ABSTRACT
Mycobacterium tuberculosis persists inside granulomas in the human lung. Analysis of the metabolic composition of granulomas from guinea pigs revealed that one of the organic acids accumulating in the course of infection is acetate (B. S. Somashekar, A. G. Amin, C. D. Rithner, J. Troudt, R. Basaraba, A. Izzo, D. C. Crick, and D. Chatterjee, J Proteome Res 10:4186–4195, 2011, doi:http://dx.doi.org/10.1021/pr2003352), which might result either from metabolism of the pathogen or might be provided by the host itself. Our studies characterize a metabolic pathway by which M. tuberculosis generates acetate in the cause of fatty acid catabolism. The acetate formation depends on the enzymatic activities of Pta and AckA. Using actyl coenzyme A (acetyl-CoA) as a substrate, acetyl-phosphate is generated and finally dephosphorylated to acetate, which is secreted into the medium. Knockout mutants lacking either the pta or ackA gene showed significantly reduced acetate production when grown on fatty acids. This effect is even more pronounced when the glyoxylate shunt is blocked, resulting in higher acetate levels released to the medium. The secretion of acetate was followed by an assimilation of the metabolite when other carbon substrates became limiting. Our data indicate that during acetate assimilation, the Pta-AckA pathway acts in concert with another enzymatic reaction, namely, the acetyl-CoA synthetase (Acs) reaction. Thus, acetate metabolism might possess a dual function, mediating an overflow reaction to release excess carbon units and resumption of acetate as a carbon substrate.
IMPORTANCE During infection, host-derived lipid components present the major carbon source at the infection site. β-Oxidation of fatty acids results in the formation of acetyl-CoA. In this study, we demonstrate that consumption of fatty acids by Mycobacterium tuberculosis activates an overflow mechanism, causing the pathogen to release excess carbon intermediates as acetate. The Pta-AckA pathway mediating acetate formation proved to be reversible, enabling M. tuberculosis to reutilize the previously secreted acetate as a carbon substrate for metabolism.
INTRODUCTION
Mycobacterium tuberculosis is still a major health threat in nonindustrialized countries. Worldwide 1.5 million people die each year due to tuberculosis, and the rising number of multiresistant strains requires the development of new antibiotics (1). The great success of the pathogen is based on its ability to persist inside the human host for decades or even lifelong. Persistence is characterized by a substantial reduction of the bacterial metabolism, accompanied by low ATP levels and little or no replication (2). Therefore, a major point of interest in tuberculosis research is the adaptation of the central carbon metabolism of M. tuberculosis to the human host. During chronic infection, the metabolism of M. tuberculosis mainly depends on β-oxidation of fatty acids, which was first observed by Bloch and Segal in 1956, who demonstrated that M. tuberculosis isolates from infected mice prefer fatty acids over glycolytic substrates (3). This observation has been confirmed by additional studies, showing that the genome of M. tuberculosis encodes a high copy number of genes involved in β-oxidation (4), of which a significant amount is induced during the infection of mouse macrophages (5) and human patients (6). Furthermore, mutant strains impaired in the acquisition (7) or utilization (8) of fatty acids are attenuated in mice. Thus, lipids and fatty acids derived from vacuolar membrane components present a major carbon source during infection. Current studies suggest that the energy sources acquired and metabolized by M. tuberculosis are more diverse and besides fatty acids include cholesterol (9), carbohydrates (10), and amino acids (11–13). These different carbon substrates can be cometabolized (14) and might indicate that access to nutrients is not as limited as previously thought. Considering these aspects, our study will focus on the metabolism of the organic acid acetate and thereby contribute to our current knowledge about the diversity of carbon utilization in M. tuberculosis.
Acetate is the anion of a short-chain fatty acid, which can be found in small amounts in the tissue extracts of organs and in the serum of healthy mice (15). Metabolic analysis of the granulomas of guinea pigs revealed that besides lipid components, different amino acids and organic acids, such as acetate, accumulate during an M. tuberculosis infection (16). Although it is generally believed that most of the detected metabolites are derived from the metabolism of the host, they might also be produced by the pathogen itself. That acetyl coenzyme A (acetyl-CoA) is a main product of fatty acid degradation led us to hypothesize that the accumulating acetate might be formed in the course of acetyl-CoA processing by M. tuberculosis. Evidence for acetate formation by M. tuberculosis is provided by the presence of two genes that are homologous to phosphotransacetylase (Pta) and acetate kinase (AckA) in Escherichia coli (4). For E. coli, it is known that acetate formation occurs in the absence of oxygen, when tricarboxylic acid (TCA) cycle activity is downregulated and carbon flow is directed through fermentative pathways (17). However, M. tuberculosis is characterized as a nonfermentative pathogen. Nonetheless, acetate might be generated in a process called the Crabtree effect. In this process, excess glycolytic substrates inhibit the respiration and the TCA cycle under aerobic conditions (18). Thus, acetate might be produced as a by-product during growth of M. tuberculosis on excess carbon substrates, when flow in the TCA cycle is limited (19).
Acetate production leads to the regeneration of coenzyme A, the redirection of carbon flux to relieve the TCA cycle, and ATP generation by substrate-level phosphorylation (20). On the other side, secreted acetate might accumulate in the environment. This process might be subsequently followed by diffusion of acetate across the membrane, leading to a toxic acidification of the cytoplasm (21). Thus, the reuptake of the previously secreted acetate and its incorporation into the central metabolism enable M. tuberculosis to remove a potentially toxic compound and provide an additional carbon source for the pathogen. Actually, whether M. tuberculosis is able to produce acetate is unknown, while M. tuberculosis assimilation of acetate from the medium has already been described (22). The pathways by which M. tuberculosis activates acetate to acetyl-CoA are rather undefined. In this study, we show that, depending on the carbon diet, M. tuberculosis is able to generate and secrete acetate, and we examine the pathways involved in both acetate dissimilation and assimilation. To make a contribution to our current understanding of the carbon metabolism of M. tuberculosis, we analyzed the potential function of acetate metabolism during consumption of different carbon sources.
MATERIALS AND METHODS
Strains and media.
For acetate formation experiments, M. tuberculosis H37Rv (ATCC 25618) and its derivative mutant strains were grown in Middlebrook 7H9 liquid medium (Difco Laboratories, Detroit, MI) supplemented with 0.5% bovine serum albumin (BSA) fraction V (Applichem, Darmstadt, Germany), which contains 1% fatty acids, 14 mM sodium chloride, and 0.05% Tween 80. To analyze growth in a fatty acid-free medium, BSA containing less than 0.01% fatty acids (Applichem, Darmstadt, Germany) was used, and Tween 80 was replaced by 0.05% tyloxapol. Glucose, glycerol, fatty acids, or pyruvate was added as a carbon substrate at the indicated concentrations. Middlebrook 7H10 agar supplemented with 5.8 mM sodium chloride, 0.2% bovine serum albumin fraction V, 4.4 mM glucose, 50 mM glycerol, 0.0001% catalase, and 0.06% oleic acid was used as the solid medium. Acetate assimilation experiments were performed in a chemically defined medium containing 7.3 mM KH2PO4, 17.6 mM Na2HPO4, 190.8 μM ferric ammonium citrate, 2 mM MgSO4, 3.4 μM CaCl2, 0.35 μM ZnSO4, 50 mM asparagine, 0.05% tyloxapol, and acetate as the carbon source at the indicated concentrations (9). Additionally, for culture of complemented strains, 50 μg/ml hygromycin B was supplied. For experiments, cells were grown to the mid-log phase (optical density at 600 nm [OD600] of 0.5 to 0.8) in 7H9 medium containing 10 mM glucose and 50 mM glycerol, washed twice in the respective broth without an additional carbon source, and then diluted to an initial OD600 of 0.05 in fresh medium supplemented with the carbon substrates indicated for the experiment. Cultivation under well-aerated, fast-growth conditions was performed in flasks with a headspace/medium ratio of 3:1 at 70 rpm and 37°C, while cultivation under slow-growth conditions was performed in standing 15-ml polystyrene tubes with conical bottoms (Greiner Bio-One) with a headspace/medium ratio of 1:2. For acetate detection and survival assays, samples were taken at the indicated time points, and serial dilutions were plated on 7H10 agar plates to determine the CFU.
Generation and complementation of ΔackA, Δpta, and Δacs deletion mutants in M. tuberculosis.
By screening a genomic cosmid library (23) via colony blot analysis, subgenomic fragments containing the pta-ackA operon or acs gene were isolated and utilized for further cloning. The M. tuberculosis strains ND1 (ΔackA), ND7 (Δpta), and ND9 (Δacs) were generated via homologous recombination (24) in M. tuberculosis H37Rv. pta (Rv0408), ackA (Rv0409), and acs (Rv3667) deletions with flanking regions of ∼1,000 bp were inserted into a suicide plasmid pYUB657 (24) containing a hygromycin resistance cassette for positive selection and the sacB gene, encoding a levansucrase, for negative selection. Following transformation of M. tuberculosis, hygromycin-resistant clones were isolated and tested for cointegration of the plasmid containing a defective copy of either ackA, pta, or acs. Subsequently, bacteria were grown to the late log phase without selective pressure, and clones that had undergone double crossover were selected on agar plates containing 2% sucrose. ND1 (ΔackA), contains an unmarked 666-bp deletion from bp 494599 to 493932 within the ackA gene; ND7 (Δpta), contains an unmarked 1,621-bp deletion from bp 493415 to 491794 within the pta gene, and ND9 (Δacs) contains an unmarked 897-bp deletion from bp 4108657 to 4109553 within the acs gene. The resulting mutant strains were validated by Southern blots (see Fig. S1 in the supplemental material). Polar effects of the pta deletion on ackA were examined through enzyme activity assay of AckA function (Table 1). Polar effects of the ackA or acs deletions are unlikely because there are no downstream genes transcribed from the same strand.
TABLE 1.
Pta mediates phosphotransacetylase and AckA acetate kinase activity
| Enzyme and strain | Activity (U/mg protein)a |
|---|---|
| Pta | |
| WT | 0.338 |
| Δpta mutant | <0.01 |
| Δpta::pND11+ complemented strain | 0.266 |
| AckA | |
| WT | 0.948 |
| ΔackA mutant | <0.01 |
| ΔackA::pND11+ complemented strain | 0.760 |
To confirm the functionality of the Pta and AckA enzymes, the M. tuberculosis H37Rv wild type, the knockout mutants, and the complemented strains were grown on 50 mM pyruvate. Enzyme activity assays with cell extracts revealed Pta and AckA activities in the wild-type and complemented strains, while Pta activity was abolished in the Δpta mutant, and AckA activity was abolished in the ΔackA mutant. The data represent one of two independent experiments.
ND1 (ΔackA) and ND7 (Δpta) were complemented with an integrating plasmid vector, pMV306 (25), which carries pta and ackA and upstream an additional 2,000-bp genomic fragment with the putative promoter region. Complemented mutant strains are indicated by Δpta::pND11+ and ΔackA::pND11+, respectively.
Quantification of substrates and products.
For high-pressure liquid chromatography (HPLC) measurements, culture samples were centrifuged for 5 min at 8,000 × g, and supernatant was incubated at 90°C for 30 min. Pyruvate and acetate were quantified in the culture supernatant using a high-pressure liquid chromatograph (LaChrom Elite; VWR Hitachi, West Chester, PA), equipped with an Aminex HPX87-H column (300 by 7.8 mm; Bio-Rad, Hercules, CA) as the stationary phase and 12 mM H2SO4 as the mobile phase (26). The separation was performed at a flow rate of 0.5 ml min−1 at 45°C. The detection of the analytes was performed by refractive index and UV absorbance (210 nm). Quantification was carried out via external standards.
In addition, acetate formation during growth on diverse carbon substrates was enzymatically detected using the acetate kit from r-biopharm (Darmstadt, Germany). Therefore, 1-ml samples of the culture were centrifuged for 5 min at 16,200 × g, and the supernatant was used as the substrate for acetate detection.
Pta and AckA activity assays.
M. tuberculosis was grown under well-aerated conditions to an OD600 of 1.5 to 2.0. The cultures were washed twice in washing buffer (50 mM Tris-HCl [pH 7.0]), resuspended in 0.75 ml buffer (50 mM Tris-HCl [pH 7], 10 mM MgCl2, 1 mM EDTA, 1 mM dithiothreitol [DTT], 30% glycerol), and transferred into a lysing matrix B tube (MP Biomedicals, Santa Ana, CA). The cells were homogenized in five steps for 25 s in a Mini-Beadbeater-8 (BioSpec Products, Bartlesville, OK) and were chilled on ice for 5 min after each run. Homogenates were then centrifuged for 10 min at 16,200 × g. The supernatant was used for the enzymatic activity assay. Enzyme activity was normalized against total protein. The total protein concentration was determined using absorbance measurement at 595 nm after incubation with a dye reagent concentrate (Bio-Rad Laboratories GmbH, Munich, Germany).
Phosphotransacetylase activity was measured while converting acetyl-phosphate into acetyl-CoA as previously described (27). One hundred micrograms of protein of the whole-cell lysate was used in 1 ml reaction buffer (100 mM Tris-HCl [pH 8], 5 mM MgCl2, 0.5 mM NAD, 5 mM malic acid, 10 mM acetyl-phosphate, 0.5 mM CoA, 45 U malate dehydrogenase [Sigma], 5 U citrate synthase [Sigma]). The absorbance at 340 nm was monitored for 6 min. One unit of Pta activity corresponds to 1 μmol acetyl-CoA production per min at 25°C.
Acetate kinase activity was measured as described previously (28). The reaction mixture contained 100 mM Tris-HCl (pH 7.4), 5 mM ADP, 10 mM MgCl2, 5.5 mM glucose, 1 mM NADP, 2 mM DTT, 6 U hexokinase, 3 U glucose-6-phosphate-dehydrogenase (Sigma), and 25 μg protein from the whole-cell lysate. The absorbance at 340 nm was monitored for 3 min in the reaction mixture without substrate. Subsequently, acetyl-phosphate was added to a final concentration of 20 mM, and production of NADPH was again detected at 340 nm. One unit of acetate kinase activity was considered to transform 1 μmol of acetyl-phosphate per min at 25°C.
RESULTS
Growth on fatty acids induces acetate formation in M. tuberculosis.
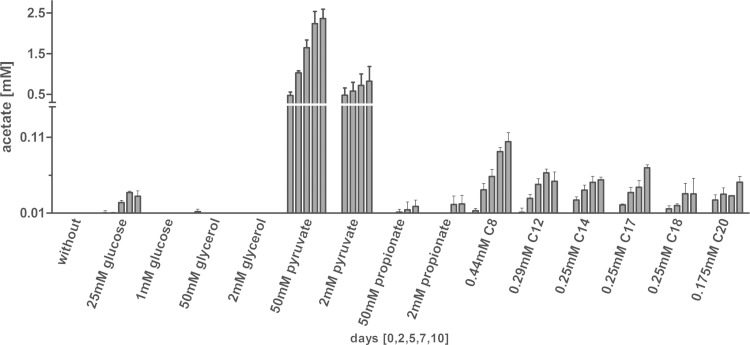
Acetate production can be found in different bacteria, mostly as a consequence of mixed acid fermentation. In contrast to E. coli, M. tuberculosis is considered to be a nonfermentative organism. Indeed, genes involved in classical fermentative pathways are lacking in the genome of M. tuberculosis (4), and products of fermentation such as lactate and formate have not been reported for M. tuberculosis so far. Furthermore, the ATP synthase is essential in mycobacteria, whereas in bacteria that are capable of fermentation, respiratory ATP production is often dispensable (29). However, besides during fermentation, E. coli also produces acetate in the presence of large amounts of glycolytic substrates. Thus, we determined whether the concentration of glycolytic carbon substrates such as glucose and glycerol had an impact on acetate formation. No induction of acetate production was observed in the presence of small amounts of glycolytic substrates, but increasing the glucose concentration stimulated at least the formation of low levels of acetate (Fig. 1). A metabolite directly leading to the generation of acetyl-CoA is pyruvate. When pyruvate was offered as a carbon substrate to growing M. tuberculosis cells, high levels of acetate were detected in the supernatant of the culture. As the pyruvate concentration in macrophages and inside granulomas is considered rather low (15, 16) and is most likely not a physiological carbon source for M. tuberculosis, we analyzed whether growth on fatty acids would also result in acetate secretion. In principle, acetyl-CoA and to a lesser extent propionyl-CoA are generated in the course of β-oxidation of fatty acids, turning fatty acids into a potential source for acetate formation. The supplementation of the medium with either odd- or even-numbered fatty acids induced acetate production in M. tuberculosis (Fig. 1). The length of the respective fatty acid had only a minor impact on acetate formation, as caprylic acid (C8) had nearly the same potential to stimulate acetate generation as lauric acid (C12), myristic acid (C14), stearic acid (C18), or arachidic acid (C20). In Fig. S2 in the supplemental material, growth curves for up to 10 days are shown to follow the multiplication rates of bacilli at various carbon sources and concentrations.
FIG 1.
Induction of acetate formation by fatty acids. M. tuberculosis H37Rv was grown in standing cultures for 10 days in the presence of different carbon sources. To analyze the impact of excess carbon substrates on acetate formation, substrates were supplied in low (0.175 to 2 mM) and high (25 to 50 mM) concentrations to the medium, and the resulting acetate production was detected in the supernatant at the indicated time points. Each bar represents the mean of three independent experiments; error bars indicate the standard deviation (SD).
As it turned out that fatty acids effect acetate secretion in M. tuberculosis, we also tested for acetate production in a less rich medium, which contained a BSA formulation free of fatty acids and tyloxapol, a nonhydrolyzable detergent, instead of Tween 80 (see Fig. S3 in the supplemental material). The experiment confirmed that acetate secretion is induced during growth on pyruvate and fatty acids, although acetate levels were reduced in comparison to levels obtained from bacilli cultured in the richer standard medium. This also shows that M. tuberculosis indeed utilizes fatty acids from BSA or Tween 80 to generate acetate. Thus, acetate production seems to depend on the presence of certain carbon substrates, with M. tuberculosis directing a part of the carbon flux toward acetate generation.
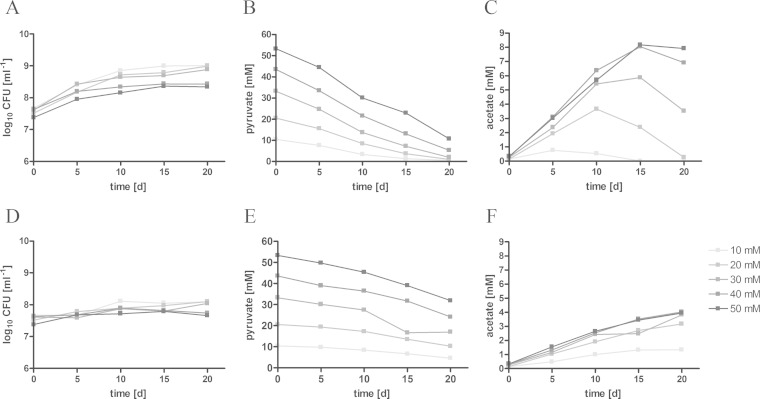
Reduced respiration has a major impact on acetate production in bacteria such as E. coli and Bacillus subtilis (17, 30). Inside granulomas, M. tuberculosis is also confronted with limited oxygen saturation, resulting in downregulation of NDH-1 and reduced oxidative phosphorylation mediated by NDH-2 (31). To determine the impact of oxygen limitation on acetate production, we compared levels of acetate secretion by M. tuberculosis at high growth rates (shaken cultures with a high headspace/medium ratio) or under slow-growth conditions (standing cultures with a low headspace/medium ratio). Pyruvate, which proved to be the best inductor of acetate formation, was offered as the carbon source under both culture conditions. Under slow-growth conditions, M. tuberculosis secreted only small amounts of acetate to the supernatant (Fig. 2). In contrast, a higher growth rate was associated with higher levels of acetate secretion. In accordance with acetate production, slow growth corresponded to reduced pyruvate assimilation, whereas pyruvate was rapidly consumed in fast-growing cultures. In fast-growing cultures, M. tuberculosis started to assimilate the previously secreted acetate when pyruvate levels dropped.
FIG 2.
Acetate formation depends on substrate concentration. M. tuberculosis H37Rv was cultivated for 20 days under fast-growth (A to C) or slow-growth (D to F) conditions. Pyruvate was provided in different concentrations (10 to 50 mM) to induce acetate formation. Growth was measured by colony counts (A and D). Pyruvate consumption (B and E) and acetate excretion (C and F) were detected in the supernatant by HPLC. The data represent one of two independent experiments.
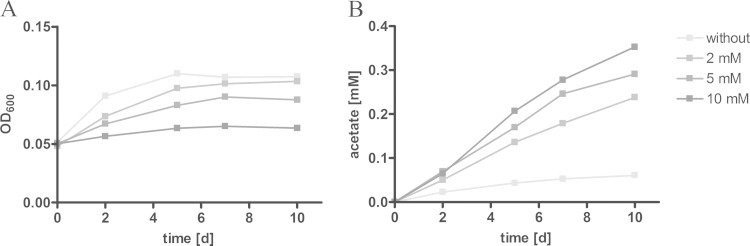
Acetate production increased under both slow- and fast-growth culture conditions when higher levels of pyruvate were supplied to the bacteria, indicating that acetate is released in an overflow reaction. In bacteria such as E. coli or Staphylococcus aureus, carbon overflow occurs as a consequence of an imbalance in carbon uptake, energy production, and biosynthesis (32, 33). To substantiate the idea that acetate formation might serve as a metabolic outlet, we cultivated M. tuberculosis under slow-growth conditions supplemented with heptadecanoic acid and inhibited the activity of isocitrate lyase (Icl1), a key enzyme of the glyoxylate shunt. The inhibition of the Icl1 was achieved by the addition of itaconate. Through inhibition of the glyoxylate shunt, M. tuberculosis loses the ability to grow on C2 substrates derived from fatty acid catabolism, due to the lack of anaplerotic precursors (34). The reduced growth in the presence of itaconate was accompanied by higher levels of acetate detected in the supernatant (Fig. 3). This observation indicates that carbon flow is directed to acetate formation when flow through the glyoxylate shunt is blocked.
FIG 3.
Inhibition of the glyoxylate shunt increases acetate dissimilation. Standing cultures of M. tuberculosis H37Rv were grown for 10 days on 0.25 mM heptadecanoic acid as the carbon substrate. To limit carbon flow through the glyoxylate shunt, the isocitrate lyase was chemically inhibited by 2, 5, or 10 mM itaconate. The impact on growth was detected via optical density of the culture (A), and the resulting acetate formation was measured enzymatically in the supernatant (B). The data represent one of three independent experiments.
Acetate dissimilation is mediated by the Pta-AckA pathway.
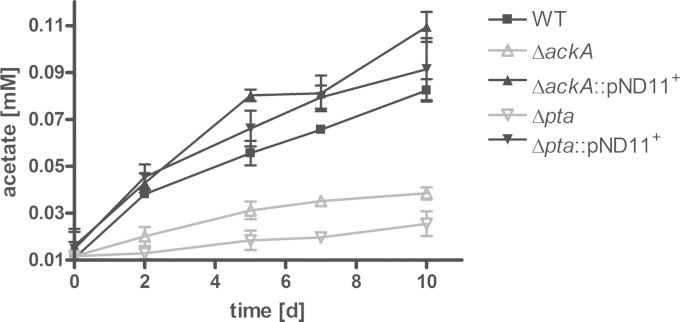
To further characterize the role of acetate metabolism in M. tuberculosis, we analyzed the pathway responsible for acetate generation. A pathway commonly used by such bacteria as E. coli, Pseudomonas aeruginosa, and S. aureus (35–38) involves the pta gene, encoding the phosphotransacetylase, and the ackA gene, encoding the acetate kinase. The genome of M. tuberculosis possesses homologous copies of these two genes. To determine whether both enzymes are functional in M. tuberculosis and mediate acetate formation from acetyl-CoA, we generated knockout strains and performed enzyme activity assays. The enzyme activities were detected in crude cell extracts from M. tuberculosis cultured on 50 mM pyruvate to stimulate acetate formation. We found that the wild-type strain exhibits phosphotransacetylase and acetate kinase activities, while the Δpta strain lacked phosphotransacetylase activity, and the ΔackA strain lacked acetate kinase activity (Table 1). The enzymatic activities of the knockout mutants were restored in complemented strains. To determine whether the Pta-AckA pathway is involved in acetate formation detected during growth on fatty acids, we measured the acetate secretion in the knockout strains cultured in the presence of heptadecanoic acid. Acetate production in both strains lacking either the pta or the ackA gene was reduced during growth on fatty acids (Fig. 4). Interestingly, the formation of acetate in the knockout strains was not completely abolished. Residual acetate accumulation might be due to additional pathways involved in acetate formation. In Corynebacterium glutamicum, two enzymes, the pyruvate oxidase, Pqo, and a CoA transferase, CtfA, convert pyruvate or acetyl-CoA, respectively, into acetate (39, 40). However, neither of the two enzymes has homologues in the annotated genome sequence of M. tuberculosis (4).
FIG 4.
Acetate production is mediated by the Pta-AckA pathway. The M. tuberculosis H37Rv wild-type strain (WT), the ΔackA and Δpta mutant strains, and the complemented strains were grown in standing cultures for 10 days, and 0.25 mM heptadecanoic acid was supplied as carbon substrate. The influence of pta or ackA deletion on acetate dissimilation was analyzed by acetate detection in the supernatant of the knockout (KO) strains. The data represent the mean from three independent experiments; error bars indicate the SD.
The role of the Pta-AckA pathway in acetate assimilation.
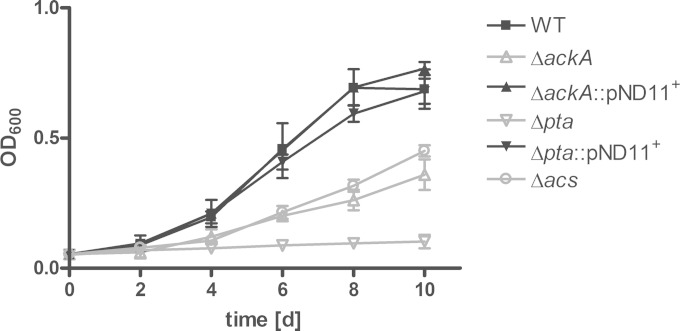
When carbon sources become limiting, E. coli starts to assimilate previously secreted acetate. This process is called “acetate switching” (41). We observed that acetate was consumed from the medium of aerobically growing M. tuberculosis when extracellular pyruvate became exhausted (Fig. 2). To analyze the impact of the Pta-AckA pathway on acetate assimilation, the M. tuberculosis wild type and the mutant strains deficient for Pta or AckA activity were grown in minimal medium supplemented with acetate as the sole carbon substrate. Interestingly, the Δpta and the ΔackA knockout strains developed different growth phenotypes, although Pta and AckA belong to the same metabolic pathway. The growth of the ackA mutant was attenuated in the presence of acetate as the sole carbon source, while growth of the pta mutant was completely abolished (Fig. 5). The observation that an M. tuberculosis strain deficient for AckA activity is still able to utilize acetate as a carbon substrate, although to a minor extent, indicates that M. tuberculosis possesses additional pathways for the activation of acetate. Additional pathways in acetate formation are known for E. coli, which produces acetate by the acetyl-CoA synthetase (Acs) reaction from acetyl-CoA. A recent publication revealed that M. tuberculosis possesses a functional Acs that is involved in acetylation of various proteins (42). Thus, conversion of acetate to acetyl-CoA by Acs might also have a role in utilization of acetate as a carbon source. Therefore, a Δacs knockout strain was generated and analyzed for growth on acetate (Fig. 5). The Δacs mutant was attenuated in growth in the presence of acetate as the sole carbon source and showed the same intermediate phenotype as the ΔackA mutant strain. Thus, acetate is activated by at least two independent pathways: the Acs pathway and the AckA-Pta pathway.
FIG 5.
The role of Acs and the Pta-AckA pathway in acetate assimilation. To analyze acetate utilization in M. tuberculosis, the wild type (WT), ΔackA, Δpta, and Δacs mutant strains, and complemented strains were grown for 10 days under fast-growth conditions. Acetate at 10 mM was supplied as the sole carbon substrate to the minimal medium, and growth was determined via optical density. The data represent the mean from three independent experiments; error bars indicate the SD.
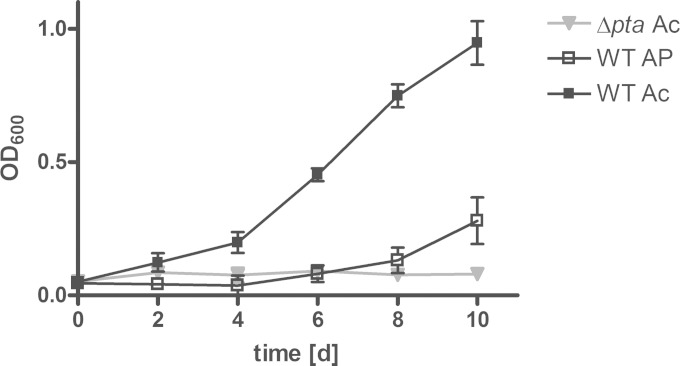
The inability of the Δpta knockout strain to convert acetate into biomass, although possessing an active acetate-activating Acs pathway, indicates a toxic effect of the accumulating intermediate acetyl-phosphate. Acetyl-phosphate is a high-energy form of phosphate and plays a crucial role in substrate phosphorylation and signal transduction (43). Abnormal regulation of signal molecules might account for the inhibitory effect of excess acetyl-phosphate (44). To analyze the impact of acetyl-phosphate on growth of M. tuberculosis, the wild type was cultured in medium supplemented with acetyl-phosphate (Fig. 6). In the presence of acetyl-phosphate, the growth of the wild-type strain was initially impaired, although growth later resumed. Resumption of growth was most likely caused by the functional phosphotransacetylase of the wild-type strain. Thus, the conversion of acetyl-phosphate to acetyl-CoA diminishes the inhibitory effect of acetyl-phosphate. These data suggest that the Pta-AckA pathway might be involved in the maintenance of the acetyl-phosphate pool and emphasize the role of the Pta-AckA pathway in acetate assimilation.
FIG 6.
Acetyl-phosphate accumulation results in restricted growth of M. tuberculosis. The M. tuberculosis H37Rv wild type (WT) and the Δpta mutant were cultivated under fast-growth conditions for 10 days. Growth was analyzed in the presence of 20 mM acetate (Ac) or 20 mM acetyl-phosphate (AP). The data represent the mean from three independent experiments; error bars indicate the SD.
DISCUSSION
In most bacteria, acetate is produced during mixed acid fermentation under anaerobic conditions or in carbon overflow during aerobic growth on excess glycolytic substrates. These characteristics of acetate production in other bacteria cannot be applied to the physiological situation of M. tuberculosis. Inside the human granuloma, M. tuberculosis faces hypoxic conditions, which promote redox stress by an intracellular increase of the NADH/NAD+ ratio. However, in contrast to E. coli, M. tuberculosis does not regenerate its NAD+ pool by mixed acid fermentation. M. tuberculosis lacks the classic fermentative pathways. Succinate secretion is an exception (45, 46). Whether succinate is the product of fermentation is still debated. Previous studies demonstrated that M. tuberculosis generates elevated amounts of succinate during growth under hypoxia (45, 46). The reductive branch of the TCA cycle, which includes the reduction of fumarate to succinate, might serve for regeneration of NAD+ (45). However, a subsequent study suggested that succinate is more likely the product of an elevated carbon flow through the glyoxylate shunt, and its secretion is substantial to balance membrane potential (46).
As acetate production in M. tuberculosis is performed in a nonfermentative manner, it might result from carbon overflow. In exponentially growing E. coli cells cultured in the presence of excess glucose, carbon units are secreted as acetate (19). Inside the phagosome of the macrophage or inside granulomas, the access to high glucose concentrations is restricted. Data about the metabolic composition of the M. tuberculosis-containing vacuoles inside macrophages are lacking, but mass spectrometry analysis determining the metabolic pool in the granulomas of guinea pigs have been performed (16). The detected levels of glucose or intermediates of the glycolysis were rather low, while free amino acids, organic acids, and lipid components accumulated during infection. These findings reflect the current concept about carbon sources utilized by M. tuberculosis in vitro and in vivo (47, 48). The low level of glycolytic substrates indicates that acetate formation in M. tuberculosis might rely on other carbon sources than glucose. Lipids and fatty acids are considered to be the main carbon sources during in vivo growth, at least in the latent phase of infection. In this study, we demonstrated that growth on fatty acids results in the formation of acetate and might therefore be the original stimulus for acetate formation.
Usually, M. tuberculosis prefers to store fatty acids in form of lipid bodies. In vitro, nonreplicating M. tuberculosis uses externally provided fatty acids to generate lipid bodies (49). Inside lipid-loaded macrophages, the pathogen acquires fatty acids from hydrolyzed cellular lipids and forms lipid inclusions, which act as an internal carbon storage (50). Thus, fatty acids stored in lipid bodies are not synthesized de novo but originate from the environment (50). Whether excess carbon units during growth on fatty acids are used for de novo fatty acid synthesis is unclear, yet fatty acid synthesis is an energy-consuming process (51). Thus, in the in vivo situation, M. tuberculosis might use host-derived fatty acids to generate lipid bodies and at the same time might perform β-oxidation of fatty acids to fuel the central carbon metabolism. Under these conditions, excess carbon units are probably not used for energy-consuming de novo synthesis of fatty acids but are rather secreted in the form of acetate.
To demonstrate that acetate is produced as a consequence of carbon overflow, we designed an experiment in which carbon flow from fatty acids via the glyoxylate shunt was blocked. Utilization of the inhibitor resulted in attenuated growth and increased secretion of acetate to the medium. An imbalance in carbon uptake and biomass formation probably caused a release of excess carbon substrates in the form of acetate. It is noteworthy that the chemical inhibitor itaconate that we used in this study is naturally produced in activated macrophages by IRG1 (52). Thus, it is intriguing to speculate that the inhibitory effect of itaconate on intracellular M. tuberculosis might contribute to increased acetate formation and acetate accumulation detectable in granulomas (16).
While analyzing different carbon sources for their potential to stimulate acetate formation, we found that M. tuberculosis secreted the highest concentrations of acetate in the presence of pyruvate. Studies on the phosphotransacetylase of E. coli revealed that pyruvate has a stimulatory effect on Pta (53). Pyruvate, which is generated through glycolysis, activates the Pta enzyme in an allosteric manner, providing E. coli with the ability to convert excess carbon units in acetate. High glycolysis rates are not observed during chronic M. tuberculosis infection (54), and even under in vitro conditions, the supply of large amounts of glucose or glycerol had nearly no effect on acetate production. The uptake of glucose might be the limiting step (55, 56). Thus, a low flux through the glycolytic pathway may avoid pyruvate accumulation and thereby minimize carbon overflow by production of acetate via the Pta-AckA pathway. In contrast, feeding of pyruvate as a carbon source to M. tuberculosis might result in high intracellular pyruvate levels and thus could explain the large amount of acetate production that we observed in our study.
Once the concentration of supplied pyruvate was consumed to a critical level, the previously secreted acetate was assimilated by M. tuberculosis. The ability of M. tuberculosis to assimilate acetate is a well-known fact. Indeed, in several studies M. tuberculosis was grown on acetate as the sole carbon source to simulate carbon flux during growth on fatty acids (57, 58). The pathways involved in acetate activation have not been completely characterized. By generating knockout strains, we were able to demonstrate that at least two pathways, the Pta-AckA pathway and the Acs reaction, are involved in acetate activation in M. tuberculosis. Although a lot of effort has been made to describe acetate-dissimilating pathways in different bacteria (35, 37, 59), so far, redundancy of acetate-activating pathways has been described only for E. coli. The presence of two different pathways to assimilate acetate is intriguing. These findings might indicate that acetate could serve as a carbon source and maintain metabolism in a carbon-limited environment, although the relevance of acetate activation during an M. tuberculosis infection is difficult to ascertain because of the multiple carbon sources available at the infection site. Thus, acetate might represent one carbon substrate that is cometabolized among others during the infection. Compartmentalized cometabolism has recently been described for M. tuberculosis (14), demonstrating that a carbon repression system is missing in M. tuberculosis and instead different carbon substrates are catabolized simultaneously.
The Pta-AckA pathway not only is a valuable release of excess carbon units, but it also is involved in the maintenance of the acetyl-phosphate pool. Acetyl-phosphate is a high-energy substrate that stores phosphate residues. As acetyl-phosphate is used as a substrate to phosphorylate proteins, it is supposed to be a global signal molecule. Phosphorylation of response regulators of two-component signal transduction systems by acetyl-phosphate has been described for numerous systems in various organisms (20). For M. tuberculosis, it was demonstrated that the response regulator MprA is phosphorylated in the presence of acetyl-phosphate (60). MprA/MprB is a two-component system that is involved in the establishment and maintenance of the latent infectious state (61). This observation directly links the acetyl-phosphate level in the cytosol to dormancy control. Thus, intracellular accumulation of acetyl-phosphate might disturb global signal transduction and has a toxic effect on the cells (62, 63), whereas lack of acetyl-phosphate might result in reduced phosphorylation of response regulators and thereby in altered gene expression. The inability of the Δpta knockout strain to grow in the presence of acetate might therefore result from an intracellular increase in the acetyl-phosphate.
As catabolism of fatty acids induces acetate production in vitro, the acetate overflow might be of biological relevance in vivo, as fatty acids are considered to be the major carbon source at the infection site. Published transposon site hybridization (TraSH) data from transposon mutant libraries revealed that acs, ackA, and pta were not essential for survival of M. tuberculosis in murine macrophages (64) or mice (65). However, predictions of TraSH data studies are limited to strong phenotypes due the resolution and quantitative accuracy of the method (66). Moreover, it seems reasonable that the balance of carbon uptake, biomass formation, and carbon release is dependent on different metabolic pathways. Thus, the potential for significance of the Pta-AckA pathway might be masked by redundant pathways. Finally, the in vivo relevance of the Pta-AckA pathway might be revealed only within the defined environment of a granuloma. Thus, it would be interesting to check for the survival of the knockout mutants in the guinea pig model, as the physiology of guinea pig granulomas is closer to that of structures in the human lung. That the access to nutrients might be more restricted inside the solid structure that is typical for granulomas in guinea pigs and fatty acid metabolism, including acetate secretion, becomes more important in this model. Indeed, acetate accumulation has been detected so far only in M. tuberculosis-infected guinea pigs (16). In this study, we present for the first time that M. tuberculosis is able to generate acetate. Acetate secretion was dependent on the supplied carbon substrate. It was absent in the presence of glycolytic substrates but stimulated during growth on fatty acids. This process was mediated by the Pta-AckA pathway. Once other carbon substrates became limiting, acetate was reconsumed from the medium. Thus, we determined the pathway by which acetate is produced, and we demonstrated that the Pta-AckA pathway and the Acs reaction are redundant pathways for acetate activation.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sebastian Suerbaum for support. We thank Mascha Schmidt for technical support.
This work was supported by the Niedersächsischer Verein zur Bekämpfung der Tuberkulose, Lungen- und Bronchialerkrankungen, by the International Research Training Group 1273 to S.B., and by the German Research Foundation (DFG) through grants SFB 587 and SFB 900 to F.-C.B., N.R., and S.B. R.B. and C.W. acknowledge support by the DFG through SPP1316.
All authors declare that there are no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00259-15.
REFERENCES
- 1.Zumla A, George A, Sharma V, Herbert N, Baroness Masham of Ilton, Oxley A, Oliver M. 2015. The WHO 2014 global tuberculosis report—further to go. Lancet Glob Health 3:e10-2. doi: 10.1016/S2214-109X(14)70361-4. [DOI] [PubMed] [Google Scholar]
- 2.Rao SP, Alonso S, Rand L, Dick T, Pethe K. 2008. The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 105:11945–11950. doi: 10.1073/pnas.0711697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloch H, Segal W. 1956. Biochemical differentiation of Mycobacterium tuberculosis grown in vivo and in vitro. J Bacteriol 72:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE III, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 5.Rohde KH, Veiga DF, Caldwell S, Balazsi G, Russell DG. 2012. Linking the transcriptional profiles and the physiological states of Mycobacterium tuberculosis during an extended intracellular infection. PLoS Pathog 8:e1002769. doi: 10.1371/journal.ppat.1002769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Timm J, Post FA, Bekker LG, Walther GB, Wainwright HC, Manganelli R, Chan WT, Tsenova L, Gold B, Smith I, Kaplan G, McKinney JD. 2003. Differential expression of iron-, carbon-, and oxygen-responsive mycobacterial genes in the lungs of chronically infected mice and tuberculosis patients. Proc Natl Acad Sci U S A 100:14321–14326. doi: 10.1073/pnas.2436197100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raynaud C, Guilhot C, Rauzier J, Bordat Y, Pelicic V, Manganelli R, Smith I, Gicquel B, Jackson M. 2002. Phospholipases C are involved in the virulence of Mycobacterium tuberculosis. Mol Microbiol 45:203–217. doi: 10.1046/j.1365-2958.2002.03009.x. [DOI] [PubMed] [Google Scholar]
- 8.McKinney JD, Honer zu Bentrup K, Munoz-Elias EJ, Miczak A, Chen B, Chan WT, Swenson D, Sacchettini JC, Jacobs WR Jr, Russell DG. 2000. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406:735–738. doi: 10.1038/35021074. [DOI] [PubMed] [Google Scholar]
- 9.Pandey AK, Sassetti CM. 2008. Mycobacterial persistence requires the utilization of host cholesterol. Proc Natl Acad Sci U S A 105:4376–4380. doi: 10.1073/pnas.0711159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marrero J, Trujillo C, Rhee KY, Ehrt S. 2013. Glucose phosphorylation is required for Mycobacterium tuberculosis persistence in mice. PLoS Pathog 9:e1003116. doi: 10.1371/journal.ppat.1003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beste DJ, Noh K, Niedenfuhr S, Mendum TA, Hawkins ND, Ward JL, Beale MH, Wiechert W, McFadden J. 2013. 13C-flux spectral analysis of host-pathogen metabolism reveals a mixed diet for intracellular Mycobacterium tuberculosis. Chem Biol 20:1012–1021. doi: 10.1016/j.chembiol.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gouzy A, Larrouy-Maumus G, Bottai D, Levillain F, Dumas A, Wallach JB, Caire-Brandli I, de Chastellier C, Wu TD, Poincloux R, Brosch R, Guerquin-Kern JL, Schnappinger D, Sorio de Carvalho LP, Poquet Y, Neyrolles O. 2014. Mycobacterium tuberculosis exploits asparagine to assimilate nitrogen and resist acid stress during infection. PLoS Pathog 10:e1003928. doi: 10.1371/journal.ppat.1003928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gouzy A, Larrouy-Maumus G, Wu TD, Peixoto A, Levillain F, Lugo-Villarino G, Guerquin-Kern JL, de Carvalho LP, Poquet Y, Neyrolles O. 2013. Mycobacterium tuberculosis nitrogen assimilation and host colonization require aspartate. Nat Chem Biol 9:674–676. doi: 10.1038/nchembio.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Carvalho LP, Fischer SM, Marrero J, Nathan C, Ehrt S, Rhee KY. 2010. Metabolomics of Mycobacterium tuberculosis reveals compartmentalized co-catabolism of carbon substrates. Chem Biol 17:1122–1131. doi: 10.1016/j.chembiol.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Shin JH, Yang JY, Jeon BY, Yoon YJ, Cho SN, Kang YH, Ryu DH, Hwang GS. 2011. 1H NMR-based metabolomic profiling in mice infected with Mycobacterium tuberculosis. J Proteome Res 10:2238–2247. doi: 10.1021/pr101054m. [DOI] [PubMed] [Google Scholar]
- 16.Somashekar BS, Amin AG, Rithner CD, Troudt J, Basaraba R, Izzo A, Crick DC, Chatterjee D. 2011. Metabolic profiling of lung granuloma in Mycobacterium tuberculosis infected guinea pigs: ex vivo 1H magic angle spinning NMR studies. J Proteome Res 10:4186–4195. doi: 10.1021/pr2003352. [DOI] [PubMed] [Google Scholar]
- 17.Böck A, Sawers G. 1996. Fermentation, p 262–282. In Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (ed), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, 2nd ed, vol 1 ASM Press, Washington, DC. [Google Scholar]
- 18.Crabtree HG. 1929. Observations on the carbohydrate metabolism of tumours. Biochem J 23:536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holms H. 1996. Flux analysis and control of the central metabolic pathways in Escherichia coli. FEMS Microbiol Rev 19:85–116. doi: 10.1111/j.1574-6976.1996.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 20.Wolfe AJ. 2005. The acetate switch. Microbiol Mol Biol Rev 69:12–50. doi: 10.1128/MMBR.69.1.12-50.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baronofsky JJ, Schreurs WJ, Kashket ER. 1984. Uncoupling by acetic acid limits growth of and acetogenesis by Clostridium thermoaceticum. Appl Environ Microbiol 48:1134–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parvin R, Pande SV, Venkitasubramanian TA. 1966. Acetate metabolism in mycobacteria. Can J Biochem 44:355–361. doi: 10.1139/o66-042. [DOI] [PubMed] [Google Scholar]
- 23.Bange FC, Collins FM, Jacobs WR Jr. 1999. Survival of mice infected with Mycobacterium smegmatis containing large DNA fragments from Mycobacterium tuberculosis. Tuber Lung Dis 79:171–180. doi: 10.1054/tuld.1998.0201. [DOI] [PubMed] [Google Scholar]
- 24.Pavelka MS Jr, Jacobs WR Jr. 1999. Comparison of the construction of unmarked deletion mutations in Mycobacterium smegmatis, Mycobacterium bovis bacillus Calmette-Guerin, and Mycobacterium tuberculosis H37Rv by allelic exchange. J Bacteriol 181:4780–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stover CK, de la Cruz VF, Bansal GP, Hanson MS, Fuerst TR, Jacobs WR Jr, Bloom BR. 1992. Use of recombinant BCG as a vaccine delivery vehicle. Adv Exp Med Biol 327:175–182. doi: 10.1007/978-1-4615-3410-5_19. [DOI] [PubMed] [Google Scholar]
- 26.Becker J, Klopprogge C, Wittmann C. 2008. Metabolic responses to pyruvate kinase deletion in lysine producing Corynebacterium glutamicum. Microb Cell Fact 7:8. doi: 10.1186/1475-2859-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Dyk TK, LaRossa RA. 1987. Involvement of ack-pta operon products in alpha-ketobutyrate metabolism by Salmonella typhimurium. Mol Gen Genet 207:435–440. doi: 10.1007/BF00331612. [DOI] [PubMed] [Google Scholar]
- 28.Aceti DJ, Ferry JG. 1988. Purification and characterization of acetate kinase from acetate-grown Methanosarcina thermophila. Evidence for regulation of synthesis. J Biol Chem 263:15444–15448. [PubMed] [Google Scholar]
- 29.Cook GM, Hards K, Vilcheze C, Hartman T, Berney M. 2014. Energetics of respiration and oxidative phosphorylation in mycobacteria. Microbiol Spectr 2:10.1128/microbiolspec.MGM2-0015–2013. doi: 10.1128/microbiolspec.MGM2-0015-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cruz Ramos H, Hoffmann T, Marino M, Nedjari H, Presecan-Siedel E, Dreesen O, Glaser P, Jahn D. 2000. Fermentative metabolism of Bacillus subtilis: physiology and regulation of gene expression. J Bacteriol 182:3072–3080. doi: 10.1128/JB.182.11.3072-3080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi L, Sohaskey CD, Kana BD, Dawes S, North RJ, Mizrahi V, Gennaro ML. 2005. Changes in energy metabolism of Mycobacterium tuberculosis in mouse lung and under in vitro conditions affecting aerobic respiration. Proc Natl Acad Sci U S A 102:15629–15634. doi: 10.1073/pnas.0507850102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veit A, Polen T, Wendisch VF. 2007. Global gene expression analysis of glucose overflow metabolism in Escherichia coli and reduction of aerobic acetate formation. Appl Microbiol Biotechnol 74:406–421. doi: 10.1007/s00253-006-0680-3. [DOI] [PubMed] [Google Scholar]
- 33.Seidl K, Muller S, Francois P, Kriebitzsch C, Schrenzel J, Engelmann S, Bischoff M, Berger-Bachi B. 2009. Effect of a glucose impulse on the CcpA regulon in Staphylococcus aureus. BMC Microbiol 9:95. doi: 10.1186/1471-2180-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eoh H, Rhee KY. 2014. Methylcitrate cycle defines the bactericidal essentiality of isocitrate lyase for survival of Mycobacterium tuberculosis on fatty acids. Proc Natl Acad Sci U S A 111:4976–4981. doi: 10.1073/pnas.1400390111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaplan NO, Lipmann F. 1948. The acetyl precursor in pyruvate synthesis in Escherichia coli. J Biol Chem 176:459. [PubMed] [Google Scholar]
- 36.Rose IA, Grunberg-Manago M, Korey SR, Ochoa S. 1954. Enzymatic phosphorylation of acetate. J Biol Chem 211:737–756. [PubMed] [Google Scholar]
- 37.Eschbach M, Schreiber K, Trunk K, Buer J, Jahn D, Schobert M. 2004. Long-term anaerobic survival of the opportunistic pathogen Pseudomonas aeruginosa via pyruvate fermentation. J Bacteriol 186:4596–4604. doi: 10.1128/JB.186.14.4596-4604.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sadykov MR, Thomas VC, Marshall DD, Wenstrom CJ, Moormeier DE, Widhelm TJ, Nuxoll AS, Powers R, Bayles KW. 2013. Inactivation of the Pta-AckA pathway causes cell death in Staphylococcus aureus. J Bacteriol 195:3035–3044. doi: 10.1128/JB.00042-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schreiner ME, Riedel C, Holatko J, Patek M, Eikmanns BJ. 2006. Pyruvate:quinone oxidoreductase in Corynebacterium glutamicum: molecular analysis of the pqo gene, significance of the enzyme, and phylogenetic aspects. J Bacteriol 188:1341–1350. doi: 10.1128/JB.188.4.1341-1350.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veit A, Rittmann D, Georgi T, Youn JW, Eikmanns BJ, Wendisch VF. 2009. Pathway identification combining metabolic flux and functional genomics analyses: acetate and propionate activation by Corynebacterium glutamicum. J Biotechnol 140:75–83. doi: 10.1016/j.jbiotec.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 41.el-Mansi EM, Holms WH. 1989. Control of carbon flux to acetate excretion during growth of Escherichia coli in batch and continuous cultures. J Gen Microbiol 135:2875–2883. [DOI] [PubMed] [Google Scholar]
- 42.Noy T, Xu H, Blanchard JS. 2014. Acetylation of acetyl-CoA synthetase from Mycobacterium tuberculosis leads to specific inactivation of the adenylation reaction. Arch Biochem Biophys 550-551:42–49. doi: 10.1016/j.abb.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lipmann F. 1941. Advances in enzymology and related areas of molecular biology, p 99–162. John Wiley & Sons, Inc., Hoboken, NJ. [Google Scholar]
- 44.Presecan-Siedel E, Galinier A, Longin R, Deutscher J, Danchin A, Glaser P, Martin-Verstraete I. 1999. Catabolite regulation of the pta gene as part of carbon flow pathways in Bacillus subtilis. J Bacteriol 181:6889–6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watanabe S, Zimmermann M, Goodwin MB, Sauer U, Barry CE III, Boshoff HI. 2011. Fumarate reductase activity maintains an energized membrane in anaerobic Mycobacterium tuberculosis. PLoS Pathog 7:e1002287. doi: 10.1371/journal.ppat.1002287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eoh H, Rhee KY. 2013. Multifunctional essentiality of succinate metabolism in adaptation to hypoxia in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 110:6554–6559. doi: 10.1073/pnas.1219375110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ehrt S, Rhee K. 2013. Mycobacterium tuberculosis metabolism and host interaction: mysteries and paradoxes. Curr Top Microbiol Immunol 374:163–188. doi: 10.1007/82_2012_299. [DOI] [PubMed] [Google Scholar]
- 48.Rhee KY, de Carvalho LP, Bryk R, Ehrt S, Marrero J, Park SW, Schnappinger D, Venugopal A, Nathan C. 2011. Central carbon metabolism in Mycobacterium tuberculosis: an unexpected frontier. Trends Microbiol 19:307–314. doi: 10.1016/j.tim.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez JG, Hernandez AC, Helguera-Repetto C, Aguilar Ayala D, Guadarrama-Medina R, Anzola JM, Bustos JR, Zambrano MM, Gonzalez-Y-Merchand J, Garcia MJ, Del Portillo P. 2014. Global adaptation to a lipid environment triggers the dormancy-related phenotype of Mycobacterium tuberculosis. mBio 5(3):e01125-14. doi: 10.1128/mBio.01125-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daniel J, Maamar H, Deb C, Sirakova TD, Kolattukudy PE. 2011. Mycobacterium tuberculosis uses host triacylglycerol to accumulate lipid droplets and acquires a dormancy-like phenotype in lipid-loaded macrophages. PLoS Pathog 7:e1002093. doi: 10.1371/journal.ppat.1002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vilcheze C, Hartman T, Weinrick B, Jacobs WR Jr. 2013. Mycobacterium tuberculosis is extraordinarily sensitive to killing by a vitamin C-induced Fenton reaction. Nat Commun 4:1881. doi: 10.1038/ncomms2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michelucci A, Cordes T, Ghelfi J, Pailot A, Reiling N, Goldmann O, Binz T, Wegner A, Tallam A, Rausell A, Buttini M, Linster CL, Medina E, Balling R, Hiller K. 2013. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci U S A 110:7820–7825. doi: 10.1073/pnas.1218599110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki T. 1969. Phosphotransacetylase of Escherichia coli B, activation by pyruvate and inhibition by NADH and certain nucleotides. Biochim Biophys Acta 191:559–569. doi: 10.1016/0005-2744(69)90349-0. [DOI] [PubMed] [Google Scholar]
- 54.Shi L, Sohaskey CD, Pfeiffer C, Datta P, Parks M, McFadden J, North RJ, Gennaro ML. 2010. Carbon flux rerouting during Mycobacterium tuberculosis growth arrest. Mol Microbiol 78:1199–1215. doi: 10.1111/j.1365-2958.2010.07399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mailaender C, Reiling N, Engelhardt H, Bossmann S, Ehlers S, Niederweis M. 2004. The MspA porin promotes growth and increases antibiotic susceptibility of both Mycobacterium bovis BCG and Mycobacterium tuberculosis. Microbiology 150:853–864. doi: 10.1099/mic.0.26902-0. [DOI] [PubMed] [Google Scholar]
- 56.Titgemeyer F, Amon J, Parche S, Mahfoud M, Bail J, Schlicht M, Rehm N, Hillmann D, Stephan J, Walter B, Burkovski A, Niederweis M. 2007. A genomic view of sugar transport in Mycobacterium smegmatis and Mycobacterium tuberculosis. J Bacteriol 189:5903–5915. doi: 10.1128/JB.00257-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Munoz-Elias EJ, Upton AM, Cherian J, McKinney JD. 2006. Role of the methylcitrate cycle in Mycobacterium tuberculosis metabolism, intracellular growth, and virulence. Mol Microbiol 60:1109–1122. doi: 10.1111/j.1365-2958.2006.05155.x. [DOI] [PubMed] [Google Scholar]
- 58.Marrero J, Rhee KY, Schnappinger D, Pethe K, Ehrt S. 2010. Gluconeogenic carbon flow of tricarboxylic acid cycle intermediates is critical for Mycobacterium tuberculosis to establish and maintain infection. Proc Natl Acad Sci U S A 107:9819–9824. doi: 10.1073/pnas.1000715107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grundy FJ, Waters DA, Allen SH, Henkin TM. 1993. Regulation of the Bacillus subtilis acetate kinase gene by CcpA. J Bacteriol 175:7348–7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zahrt TC, Wozniak C, Jones D, Trevett A. 2003. Functional analysis of the Mycobacterium tuberculosis MprAB two-component signal transduction system. Infect Immun 71:6962–6970. doi: 10.1128/IAI.71.12.6962-6970.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zahrt TC, Deretic V. 2001. Mycobacterium tuberculosis signal transduction system required for persistent infections. Proc Natl Acad Sci U S A 98:12706–12711. doi: 10.1073/pnas.221272198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gueriri I, Bay S, Dubrac S, Cyncynatus C, Msadek T. 2008. The Pta-AckA pathway controlling acetyl phosphate levels and the phosphorylation state of the DegU orphan response regulator both play a role in regulating Listeria monocytogenes motility and chemotaxis. Mol Microbiol 70:1342–1357. doi: 10.1111/j.1365-2958.2008.06496.x. [DOI] [PubMed] [Google Scholar]
- 63.Ramos-Montanez S, Kazmierczak KM, Hentchel KL, Winkler ME. 2010. Instability of ackA (acetate kinase) mutations and their effects on acetyl phosphate and ATP amounts in Streptococcus pneumoniae D39. J Bacteriol 192:6390–6400. doi: 10.1128/JB.00995-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rengarajan J, Bloom BR, Rubin EJ. 2005. Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proc Natl Acad Sci U S A 102:8327–8332. doi: 10.1073/pnas.0503272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sassetti CM, Rubin EJ. 2003. Genetic requirements for mycobacterial survival during infection. Proc Natl Acad Sci U S A 100:12989–12994. doi: 10.1073/pnas.2134250100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.DeJesus MA, Zhang YJ, Sassetti CM, Rubin EJ, Sacchettini JC, Ioerger TR. 2013. Bayesian analysis of gene essentiality based on sequencing of transposon insertion libraries. Bioinformatics 29:695–703. doi: 10.1093/bioinformatics/btt043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.