Abstract
Duodenal adenocarcinoma is a rare malignancy and carries a poor prognosis. The role of adjuvant therapy and the optimal chemotherapy regimen remain largely unclear. Treatment with trastuzumab results in prolonged survival in gastroesophageal cancer if human epidermal growth factor receptor 2 (HER2) is overexpressed or amplified in tumor cells. However, unlike gastric adenocarcinomas, duodenal cancers seem to rarely harbor HER2 amplification or overexpression. We report the case of a patient with HER2-positive stage III duodenal adenocarcinoma who has received adjuvant chemotherapy including trastuzumab.
Key Words: Duodenal adenocarcinoma, Trastuzumab, Human epidermal growth factor receptor 2 amplification
Introduction
Small bowel cancers are rare and represent less than 5%percnt; of gastrointestinal cancers. The 4 most important histological types of cancer in the small bowel are adenocarcinomas, neuroendocrine tumors, gastrointestinal stromal tumors, and lymphomas. Small bowel adenocarcinoma (SBA) represents approximately 40%percnt; of small bowel cancers and carries a poor prognosis, with a 5-year overall survival ranging from 26 to 40%percnt; [1, 2, 3, 4, 5, 6, 7]. The duodenum is the most frequently involved segment, accounting for 55–82%percnt; of the cases, followed by the jejunum and the ileum [3].
The prognosis of SBA appears to be in between that of colon and gastric cancers, and complete surgical resection with locoregional lymphadenectomy remains the only potentially curative treatment [7]. Lymph node invasion is considered the main prognostic factor for localized SBA [5, 7]. In addition, the number of lymph nodes assessed and the number of positive lymph nodes seem to be of prognostic value. Patients with stage III SBA and ≥3 invaded lymph nodes have a worse 5-year disease-free survival rate than patients with 1 or 2 invaded lymph nodes (37 vs. 57%percnt;) [8]. However, even for patients with a high risk of recurrence after surgical resection, the role of adjuvant therapy remains largely unclear. Moreover, the optimal chemotherapy regimen is not well established for duodenal adenocarcinoma.
Human epidermal growth factor receptor 2 (HER2) overexpression or amplification has been reported in many epithelial malignancies. The role of HER2-directed therapy and its success in breast cancer patients has led to the evaluation of protein overexpression, gene amplification, and antitumor activity of trastuzumab, an anti-HER2 antibody, in multiple tumor types, including colorectal and gastric adenocarcinomas [9, 10]. Gene amplification and overexpression of HER2 have been reported in approximately 15%percnt; of gastric cancers. Treatment with trastuzumab results in prolonged survival in gastroesophageal cancer if HER2 is overexpressed or amplified in tumor cells [11]. However, HER2 status has not been well investigated in primary small intestinal adenocarcinoma, probably because of its rarity [12]. Unlike gastric adenocarcinomas, duodenal cancers seem to rarely harbor HER2 amplification or overexpression [12, 13, 14].
We report the case of a patient with HER2-positive stage III duodenal adenocarcinoma who has received adjuvant chemotherapy, including trastuzumab.
Case Report
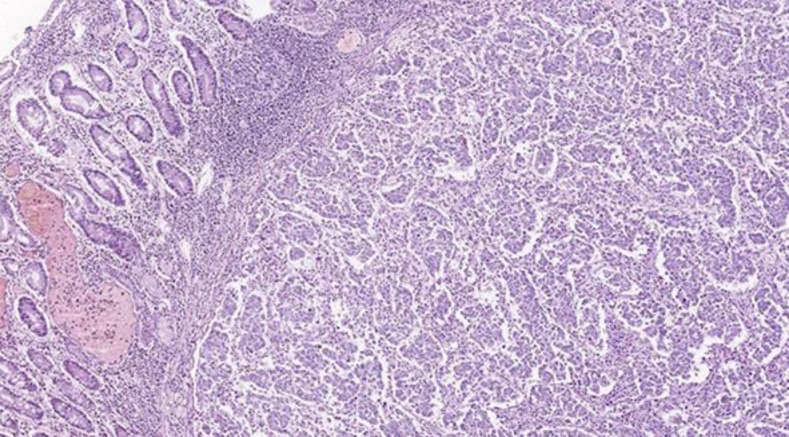
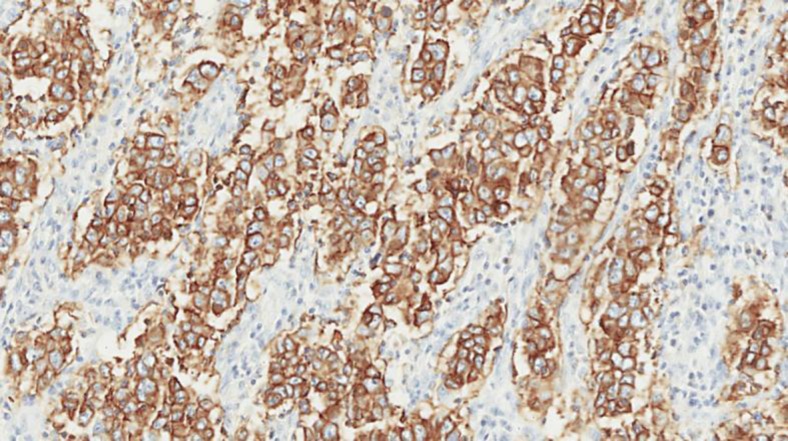
A 51-year-old, previously healthy man presented during routine follow-up with anemia (hemoglobin 9.1 g/dl). An upper endoscopy was requested, which showed an ulcerated lesion in the third portion of the duodenum. Biopsy was consistent with duodenal adenocarcinoma. Staging computed tomography (CT) showed segmental wall thickening of the third duodenal portion, measuring 6 cm in length, without a clear cleavage plan with the pancreatic head. Gastroduodenopancreatectomy was performed, and the pathology confirmed a pT2 pN1 (5 out of 19 lymph nodes positive), poorly differentiated adenocarcinoma of the duodenum (fig. 1). Immunohistochemistry stained positive for HER2 (3%plus;; fig. 2).
Fig. 1.
Hematoxylin and eosin (×20) stain showing poorly differentiated duodenal adenocarcinoma.
Fig. 2.
Immunohistochemistry staining positive for HER2 (3%plus;).
Two months after the surgery, the patient was started on adjuvant chemotherapy with infusional 5-fluorouracil (5-FU) 800 mg/m2 from day 1 to 5 and cisplatin 80 mg/m2 on day 1 every 3 weeks for 6 cycles. Trastuzumab was administered as an intravenous infusion of 6 mg/kg (initial loading dose of 8 mg/kg) every 3 weeks during 6 cycles, followed by trastuzumab maintenance every 3 weeks until completing 1 year. During the first cycle, the patient developed grade 2 oral mucositis, and after the second cycle, a dose reduction of 5-FU to 650 mg/m2 from day 1 to 5 was carried out. On the fifth cycle of chemotherapy, the cisplatin dose had to be reduced to 60 mg/m2 due to progressive tinnitus. The patient successfully completed the planned 6 cycles of cisplatin, 5-FU, and trastuzumab and is currently receiving maintenance trastuzumab every 3 weeks for 1 year. The last CT scan showed no evidence of disease recurrence.
Discussion
Studies conducted on the management of SBA, such as adjuvant chemotherapy and radiation therapy, are limited by small sample sizes and series that are retrospective. Clinical trials to determine optimal treatment regimens for patients with duodenal adenocarcinoma have been limited due to its low incidence. As a result, patients with those malignancies are frequently treated with either colorectal or gastric cancer drug regimens [15, 16]. The absence of a well-established adjuvant treatment guideline for duodenal adenocarcinoma and the resultant poor patient survival underscore the importance of better understanding its biology in order to develop new therapeutic strategies.
HER2 is a transmembrane tyrosine kinase receptor, member of the HER family (HER1–4). HER2 function and signaling activity requires receptor dimerization followed by transactivation of the tyrosine kinase portion of the dimer. Phosphorylation leads to recruitment and activation of downstream proteins, and the signaling cascade is initiated. Gene amplification induces protein overexpression in cell membranes and regulates signal transduction in cellular processes, such as proliferation, differentiation, and cell survival. Aberrant HER2 expression or function has been associated in carcinogenesis of breast, ovarian, gastric, prostate, salivary gland, and lung tumors [17].
In HER2-positive breast cancer, trastuzumab has shown a survival advantage in both early and metastatic scenarios and is currently the standard of care [18, 19, 20]. There is growing evidence that HER2 is an important biomarker and key driver of tumorigenesis in gastric cancer, with studies showing amplification or overexpression in 7–34%percnt; of tumors [11, 21]. In preclinical models of gastric cancer, trastuzumab showed at least additive antitumor effects when combined with capecitabine or cisplatin, or both [22]. In the pivotal phase III ToGA trial, the addition of trastuzumab to cisplatin and 5-FU or capecitabine in patients with HER2-positive tumors confirmed a better overall survival (13.5 vs. 11.1 months, p = 0.0048) when compared to chemotherapy alone [11].
The prevalence of HER2 overexpression and amplification in SBA has only been investigated in few studies. In the one by Overman et al. [13], only 1 out of 54 (1.7%percnt;) tumors expressed HER2 on immunohistochemistry. Similarly, in the study by Aparicio et al. [14], HER2 expression was observed in only 2 of 51 (3.9%percnt;) cases, both of them in the ileum, while no HER2 expression was found in 32 patients with duodenal tumors. Another study conducted immunohistochemical analysis and fluorescence in situ hybridization (FISH) for HER2 on 49 primary nonampullar small intestinal adenocarcinomas. The authors showed a complete lack of HER2 protein expression in 47 cases (96%percnt;) by immunohistochemical analysis. Only 2 cases (4%percnt;) showed a 1%plus; staining pattern. On FISH, none of the tumors, including those with 1%plus; HER2 immunoreactivity, exhibited HER2 gene amplification [12].
We describe the case of a patient whose resected stage III duodenal adenocarcinoma stained positive (3%plus;) for HER2. Based on the potential role of HER2 as a driver of tumorigenesis and the beneficial effect of anti-HER2 therapy in other malignancies, we discussed with the patient the potential advantages of combining trastuzumab to cisplatin and 5-FU for 6 cycles followed by trastuzumab maintenance for 1 year in the adjuvant setting in order to reduce the risk of recurrence. Based on extrapolation from the ToGa trial results in the advanced scenario, the patient agreed to receive adjuvant anti-HER2 therapy. Adjuvant trastuzumab has proven benefits for patients with breast cancer and is still under study for gastroesophageal malignancies [23, 24]. The question whether adjuvant anti-HER2 therapy in combination with chemotherapy reduces the risk of duodenal adenocarcinoma recurrence will probably never be answered, given the rarity of those cases.
Statement of Ethics
The corresponding author acknowledges that she is responsible for complying with ethical requirements and declares that the patient was correctly informed and written informed consent was obtained; the confidentiality of the patient was strictly preserved; the patient was informed about the submission of the manuscript and will be acquainted when the article is published.
Disclosure Statement
The authors declare that they have nothing to disclose.
References
- 1.Laforest A, Aparicio T, Zaanan A, et al. ERBB2 gene as a potential therapeutic target in small bowel adenocarcinoma. Eur J Cancer. 2014;50:1740–1746. doi: 10.1016/j.ejca.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Lepage C, Bouvier AM, Manfredi S, et al. Incidence and management of primary malignant small bowel cancers: a well-defined French population study. Am J Gastroenterol. 2006;101:2826–2832. doi: 10.1111/j.1572-0241.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- 3.Bilimoria KY, Bentrem DJ, Wayne JD, et al. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249:63–71. doi: 10.1097/SLA.0b013e31818e4641. [DOI] [PubMed] [Google Scholar]
- 4.Cao J, Zuo Y, Lv F, et al. Primary small intestinal malignant tumors: survival analysis of 48 postoperative patients. J Clin Gastroenterol. 2008;42:167–173. doi: 10.1097/01.mcg.0000248014.78020.7a. [DOI] [PubMed] [Google Scholar]
- 5.Dabaja BS, Suki D, Pro B, et al. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer. 2004;101:518–526. doi: 10.1002/cncr.20404. [DOI] [PubMed] [Google Scholar]
- 6.Howe JR, Karnell LH, Menck HR, et al. the American College of Surgeons Commission on Cancer and the American Cancer Society: Adenocarcinoma of the small bowel: review of the National Cancer Data Base, 1985–1995. Cancer. 1999;86:2693–2706. doi: 10.1002/(sici)1097-0142(19991215)86:12<2693::aid-cncr14>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 7.Talamonti MS, Goetz LH, Rao S, et al. Primary cancers of the small bowel: analysis of prognostic factors and results of surgical management. Arch Surg. 2002;137:564–570. doi: 10.1001/archsurg.137.5.564. [DOI] [PubMed] [Google Scholar]
- 8.Overman MJ, Hu CY, Wolff RA, et al. Prognostic value of lymph node evaluation in small bowel adenocarcinoma: analysis of the surveillance, epidemiology, and end results database. Cancer. 2010;116:5374–5382. doi: 10.1002/cncr.25324. [DOI] [PubMed] [Google Scholar]
- 9.Järvinen TA, Liu ET. HER-2/neu and topoisomerase II alpha – simultaneous drug targets in cancer. Comb Chem High Throughput Screen. 2003;6:455–470. doi: 10.2174/138620703106298635. [DOI] [PubMed] [Google Scholar]
- 10.Ménard S, Pupa SM, Campiglio M, et al. Biologic and therapeutic role of HER2 in cancer. Oncogene. 2003;22:6570–6578. doi: 10.1038/sj.onc.1206779. [DOI] [PubMed] [Google Scholar]
- 11.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 12.Chan OT, Chen ZM, Chung F, et al. Lack of HER2 overexpression and amplification in small intestinal adenocarcinoma. Am J Clin Pathol. 2010;134:880–885. doi: 10.1309/AJCPK6QHNNOEMJIM. [DOI] [PubMed] [Google Scholar]
- 13.Overman MJ, Pozadzides J, Kopetz S, et al. Immunophenotype and molecular characterisation of adenocarcinoma of the small intestine. Br J Cancer. 2010;102:144–150. doi: 10.1038/sj.bjc.6605449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aparicio T, Svrcek M, Zaanan A, et al. Small bowel adenocarcinoma phenotyping, a clinicobiological prognostic study. Br J Cancer. 2013;109:3057–3066. doi: 10.1038/bjc.2013.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singhal N, Singhal D. Adjuvant chemotherapy for small intestine adenocarcinoma. Cochrane Database Syst Rev. 2007;18:CD005202. doi: 10.1002/14651858.CD005202.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zouhairi ME, Venner A, Charabatya, et al. Small bowel adenocarcinoma. Curr Treat Options Oncol. 2008;9:388–399. doi: 10.1007/s11864-009-0098-0. [DOI] [PubMed] [Google Scholar]
- 17.Hudis CA. Trastuzumab – mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 18.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 19.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 20.Smith I, Procter M, Gelber RD, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 21.Tanner M, Hollmen M, Junttila TT, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase II alpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273–278. doi: 10.1093/annonc/mdi064. [DOI] [PubMed] [Google Scholar]
- 22.Fujimoto-Ouchi K, Sekiguchi F, Yasuno H, et al. Antitumor activity of trastuzumab in combination with chemotherapy in human gastric cancer xenograft models. Cancer Chemother Pharmacol. 2007;59:795–805. doi: 10.1007/s00280-006-0337-z. [DOI] [PubMed] [Google Scholar]
- 23.A Study of the Combination of Oxaliplatin, Capecitabine and Herceptin (Trastuzumab) and Chemoradiotherapy in the Adjuvant Setting in Operated Patients with HER2%plus; Gastric or Gastro-Esophageal Junction Cancer (TOXAG Study). http://www.clinicaltrials.gov/show/NCT01748773.
- 24.T-XELOX in HER2-Positive Stage III Gastric Cancer after D2 Gastrectomy. https://clinicaltrials.gov/ct2/show/NCT02250209.