Abstract
A 45-year-old female developed neurological symptoms and elevated diastolic blood pressure while on bevacizumab (Avastin) and gemcitabine for recurrent carboplatin-resistant high-grade serous ovarian cancer. A brain MRI diagnosed our patient with posterior reversible encephalopathy syndrome. We are discussing her presenting symptoms in this paper as well as the management and the outcome. We emphasize the importance of keeping this rare but very serious complication in all patients receiving bevacizumab.
Key Words: Posterior reversible encephalopathy syndrome, Bevacizumab
Introduction
Posterior reversible encephalopathy syndrome (PRES), also known as reversible posterior leukoencephalopathy syndrome, is a distinct clinic-radiological entity that was initially described by Hinchey et al. [1] in 1996. It describes a neurological syndrome with presenting symptoms ranging from headache, altered mental state, seizures, and visual loss to loss of consciousness, coma or even death [2].
The term describes a potentially reversible imaging appearance and symptomatology that is shared by a diverse array of causes. The mechanism is not exactly understood, but it is thought to be related to a hyperperfusion state with a blood-brain barrier breakdown. This leads to the extravasation of fluid, potentially containing blood or macromolecules, resulting in cortical or subcortical edema. Alternatively, vasospasms may precipitate the reversible edema, leading to cytotoxic edema if left untreated. It may also cause focal reversible vasogenic edema involving predominantly the parietal and occipital lobes [3].
The typical imaging findings of PRES are most apparent as hyperintensity on fluid attenuated inversion recovery (FLAIR) images in the parieto-occipital and posterior frontal cortical and subcortical white matter. Less commonly, the brainstem, basal ganglia and cerebellum are involved [3].
PRES has been described in association with severe hypertension, preeclampsia, transplantation, and less frequently, following the administration of immunosuppressive agents and cytotoxic drugs. The pathophysiology of PRES implicates endothelial dysfunctions especially in cases associated with preeclampsia or cytotoxic therapies, which may have a direct toxicity on vascular endothelium leading to capillary leakage, the disruption of the blood-brain barrier as well as axonal swelling, which may then trigger a vasogenic edema [4, 5, 6]. PRES has also been reported in association with the antivascular endothelial growth factor monoclonal antibody bevacizumab (BEV). BEV is believed to decrease tumor perfusion, vascular density and interstitial fluid pressure [7, 8].
We herein present a case of PRES secondary to BEV therapy and discuss salient aspects in its diagnosis and management.
Case Report
A 45-year-old female was diagnosed with high-grade serous ovarian cancer stage IIIC with omental involvement and malignant ascites. She was known to have mild hypertension controlled with medication. She received 3 cycles of paclitaxel and carboplatin as a neoadjuvant chemotherapy followed by debulking total abdominal hysterectomy and bilateral salpingo-oophorectomy. She was then given 3 more cycles of the same protocol as adjuvant chemotherapy.
After 2 months, the patient presented with a disease recurrence in the form of an increase in her CA125 tumor marker as well as evident intra-abdominal lymph nodes and malignant ascites. She was started on the single non-carboplatin chemotherapeutic agent named gemcitabine with a dose of 700 mg/m2 on days 1 and 8 every 3 weeks (plus BEV 15 mg/kg every 3 weeks given for 2 cycles). On day 4 after her last cycle, she started to develop generalized fatigue, increasing nausea, vomiting and drowsiness. Two days later, she experienced seizures at home and was brought to urgent care. She was found to have a systolic blood pressure in the range of 150–180 mm Hg and a diastolic blood pressure in the range of 120–125 mm Hg. Her level of consciousness was decreased. This progressed to non-compulsive seizures necessitating a prompt intravenous anticonvulsive medication, intubation and admission to the ICU. A CT of the brain showed a hypodense area in the temporal occipital lobes, therefore raising the possibility of ischemia. An MRI was recommended and this showed classic MRI signals for PRES (fig. 1). During her stay in the ICU, the patient was thoroughly examined for other clinical possibilities such as septicemia, encephalitis, metabolic seizures as well as cerebrovascular insults. These were all excluded.
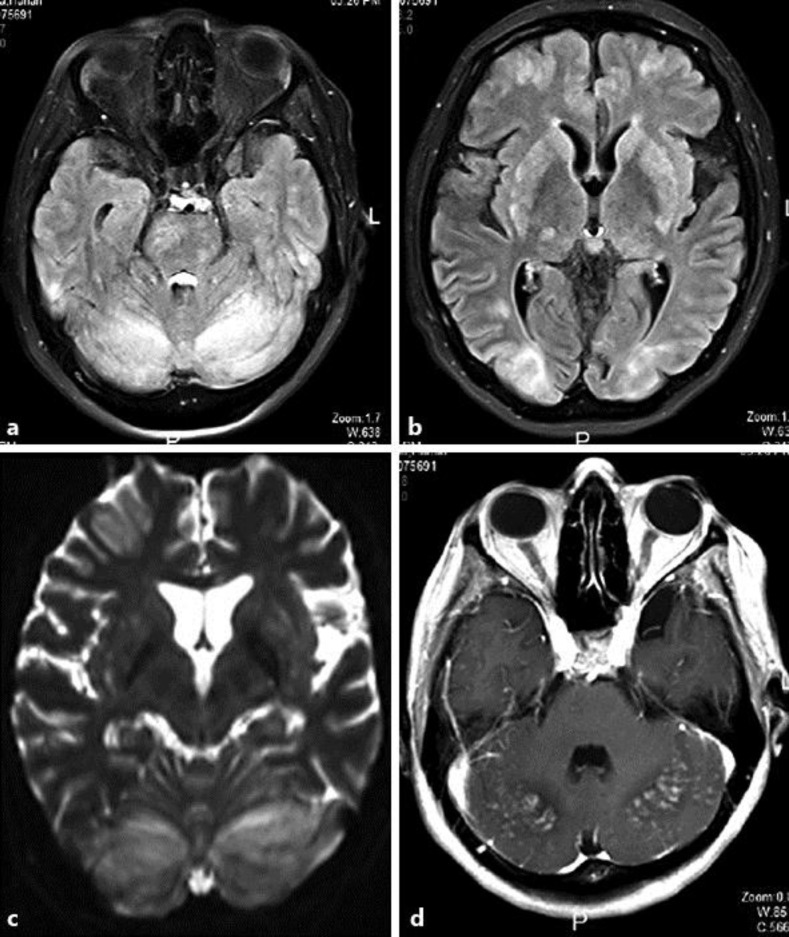
Fig. 1.
MRI FLAIR sequence axial cuts (a, b) and ADC map (c) showing bilateral cerebellar, occipital cortical and subcortical as well as bilateral basal ganglia, frontal cortical, thalamic and brainstem patches and spots of abnormal bright signal (vasogenic edema) with a high ADC value. An MRI post contrast axial cut (d) shows posterior cerebellar patchy nodular and leptomeningeal enhancement.
The patient was kept intubated for 2 weeks in the ICU with blood pressure control and supportive measures. No more chemotherapy was administered. After extubation, the patient remained obtunded with a Glasgow coma scale of 11–12. Repeated MRI images after 2 weeks showed a decrease in all white matter abnormal T2 signals. After 4 weeks, another MRI showed an almost complete resolution of the previously seen white matter T2-hyperintense patches with few residual foci of microhemorrhages in the cerebellum (fig. 2). A few days later, the patient developed aspiration pneumonia and respiratory failure and could not be resuscitated.
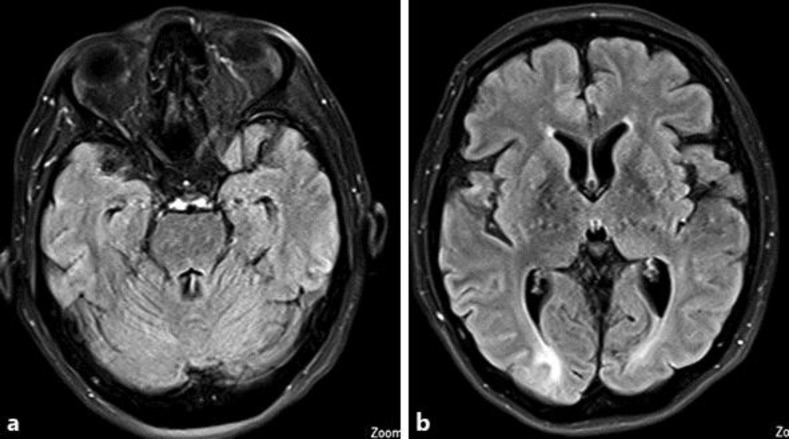
Fig. 2.
MRI FLAIR sequence axial cuts (a, b) 21 days after therapy for PRES, showing almost a total resolution of the edema bright signal apart from the small residue at occipital regions.
Discussion
BEV has been approved by the Food and Drug Administration as well as the European Society of Medical Oncology for the therapy for various solid tumor malignancies because it has shown to improve survival and the rate of tumor regression in colorectal cancer [9], non-small cell lung cancer [10], renal cell carcinoma [11], recurrent glioblastoma multiforme, and most recently, epithelial ovarian cancer [12].
Our patient had epithelial ovarian cancer. According to the Vancouver Consensus of 2010, she is considered to have carboplatin-resistant recurrences since this occurred within 6 months of the initial chemotherapy completion. She was started on gemcitabine according to the guidelines of the European Society of Medical Oncology and the National Comprehensive Cancer Network (NCCN). BEV was added in hope of a better survival according to the recent Aurelia trial. Although generally well tolerated, the use of BEV-based combination chemotherapy is associated with a risk of grade 3 hypertension in up to 16%percnt; of patients. This is possibly secondary to vasospasms, which may lead to endothelial dysfunction. The disrupted blood-brain barrier caused a vasogenic edema of the posterior cerebral white matter and PRES in 1%percnt; of patients [13]. It is postulated that BEV could induce vasospasms leading to PRES without causing significant hypertension [14]. We believe this was the case in our patient. It is important to differentiate this syndrome from acute cerebral ischemia or thrombotic phenomena which are also associated with BEV [14].
Gemcitabine is a commonly used chemotherapeutic agent for a variety of tumors. Although this nucleoside analog antineoplastic agent is similar in structure to cytarabine, central nervous system toxicities have rarely been attributed to it [15]. There are a few reports in the literature associating gemcitabine with PRES. However, we have used gemcitabine in a large number of patients over many years without such a case, and we therefore tend to believe that BEV was probably the more likely cause of PRES in our case. Nonetheless, it remains possible that BEV alone might not cause PRES, but does so when combined with other chemotherapeutic agents.
Conclusion
BEV is widely used for many indications in today's oncology practice. A high level of suspicion for PRES is advisable in patients who develop headache, confusion, visual disturbance or seizure during its use, either as a monotherapy or in combination.
PRES is a potentially reversible condition. However, failure to recognize the syndrome and a withdrawal of the offending agent may result in catastrophic CNS injury or death. The increase in reported cases of PRES warrants further investigations into its risk factors and pathophysiological mechanisms as well as further education of oncology health care providers.
Statement of Ethics
Consent to publish this case report was obtained from the patient's husband.
Disclosure Statement
None of the authors has any conflicts of interest regarding this case report. No sponsorship or funding arrangement regarding this case report was received.
References
- 1.Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 2.Garg RK. Posterior leukoencephalopathy syndrome. Postgrad Med J. 2001;77:24–28. doi: 10.1136/pmj.77.903.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85:427–432. doi: 10.4065/mcp.2009.0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartynski WS. Posterior reversible encephalopathy syndrome: part 2. Controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29:1043–1049. doi: 10.3174/ajnr.A0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seet RC, Rabinstein AA, Lindell PE, Uhm JH, Wijdicks EF. Cerebrovascular events after bevacizumab treatment: an early and severe complication. Neurocrit Care. 2011;15:421–427. doi: 10.1007/s12028-011-9552-5. [DOI] [PubMed] [Google Scholar]
- 6.Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995;108:2369–2379. doi: 10.1242/jcs.108.6.2369. 2. [DOI] [PubMed] [Google Scholar]
- 7.Glusker P, Recht L, Lane B. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006;354:980–981. doi: 10.1056/NEJMc052954. [DOI] [PubMed] [Google Scholar]
- 8.Ozcan C, Wong SJ, Hari P. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006;354:981–982. [PubMed] [Google Scholar]
- 9.Lievre A, Samalin E, Mitry E, et al. Bevacizumab plus FOLFIRI or FOLFOX in chemotherapy-refractory patients with metastatic colorectal cancer: a retrospective study. BMC Cancer. 2009;9:347. doi: 10.1186/1471-2407-9-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandler A, Yi J, Dahlberg S, et al. Treatment outcomes by tumor histology in Eastern Cooperative Group Study E4599 of bevacizumab with paclitaxel/carboplatin for advanced non-small cell lung cancer. J Thorac Oncol. 2010;5:1416–1423. doi: 10.1097/JTO.0b013e3181da36f4. [DOI] [PubMed] [Google Scholar]
- 11.Escudier B, Bellmunt J, Négrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol. 2010;28:2144–2150. doi: 10.1200/JCO.2009.26.7849. [DOI] [PubMed] [Google Scholar]
- 12.Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302–1308. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 13.Lau PC, Paunipagar B. Posterior reversible encephalopathy syndrome with bevacizumab. Hong Kong Med J. 2011;17:80–81. [PubMed] [Google Scholar]
- 14.Rabinstein AA, Mandrekar J, Merrell R, Kozak OS, Durosaro O, Fugate J. Blood pressure fluctuations in posterior reversible encephalopathy syndrome. J Stroke Cerebrovasc Dis. 2012;21:254–258. doi: 10.1016/j.jstrokecerebrovasdis.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Cioffi P, Laudadio L, Nuzzo A, et al. Gemcitabine-induced posterior reversible encephalopathy syndrome: a case report. J Oncol Pharm Pract. 2012;18:299–302. doi: 10.1177/1078155211424628. [DOI] [PubMed] [Google Scholar]