for Sir2 in the control of acetyl-CoA synthetase. Curr Opin Microbiol 7:115–119. doi: 10.1016/j.mib.2004.02.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.