Abstract
Background
Organophosphorus pesticides are widely used throughout the world. Because of their ease of availability, organophosphorus compounds are commonly used for self-poisoning in developing countries. The acute effects of exposure to organophosphorus pesticides are well known, but the chronic effects are unclear. Recent studies suggest that abnormalities of the central and peripheral nervous systems persisted for up to 5 years after acute poisoning due to a single large dose of organophosphates (OPs). However, the long-term effects on cardiovascular diseases are poorly understood.
Methodology/Principal Findings
An OPs-exposed cohort (N = 7,561) and an age- and gender-matched control cohort (N = 30,244), both identified from the National Health Insurance Research Database, were compared. We utilized the multivariable Cox proportional model to estimate the risks of developing arrhythmia, coronary artery disease (CAD) and congestive heart failure (CHF). The patients with acute poisoning from OPs had higher incidence rates of arrhythmia (5.89 vs. 3.61 per 1,000 person-years), CAD (9.10 vs. 6.88 per 1,000 person-years), and CHF (3.89 vs. 2.98 per 1,000 person-years) compared with that of the non-OPs poisoning cohort, with a crude subhazard ratio (SHR) of 1.40, 1.13, and 1.12, respectively. Additionally, a significantly higher risk of arrhythmia was observed in the OPs poisoning cohort (adjusted SHR = 1.25) compared with the non-OPs poisoning cohort, particularly in male patients (adjusted SHR = 1.33) and those under 49 years of age (adjusted SHR = 3.16). After accounting for the competing risks of death, there was a higher risk of arrhythmia and CAD during a three year follow-up period (adjusted SHR = 1.50 for arrhythmia; adjusted SHR = 1.10 for CAD). We also found an adjusted SHR of 1.36 associated with developing CHF after 6 years of follow-up for OPs poisoning cohort.
Conclusions
Acute OPs poisoning may continuously impact human health through mechanisms that are unclear. Any supportive measurements that could contribute to a reduction in the risk of heart disease may be beneficial in cases of OPs poisoning survivors.
Introduction
Organophosphates (OPs) poisoning is an important public health problem in developing countries, because such compounds are widely used for the control of agricultural, industrial and domestic pests, and also leads to large numbers of cases of toxic effects on humans. Eddleston et al. indicated that organophosphorus pesticide self-poisoning kills an estimated 200,000 people every year [1]. Although most of such deaths occur in developing countries, this poisoning is also an important cause of fatal self-poisoning in developed countries [2]. Additionally, OPs can lead to a high potential of environmental pollution and increased health risks for the OPs poisoning patients who survive.
Acute OPs poisoning can cause acute cholinergic dysfunction, muscle weakness, seizures, coma, and respiratory failure. OPs stimulate both nicotinic and muscarinic acetylcholine receptors, as well as adrenergic receptors through the inhibition of acetylcholinesterase, which leads to the accumulation of acetylcholine and development of severe functional damage within both the central and peripheral nervous systems [1, 3]. Respiratory paralysis and cardiac arrest are considered to be the most common causes of death in acute OPs poisoning patients [4, 5]. Acute OPs poisoning is associated with three phases of cardiac manifestation. Firstly, there is a period of increased sympathetic tone, which is followed by a prolonged parasympathetic phase. Lastly, QT prolongation is followed by torsade de points ventricular tachycardia and ventricular fibrillation [6, 7].
Several studies have illustrated the long-term effects of OPs poisoning. Delayed polyneuropathy induced by OPs, but not carbamate, has been noted to be a harsh sequelae that results from exposure to certain OPs [8] and is characterized by distal degeneration of some axons of both the peripheral and central nervous systems occurring 1–4 weeks after single large dose acute exposures. Significant inhibition of neuropathy target esterases is potentially an etiological attribution of OPs-induced peripheral neuropathy. Few studies have also examined the long-term neuropsychiatric adverse effects of OPs after acute intoxication or multiple exposures to lower doses; however, those that have been conducted found increased occurrence of neurological or psychiatric presentations and poorer performance on standardized neuropsychological tests [9–12]. Few studies have illustrated an increase in the vulnerability of developing arrhythmias after accidental or terror-related OPs intoxication in experimental animals [13, 14]. No studies have demonstrated the long-term effects on the cardiovascular system after survival of OPs poisoning in humans, which is due to the difficulties in follow-up data collection.
Acute coronary syndrome and associated ventricular arrhythmia is one of the most prevalent causes of death in industrialized countries, as well as in developing countries [15, 16]. Despite a substantial amount being known and reported about classic cardiovascular risk factors such as family history, ethnicity, age, smoking, hypertension, dyslipidemia, diabetes, obesity, sedentary lifestyle, and dietary factors, there are still contentious areas that are not supported with conclusive evidence [17]. To improve the understanding of the long-term effects of OPs poisoning on cardiovascular diseases, we used a large population-based dataset to study the association between cardiovascular diseases (CVDs) and OPs poisoning. Our study provides explicit information on the relationship between OPs poisoning and CVDs, especially arrhythmia, coronary artery disease and congestive heart failure. This comprehensive investigation indicate that OPs poisoning could be a risk factor for the development of CVDs.
Methods
Data Source
This retrospective cohort study was conducted using Taiwan's National Health Insurance Research Database (NHIRD). The Taiwan National Health Insurance (NHI), a government-operated, universal health program established in 1995, has covered approximately 99% of the overall population and has been contracted by 97% of the hospitals and clinics nationwide. National Health Research Institute (NHRI) built and managed the NHIRD, which processed reimbursement claim data from the NHI program. The database contains comprehensive information on insured subjects, including dates of clinical visits, diagnostic codes, details about prescriptions, and expenditure amounts. Details of the database are presented on the NHRI website (http://www.nhi.gov.tw/english/index.aspx). Personal identifiers are encrypted for privacy protection, but all data sets can be linked to each other with the unique and anonymous identifiers created by NHRI. The present study was approved by the Institutional Review Board (IRB) of China Medical University and Hospital (CMU-REC-101-012).
Participants
The OPs poisoning cohort was identified from insured individuals with OPs poisoning (ICD-9-CM code 989.3) with an initial hospitalization between 2000 and 2011. The date of hospitalization diagnosis for OPs poisoning was designated as the index date. In both cohorts, individuals with a history of arrhythmia (ICD-9-CM code 427, 758.0, 758.1), coronary artery disease (CAD) (ICD-9-CM code 410–414), and congestive heart failure (CHF) (ICD-9-CM code 428) before the index date, or with incomplete age or sex information, were excluded. For each patient with OPs poisoning, four patients without OPs poisoning was randomly selected for the non-OPs poisoning cohort, using frequency matching method to insure both cohorts had same distributions over strata of sex, age (every 5-y span), and index year of OPs poisoning. Our further data analysis showed that the cumulative censoring rate over 12 years (2000–2011) was 24.0% in the OPs poisoning cohort, which was higher than that in the non-OPs poisoning cohort (10.0%). However, we did not have death records in the insurance data bases. The possible reasons for the discontinuity of national health insurance include death, withdrawal of insurance, immigration, prison sentence, etc.
Main Outcome and Co-morbidities
Each of the study subjects was followed until a diagnosis of arrhythmia, CAD, or CHF was made, until the patients were censored for loss to follow-up, death, withdrawal from the database, or the end of 2011, whichever event occurred first. We also incorporated inpatient diagnosis records to ascertain the baseline comorbidities, including diabetes (ICD-9-CM 250), hypertension (ICD-9-CM 401–405), hyperlipidemia (ICD-9-CM 272), and chronic obstructive pulmonary disease (COPD) (ICD-9-CM codes 490–492, 494, 496).
Statistical Analysis
The distributions of demographic characteristics were compared between the OPs poisoning cohort and the non-OPs poisoning cohort. A Chi-square test was used for categorical variables, and a Mann-Whitney U test was used for continuous variables. Gender-, age- and comorbidity-specific incidences of each cardiovascular event were calculated for both cohorts. We used the Fine and Gray model [18], which extends the univariable and multivariable standard Cox proportional hazard regression model, to estimate the subhazard ratios (SHR) and 95% confidence interval (CI) for assessing the effects of OPs poisoning, the risk of CVDs for the OPs poisoning cohort, comparing with the non-OPs cohort, after accounting for the competing risks of death. The identification of death events was based on the discharge due to death, lost to follow up, or withdrew from insurance system in the NHIRD. The multivariable models were simultaneously adjusted for demographic status and co-morbidities of diabetes, hypertension, hyperlipidemia, and COPD. The Cox models were also used to evaluate whether OPs poisoning interacted with age (stratified ages into 2 levels ≤ 49 years and > 49 years) and with selected comorbidity (comorbidities into yes and no) in the association with the CVD. Additional analysis was conducted to assess whether the association between the risks of CVD and OP poisoning varied during the follow-up period. Risks of CVDs were estimated for three periods: ≤3 years, 4–6 years, and >6 years. We compared the Kaplan-Meier analyses to competing risk cumulative incidence of cardiovascular events between the OPs poisoning cohort and the non-OPs poisoning cohort using the Aalen-Johansen estimator [19]. All data analyses were conducted using SAS software (version 9.2 for Windows; SAS Institute Inc., Cary, NC, USA). A P value< .05 was considered statistically significant.
Results
We selected 7,561 patients with OPs poisoning for our study cohort, and 30,244 control patients without OPs poisoning for our comparison cohort (Table 1). The median follow-up time of occurrence of arrhythmia were 5.94 (IQR = 3.96–9.63) years and 7.12 (IQR = 3.96–9.63) years in the OPs poisoning and non-OPs poisoning cohorts, respectively. The median follow-up duration for coronary artery disease was 5.81 (IQR = 2.10–9.03) years for the OPs poisoning cohort and 7.04 (IQR = 3.79–9.54) years for the non-OPs poisoning cohort. The median follow-up period for congestive heart failure was 6.07 (IQR = 2.23–9.15) years for the OPs poisoning cohort and 7.21 (IQR = 3.99–9.63) years for the non-OPs poisoning cohort.
Table 1. Number of events of OPs poisoning.
| OPs poisoning | ||
|---|---|---|
| Year | No | Yes |
| 2000 | 3844 | 961 |
| 2001 | 3896 | 974 |
| 2002 | 3840 | 960 |
| 2003 | 3420 | 855 |
| 2004 | 2888 | 722 |
| 2005 | 2596 | 649 |
| 2006 | 2308 | 577 |
| 2007 | 1884 | 471 |
| 2008 | 1612 | 403 |
| 2009 | 1452 | 363 |
| 2010 | 1308 | 327 |
| 2011 | 1196 | 299 |
| Total | 30244 | 7561 |
Among the study participants, 51.3% were older than 50 years of age, and 70.9% were men. The median age (IQR) of the OPs poisoning cohort and the non-OPs poisoning cohort were 50.5 (IQR = 39.0–63.6) years old and 50.3 (IQR = 38.6–62.9) years old, respectively. Compared with the non-OPs poisoning cohort, co-morbidity of diabetes (10.6% vs. 2.91%, p<0.001), hypertension (14.4% vs. 4.05%, p<0.001), hyperlipidemia (3.74% vs. 0.89%, p<0.001) and COPD (4.46% vs. 1.32%, p<0.001) were more common in the OPs poisoning cohort (Table 2).
Table 2. Characteristics of patients with OPs poisoning and matched patients without OPs poisoning.
| OPs poisoning | |||||
|---|---|---|---|---|---|
| Yes (N = 7561) | No (N = 30244) | ||||
| n | % | n | % | p-value | |
| Age, year | 0.99 | ||||
| ≤34 | 1313 | 17.4 | 5252 | 17.4 | |
| 35–49 | 2372 | 31.4 | 9488 | 31.4 | |
| 50–64 | 2202 | 29.1 | 8808 | 29.1 | |
| ≥ 65 | 1674 | 22.1 | 6696 | 22.1 | |
| Median (IQR) # | 50.5 | 39.0–63.6 | 50.3 | 38.6–62.9 | 0.02 |
| Gender | 0.99 | ||||
| Female | 2202 | 29.1 | 8808 | 29.1 | |
| Male | 5359 | 70.9 | 21436 | 70.9 | |
| Comorbidity | |||||
| Diabetes | 803 | 10.6 | 881 | 2.91 | <0.001 |
| Hypertension | 1085 | 14.4 | 1224 | 4.05 | <0.001 |
| Hyperlipidemia | 283 | 3.74 | 269 | 0.89 | <0.001 |
| COPD | 337 | 4.46 | 400 | 1.32 | <0.001 |
Chi-square test
#:Mann-Whitney U test
IQR denotes interquartile range
Age means age at OPs poisoning.
Table 3 shows the incidence densities and subhazard ratios of multiple cardiovascular events by gender, age and co-morbidity. Overall, the patients with OPs poisoning had higher incidence rates of arrhythmia (5.89 vs. 3.61 per 1,000 person-years), and CAD (9.10 vs. 6.88 per 1,000 person-years), than the non-OPs poisoning cohort, with a crude SHR of 1.40 (95% CI = 1.21–1.61), and 1.13 (95% CI = 1.01–1.27). However, the association between OPs poisoning and CHF was not statistically significant.
Table 3. The risk of arrhythmia, coronary artery disease (CAD), and congestive heart failure (CHF) in patients and subhazard ratio for patients with OPs poisoning compared to patients without OPs poisoning by age, sex, and comorbidity in the competing-risk regression model.
| Overall | Gender 1 | Age, year 2 | Comorbidity§ 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | ≤49 | 50–64 | ≥ 65 | No | Yes | ||||
| Arrhythmia | ||||||||||
| OPs poisoning | Event | 253 | 63 | 190 | 59 | 81 | 113 | 168 | 85 | |
| PY | 42980 | 12727 | 30253 | 23132 | 12612 | 7236 | 33965 | 9015 | ||
| Rate | 5.89 | 4.95 | 6.28 | 2.55 | 6.42 | 15.6 | 4.95 | 9.43 | ||
| Non-OPs poisoning | Event | 734 | 207 | 527 | 65 | 201 | 468 | 571 | 163 | |
| PY | 203190 | 59941 | 143249 | 105035 | 60291 | 37864 | 192296 | 10894 | ||
| Rate | 3.61 | 3.45 | 3.68 | 0.62 | 3.33 | 12.4 | 2.97 | 15 | ||
| Crude SHR†(95%) | 1.40(1.21, 1.61)*** | 1.23(0.93, 1.63) | 1.46(1.24, 1.73)*** | 3.69(2.59, 5.25)*** | 1.64(1.26, 2.12)*** | 0.99(0.80, 1.21) | 1.46(1.23, 1.74)*** | 0.55(0.42, 0.71)*** | ||
| Adjusted SHR ‡(95%) | 1.25(1.07, 1.46)*** | 1.02(0.75, 1.39) | 1.33(1.12, 1.59)*** | 3.16(2.18, 4.59)*** | 1.32(0.98, 1.78) | 0.89(0.72, 1.11) | 1.59(1.33, 1.89)*** | 0.66(0.51, 1.00) | ||
| CAD | ||||||||||
| OPs poisoning | Event | 387 | 105 | 282 | 77 | 143 | 167 | 208 | 179 | |
| PY | 42536 | 12561 | 29976 | 23072 | 12354 | 7111 | 33792 | 8743 | ||
| Rate | 9.1 | 8.36 | 9.41 | 3.34 | 11.6 | 23.5 | 6.16 | 20.5 | ||
| Non-OPs poisoning | Event | 1380 | 310 | 1070 | 161 | 493 | 726 | 1096 | 284 | |
| PY | 200499 | 59435 | 141065 | 104691 | 59056 | 36751 | 190034 | 10464 | ||
| Rate | 6.88 | 5.22 | 7.59 | 1.54 | 8.35 | 19.8 | 5.77 | 27.1 | ||
| Crude SHR†(95%) | 1.13(1.01, 1.27)* | 1.37(1.10, 1.72)** | 1.06(0.93, 1.21) | 1.94(1.47, 2.54)*** | 1.17(0.97, 1.41) | 0.92(0.79, 1.10) | 0.93(0.81, 1.08) | 0.65(0.54, 0.79)** | ||
| Adjusted SHR ‡(95%) | 0.96(0.85, 1.08) | 1.10(0.86, 1.40) | 0.92(0.80, 1.05) | 1.48(1.10, 1.99)*** | 0.97(0.79, 1.19) | 0.79(0.66, 1.00) | 0.99(0.85, 1.15) | 0.78(0.64, 1.00) | ||
| CHF | ||||||||||
| OPs poisoning | Event | 169 | 58 | 111 | 33 | 44 | 92 | 79 | 90 | |
| PY | 43427 | 12795 | 30632 | 23293 | 12770 | 7363 | 34339 | 9088 | ||
| Rate | 3.89 | 4.53 | 3.62 | 1.42 | 3.45 | 12.5 | 2.3 | 9.9 | ||
| Non-OPs poisoning | Event | 607 | 182 | 425 | 41 | 151 | 415 | 441 | 166 | |
| PY | 203748 | 60124 | 143624 | 105154 | 60429 | 38166 | 192809 | 10939 | ||
| Rate | 2.98 | 3.03 | 2.96 | 0.39 | 2.5 | 10.9 | 2.29 | 15.2 | ||
| Crude SHR†(95%) | 1.12(0.95, 1.33) | 1.29(0.96, 1.73) | 1.05(0.85, 1.29) | 3.25(2.06, 5.14)*** | 1.17(0.84, 1.64) | 0.90(0.72, 1.13) | 0.88(0.69, 1.12) | 0.56(0.43, 0.73)*** | ||
| Adjusted SHR ‡(95%) | 0.91(0.76, 1.09) | 0.96(0.70, 1.32) | 0.89(0.71, 1.10) | 2.50(1.52, 4.10)*** | 0.79(0.54, 1.15) | 0.78(0.62, 1.01) | 0.97(0.76, 1.23) | 0.70(0.54, 1.00) | ||
PY, person-years; Rate, incidence rate per 1000 person-years; Crude SHR†, relative subhazard ratio; Adjusted SHR‡, subhazard ratio adjusted for age, sex, and comorbidities of diabetes, hypertension, hyperlipidemia and COPD; Comorbidity§: Patients with any one of the comorbidities diabetes, hypertension, diabetes, hyperlipidemia and COPD were classified as the comorbidity group;
*p<0.05
**p<0.01
***P<0.001.
1Adjusted SHR was calculated by competing-risk regression model stratified by gender and adjusted for age, and comorbidities of diabetes, hypertension, hyperlipidemia and COPD.
2Adjusted SHR was calculated by competing-risk regression model stratified by age, and adjusted for sex, and comorbidities of diabetes, hypertension, hyperlipidemia and COPD.
3Adjusted SHR was calculated by competing-risk regression model stratified by comorbidity and adjusted for age, and sex.
Multivariable competing-risks regression models for the risk of arrhythmia showed a significantly higher risk in the OPs poisoning cohort (Adjusted SHR = 1.25, 95% CI = 1.07–1.39) compared with the non-OPs poisoning cohort. The risk of arrhythmia was higher in men with OPs poisoning than in men without OPs poisoning (Adjusted SHR = 1.33, 95% CI = 1.12–1.39). The age-specific risk analyses showed that patients with OPs poisoning aged ≤ 49 years exhibited a significantly higher risk of arrhythmia than patients aged ≤ 49 years without OPs poisoning (adjusted SHR = 3.16; 95% CI, 2.18–4.59). In patients without co-morbidity, the risk of arrhythmia was 1.59-fold higher in the OPs poisoning cohort than in the non-OPs poisoning cohort (95%CI = 1.33–1.89). The age-specific analysis indicated that the OPs poisoning cohort exhibited a significantly higher risk of CAD than did the non-OPs poisoning cohort in ≤49-year age group (adjusted SHR = 1.48, 95% CI = 1.10–1.99). The younger patient group had a adjusted SHR of 2.50 developing CHF (95% CI = 1.52–4.10) in the OPs poisoning cohort compared to the non-OPs poisoning cohort.
In this study, the interaction measures between OPs poisoning and age, and between OPs poisoning and comorbidity associating with developing arrhythmia, CAD, and CFH (Table 4). Compared with patients ≤49 years and without OPs poisoning, patients >49 years and without OPs poisoning were associated with an increased risk of arrhythmia (adjusted SHR = 9.93, 95% CI, 7.69–12.8), followed by patients >49 years and with OPs poisoning (adjusted SHR = 9.64, 95% CI, 7.17–13.0) and patients ≤49 years and with OPs poisoning (adjusted SHR = 3.45, 95% CI, 2.43–4.91; interaction P < .001). Furthermore, relative to the non-OPs poisoning cohort without any comorbidity, the patients with any comorbidity were at a much higher risk of arrhythmia (adjusted SHR = 3.04, 95% CI = 2.11–4.37), followed by OPs poisoning patients and with comorbidity (adjusted SHR = 1.67, 95% CI, 1.13–2.46) and OPs poisoning patients and with any comorbidity (adjusted SHR = 1.46, 95% CI, 1.13–2.46; interaction P < .001). The risk of coronary artery disease and congestive heart failure had same trend which the effect between OPs poisoning and age or comorbidity. Though risk factors of age and comorbidity were important effect in CVD, the OPs poisoning effect to CVD still had statistically significant.
Table 4. Competing-risk regression model for the risk of coronary artery disease (CAD), and congestive heart failure (CHF) associated OPs poisoning with interaction of comorbidity.
| Age ≤ 49 | Age > 49 | Non-comorbidity | Comorbidity§ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-OPs poisoning | OPs poisoning | Non-OPs poisoning | OPs poisoning | Non-OPs poisoning | OPs poisoning | Non-OPs poisoning | OPs poisoning | |||
| Arrhythmia | ||||||||||
| Number | 14740 | 3685 | 15504 | 3878 | 28135 | 5640 | 2109 | 1921 | ||
| Event | 65 | 59 | 669 | 194 | 571 | 168 | 163 | 85 | ||
| Adjusted SHR† | 1(Reference) 1 | 3.45(2.43, 4.91)*** | 9.93(7.69, 12.8)*** | 9.64(7.17, 13.0)*** | 1(Reference) 2 | 1.46(1.23, 1.74)*** | 3.04(2.11, 4.37)*** | 1.67(1.13, 2.46)*** | ||
| p-value & | <0.001 | <0.001 | ||||||||
| CAD | ||||||||||
| Number | 14740 | 3685 | 15504 | 3878 | 28135 | 5640 | 2109 | 1921 | ||
| Event | 161 | 77 | 1219 | 310 | 1096 | 208 | 284 | 179 | ||
| Adjusted SHR† | 1(Reference) 1 | 1.78(1.35, 2.33)*** | 7.25(6.14, 8.55)*** | 5.87(4.80, 7.19)*** | 1(Reference) 2 | 0.99(0.85, 1.15) | 2.06(1.79, 2.37)*** | 1.78(1.51,2 .10)*** | ||
| p-value & | <0.001 | 0.004 | ||||||||
| CHF | ||||||||||
| Number | 14740 | 3685 | 15504 | 3878 | 28135 | 5640 | 2109 | 1921 | ||
| Event | 41 | 33 | 566 | 136 | 441 | 79 | 166 | 90 | ||
| Adjusted SHR† | 1(Reference) 1 | 2.86(1.81, 4.53)*** | 12.6(9.18, 17.4)*** | 8.89(6.17, 12.8)*** | 1(Reference) 2 | 0.96(0.76, 1.23) | 2.53(2.08, 3.07)*** | 2.00(1.58, 2.53)*** | ||
| p-value & | <0.001 | 0.01 | ||||||||
Adjusted SHR†:adjusted for age and sex.
&p-value for interaction;
*p<0.05
**p<0.01
***P<0.001.
Comorbidity§: Patients with any one of the comorbidities diabetes, hypertension, diabetes, hyperlipidemia and COPD were classified as the comorbidity group.
1Adjusted SHR was calculated by competing-risk regression model adjusted for sex, and comorbidities of diabetes, hypertension, hyperlipidemia and COPD.
2Adjusted SHR was calculated by competing-risk regression model adjusted for age and sex.
Furthermore, the adjusted subhazard ratio varied during the length of follow-up period after OPs poisoning diagnosed (Table 5). The adjusted SHR for arrhythmia was significant in different follow-up durations (≤ 3 years, SHR = 1.50, 95% CI = 1.18–3.92; > 6 years, SHR = 1.40, 95% CI = 1.04–1.89). The incidence density rates of CHF increased with increasing follow-up periods in both cohorts. We found a 1.36-fold significantly higher relative risk of developing CHF after a 6 year follow-up period (95% CI = 1.01–1.84).
Table 5. Trends of cardiovascular event risks by stratified follow-up years in the competing-risk regression model.
| OPs poisoning | ||||||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | |||||||
| Follow time, years | Event | PY | Rate | Event | PY | Rate | Crude SHR† (95% CI) | Adjusted SHR‡ (95% CI) |
| Arrhythmia | ||||||||
| ≤3 | 109 | 18017 | 6.05 | 250 | 82139 | 3.04 | 1.76(1.41, 2.21)*** | 1.50(1.18,3.92)** |
| 4–6 | 80 | 13580 | 5.89 | 251 | 64485 | 3.89 | 1.26(0.97, 1.62) | 1.13(0.87, 1.47) |
| >6 | 64 | 11383 | 5.62 | 233 | 56566 | 4.12 | 1.34(1.02, 1.77)* | 1.40(1.04, 1.89)* |
| CAD | ||||||||
| ≤3 | 178 | 17954 | 9.91 | 536 | 81712 | 6.56 | 1.34(1.13, 1.59)** | 1.10(0.93, 1.31) |
| 4–6 | 119 | 13406 | 8.88 | 464 | 63496 | 7.31 | 1.01(0.83, 1.24) | 0.91(0.74, 1.12) |
| >6 | 90 | 11176 | 8.05 | 380 | 55290 | 6.87 | 1.15(0.91, 1.45) | 1.06(0.83, 1.34) |
| CHF | ||||||||
| ≤3 | 63 | 18111 | 3.48 | 203 | 82210 | 2.47 | 1.25(0.94, 1.66) | 1.02(0.76, 1.37) |
| 4–6 | 45 | 13744 | 3.27 | 211 | 64653 | 3.26 | 0.84(0.61, 1.16) | 0.70(0.50, 1.00) |
| >6 | 61 | 11572 | 5.27 | 193 | 56886 | 3.39 | 1.53(1.15, 2.04)** | 1.36(1.01, 1.84)* |
PY, person-years; Rate, incidence rate per 1,000 person-years; Crude SHR†, relative subhazard ratio;
Adjusted SHR‡, subhazard ratio adjusted for age, sex, and comorbidities of diabetes, hypertension, hyperlipidemia and COPD.
*p<0.05
**p<0.01
***P<0.001.
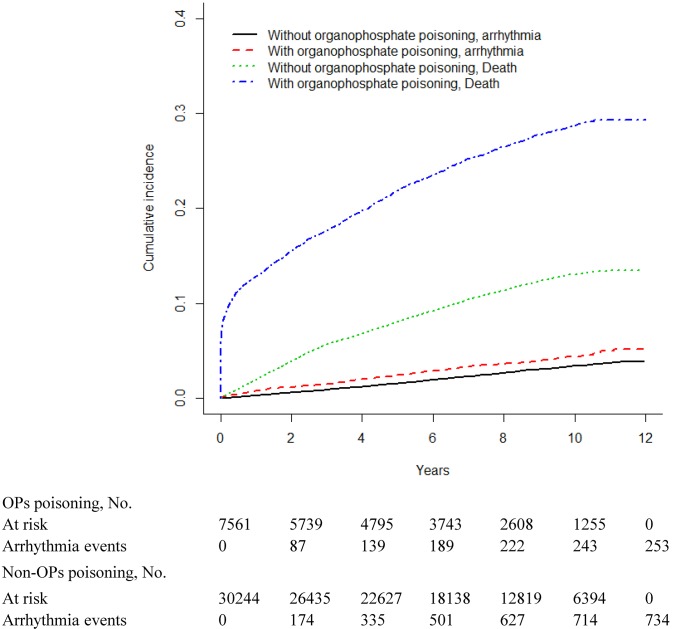
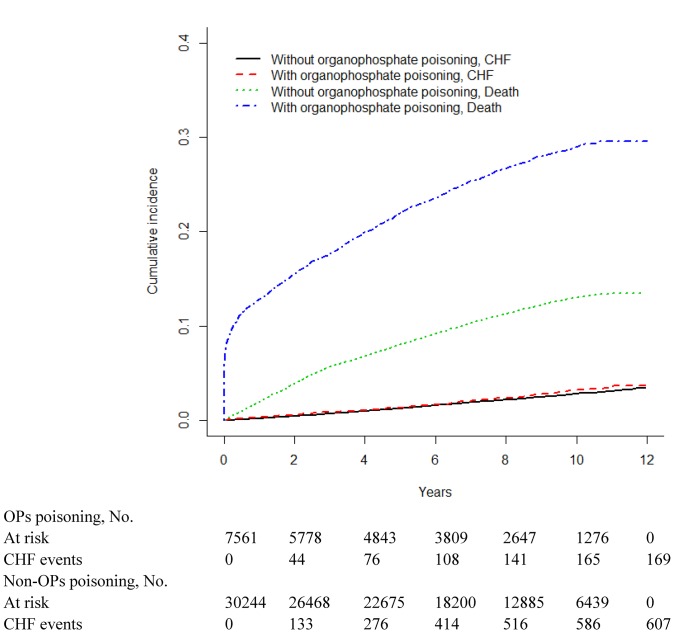
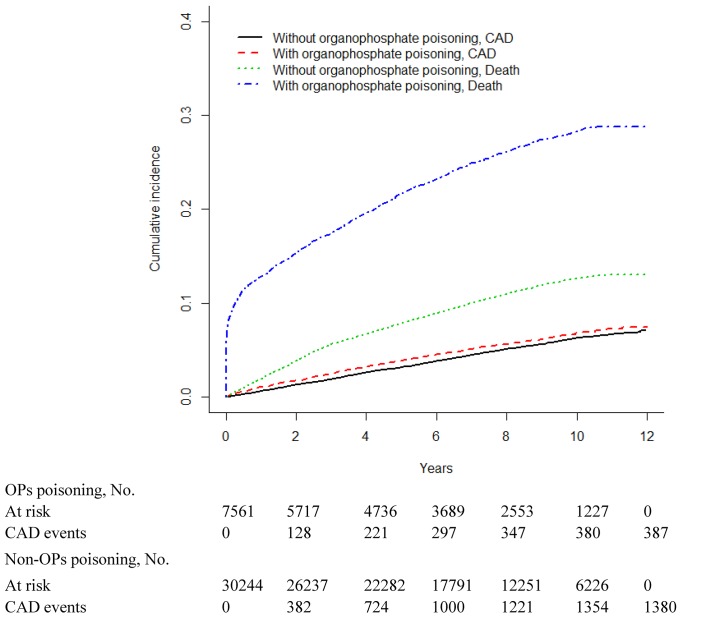
Figs 1–3 show the cumulative incidence curve of arrhythmia, CAD and CHF for the 2 cohorts after accounting for death as the competing risk indicated that the incidence of arrhythmia, CAD and CHF was higher among OPs poisoning patients than among non-OPs poisoning patients. Table 6 showed the survival analysis inference for each cohort. The 3-year and 5-year cumulative incidence rates were both higher among OPs poisoning patients than that among non-OPs poisoning patients.
Fig 1. Cumulative incidence of arrhythmia compared between with and without organophosphate poisoning.
Fig 3. Cumulative incidence of congestive heart failure (CHF) compared between with and without organophosphate poisoning.
Table 6. Cumulative incidence rates among different period.
| OPs poisoning | ||||
|---|---|---|---|---|
| No | Yes | |||
| Follow-up duration, year | % | 95% CI | % | 95% CI |
| Arrhythmia | ||||
| 3 year | 0.90 | (0.58–1.41) | 1.74 | (0.95–3.17) |
| 5 year | 1.63 | (1.28–2.09) | 2.93 | (2.04–4.19) |
| CAD | ||||
| 3 year | 1.95 | (1.42–2.67) | 2.90 | (1.94–4.32) |
| 5 year | 3.34 | (2.78–4.02) | 4.56 | (3.52–5.89) |
| CHF | ||||
| 3 year | 0.74 | (0.40–1.38) | 1.02 | (0.41–2.55) |
| 5 year | 1.34 | (0.95–1.89) | 1.57 | (0.87–2.86) |
Fig 2. Cumulative incidence of coronary artery disease (CAD) compared between with and without organophosphate poisoning.
Discussion
Heart attack due to CAD and its related complications remains one of the most prevalent causes of death worldwide. Several risk factors were established or suggested after extensive evidenced-based studies [20]. The strongest predictors of 10-year risk are age, sex, race, total cholesterol, high-density lipoprotein cholesterol (HDL-C), blood pressure, blood-pressure treatment status, diabetes, and current smoking status. The identification of novel risk factors for CVD is critical to improve our understanding of disease biology and to prevent cardiovascular morbidities and mortality. In the population-based cohort study presented herein, we found that OPs poisoning is a significant risk factor for cardiovascular diseases such as arrhythmia, CAD, or CHF. To our knowledge, there are no published reports regarding the potential adverse effects on heart health in a large population-based cohort of acute OPs poisoning.
In this community-based cohort study, we found that the estimated incidence density rates of any type of arrhythmia in cases of OPs poisoning were 1.6 times that of non-OPs poisoning individuals after age, sex, and comorbidity conditions were adjusted, although cases of OPs poisoning were significantly associated with chronic diabetes mellitus, hypertension, hyperlipidemia and COPD. It is also surprising that the vulnerability of arrhythmia lasted for more than 6 years.
The primary mechanism of OPs toxicity has been well studied and is known as inhibition of the acetylcholinesterase (AChE) enzyme. AChE is found in synapses, where it degrades the neurotransmitter acetylcholine and produces choline and acetate, a reaction important for controlling the operation of cardiac muscles–is the representative system affected by OPs. In a study conducted by Jayasinghe and Pathirana [21], without significant residual, autonomic dysfunction of cardiovascular system were found in 66 cases from a cohort study after 6 weeks follow-up acute OPs poisoning. Other toxic effects, such as oxidative stress induced by acute OPs poisoning or continuous reduction in M2 auto-receptor system, might explain the long term adverse effects of OPs on heart rhythms [11, 22, 23].
Additionally, excessive acetylcholine has been noted to be a potential etiological factor for reducing the threshold of epinephrine-induced arrhythmias after OPs poisoning in human and animal studies [13, 24]. Allon et al. indicated that the effect of locally released acetylcholine on epinephrine-induced arrhythmias could last for 6 months, and this may partly explain the delayed mortality observed in OPs poisoning patients [13].
In this large population-based study, we also found a possible correlation between acute OPs poisoning and ischemic heart diseases. The estimated incidence density rates of cardiovascular diseases (CAD and CHF) in cases of OPs poisoning were 1.3 times higher than those of non-OPs poisoning. After adjustment for age, sex, and co-morbidity conditions, the risk of CAD in cases of OPs poisoning remained significant (adjusted HR = 0.96, 95% CI = 0.85–1.08), but not for CHF (adjusted HR = 0.91, 95% CI = 0.76–1.09). The vulnerability of CAD or CHF in cases of OPs poisoning were also noted to last for 3 years, but not longer than arrhythmia. Additional, different mechanisms should be considered.
In ischemic heart disease, several risk factors have been studied and elucidated. Chronic disorders, such as diabetes mellitus, hypertension or hyperlipidemia, were considered to be modifiable factors for CAD [20]. In our cohort, acute OPs poisoning patients were noted to be associated with a higher rate of these chronic disorders and may compromise the cardiac effects of OPs after more than 3 years have passed since poisoning. OPs can lead to long-term inhibition of AChE and consumption of paraoxonase (PON1) by at least two mechanisms: first, plasma AChE binds OPs poisons; second, OPs are bioactivated to highly toxic oxon forms by cytochrome P450s, which is followed by destruction by hydrolysis by PON1 to harmless products [25]. PON1 may confer protection against damage of vessel walls by antioxidation and by destroying oxidation products [26]. Both the peroxidation of LDLs and secondary inflammatory responses are key steps in the initiation of atherogenesis [27]. Atherogenesis is the major cause of CAD and CHF. Thus, a decrease of PON1 activity caused by detoxification OPs is implicated in the pathogenesis of atherosclerosis and CVD [28].
It is also worth noting that the effect of OPs poisoning on CVD appears to be significant among relatively young populations. People who were exposed to OPs poisoning at 49 years of age or younger had a 1.48–3.16 times higher risk of CVD than those who were not exposed, while the hazards were not significantly different between individuals with and without OPs poisoning in individuals aged 50 years old or older. This is not unexpected because relatively older age (male > 45, female > 55) is a conventional risk factor for atherosclerotic cardiovascular disease, as stated in the American College of Cardiology and the World Heart Federation 2013 guidelines [20]. Thus, in older patients, the effect of OPs poisoning on CVDs could be masked by the homogeneous condition between the two groups. However, the information obtained from the large population-based cohort is important for the prevention of cardiac diseases in younger patients who survived acute OPs poisoning.
The CVD is the leading cause of death worldwide and with sophisticated mechanisms. Our findings suggest that OPs poisoning is another factor associated with the CVD risk. The CVD risk has been associated with traditional lifestyle factors of tobacco use, unhealthy diet and obesity, physical inactivity and harmful use of alcohol. It may be critical for clinical implication for patients with OPs exposure on the management of risk factors to prevent CVD. This may be particularly important for agriculture population at higher risk of OPs exposure.
The main strength of the present study is its population-based design and its generalizability. However, several limitations should be considered. First, administrative database studies are potentially prone to errors arising from the diagnostic code. The ICD-9-CM code 989.3 describes the toxic effects of organophosphate and carbamate. Carbamate poisoning shares clinical presentations of organophosphate poisoning with a shorter course due to its reversible inhibition of acetylcholinesterases. Delayed neuropathy due to carbamate intoxication is very rare and should always be disregarded [29], and the same is true of its late cardiac effects. The relative risk of long term cardiovascular diseases of OPs poisoning may be greater if the carbamate poisoning cases were excluded. The late cardiovascular complications of the OP poisoning may also be due to the continued occupational exposure to the low-level OP poisoning [30, 31]. Mills et al. [32] have observed little evidence of increased risk of cardiovascular complications associated with the occupational use of pesticides. Second, information on the lifestyle and behavior of patients is lacking in the NHIRD; thus, it was impossible to adjust for health- and behavior-related factors such as smoking, alcohol consumption, dietary habits, exercise, physical activity level, socioeconomic status, and body mass index, which are all potential confounding factors. Third, there are various types of organophosphates with different potencies and doses involved in toxic exposure among the patients, which may cause variable results. Fourth, habits of smoking, hypertension, high blood cholesterol, DM, obese and positive family history are noted to be major risk factors of CVD. COPD is a newly suggested to be a modifiable risk factor of CVD. In OPs cases, aspiration pneumonia was frequently noted to be a complication. So, in study of the possible correlation between OPs intoxication and CVD development, we used these 4 diseases as comorbidity. As we know, several factors, individual or combined, might contribute to these 4 diseases and not suitable to put together in the manuscript.
Conclusions
In summary, this population-based retrospective cohort study has demonstrated a significant relationship between acute OPs poisoning and the risk of developing CVDs, which could persist for over 6 years, even for those with comorbidity of diabetes mellitus, hypertension and hyperlipidemia. Acetylcholine accumulation in nerve endings might be associated with the persistent cardiac injury for patients with OPs poisoning. The OPs are widely used in farms, industry and by consumers, and may cause neurological sequalae of acute poisoning, leading to cardiovascular impairment. Additional supportive measurements are essential in reducing the risk of heart disease. Furthermore, future studies are needed to investigate the chronic risk sequelae and possible mechanisms not only for heart diseases but also other organs associated with OPs poisoning.
Data Availability
Ethical restrictions placed on the data by the National Health Insurance Research Group prevent public sharing of participant-level data. Data can be obtained by any interested researcher from the National Health Insurance Research database at: http://nhird.nhri.org.tw/en/index.htm.
Funding Statement
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (DOH102-TD-B-111-004) and (MOHW104-TDU-B-212-113002). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Eddleston M, Buckley NA, Eyer P, Dawson AH. Management of acute organophosphorus pesticide poisoning. Lancet. 2008; 371: 597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bruyndonckx RB, Meulemans AI, Sabbe MB, Kumar AA, Delooz HH. Fatal intentional poisoning cases admitted to the University Hospitals of Leuven, Belgium, from 1993 to 1996. Eur J Emerg Med. 2002; 9: 238–243. [DOI] [PubMed] [Google Scholar]
- 3. Bird S. Organophosphate and carbamate poisoning. UpToDate. 2014; 14: 339. [Google Scholar]
- 4. Fukushima H, Watanabe T, Asai H, Yada N, Ito S, Seki T, et al. Out-of-hospital cardiac arrest caused by acute intoxication. Chudoku Kenkyu. 2010; 23: 41–46. [PubMed] [Google Scholar]
- 5. Aghabiklooei A, Mostafazadeh B, Farzaneh E, Morteza A. Does organophosphate poisoning cause cardiac injury?. Pak J Pharm Sci. 2013; 26: 1247–1250. [PubMed] [Google Scholar]
- 6. Ludomirsky A, Klein HO, Sarelli P, Becker B, Hoffman S, Taitelman U, et al. Q-T prolongation and polymorphous (“torsade de pointes”) ventricular arrhythmias associated with organophosphorus insecticide poisoning. Am J Cardiol. 1982; 49: 1654–1658. [DOI] [PubMed] [Google Scholar]
- 7. Karki P, Ansari JA, Bhandari S, Koirala S. Cardiac and electrocardiographical manifestations of acute organophosphate poisoning. Singap Med J. 2004; 45: 385–386. [PubMed] [Google Scholar]
- 8. Lotti M, Moretto A. Organophosphate-induced delayed polyneuropathy. Toxicol Rev. 2005; 24: 37–49. [DOI] [PubMed] [Google Scholar]
- 9. London L, Myers JE, Nell V, Taylor T, Thompson ML. An investigation into neurologic and neurobehavioral effects of long-term agrichemical use among deciduous fruit farm workers in the Western Cape, South Africa. Environ Res. 1997; 73: 132–145. [DOI] [PubMed] [Google Scholar]
- 10. Yanagisawa N, Morita H, Nakajima T. Sarin experiences in Japan: acute toxicity and long-term effects. J Neurol Sci. 2006; 249: 76–85. [DOI] [PubMed] [Google Scholar]
- 11. Allon N, Chapman S, Egoz I, Rabinovitz I, Kapon J, Weissman BA, et al. Deterioration in brain and heart functions following a single sub-lethal (0.8 LCt50) inhalation exposure of rats to sarin vapor: a putative mechanism of the long term toxicity. Toxicol Appl Pharmacol. 2011; 253: 31–37. 10.1016/j.taap.2011.03.007 [DOI] [PubMed] [Google Scholar]
- 12. Blanc-Lapierre A, Bouvier G, Gruber A, Leffondré K, Lebailly P, Fabrigoule C, et al. Congnitive disorders and occupational exposure to organophosphates: results from the PHYTONER study. Am J Epidemiol. 2013; 177: 1086–1096. 10.1093/aje/kws346 [DOI] [PubMed] [Google Scholar]
- 13. Allon N, Rabinovitz I, Manistersky E, Weissman BA, Grauer E. Acute and long-lasting cardiac changes following a single whole-body exposure to sarin vapor in rats. Toxicol Sci. 2005; 87: 385–390. [DOI] [PubMed] [Google Scholar]
- 14. McDonough JH Jr, Dochterman LW, Smith CD, Shih TM. Protection against nerve agent-induced neuropathology, but not cardiac pathology, is associated with the anticonvulsant action of drug treatment. Neurotoxicology. 1995; 16: 123–132. [PubMed] [Google Scholar]
- 15. Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, et al. ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006; 114: e385–e484. [DOI] [PubMed] [Google Scholar]
- 16. Chugh SS, Reinier K, Teodorescu C, Evanado A, Kehr E, Al Samara M, et al. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis. 2008; 51: 213–228. 10.1016/j.pcad.2008.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barrios V, Gómez-Huelgas R, Rodríguez R, Pablos-Velasco P. Adiponectin: an emerging cardiovascular risk factor. The REFERENCE study. Rev Esp Cardiol. 2008; 61: 1159–1167. [PubMed] [Google Scholar]
- 18. Fine J, Gray RA. Proportional Hazards Model for the Subdistribution of a Competing Risk. J. Amer. Statist. Assoc. 1999; 94: 446. [Google Scholar]
- 19. Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999; 18: 695–706. [DOI] [PubMed] [Google Scholar]
- 20.World Heart Federation. Cardiovascular disease—risk factors. Available: http://www.world-heart-federation.org/fileadmin/user_upload/documents/Fact_sheets/2012/PressBackgrounderApril2012RiskFactors.pdf.
- 21. Jayasinghe SS, Pathirana KD. Autonomic Function following Acute Organophosphorus Poisoning: A Cohort Study. PLOS ONE. 2012; 7: e37987 10.1371/journal.pone.0037987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amara IB, Soudani N, Hakim A, Troudi A, Zeghal KM, Boudawara T, et al. Protective effects of vitamin E and selenium against dimethoate-induced cardiotoxicity in vivo: biochemical and histological studies. Environ Toxicol. 2013; 28: 630–643. 10.1002/tox.20759 [DOI] [PubMed] [Google Scholar]
- 23. Velmurugan G, Venkatesh Babu DD, Ramasamy S. Prolonged monocrotophos intake induces cardiac oxidative stress and myocardial damage in rats. Toxicology. 2013; 307: 103–108. 10.1016/j.tox.2012.11.022 [DOI] [PubMed] [Google Scholar]
- 24. Lyzhnikov EA, Savina AS, Shepeler VM. On pathogenesis of cardiac rhythm and conductivity disorder in cases of acute insecticide poisonings. Kardiologia (USSR). 1975; 15 : 126–129. [PubMed] [Google Scholar]
- 25. Furlong CE. Genetic variability in the cytochrome P450-paraoxonase 1 (PON1) pathway for detoxication of organophosphorus compounds. J Biochem Mol Toxicol. 2007; 21: 197–205. [DOI] [PubMed] [Google Scholar]
- 26. Xiong XM, Dai W, Li P, Wu SJ, Hu M, Liu LY. Subchronic toxicity organophosphate insecticide- induced damages on endothelial function of vessels in rabbit by inhibiting antioxidases. Prog Biochem Biophy. 2010;S 37: 1232–1239. [Google Scholar]
- 27. Voetsch B, Benke KS, Damasceno BP, Siqueira LH, Loscalzo J. Paraoxonase 192 Gln-> Arg polymorphism: an independent risk factor for nonfatal arterial ischemic stroke among young adults. Stroke. 2002; 33: 1459–1464. [DOI] [PubMed] [Google Scholar]
- 28. Xiao ZJ, Chen J, Sun Y, Zheng ZJ. Lack of association between the Paraoxonase 1 Q/R192 single nucleotide polymorphism and stroke in a Chinese cohort. Acta Neurol Belg. 2009; 109: 205–209. [PubMed] [Google Scholar]
- 29. Lotti M, Moretto A. Do carbamates cause polyneuropathy? Muscle Nerve. 2006; 34: 499–502. [DOI] [PubMed] [Google Scholar]
- 30. Mostafalou S, Abdollahi M. Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicol Appl Pharmacol. 2013; 268: 157–177. 10.1016/j.taap.2013.01.025 [DOI] [PubMed] [Google Scholar]
- 31. Dayton SB, Sandler DP, Blair A, Alavanja M, Beane Freeman LE, Hoppin JA. Pesticide use and myocardial infraction incidence among farm women in the agricultural health study. J Occup Environ Med. 2010; 52: 693–697. 10.1097/JOM.0b013e3181e66d25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mills KT, Blair A, Freeman LE, Sandler DP, Hoppin JA. Pesticides and myocardial infarction incidence and mortality among male pesticide applicators in the Agricultural Health Study. Am J Epidemiol. 2009; 170: 892–900. 10.1093/aje/kwp214 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Ethical restrictions placed on the data by the National Health Insurance Research Group prevent public sharing of participant-level data. Data can be obtained by any interested researcher from the National Health Insurance Research database at: http://nhird.nhri.org.tw/en/index.htm.