Abstract
β-D-galactofuranose (Galf) is a component of polysaccharides and glycoconjugates and its transferase has been well analyzed. However, no β-D-galactofuranosidase (Galf-ase) gene has been identified in any organism. To search for a Galf-ase gene we screened soil samples and discovered a strain, identified as a Streptomyces species by the 16S ribosomal RNA gene analysis, that exhibits Galf-ase activity for 4-nitrophenyl β-D-galactofuranoside (pNP-β-D-Galf) in culture supernatants. By draft genome sequencing of the strain, named JHA19, we found four candidate genes encoding Galf-ases. Using recombinant proteins expressed in Escherichia coli, we found that three out of four candidates displayed the activity of not only Galf-ase but also α-L-arabinofuranosidase (Araf-ase), whereas the other one showed only the Galf-ase activity. This novel Galf-specific hydrolase is encoded by ORF1110 and has an optimum pH of 5.5 and a Km of 4.4 mM for the substrate pNP-β-D-Galf. In addition, this enzyme was able to release galactose residue from galactomannan prepared from the filamentous fungus Aspergillus fumigatus, suggesting that natural polysaccharides could be also substrates. By the BLAST search using the amino acid sequence of ORF1110 Galf-ase, we found that there are homolog genes in both prokaryotes and eukaryotes, indicating that Galf-specific Galf-ases widely exist in microorganisms.
Introduction
β-D-galactofuranose (Galf) is a constituent of polysaccharides and glycoconjugates that are present on the surface of the cell wall in many pathogenic bacteria and eukaryotes [1,2]. Galf is present in bacteria, filamentous fungi, trypanosomatids and nematodes, but not in yeasts nor in mammals [3,4]. Because Galf is known to be immunogenic to mammals [5–8], it is now a target molecule for anti-fungal reagents to suppress pathogenicity [1,2,4,9,10].
In certain filamentous fungi, Galf is found in galactomannan (GM), galactomannoproteins modified with N-glycans and O-glycans and glycolipids [1,2,11–16]. Filamentous fungi enzymes involved in Galf-containing oligosaccharide synthesis have been well studied, especially in Aspergillus. For instance, in the model filamentous fungus Aspergillus nidulans, at the initial step of Galf-sugar chain synthesis, UDP-glucose, which is a donor substrate for α- and β-glucan synthesis, is converted to UDP-galactopyranose (UDP-Galp) by the UDP-glucose-4-epimerase UgeA [17]. Then, UDP-Galp is converted to UDP-Galf by the UDP-Galf mutase UgmA (GlfA in Aspergillus fumigatus) [18–20]. These reactions occur in the cytoplasm. UDP-Galf is subsequently transported into the Golgi lumen by the UDP-Galf transporter UgtA (GlfB in A. fumigatus) which localized in the Golgi membrane [21,22]. To identify a Galf transferase gene in A. nidulans, we previously conducted reverse-genetics and biochemical approaches. We identified a gene named gfsA that encodes the Galf transferase localized to Golgi which function is to attach UDP-Galf onto the O-glycan chain [23]. ΔugmA, ΔugtA and ΔgfsA strains exhibit retarded hyphal morphology, suggesting that the Galf biosynthetic pathway is crucial for cell growth [19,22,23].
While the molecular mechanisms of the biosynthesis of Galf-containing sugar chains have been analyzed, enzymes involved in degradation and metabolism of Galf-oligosaccharides are not well known [1]. One such enzymes is β-D-galactofuranosidase (Galf-ase), which can release Galf from polysaccharides and glycoconjugates. There are reports about the purification of exo- and endo-Galf-ases from the culture supernatant of several microorganisms [24–29]. However, no Galf-ase gene has been identified. α-L-arabinofuranosidase (Araf-ase), which hydrolyzes α-L-arabinofuranoside (Araf), is structurally similar to Galf, and Araf-ase-encoding genes have been identified in Aspergillus species [10,30–37]. In Aspergillus niger, Araf-ases, which belong to glycosyl hydrolase family 51 (GH51) and 54 (GH54), have both Araf-ase and Galf-ase activities [30]. However, no gene encoding a Galf-ase-specific enzyme has been reported yet [38].
In this study, we screened soil samples for microorganisms that exhibit Galf-ase activity. The screen allowed us to identify a novel gene that encodes a Galf-ase-specific enzyme, which does not exhibit any Araf-ase activity.
Materials and Methods
Microorganism, cultivation and microscopy
Bacteria were isolated from soil in Kagawa University, Japan. Since the area of the university is public, no specific permission was required to collect samples that did not include any endangered nor protected species. The isolated strain JHA19 (material number, QM2015–0042) has been deposited in the Material Management Center (MMC; http://mmc-u.jp/en/). YMG medium (0.4% yeast extract, 1% malt extract, 0.4% glucose and 2% agar, pH 7.3) was used for bacterial growth on plates and in liquid cultures, which were performed at 30°C with shaking at 200 rpm. Cells of the isolated strain JHA19 were cultured in YMG liquid medium for 3 days and observed under an Eclipse 80i microscope (Nikon) with a Plan Apo 100x/1.40 NA oil objective lens (Nikon). Images were acquired with a CoolSNAP EZ CCD camera (Photometrics) and the software MetaVue (Molecular Devices).
Preparation of 4-nitrophenyl β-D-galactofuranoside
4-nitrophenyl β-D-galactofuranoside (pNP-β-D-Galf) was synthesized as described previously [39,40] with some modifications as follows: Galactose (1.80 g, 10 mmol) was stirred at 70°C in pyridine (30 mL, 3.7 mmol) for 1 h, and then acetylated with acetic anhydride (5.82 mL, 61.5 mmol) for 12 h. The mixture was purified conventionally, to give per-O-acetyl-α,β-D-Galf (syrup, 3.78 g, 97% yield, including per-acetyl Galp as isomer). pNP (3.92 g, 28.2 mmol) was added to a solution of per-O-acetyl-α,β-D-Galf (3.78 g, 9.7 mmol, including per-acetyl Galp) in dry CH3CN (50 ml) cooled to 0°C. After 10 min of stirring, BF3-Et2O (3.8 mL, 30 mmol) was added. After 24 h, the reaction mixture was diluted with ethyl acetate, extracted with saturated NaHCO3 aq. until neutralization was completed, washed brine, and dried with MgSO4. After filtration, the solvent was evaporated under vacuum, and then syrupy liquid pNP-2,3,5,6-tetra-O-acetyl-β-D-Galf was obtained. For analytical purposes, a part of the sample was purified by column chromatography, however most of sample was deacetylated without purification. Syrupy material pNP-2,3,5,6-tetra-O-acetyl-β-D-Galf was suspended in 1.0 mol/L CH3ONa in CH3OH (30 ml), and stirred at room temperature during 12 h. The reaction mixture was evaporated under vacuum, and purified by silica-gel column chromatography (CHCl3/CH3OH, 4:1) to give colorless solid (pNP-β-D-Galf, 0.24 g, 8% yield, over two steps from per-O-acetyl-α,β-D-Galf to pNP-β-D-Galf).
Enzyme assay
Galf-ase and Araf-ase activity was determined using pNP-β-D-Galf or pNP-α-L-Araf as a substrate, respectively. The enzyme solution was prepared in 45 μL, which was mixed with 2.5 μL of 10 mM substrate and 2.5 μL of 1 M acetate buffer, pH 4.5. After incubation for the appropriate time at 37°C, 50 μL of 1 M sodium carbonate was added to terminate the reaction, and the liberated pNP was determined from absorbance at 405 nm. One unit (U) of enzyme activity was defined as the amount of enzyme required to liberate 1 mmol of pNP per min [24,28]. The activity of exoglycosidases was assessed using appropriate pNP-glycosides (α-D-Xyl and β-D-Xyl from Seikagaku; the others from Sigma).
Preparation of genomic DNA
Genomic DNA of strain JHA19 was extracted as described previously with certain modifications [41]. After culture in 100 mL YMG medium at 30°C for 1 week, the culture of the strain JHA19 was centrifuged at 5000 rpm for 15 min and the cell pellet was resuspended in 5 mL TE10 (10 mM Tris-HCl, 10 mM EDTA, pH 8.0). After addition of 10 mg lysozyme (Wako) and 10 mg achromopeptidase (Wako), the cell suspension was incubated at 37°C for 20 min. The resultant sample was added with 100 μL TE10, 2.5 ml EDTA (0.5 M, pH 8.0), 1.25 mL 10% (w/v) SDS and 125 μL proteinase K (20 mg/mL) (Wako) and incubated overnight at 37°C. After another incubation at 65°C for 5 min, 20 mL TE10 was added. Ten mL of the resultant sample was taken and mixed with 20 mL TE10, 2 mL 3 M sodium acetate and 20 mL phenol/chloroform (1:1, v/v) by gently rotating for 30 min. After centrifugation at 4500 rpm for 20 min, the aqueous phase was divided into two tubes. Each tube was added with 2.5 volume 100% ethanol and centrifuged at 4500 rpm for 10 min. The pellet was dried and suspended in 10 mL TE. Those two genomic DNA suspension tubes were combined into one tube, which was added with 10 μL RNase (10 mg/mL) and incubated at 37°C for 30 min. The resultant sample was added with 200 μL 10% (w/v) SDS and 50 μL proteinase K (10 mg/mL) and incubated at 55°C for 1 h. After addition of 2 mL 3 M sodium acetate, the sample was mixed with 20 mL phenol/chloroform by gently rotating for 30 min. After centrifugation at 4500 rpm for 20 min, the aqueous phase was divided into a few tubes. Each tube was added with 3 volume 100% ethanol and centrifuged at 4500 rpm for 20 min, and the pellet was dried and suspended in 1 mL TE, which was used as the genomic DNA sample.
16S ribosomal RNA gene analysis
16S rRNA gene sequence was amplified by PCR from the genomic DNA sample of strain JHA19 using universal primers listed in S1 Table. The DNA sequence of the PCR product was applied to a BLAST search, and the strain species was identified.
Whole-genome sequencing analysis
Whole-genome shotgun sequencing of the strain JHA19 was conducted using an FLX454 sequencer (Illumina). As a result, 252 Mbp was generated from 6x105 sequencing reads, which gave 32.7 fold-coverage. For sequence assembling, the program Newbler version 2.7 was used, and 70 contigs were generated. The genome annotation was performed with both Glimmer version 3.02b and BLAST 2.2.26. More detailed information will be presented elsewhere.
Preparation of recombinant Galf-ase proteins
To construct recombinant expression plasmids, four candidate Galf-ase genes were amplified by PCR using the DNA polymerase PrimeStarGXL (Takara), primers shown in S1 Table and genomic DNA of JHA19 as a template. An EcoRI digested pET50b vector and amplified DNA were ligated with In-Fusion HD Cloning Kit (Takara).
Escherichia coli BL21(DE3)CodonPlus strain transformed with each Galf-ase expression plasmid was precultured in LB medium (Miller, Merck) at 37°C overnight. OD600 of cells was adjusted to 0.05 and cultured until OD600 = 0.8, added with 100 mM IPTG and cultured overnight at 15°C. Cells were centrifuged at 7000 rpm for 7 min, resuspended in 5 mL 20 mM MOPS (pH 8.0) and lysed by ultrasonication on ice. The cell lysates were centrifuged at 15000 rpm for 10 min at 4°C and the supernatants were applied to a HisTrapTM FF 1 mL column (GE Healthcare). Recombinant protein purification was performed according to the manufacturer’s instructions.
Preparation of galactomannan from Aspergillus fumigatus
Galactomannan (GM) was prepared from A. fumigatus essentially as described previously with some modifications [27]. Conidia were harvested from a plate of minimal medium (1% glucose, 0.6% NaNO3, 0.052% KCl, 0.052% MgSO4・7H2O, 0.152% KH2PO4, biotin (trace) and Hunter’s trace elements, pH 6.5), where the A. fumigatus A1163 (CEA10) strain was grown at 37°C for 3 days. The collected conidia were inoculated in a 500 mL Sakaguchi flask with 100 mL YNB medium supplemented with galactose (YNBG medium; 0.67% yeast nitrogen base, 0.5% (NH4)2SO4, 9% galactose) and precultured at 37°C for 24 h. The preculture was transfered in a 5 L round-bottom flask with 1 L YNBG medium and cultured at 37°C for 14 days. Thereafter, cells from 4.4 L culture were added with formaldehyde at a final concentration of 1% and left for 24 h. After centrifugation, the supernatant was dialyzed with water for 3 days, then evaporated and lyophilized. The resultant sample was dissolved in 5 mL 20 mM phosphate buffer (pH 7.0), applied to TOYOPEARL DEAE-650 (TOSOH) and sequentially eluted with water, 0.5 M and 1 M NaCl solutions in 20 mM phosphate buffer (pH 7.0). The water eluate was dialyzed with 10 mM and 5 mM phosphate buffer (pH 7.0) and water overnight, for 6 h and 1 h, respectively. The resultant solution was evaporated and lyophilized, then used as the GM sample.
TLC analysis
N-terminal tags (2xHis6 and Nus) were cleaved off the recombinant ORF1110 protein using HRV3C protease (Novagen) and removed by chromatography on a HisTrapTM FF 1 mL column. The flow-through sample was concentrated to 22.5 μL (7.6 mU/μL) and incubated with 25 μL GM (1 mg/μL) and 2.5 μL acetate buffer (1 M, pH 4.5) at 37°C for 24 h. The sample was then separated by TLC using a TLC Silica gel 60 plate (Millipore) and 1-butanol/ethanol/water (2:1:1, v/v/v) as solvent. For detection the TLC plate was sprayed with 0.2% orcinol and 10% methanol/sulfuric acid and baked at 120°C for 10 min.
ELISA
To analyze Galf-ase activity of the ORF1110 protein by ELISA, Platelia Aspergillus Ag EIA Kit (Bio-Rad) was used according to the manufacturer’s instructions. Briefly, 50 μL of positive control containing GM, 0.5 μL of 7.5 mU ORF1110 Galf-ase and 1 μL of acetate buffer (1 M, pH 4.5) were mixed in a total volume of 100 μL and incubated at 37°C for 0, 1, 3 or 6 h. The resultant samples were diluted four times and their absorbance at 450 nm was measured.
Bioinformatic analysis
BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) searches were conducted using sequences of 16S rRNA gene or ORF1110 Galf-ase of strain JHA19. Retrieved sequences were subjected to the program CLUSTAL W (http://clustalw.ddbj.nig.ac.jp/index.php?lang=ja) [42] and their clustering was performed using the neighbor-joining method. Domain search for amino acid sequences was performed using the program Pfam (http://pfam.xfam.org/). Prediction of GH family was carried out using the program CAT (http://mothra.ornl.gov/cgi-bin/cat/cat.cgi?tab=Home).
Accession numbers
ORF0232, ORF1110, ORF2125 and ORF2812 have been deposited at DDBJ/EMBL/GenBank under the accession nos. LC073693, LC073694, LC073695 and LC073696, respectively.
Results
Identification of a soil microorganism that exhibits Galf-ase activity
To search for a Galf-specific Galf-ase, we isolated 282 bacterial strains, mainly actinomycetes, from soil samples. Culture supernatants of three isolated strains, named JHA19, JHA26 and EMA216, exhibited Galf-ase activity using pNP-β-D-Galf as a substrate. In addition to the Galf-ase activity, we detected the activities of β-galactosidase (pyranose form), α-mannosidase, β-N-acetylgalactosaminidase and β-N-acetylglucosaminidase from the culture supernatant of JHA19 using the corresponding pNP-glycosides as substrates. Since the activity of Galf-ase was higher than that of Araf-ase, which was hardly detected in the culture supernatant of JHA19, it suggested that this strain might harbor enzyme(s) specific for Galf-ase. Therefore, we chose strain JHA19 for further enzymatic characterization.
Strain JHA19 displayed filamentous growth on a plate (Fig 1A) and appeared like a Gram-positive and bacillary bacterium (Fig 1B), suggesting that it belongs to the Streptomyces species. To further identify this strain, we performed a BLAST search based on the 16S rRNA gene sequence, and found that it shows 99% identity to Streptomyces coelicolor, S. albogriseolus, S. tendae, S. ambofaciens and S. lividans (Fig 1C). This result clearly demonstrated that strain JHA19 belongs to the Streptomyces species.
Fig 1. Identification of the strain JHA19.
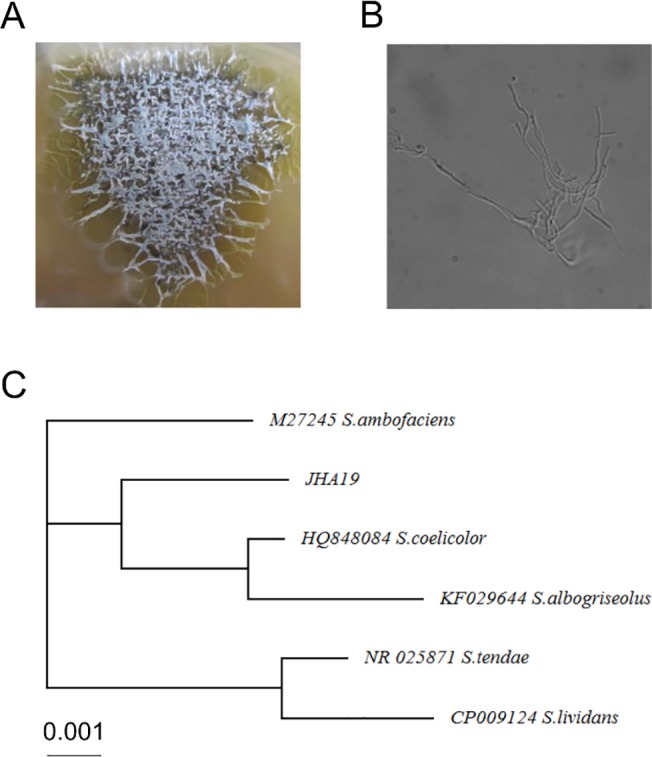
Strain JHA19 (A) grown on a plate and (B) observed under microscope. (C) Phylogenetic tree of 16S rRNA gene sequences from Streptomyces species constructed using the neighbor-joining method. The 16S rRNA gene sequence of strain JHA19 was amplified by PCR using universal primers and genomic DNA as a template, determined by DNA sequencing and then subjected to the program CLUSTAL W for the phylogenetic analysis. The DDBJ accession numbers of the sequences used for phylogenetic comparisons are depicted.
Exploration of candidate Galf-ase genes in strain JHA19
To search for genes encoding Galf-ases, we conducted a whole-genome shotgun sequencing of strain JHA19. We determined most of the genome sequence, the details of which will be reported elsewhere. We searched the sequence for ORFs that showed high sequence similarity to known furanosidase genes and found four Galf-ase candidates named ORF0232, ORF1110, ORF2125 and ORF2812 (Fig 2; Table 1). Based on a domain search using Pfam, we predicted that ORF0232, ORF2125 and ORF2812 may have Araf-ase activity because they show the highest similarity to reported Araf-ases. Indeed, the ORF0232 protein includes glycosyl hydrolases family 62 domain whose known activity is Araf-ase, the ORF2125 protein contains an Araf-ase C-terminus domain and the ORF2812 protein also has an Araf-ase B domain (AbfB), which is typically seen in GH54 Araf-ases [31,37,43]. Furthermore, a BLAST search revealed that ORF1110 has the highest similarity to a gene encoding an uncharacterized GH2 family protein which contains an AbfB domain based on the program CAT. Therefore, we further analyzed these four candidate genes, including ORF1110.
Fig 2. Domains of ORF0232, 1110, 2125 and 2812 proteins.
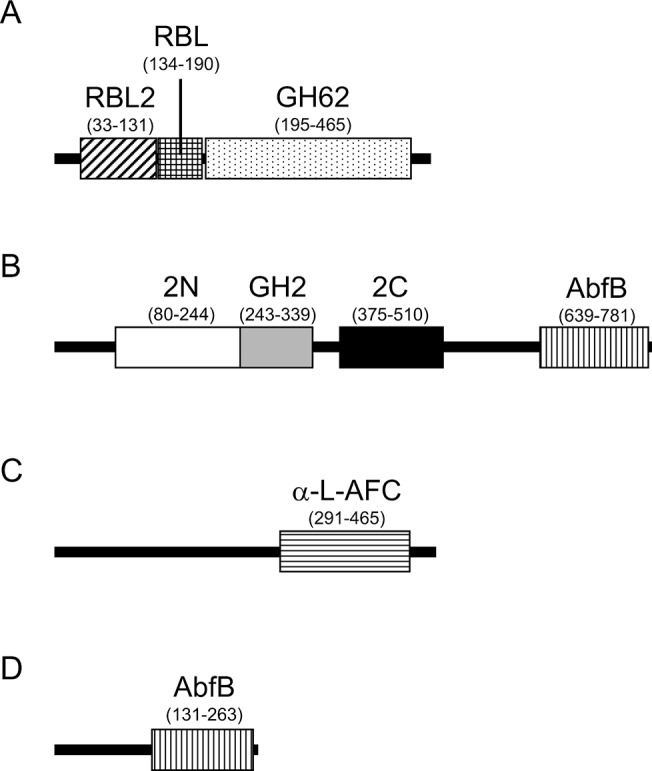
The program Pfam was used to predict domains on each ORF protein. The corresponding amino acid numbers of each domain are indicated in parenthesis. (A) ORF0232: RBL2, Ricin-type beta-trefoil lectin domain-like; RBL, Ricin-type beta-trefoil lectin domain; GH62, Glycosyl hydrolases family 62 domain. (B) ORF1110: 2N, Glycosyl hydrolases family 2, sugar binding domain (Galactose-binding domain-like superfamily); GH2, Glycosyl hydrolases family 2 domain; 2C, Glycosyl hydrolases family 2, TIM barrel domain (Tim barrel glycosyl hydrolase superfamily); AbfB, α-L-arabinofuranosidase B domain. (C) ORF2125: α-L-AFC, α-L-arabinofuranosidase C-terminus domain. (D) ORF2812: AbfB, α-L-arabinofuranosidase B domain.
Table 1. Candidate genes for β-D-galactofuranosidase in strain JHA19.
| ORF | Homolog a | Identity (%) | GH b | Size (aa) |
|---|---|---|---|---|
| 0232 | α-L-arabinofuranosidase [WP_037890671.1 (Streptomyces viridochromogenes)] | 86 | 62 | 495 |
| 1110 | hydrolase [WP_030950552.1 (Streptomyces sp. NRRL F-5140)] | 91 | 2 | 786 |
| 2125 | α-N-arabinofuranosidase [XP_010042338.1 (Streptomyces chartreusis)] | 86 | 51 | 502 |
| 2812 | α-L-arabinofuranosidase [WP_030948980.1 (Streptomyces sp. NRRL F-5140)] | 59 | - | 268 |
a Based on BLAST searches using the amino acid sequences of the four ORFs, the corresponding homologs with the highest degree of identity are shown.
b Based on CAT program predictions.
Enzymatic activities of recombinant proteins
We introduced ORF0232, ORF1110, ORF2125 and ORF2812 sequences into an E. coli expression vector lacking lacZ to circumvent a potential risk of contamination of subsequent enzymatic assays by β-galactosidase. The recombinant proteins were expressed and purified by a Ni affinity column. We first confirmed that samples from E. coli cells harboring an empty vector had no enzymatic activity for pNP-α-L-Araf nor pNP-β-D-Galf (data not shown). Recombinant proteins expressed from ORF0232, ORF2125 and ORF2812 showed Araf-ase activity for pNP-α-L-Araf as a substrate, like their homologs (Fig 3A, 3C and 3D). In addition, we measured the ratio of the activity of Araf-ase to Galf-ase, and found that ORF0232, ORF2125 and ORF2812 proteins exhibited the activity for both Araf-ase and Galf-ase. AbfA and AbfB in A. niger also showed both Araf-ase and Galf-ase activities, but the activity of Galf-ase was 10-fold less than that of Araf-ase, unlike proteins of ORF0232, ORF2125 and ORF2812 [30]. Although homologs of ORF0232, ORF2125 and ORF2812 are reported as Araf-ases, these recombinant proteins also displayed the Galf-ase activity, suggesting that enzymes reported as Araf-ases might generally exhibit the Galf-ase activity. In contrast, the recombinant protein of ORF1110 exhibited Galf-ase activity only, but not Araf-ase activity, suggesting that this GH2 family protein is a Galf-specific Galf-ase (Fig 3B). Thus, we focused on examining chemoenzymatic characteristics of the ORF1110 protein.
Fig 3. Galf-ase and Araf-ase activities of recombinant proteins.
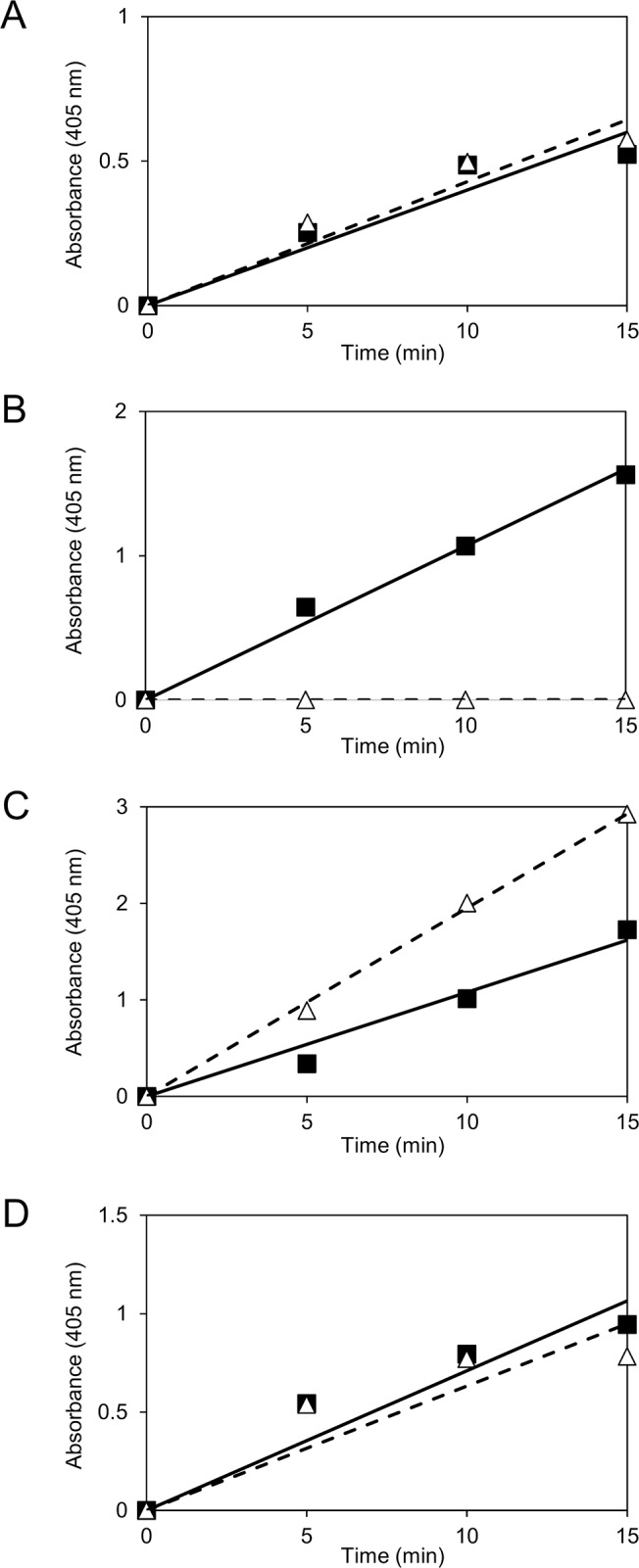
Galf-ase and Araf-ase activities were determined using pNP-β-D-Galf (closed square, solid line) and pNP-α-L-Araf (open triangle, dotted line) as substrates, respectively. The recombinant proteins of each ORF were used; (A), ORF0232; (B), ORF1110; (C), ORF2125; (D), ORF2812. Note that only the ORF1110 protein showed Galf-ase specific activity. The activity ratios of Araf-ase to Galf-ase are as follows: ORF0232, 1.1:1; ORF2125, 1.8:1; ORF2812, 0.89:1.
Chemoenzymatic properties of ORF1110 encoded Galf-ase
To determine the substrate specificity of the recombinant ORF1110 protein, we measured hydrolytic activity using a variety of pNP-glycosides in their pyranose form (β-D-Gal, α-D-Gal, β-D-Glc, β-D-Man, α-D-Man, β-D-Xyl, α-D-Xyl, β-D-GalNAc and β-D-GlcNAc). No activity was observed with any of these substrates, except with pNP-β-D-Galf, confirming that this enzyme specifically hydrolyzes β-D-Galf.
The optimum pH for ORF1110 Galf-ase activity was found to be 5.5 (Fig 4A). The thermal stability of the enzyme was examined by heating it at various temperatures for 10 min. The enzyme was found to be stable at temperatures up to 40°C.
Fig 4. Optimum pH and inhibitory effect of L-arabino-1,4-lactone on Galf-ase activity of ORF1110 protein.
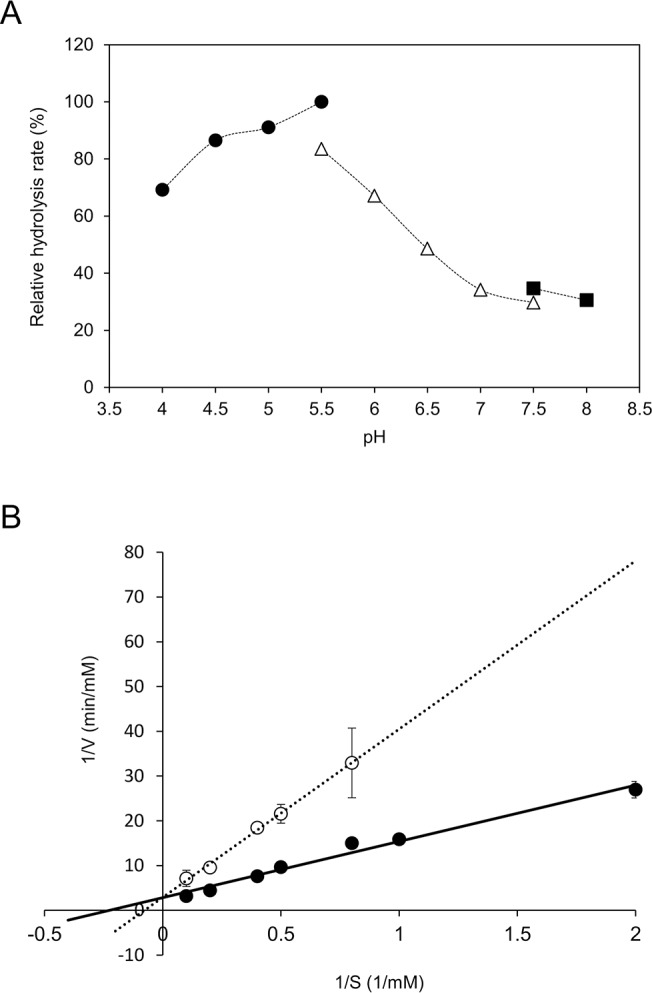
(A) The optimum pH of ORF1110 protein for Galf-ase activity was determined using buffers of sodium acetate-acetic acid (4–5.5, closed circle), potassium phosphate (5.5–7.5, open triangle) and MOPS (7.5–8, closed square). (B) Competitive inhibition of Galf-ase activity by L-arabino-1,4-lactone was analyzed with (open circle) or without (closed circle) the inhibitor. Each data point indicates the mean with standard deviation (error bars) from three experiments.
The activity of the Araf-ase TtAFase belonging to the GH2 family in Thermotoga thermarum was reported to be highly inhibited by addition of either Cu2+ or Zn2+ [44]. Hence, we investigated the effects of metal ions (at a concentration of 5 mM) on the Galf-ase activity of ORF1110. We found that the ORF1110 Galf-ase activity was mostly inactivated by addition of Cu2+, Zn2+ and EDTA to 6.5%, 49% and 55% of its original activity, respectively.
Next, we examined the effect of the substrate pNP-β-D-Galf concentration on the initial velocity of the enzyme reaction. The apparent Km and Vmax were 4.4 mM and 0.35 mM/min, respectively. Even though this protein does not have Araf-ase activity, it exhibited competitive inhibition by L-arabino-1,4-lactone, an Araf-ase inhibitor (Ki, 51 mM) (Fig 4B) [45]. This suggests that there may be different substrate recognition mechanism between Galf-ase and Araf-ase at the active site.
Crucial amino acid residues of ORF1110 Galf-ase
The recombinant ORF1110 protein lacking an AbfB domain exhibited almost the same Galf-ase activity as the full-length protein, suggesting that this domain is likely not required for the Galf-ase activity (data not shown).
Since the protein encoded by ORF1110 shows low similarity to well-known Araf-ases, it is not possible to predict which amino acid residues are crucial for its enzymatic activity by sequence comparison. Thus, we first used a sequence alignment of the GH2 family proteins that show higher sequence similarities to the ORF1110 Galf-ase (Fig 5). The sequence alignment revealed a number of conserved aspartic acid and glutamic acid residues. Using site directed mutagenesis we individually changed each conserved residues to alanine in ORF1110 and measured the effect of the mutations on Galf-ase activity. Most mutations had an effect on Galf-ase activity with D423A and E464A having the most drastic effect, suggesting that the glycosyl hydrolase 2C domain is the catalytic center of this enzyme, and that several amino acid residues are involoved (Table 2).
Fig 5. Alignment of ORF1110 Galf-ase and its homologs.
Alignment of partial ORF1110 Galf-ase sequence and corresponding regions of its homologs is shown. The conserved amino acid residues indicated with asterisks were mutated into alanine. The DDBJ accession numbers are as follows: S. griseus, WP012382063; A. mediterranei, WP013223544; Bacillus sp., WP031315417.
Table 2. Relative Galf-ase activity of recombinant wild-type and mutant ORF1110 proteins.
| ORF1110 protein | Relative activity (%) a |
|---|---|
| WT | 100 |
| D183A | 80.7 |
| D201A | 15.5 |
| D330A | 6.5 |
| D336A | 100 |
| D372A | 95.1 |
| D386A | 118 |
| E405A | 9.7 |
| D414A | 66.6 |
| D423A | 1.8 |
| E464A | 2.6 |
| D482A | 75.4 |
| D500A | 9.8 |
| D508A | 33.5 |
| E530A | 15.2 |
| D590A | 62.7 |
| E592A | 54.7 |
| E594A | 11.3 |
| D602A | 107 |
a The relative Galf-ase activity was analyzed using purified recombinant proteins and pNP-β-D-Galf as a substrate.
The relative activity of the WT protein was set as 100.
ORF1110 Galf-ase can hydrolyze Aspergillus fumigatus GM
Lastly, we tested whether ORF1110 Galf-ase could catalyze not only the artificial substrate pNP-β-D-Galf but also a natural Galf-containing oligosaccharide. β-D-Galf exists in glycan parts at the cell surface of Aspergillus species. Thus, we extracted GM, including Galf chains, from A. fumigatus strain A1163 (CEA10) and analyzed Galf-ase activity by TLC (Fig 6A). The results indicated that Gal was released from the GM sample, suggesting that ORF1110 Galf-ase can hydrolyze a natural GM oligosaccharide from A. fumigatus (Fig 6B).
Fig 6. TLC analysis of Galf-ase activity for GM.
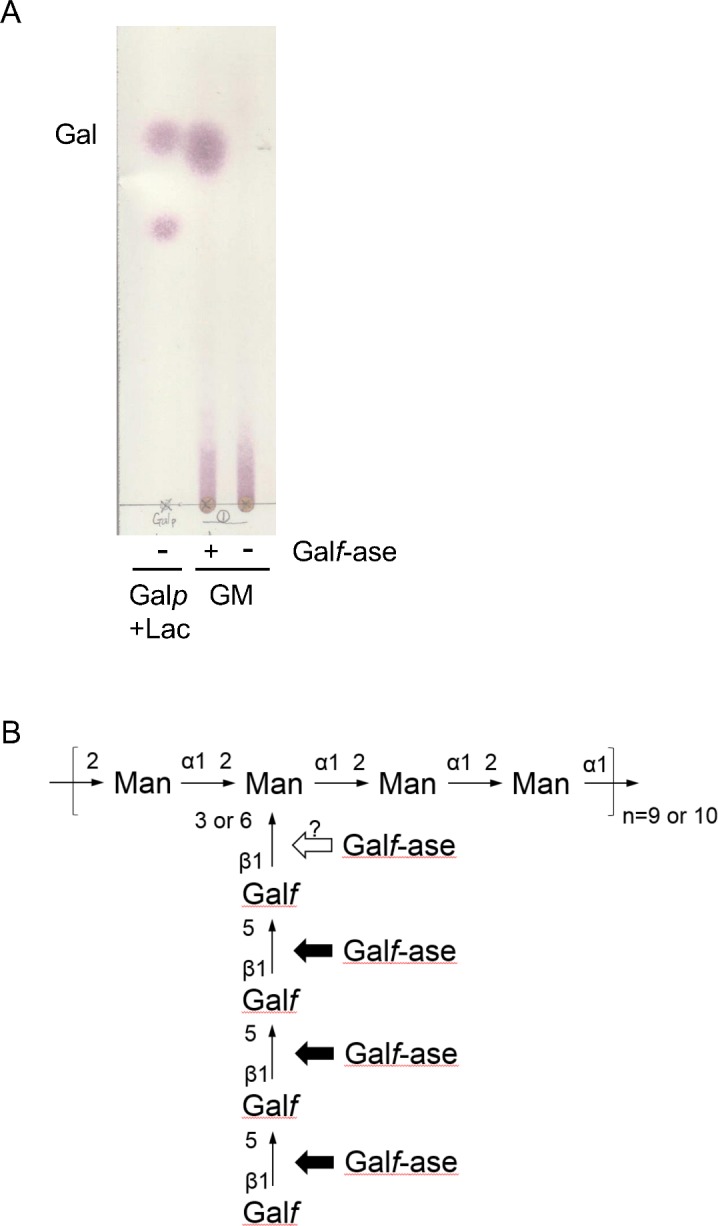
(A) The recombinant ORF1110 Galf-ase was mixed with GM prepared from A. fumigatus and incubated at 37°C for 24 h. The resultant sample was subjected to TLC analysis. As references, galactopyranose (Galp) and lactose (Lac) were spotted. As a negative control, a sample without recombinant ORF1110 Galf-ase was spotted. GM, galactomannan. (B) A schematic diagram of the predicted partial structure of A. fumigatus GM proposed previously [7]. The ORF1110 Galf-ase seems to catalyze terminal Galf residues of A. fumigatus GM.
Discussion
In this study, we have isolated a strain of Streptomyces which possesses Galf-specific Galf-ase encoded by ORF1110. To our best knowledge, this is the first report about Galf-specific Galf-ase that does not also exhibit Araf-ase activity. Since we found that the ORF1110 Galf-ase belongs to GH2, which generally has a β-D-galactosidase activity, we examined hydrolase activity of the ORF1110 enzyme towards pNP-β-D-galactopyranoside. However, no activity was detected, suggesting that the ORF1110 enzyme activity is specific to furanose substrates.
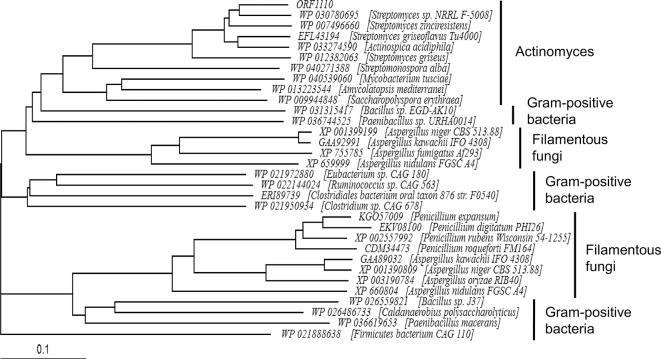
BLAST search suggested that ORF1110 protein-like Galf-ases exist in a wide range of organisms from bacteria to eukaryotes (Fig 7). We cloned an ORF1110 homologous gene in Streptomyces griseus and confirmed that the derived recombinant protein exhibited Galf-specific Galf-ase activity (unpublished data). In Aspergillus species, there are also genes corresponding to ORF1110. Since the Galf biosynthetic pathway is important for their hyphal growth, Galf-degradation and metabolism pathways regulated by Galf-specific Galf-ase would be also crucial for fungal physiology. However, little is known about molecular mechanisms of Galf-degradation and metabolism. Therefore, it would be interesting to investigate the physiological functions of genes encoding Galf-specific Galf-ases in Aspergillus species.
Fig 7. Phylogenetic tree of ORF1110 Galf-ase homologs.
The amino acid sequences of hydrolases belonging to the GH2 family were retrieved by a BLAST search using ORF1110 Galf-ase sequence and phylogenetically analyzed using the program CLUSTAL W. The DDBJ accession numbers of sequences used are shown. Note that ORF1110 Galf-ase homologs are widely distributed in Gram-positive bacteria and filamentous fungi.
It was reported that Araf-ases AbfA and AbfB in A. niger, belonging to the GH51 and GH54, respectively, exhibit activities of both Araf-ase and Galf-ase [30]. Although both pNP-β-D-Galf and pNP-α-L-Araf are recognized as substrates by AbfB, affinity for pNP-β-D-Galf is lower resulting in less Galf-ase activity compared to Araf-ase. Considering that the ORF1110 protein exhibits only Galf-ase activity, almost no Araf-ase activity, and shows the competitive inhibition by L-arabino-1,4-lactone, the C6 atom of Galf in the substrate pNP-β-D-Galf appears to be crucial in the hydrogen bonding required for the proper positioning of the substrate on the catalytic site. pNP-α-L-Araf, which structure is similar to that of pNP-β-D-Galf, would enter the active site of the ORF1110 Galf-ase, but pNP-α-L-Araf may exhibit less hydrogen bonding due to lack of the C6 atom, resulting in lower activity of Araf-ase than Galf-ase. The structural analysis of the ORF1110 Galf-ase will be required to reveal the details of the catalytic mechanism.
We confirmed the Galf-ase activity of the ORF1110 protein for A. fumigatus GM in two ways: One was by detecting non reducing terminal Galf by TLC analysis, and the other was by observing a 40% reduction in ELISA assays using EB-A2 antibody (data not shown). Although we could not find to analyze as a candidate Galf-ase, we found another putative hydrolase gene adjacent to ORF1110 in the JHA19 genome. This predicted hydrolase belongs to GH2 and contains signal peptide like the ORF1110 Galf-ase. These information suggests that this putative hydrolase might be simultaneously expressed with ORF1110 to function together with the ORF1110 Galf-ase. Using A. fumigatus GM as a substrate, it was shown that the culture supernatant of A. fumigatus, unlike ORF1110 Galf-ase, produced several bands on TLC, suggesting that there might be not only exo-Galf-ase but also endo-Galf-ase activity in the fungus [27]. In addition, a detailed structural analysis of the sugar chain on glycoproteins demonstrated that β-1,2- and β-1,6-linked Galf, except for β-1,5-linked Galf, also exist [27]. Further work will be needed to determine which linkage of Galf is hydrolyzed by ORF1110 Galf-ase.
In conclusion, we have characterized a novel Galf-specific Galf-ase encoded by ORF1110 in strain JHA19. Considering that ORF1110 Galf-ase homologs are widely present and Galf residues are present on the cell surface of pathogenic microbes such as A. fumigatus, it is crucial to further understand the molecular mechanisms driving Galf-catalyzing enzymes for establishing novel pharmaceutical therapy against fungal pathogens.
Supporting Information
(DOCX)
Acknowledgments
We thank Dr. Mitsuaki Tabuchi of Kagawa University, for the kind gift of bacterial strains, and Dr. Takane Katayama of Kyoto University, for the gift of ΔlacZ E. coli strain. We also appreciate Dr. Masatoshi Goto of Kyushu University, for continuous discussions. This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan (K.T.) and (Y.H.).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by JSPS KAKENHI (https://www.jsps.go.jp/english/index.html) Grant Numbers 26292054 to K. Takegawa and 26892022 to YH. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Richards MR, Lowary TL (2008) Chemistry and biology of galactofuranose-containing polysaccharides. Chembiochem 10: 1920–1938. [DOI] [PubMed] [Google Scholar]
- 2. Tefsen B, Ram AF, van Die I, Routier FH (2012) Galactofuranose in eukaryotes: aspects of biosynthesis and functional impact. Glycobiology 22: 456–469. 10.1093/glycob/cwr144 [DOI] [PubMed] [Google Scholar]
- 3. Peltier P, Euzen R, Daniellou R, Nugier-Chauvin C, Ferrières V (2008) Recent knowledge and innovations related to hexofuranosides: structure, synthesis and applications. Carbohydr Res 343: 1897–1923. 10.1016/j.carres.2008.02.010 [DOI] [PubMed] [Google Scholar]
- 4. Latgé JP (2009) Galactofuranose containing molecules in Aspergillus fumigatus . Med Mycol 47: S104–9. 10.1080/13693780802258832 [DOI] [PubMed] [Google Scholar]
- 5. Morelle W, Bernard M, Debeaupuis JP, Buitrago M, Tabouret M, Latgé JP (2005) Galactomannoproteins of Aspergillus fumigatus . Eukaryot Cell 4: 1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reiss E, Lehmann PF (1979) Galactomannan antigenemia in invasive aspergillosis. Infect Immun 25: 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Latgé JP, Kobayashi H, Debeaupuis JP, Diaquin M, Sarfati J, Wieruszeski JM, et al. (1994) Chemical and immunological characterization of the extracellular galactomannan of Aspergillus fumigatus . Infect Immun 62: 5424–5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yuen KY, Chan CM, Chan KM, Woo PC, Che XY, Leung AS, et al. (2001) Characterization of AFMP1: a novel target for serodiagnosis of aspergillosis. J Clin Microbiol 39: 3830–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alam MK, El-Ganiny AM, Afroz S, Sanders DA, Liu J, Kaminskyj SG (2012) Aspergillus nidulans galactofuranose biosynthesis affects antifungal drug sensitivity. Fungal Genet Biol 49: 1033–1043. 10.1016/j.fgb.2012.08.010 [DOI] [PubMed] [Google Scholar]
- 10. Futagami T, Mori K, Yamashita A, Wada S, Kajiwara Y, Takashita H, et al. (2011) Genome sequence of the white koji mold Aspergillus kawachii IFO 4308, used for brewing the Japanese distilled spirit shochu. Eukaryot Cell 10: 1586–1587. 10.1128/EC.05224-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fontaine T, Simenel C, Dubreucq G, Adam O, Delepierre M, Lemoine J, et al. (2000) Molecular organization of the alkali-insoluble fraction of Aspergillus fumigatus cell wall. J Biol Chem 275: 27594–25607. [DOI] [PubMed] [Google Scholar]
- 12. Wallis GL, Easton RL, Jolly K, Hemming FW, Peberdy JF (2001) Galactofuranoic-oligomannose N-linked glycans of alpha-galactosidase A from Aspergillus niger . Eur J Biochem 268: 4134–4143. [DOI] [PubMed] [Google Scholar]
- 13. Wallis GL, Swift RJ, Hemming FW, Trinci AP, Peberdy JF (1999) Glucoamylase overexpression and secretion in Aspergillus niger: analysis of glycosylation. Biochim Biophys Acta 1472: 576–586. [DOI] [PubMed] [Google Scholar]
- 14. Leitao EA, Bittencourt VC, Haido RM, Valente AP, Peter-Katalinic J, Letzel M, et al. (2003) Beta-galactofuranose-containing O-linked oligosaccharides present in the cell wall peptidogalactomannan of Aspergillus fumigatus contain immunodominant epitopes. Glycobiology 13: 681–692. [DOI] [PubMed] [Google Scholar]
- 15. Goto M (2007) Protein O-glycosylation in fungi: diverse structures and multiple functions. Biosci Biotechnol Biochem 71: 1415–1427. [DOI] [PubMed] [Google Scholar]
- 16. Lamarre C, Beau R, Balloy V, Fontaine T, Wong Sak Hoi J, Guadagnini S, et al. (2009) Galactofuranose attenuates cellular adhesion of Aspergillus fumigatus . Cell Microbiol 11: 1612–1623. 10.1111/j.1462-5822.2009.01352.x [DOI] [PubMed] [Google Scholar]
- 17. El-Ganiny AM, Sheoran I, Sanders DA, Kaminskyj SG (2010) Aspergillus nidulans UDP-glucose-4-epimerase UgeA has multiple roles in wall architecture, hyphal morphogenesis, and asexual development. Fungal Genet Biol 47: 629–635. 10.1016/j.fgb.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 18. Damveld RA, Franken A, Arentshorst M, Punt PJ, Klis FM, van den Hondel CA, et al. (2008) A novel screening method for cell wall mutants in Aspergillus niger identifies UDP-galactopyranose mutase as an important protein in fungal cell wall biosynthesis. Genetics 178: 873–881. 10.1534/genetics.107.073148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. El-Ganiny AM, Sanders DA, Kaminskyj SG (2008) Aspergillus nidulans UDP-galactopyranose mutase, encoded by ugmA plays key roles in colony growth, hyphal morphogenesis, and conidiation. Fungal Genet Biol 45: 1533–1542. 10.1016/j.fgb.2008.09.008 [DOI] [PubMed] [Google Scholar]
- 20. Schmalhorst PS, Krappmann S, Vervecken W, Rohde M, Müller M, Braus GH, et al. (2008) Contribution of galactofuranose to the virulence of the opportunistic pathogen Aspergillus fumigatus . Eukaryot Cell 7: 1268–1277. 10.1128/EC.00109-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Engel J, Schmalhorst PS, Dörk-Bousset T, Ferrières V, Routier FH (2009) A single UDP-galactofuranose transporter is required for galactofuranosylation in Aspergillus fumigatus . J Biol Chem 284: 33859–33868. 10.1074/jbc.M109.070219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Afroz S, El-Ganiny AM, Sanders DA, Kaminskyj SG (2011) Roles of the Aspergillus nidulans UDP-galactofuranose transporter, UgtA in hyphal morphogenesis, cell wall architecture, conidiation, and drug sensitivity. Fungal Genet Biol 48: 896–903. 10.1016/j.fgb.2011.06.001 [DOI] [PubMed] [Google Scholar]
- 23. Komachi Y, Hatakeyama S, Motomatsu H, Futagami T, Kizjakina K, Sobrado P, et al. (2013) GfsA encodes a novel galactofuranosyltransferase involved in biosynthesis of galactofuranose antigen of O-glycan in Aspergillus nidulans and Aspergillus fumigatus . Mol Microbiol 90: 1054–1073. 10.1111/mmi.12416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wallis GL, Hemming FW, Peberdy JF (2001) An extracellular beta-galactofuranosidase from Aspergillus niger and its use as a tool for glycoconjugate analysis. Biochim Biophys Acta 1525: 19–28. [DOI] [PubMed] [Google Scholar]
- 25. Rietschel-Berst M, Jentoft NH, Rick PD, Pletcher C, Fang F, Gander JE (1977) Extracellular exo-beta-galactofuranosidase from Penicillium charlesii: isolation, purification, and properties. J Biol Chem 252: 3219–3226. [PubMed] [Google Scholar]
- 26. Ramli N, Fujinaga M, Tabuchi M, Takegawa K, Iwahara S (1995) Isolation and characterization of a novel endo-beta-galactofuranosidase from Bacillus sp. Biosci Biotechnol Biochem 59: 1856–1860. [DOI] [PubMed] [Google Scholar]
- 27. Kudoh A, Okawa Y, Shibata N (2015) Significant structural change in both O- and N-linked carbohydrate moieties of the antigenic galactomannan from Aspergillus fumigatus grown under different culture conditions. Glycobiology 25: 74–87. 10.1093/glycob/cwu091 [DOI] [PubMed] [Google Scholar]
- 28. Cousin MA, Notermans S, Hoogerhout P, Van Boom JH (1989) Detection of beta-galactofuranosidase production by Penicillium and Aspergillus species using 4-nitrophenyl beta-D-galactofuranoside. J Appl Bacteriol 66: 311–317. [DOI] [PubMed] [Google Scholar]
- 29. Van Bruggen-Van Der Lugt AW, Kamphuis HJ, De Ruiter GA, Mischnick P, Van Boom JH, Rombouts FM (1992) New structural features of the antigenic extracellular polysaccharides of Penicillium and Aspergillus species revealed with exo-beta-D-galactofuranosidase. J Bacteriol 174: 6096–6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tefsen B, Lagendijk EL, Park J, Akeroyd M, Schachtschabel D, Winkler R, et al. (2012) Fungal α-arabinofuranosidases of glycosyl hydrolase families 51 and 54 show a dual arabinofuranosyl- and galactofuranosyl-hydrolyzing activity. Biol Chem 393: 767–775. 10.1515/hsz-2012-0134 [DOI] [PubMed] [Google Scholar]
- 31. Flipphi MJ, van Heuvel M, van der Veen P, Visser J, de Graaff LH (1993) Cloning and characterization of the abfB gene coding for the major alpha-L-arabinofuranosidase (ABF B) of Aspergillus niger . Curr Genet 24: 525–532. [DOI] [PubMed] [Google Scholar]
- 32. Gielkens M, González-Candelas L, Sánchez-Torres P, van de Vondervoort P, de Graaff L, Visser J, et al. (1999) The abfB gene encoding the major alpha-L-arabinofuranosidase of Aspergillus nidulans: nucleotide sequence, regulation and construction of a disrupted strain. Microbiology 145: 735–741. [DOI] [PubMed] [Google Scholar]
- 33. Koseki T, Okuda M, Sudoh S, Kizaki Y, Iwano K, Aramaki I, et al. (2003) Role of two alpha-L-arabinofuranosidases in arabinoxylan degradation and characteristics of the encoding genes from shochu koji molds, Aspergillus kawachii and Aspergillus awamori . J Biosci Bioeng 96: 232–241. [DOI] [PubMed] [Google Scholar]
- 34. Zhao G, Yao Y, Qi W, Wang C, Hou L, Zeng B, et al. (2012) Draft genome sequence of Aspergillus oryzae strain 3.042. Eukaryot Cell 11: 1178 10.1128/EC.00160-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao G, Yao Y, Hou L, Wang C, Cao X (2014) Draft Genome Sequence of Aspergillus oryzae, an Increased Acid Protease Production Strain. Genome Announc 2: 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matsumura K, Obata H, Hata Y, Kawato A, Abe Y, Akita O (2004) Isolation and characterization of a novel gene encoding alpha-L-arabinofuranosidase from Aspergillus oryzae . J Biosci Bioeng 98: 77–84. [DOI] [PubMed] [Google Scholar]
- 37. Crous JM, Pretorius IS, van Zyl WH (1996) Cloning and expression of the alpha-L-arabinofuranosidase gene (ABF2) of Aspergillus niger in Saccharomyces cerevisiae . Appl Microbiol Biotechnol 46: 256–260. [DOI] [PubMed] [Google Scholar]
- 38. Chlubnova I, Legentil L, Dureau R, Pennec A, Almendros M, Daniellou R, et al. (2012) Specific and non-specific enzymes for furanosyl-containing conjugates: biosynthesis, metabolism, and chemo-enzymatic synthesis. Carbohydr Res 356: 44–61. 10.1016/j.carres.2012.04.002 [DOI] [PubMed] [Google Scholar]
- 39. Varela O, Marino C, Lederkremer RM (1986) Synthesis of p-nitrophenyl-β-D-galactofuranoside. A convenient substrate for β-D-galactofuranosidase. Carbohydr Res 155: 247–251. [DOI] [PubMed] [Google Scholar]
- 40. Marino C, Mariño K, Miletti L, Alves MJM, Colli W, Lederkremer RM (1998) 1-Thio-β-D-galactofuranosides: synthesis and evaluation as β-D-galactofuranosidase inhibitors. Glycobiology 8: 901–904. [DOI] [PubMed] [Google Scholar]
- 41. Nikodinovic J, Barrow KD, Chuck JA (2003) High yield preparation of genomic DNA from Streptomyces . Biotechniques 35: 932–936. [DOI] [PubMed] [Google Scholar]
- 42. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948. [DOI] [PubMed] [Google Scholar]
- 43. Gielkens M, González-Candelas L, Sánchez-Torres P, van de Vondervoort P, de Graaff L, Visser J, et al. (1999) The abfB gene encoding the major α-L-arabinofuranosidase of Aspergillus nidulans: nucleotide sequence, regulation and construction of a disrupted strain. Microbiology 145: 735–741. [DOI] [PubMed] [Google Scholar]
- 44. Shi H, Zhang Y, Xu B, Tu M, Wang F (2014) Characterization of a novel GH2 family α-L-arabinofuranosidase from hyperthermophilic bacterium Thermotoga thermarum . Biotechnol Lett 36: 1321–1328. 10.1007/s10529-014-1493-6 [DOI] [PubMed] [Google Scholar]
- 45. Schwabe K, Grossmann A, Fehrmann B, Tschiersch B (1978) Inhibition of alpha-L-arabinofuranosidase (Aspergillus niger) and non-inhibition of alpha-L-arabinopyranosidase (almond emulsin and barley) by L-arabinono-1,4-lactone. Carbohydr Res 67: 541–544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.