Abstract
Lyme neuroborreliosis has several different clinical manifestations in children, of which facial nerve palsies, meningitis and radiculopathies are the most common. Transverse myelitis (TM) secondary to Lyme disease has been reported in rare occasions, typically presenting with severe weakness, sensory abnormalities and autonomic dysfunction. We present the case of a 16-year-old male who developed acute left peripheral facial palsy and longitudinal extensive TM secondary to Lyme disease. Remarkably, the patient reported only mild symptoms with severe back pain in the absence of profound signs of myelopathy. We reviewed the medical literature and analyzed the clinical features of pediatric patients with Borrelia burgdorferi-related TM.
Key Words: Transverse myelitis, Longitudinal extensive transverse myelitis, Myelopathy, Lyme disease
Introduction
Lyme disease is the most common vector-associated infectious disease in North America, with a bimodal distribution in both young childhood and adulthood [1]. Transmission of the bacterium Borrelia burgdorferi occurs via infected Ixodes scapularis ticks. Clinical manifestations initially may show the classic erythema chronicum migrans with subsequent spread to other organ systems. This can lead to carditis, arthritis as well as cranial nerve neuropathies (most commonly affecting VII cranial nerve), meningitis or radiculopathy [2]. Lyme disease infection of the nervous system rarely manifests as acute transverse myelitis (TM) with myelopathy [3, 4]. TM is a focal inflammatory disorder of the spinal cord resulting in motor, sensory, and autonomic dysfunction presenting over hours to days. TM is commonly a consequence of acute demyelinating diseases, but other inflammatory, infectious, ischemic and paraneoplastic etiologies are possible. Longitudinal extensive transverse myelitis (LETM) refers to spinal cord lesions that extend over three or more vertebral segments, have a central location within the spinal cord and cause T2 hyperintensity/signal abnormality on magnetic resonance imaging (MRI) of the spine [5]. Lyme neuroborreliosis is not commonly considered in the differential diagnosis of TM, but several reports have indicated its association with Lyme disease in children. Importantly, all pediatric cases of acute TM associated with Lyme infection reported in the literature present with the unique occurrence of LETM in neuroimaging. We present the case of a 16-year-old male who developed acute left peripheral facial palsy and LETM secondary to Lyme disease.
Case Presentation
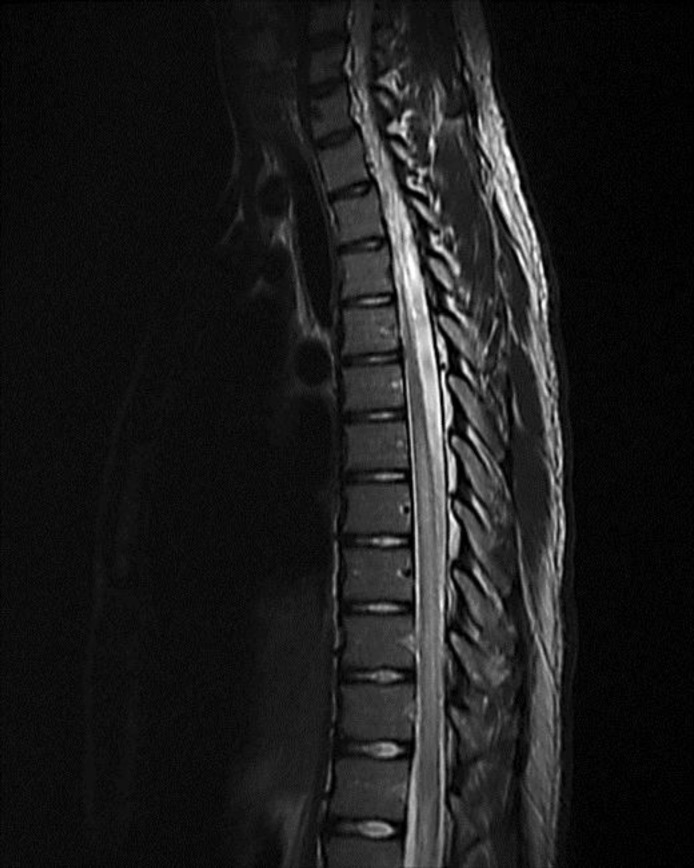
A 16-year-old male presented to our emergency department with a 3-week history of severe lower back pain radiating around his right flank and abdomen, which became more intense 1 week prior to evaluation. He denied trauma, bowel or bladder incontinence, sexual dysfunction, focal limb weakness or paresthesias. Additionally, he developed left facial weakness 5 days prior to hospital presentation consistent with peripheral VII nerve palsy evaluated by his primary care physician. The patient's history was significant for a high fever, headache and generalized body malaise 4 weeks prior to admission. Based on the history of constitutional symptoms, peripheral facial nerve palsy and endemic occurrence of Lyme disease, B. burgdorferi antibody testing was conducted. Serologic testing for antibodies to B. burgdorferi was conducted following the two-tier strategy. Both enzyme-linked immunosorbent assay and Western blot assay were positive. The patient denied other extra-neurological manifestations of Lyme disease, such as arthralgia or erythema migrans, and had no known history of a tick bite. Treatment with minocycline 100 mg twice a day for positive Lyme antibody was initiated. On hospital admission, his neurological examination was significant for left peripheral facial nerve palsy and subtle sensory level to below T7 dermatome to temperature. Strength was normal along with other sensory modalities. Due to the severity, persistence and abrupt worsening of the patient's back pain despite conservative measures and analgesics, a lumbar and thoracic MRI study without contrast was obtained. MRI revealed a diffuse signal abnormality of greater than two thirds of the cross-sectional area of the thoracic cord from T7 down almost to the level of the conus, predominantly involving the white matter consistent with LETM (fig. 1). Additional investigations included cerebrospinal fluid (CSF) analysis which demonstrated nonspecific markers of active central nervous system inflammation, including an elevated leukocyte count (225) with 75%percnt; lymphocytes and 25%percnt; monocytes, no erythrocytes, elevated protein (194 mg/dl) and normal glucose. Intrathecal production of antibodies to B. burgdorferi was demonstrated through the simultaneous measurement of serum and CSF antibodies showing higher levels in the CSF with an elevated IgG index. CSF polymerase chain reaction for herpes simplex virus, varicella zoster virus, arboviruses and enteroviruses were negative. Neuromyelitis optica (NMO) antibody levels in CSF and serum were negative as well. Myelin-oligodendrocyte glycoprotein antibodies were also negative. Other autoimmune and inflammatory markers were within normal limits. Brain MRI showed no intra- or extra-axial masses, enhancing lesions, hydrocephalus, areas of restricted diffusion or white matter abnormalities. The patient was also started on ceftriaxone 2 g intravenously every 12 hours for 21 days for probable neuroborreliosis.
Fig. 1.
Thoracic spine MRI. T2-weighted image, sagittal view, reveals extensive spinal cord signal abnormality extending multiple levels from T6 to T7.
Discussion
Children with neuroborreliosis commonly present with facial nerve neuropathy, either unilateral or bilateral, or aseptic meningitis, but the involvement of the spinal cord is very rare. Pediatric cases of neuroborreliosis with acute spinal cord involvement typically display severe symptoms of myelopathy, including flaccid limb paralysis or paresis, autonomic difficulties, areflexia, sensory disturbances and MRI findings consistent with TM [3, 6]. Importantly, all pediatric Lyme cases with acute spinal cord involvement present with MRI findings indicative of LETM [3]. The mechanism or relation of this association is unknown, but it is in line with our patient's presentation. Additionally, atypical presentations have been reported in children with Lyme LETM, including severe weight loss, vomiting, abdominal pain, malaise or respiratory failure [7, 8]. There are also cases describing patients with severe imaging findings despite minimal clinical manifestations, such as in our patient. Bigi et al. [9] described a patient who presented with headache and back pain with severe abnormalities in neuroimaging that improved after antibiotic therapy. A proposed mechanism for this discrepancy between clinical and radiological findings is the effects of early antibiotic treatment during initial Lyme infection.
The diagnosis of neuroborreliosis is particularly challenging in pediatric patients as they might present with atypical findings or nonspecific neurological features. Furthermore, the occurrence of LETM is rare, and a potentially ominous diagnosis requires assessing for other potential etiologies. LETM is often associated with inflammatory, infectious or paraneoplastic etiologies, with demyelinating disease and NMO spectrum disorders being the most common [10]. NMO is characterized by optic neuritis and myelitis along with the presence of antibodies against aquaporin-4 channels. Characteristic brain MRI abnormalities are typically encountered. Acute disseminated encephalomyelitis causes profound changes in level and content of consciousness with multifocal involvement on neuroimaging. Multiple sclerosis can be considered in the differential diagnosis; however, it tends to affect the lateral and dorsal spinal cord with concomitant multifocal involvement in the setting of absent infectious markers.
Other common inflammatory processes presenting with LETM such as systemic lupus erythematosus, Sjögren's syndrome and sarcoidosis are also part of the differential diagnosis. Antinuclear antibody levels along with other autoimmune and systemic disease markers are elevated in systemic lupus erythematosus. Sjögren's syndrome is an inflammatory condition with anti-SSA/Ro and anti-SSB/La antibodies primarily affecting the salivary and lacrimal glands. Primary neurological manifestations of the disease are uncommon. Sarcoidosis is another inflammatory condition characterized by noncaseating granulomas affecting multiple organ systems, including the frequent involvement of the pulmonary system, and early CNS involvement. Diagnosis of sarcoidosis requires CSF analysis, measurement of serum interleukine-2 receptor and chest imaging with computed tomography, MRI and fluorodeoxyglucose positron emission tomography. Tissue biopsy is necessary for a definitive diagnosis [10]. B-cell lymphoma, ependymomas and astrocytomas have also been known to cause LETM, and a neoplastic etiology should be considered when immunosuppressive treatment is ineffective. Characteristic enhancing, expanding or cystic spinal cord lesions are noted on MRI. Spinal biopsy is necessary for a definitive diagnosis. Finally, other LETM etiologies include paraneoplastic disease [11], including the presence of antibodies to amphiphysin or glutamic acid decarboxylase or collapsing response mediatory protein-5 and nutritional deficiencies.
In our patient, comprehensive CSF investigations excluded other common infectious etiologies, including bacterial infections (LETM is primarily linked to Treponema pallidum, and Mycobacteria including M. tuberculosis and M. bovis) or viral etiologies, namely herpes simplex virus, varicella zoster virus, cytomegalovirus and Ebstein-Barr virus, arbovirus or enteroviruses. Other autoimmune or inflammatory etiologists were excluded by the absence of specific serological and CSF markers or MRI abnormalities. Our patient's presentation highlights the importance of recognizing the potential involvement of the spinal cord in neuroborreliosis specifically with LETM and emphasizes the potential occurrence of mild symptoms despite severe affection of the spine on imaging studies.
Conclusions
Pediatric patients with Lyme neuroborreliosis may present with unusual symptoms such as vomiting, headaches, abdominal or lumbar pain and respiratory distress. If acute TM occurs, patients commonly present with evidence of LETM on MRI. Clinical symptoms and signs of Lyme neuroborreliosis may be subtle, even in cases with an extensive involvement of the spine on MRI, and a high level of suspicion is necessary to make the diagnosis. Lyme disease infection of the central nervous system should be on the differential diagnosis of LETM in children. Early treatment is essential to prevent complications and potential neurological deterioration.
Disclosure Statement
A.R.-Z. has received honoraria from TEVA Pharmaceuticals. S.K., N.S. and A.D. have nothing to disclose.
Acknowledgments
The Phyllis E. Dake Endowed Chair in Movement Disorders (A.R.-Z.) supported this study.
References
- 1.Shapiro ED. Clinical practice. Lyme disease. N Engl J Med. 2014;370:1724–1731. doi: 10.1056/NEJMcp1314325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esposito S, Bosis S, Sabatini C, Tagliaferri L, Principi N. Borrelia burgdorferi infection and Lyme disease in children. Int J Infect Dis. 2013;17:e153–e158. doi: 10.1016/j.ijid.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Erol I, Kilicarslan B, Saygi S, Demir S, Alehan F. Acute transverse myelitis in a child with Lyme disease and a review of literature. Pediatr Neurol. 2013;48:325–328. doi: 10.1016/j.pediatrneurol.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Baumann M, Birnbacher R, Koch J, Strobl R, Rostasy K. Uncommon manifestations of neuroborreliosis in children. Eur J Paediatr Neurol. 2010;14:274–277. doi: 10.1016/j.ejpn.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 6.Meurs L, Labeye D, Declercq I, Pieret F, Gille M. Acute transverse myelitis as a main manifestation of early stage II neuroborreliosis in two patients. Eur Neurol. 2004;52:186–188. doi: 10.1159/000081864. [DOI] [PubMed] [Google Scholar]
- 7.Gaudichon J, Sakr W, Becher S, Linard M, Kozisek S. Acute transverse myelitis and Lyme borreliosis: a case report (in French) Arch Pediatr. 2013;20:646–649. doi: 10.1016/j.arcped.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 8.Huisman TA, Wohlrab G, Nadal D, Boltshauser E, Martin E. Unusual presentations of neuroborreliosis (Lyme disease) in childhood. J Comput Assist Tomogr. 1999;23:39–42. doi: 10.1097/00004728-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Bigi S, Aebi C, Nauer C, Bigler S, Steinlin M. Acute transverse myelitis in Lyme neuroborreliosis. Infection. 2010;38:413–416. doi: 10.1007/s15010-010-0028-x. [DOI] [PubMed] [Google Scholar]
- 10.Trebst C, Raab P, Voss EV, Rommer P, Abu-Mugheisib M, Zettl UK, et al. Longitudinal extensive transverse myelitis – it's not all neuromyelitis optica. Nat Rev Neurol. 2011;7:688–698. doi: 10.1038/nrneurol.2011.176. [DOI] [PubMed] [Google Scholar]
- 11.Flanagan EP, Keegan BM. Paraneoplastic myelopathy. Neurol Clin. 2013;31:307–318. doi: 10.1016/j.ncl.2012.09.001. [DOI] [PubMed] [Google Scholar]