Abstract
Cerebral aneurysms are well known to be associated with cardiac myxomas. The mechanism of cerebral aneurysm formation remains to be elucidated. Embolization of tumor particles in the vessel wall has been proposed as the likely mechanism for aneurysm formation. Recent reports suggest interleukin-6 (IL-6) may play a role as well. We describe a patient who presented with subarachnoid hemorrhage secondary to ruptured right middle cerebral artery (MCA) aneurysm and unruptured left MCA aneurysm. Subsequently, the patient was found to have an atrial myxoma and persistently elevated serum IL-6 levels. Transcranial Doppler monitoring showed multiple emboli in the right MCA vascular territory on day 1 after surgery but no recurrent embolization during the next 2 weeks on repeated tests. Elevated IL-6 levels were noted both on day 1 and on day 30. Our findings provide evidence that IL-6 elevation and not tumor embolization is likely the culprit for aneurysm formation in some patients with atrial myxoma.
Key Words: Intracardiac myxoma, Cerebral aneurysm, Interleukin-6
Introduction
Intracardiac atrial myxomas are the most common tumors in the heart [1]. Myxomas are associated with a variety of neurological manifestations [2], including, less commonly, cerebral aneurysms [3, 4, 5]. Up to 50%percnt; of patients with atrial myxomas initially present with stroke symptoms [6]. The majority of cerebral aneurysms associated with atrial myxomas have been described as fusiform in shape, multiple or located distally [3, 7, 8]. Traditionally, the embolization of tumor particles in the vasa vasorum leading to weak subintimal tissue has been thought to be the cause of aneurysm formation [4, 5]. Previous research has brought to light the presence of elevated levels of interleukin-6 (IL-6) in patients suffering from atrial myxomas [9]. IL-6 is a proinflammatory cytokine that has also been implicated in the development of cerebral aneurysms [10].
Case Report
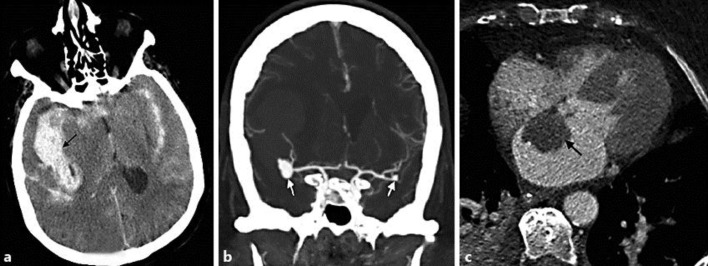
A 73-year-old Caucasian female presented to the hospital with a poor-grade subarachnoid hemorrhage. She had a history of hypertension, hyperlipidemia and coronary artery disease. Computed tomography (CT) revealed diffuse subarachnoid hemorrhage with a large right sylvian fissure clot exerting mass effect with midline shift (fig. 1a). CT angiography revealed a 9-mm lobulated aneurysm at the right middle cerebral artery (MCA) trifurcation and a small 3-mm aneurysm at the left MCA bifurcation (fig. 1b). The patient underwent right frontal pterional craniotomy, evacuation of intracerebral hematoma and clipping of the right MCA aneurysm. The patient was then treated in the intensive care unit as per the institutional protocol. After surgery, the patient initially showed signs of improvement and started localizing on the right side; however, she deteriorated on postoperative day 4 with worsening edema in the right temporal region. She underwent an emergent decompressive craniotomy and temporal lobectomy. Mild to moderate vasospasm was detected on post-bleed day 6 by CT angiography and perfusion. The patient was treated with hypertensive hypervolemic therapy. Transthoracic echocardiography revealed a left atrial mass. Findings were confirmed on a contrasted CT of the heart (fig. 1c) with a 3.2 × 2.3 cm well-circumscribed myxoma arising from the atrial septum. Transcranial Doppler monitoring was done for vasospasm and cardiac emboli on a daily basis. Temporal windows were insonated using a 2-MHz probe to record emboli in the bilateral MCA territory for 15 min. We detected 31 emboli in the right MCA area and none in the left MCA territory on day 1 after clipping, but we did not see any evidence of further emboli in the following 14 days. IL-6 levels were significantly elevated at 42.9 pg/ml (normal level <3.9 pg/ml) and persistently high 30 days later (30.0 pg/ml). The ventricular drain was weaned off after post-bleed day 14. The patient subsequently received tracheostomy and gastrostomy tubes and was transferred to a skilled care facility for ventilator wean and rehabilitation.
Fig. 1.
a Noncontrast CT of the head demonstrates diffuse subarachnoid hemorrhage with a clot in the right sylvian fissure region. b CT angiogram of the brain shows a 9-mm lobulated aneurysm at the right MCA trifurcation and a small 3-mm aneurysm at the left MCA bifurcation. c Axial high-resolution CT of the heart shows a left atrial myxoma measuring 3.2 × 2.3 cm.
Discussion
The occurrence of cerebral aneurysms in the presence of atrial myxoma is well documented in the literature, and its mechanism is still not completely clear. Myxoma embolization is considered as the predominant mechanism for cerebral aneurysm formation [4, 5]. These myxomatous aneurysms were thought to result from penetration and weakening of the vessel wall by embolized myxoma cells. Some authors have proposed that tumor cells gain entrance into the vessel wall through the vasa vasorum and subsequently destroy the cytoarchitecture leading to aneurysm formation [5]. Others believe it to be a transendothelial invasion leading to the downstream events [4]. This hypothesis was validated with histological studies demonstrating interruption of internal elastic lamina with myxoma cells [4]. But several observations contradict these hypotheses. Cerebral aneurysms have been described to occur months to years after myxoma resection [3, 11]. It is therefore more plausible that myxomas initiate the aneurysm formation cascade in an endocrine or paracrine fashion by secreting chemokines like IL-6. Our findings also support the hypothesis that IL-6 elevation, not recurrent embolism, is likely the cause of aneurysm formation in patients with atrial myxoma.
IL-6 is elevated in patients with myxoma as well as other disease conditions. It has been shown that atrial myxoma cells are capable of producing IL-6 [9, 12]. Recent research has supported the link between overproduction of IL-6 and cerebral aneurysm development [10]. Morgan et al. [10] demonstrated that the 572C/174G haplotype of IL-6 was associated with increased risk of cerebral aneurysm formation. Persistently elevated levels of IL-6 in our patient cannot be simply explained by the acute-phase response from subarachnoid hemorrhage.
Our findings suggest that persistently elevated IL-6 levels, in the absence of recurrent emboli, may be an important factor for cerebral aneurysm formation in some patients with atrial myxoma. Even though embolism of myxoma is a documented contributor to initial aneurysm formation, it is possible that subsequent aneurysms may only require endocrine signals originating from other cerebral aneurysms to grow. If this is true, elevated IL-6 levels may have a stronger correlation with future aneurysm formation than detected tumor embolization [12, 13, 14]. Cardiac myxoma resection is usually accompanied by a reduction in serum IL-6 levels. However, a few studies with new aneurysm formation after myxoma resection still showed persistently elevated IL-6 levels [3]. Further studies involving continuous transcranial Doppler monitoring in patients known to harbor atrial myxoma coupled with longitudinal, serum and cerebrospinal fluid IL-6 level determination may further elucidate the exact mechanism of aneurysm formation.
References
- 1.MacGowan SW, et al. Atrial myxoma: national incidence, diagnosis and surgical management. Ir J Med Sci. 1993;162:223–226. doi: 10.1007/BF02945200. [DOI] [PubMed] [Google Scholar]
- 2.Knepper LE, et al. Neurologic manifestations of atrial myxoma. A 12-year experience and review. Stroke. 1988;19:1435–1440. doi: 10.1161/01.str.19.11.1435. [DOI] [PubMed] [Google Scholar]
- 3.Sabolek M, et al. Multiple cerebral aneurysms as delayed complication of left cardiac myxoma: a case report and review. Acta Neurol Scand. 2005;111:345–350. doi: 10.1111/j.1600-0404.2005.00413.x. [DOI] [PubMed] [Google Scholar]
- 4.Furuya K, et al. Histologically verified cerebral aneurysm formation secondary to embolism from cardiac myxoma. Case report. J Neurosurg. 1995;83:170–173. doi: 10.3171/jns.1995.83.1.0170. [DOI] [PubMed] [Google Scholar]
- 5.Herbst M, et al. Cerebral embolism from left atrial myxoma leading to cerebral and retinal aneurysms: a case report. AJNR Am J Neuroradiol. 2005;26:666–669. [PMC free article] [PubMed] [Google Scholar]
- 6.Eddleman CS, et al. Rupture of cerebral myxomatous aneurysm months after resection of the primary cardiac tumor. Neurocrit Care. 2010;13:252–255. doi: 10.1007/s12028-010-9400-z. [DOI] [PubMed] [Google Scholar]
- 7.Tamuleviciute E, et al. Myxomatous aneurysms: a case report and literature review. Interv Neuroradiol. 2011;17:188–194. doi: 10.1177/159101991101700208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oguz KK, Firat MM, Cila A. Fusiform aneurysms detected 5 years after removal of an atrial myxoma. Neuroradiology. 2001;43:990–992. doi: 10.1007/s002340100614. [DOI] [PubMed] [Google Scholar]
- 9.Saji T, et al. Increased serum interleukin-6 in cardiac myxoma. Am Heart J. 1991;122:579–580. doi: 10.1016/0002-8703(91)91022-f. [DOI] [PubMed] [Google Scholar]
- 10.Morgan L, et al. The interleukin-6 gene −174G>C and −572G>C promoter polymorphisms are related to cerebral aneurysms. J Neurol Neurosurg Psychiatry. 2006;77:915–917. doi: 10.1136/jnnp.2005.081976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jean WC, Walski-Easton SM, Nussbaum ES. Multiple intracranial aneurysms as delayed complications of an atrial myxoma: case report. Neurosurgery. 2001;49:200–202. doi: 10.1097/00006123-200107000-00031. discussion 202–203. [DOI] [PubMed] [Google Scholar]
- 12.Takahara H, et al. Left atrial myxoma with production of interleukin 6. Nihon Kyobu Geka Gakkai Zasshi. 1992;40:326–329. [PubMed] [Google Scholar]
- 13.Koo YH, et al. Multiple fusiform cerebral aneurysms and highly elevated serum interleukin-6 in cardiac myxoma. J Korean Neurosurg Soc. 2009;45:394–396. doi: 10.3340/jkns.2009.45.6.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendoza CE, Rosado MF, Bernal L. The role of interleukin-6 in cases of cardiac myxoma. Clinical features, immunologic abnormalities, and a possible role in recurrence. Tex Heart Inst J. 2001;28:3–7. [PMC free article] [PubMed] [Google Scholar]