Abstract
Recent neuroimaging work has suggested that aggressive behaviour (AB) is associated with structural and functional brain abnormalities in processes subserving emotion processing and regulation. However, most neuroimaging studies on AB to date only contain relatively small sample sizes. To objectively investigate the consistency of previous structural and functional research in adolescent AB, we performed a systematic literature review and two coordinate-based activation likelihood estimation meta-analyses on eight VBM and nine functional neuroimaging studies in a total of 783 participants (408 [224AB/184 controls] and 375 [215 AB/160 controls] for structural and functional analysis respectively). We found 19 structural and eight functional foci of significant alterations in adolescents with AB, mainly located within the emotion processing and regulation network (including orbitofrontal, dorsomedial prefrontal and limbic cortex). A subsequent conjunction analysis revealed that functional and structural alterations co-localize in right dorsomedial prefrontal cortex and left insula. Our results are in line with meta-analytic work as well as structural, functional and connectivity findings to date, all of which make a strong point for the involvement of a network of brain areas responsible for emotion processing and regulation, which is disrupted in AB. Increased knowledge about the behavioural and neuronal underpinnings of AB is crucial for the development of novel and implementation of existing treatment strategies. Longitudinal research studies will have to show whether the observed alterations are a result or primary cause of the phenotypic characteristics in AB.
Introduction
Aggressive behaviour (AB), as observed in social disorders such as DBD (including conduct (CD) and oppositional defiant disorder (ODD)), is characterized by a repeated pattern of antisocial behaviour and severe aggression, where the basic rights of others, major age-appropriate norms or societal rules are violated [1]. Such problems can cause significant impairment in social, academic, or occupational functioning [2,3]. Clinical and subclinical forms of AB are observed in up to 14% of all girls and 16% of all boys [4]. The negative impact of aggression-related problems reaches beyond a patient’s family, ultimately affecting society as a whole (e.g. school-dropouts, delinquency, teen-pregnancies, substance abuse or difficulties integrating into work life [3,5,6]). Early conduct problems are key precursors of persistent AB and thus also predictive for ODD, CD and antisocial personality disorder in adulthood [7]. Neurodevelopmental theories [8,9,10] and longitudinal studies [11] are in line with these behavioural observations, suggesting that the presence of early brain alterations in individuals with aggressive behaviour may heighten the risk for long-lasting social impairments [12,13]. In the current paper we particularly focus on adolescents with aggressive behaviour (AB), hereby summarizing neuroimaging research in youths with either conduct problems, CD or ODD.
In recent years structural (e.g voxel-based/surface-based) and functional (e.g. fMRI/PET) neuroimaging techniques have grown into powerful tools to investigate the neuronal basis of the human brain in typically developing individuals as well as patients. It has been demonstrated that both, brain structure and function, may be modified by experience [14,15]. Activation-dependant structural plasticity can even occur after as little as seven days of training [16,17] and it is suggested to play a key role in human adaptation to environmental changes and disease. Even though neuroimaging evidence points toward a neuronal basis of AB [13,18], the overall number of research studies within this population remains relatively scarce. Furthermore, it has to be noted that AB characteristics as seen in CD and/or ODD are considered heterogeneous in respect to their pathologies. CD and ODD are frequently associated with comorbidities such as attention-deficit hyperactivity disorder (ADHD) or anxiety [19]). These comorbid disorders can differ in their pathophysiological mechanisms, some of them seem exclusive on a biological level making it possible that different developmental trajectories with varying neurobiological bases lead to the clinical manifestations of AB [20]. The vagueness of the group definition within many of the current studies on AB is thus bound to impact general conclusions drawn from it.
Even though the total number of studies is still limited, neuroanatomical and functional variations in youths with AB have been reported with increased frequency since the advent of modern neuroimaging. In particular, brain structure in AB has been investigated using voxel-based morphometry (VBM), diffusion tensor imaging (DTI) or surfaced-based morphometry. VBM studies for example have revealed differences in gray and white matter volume in brain regions including the amygdala, insula, orbitofrontal and dorsomedial prefrontal cortex (e.g. [21,22,23,24]) when comparing adolescents with AB and typically developing controls. Similarly, studies using surface-based morphometry [25,26] or DTI [27,28,29,30,31,32,33] provide evidence for structural alterations and/or impaired connectivity within brain regions involved in emotion processing, reward and empathy. Functional neuroimaging studies corroborate the structural neuroimaging literature. Cognitive paradigms employed in the investigation of AB have focused on disturbances in the emotion processing and regulation network of the brain. These tasks particularly target emotion processing/regulation [34,35,36,37,38,39,40,41,42,43], empathy [41,44,45], theory of mind [46], passive avoidance [47], decision making [48,49] or executive functioning [40,42,50]. Overall, studies point towards aberrant brain function in AB in key areas of social cognition and emotion, including prefrontal (orbitofrontal, dorsolateral and medial prefrontal cortex), limbic (e.g. amygdala, anterior insula, cingulate cortex) and temporal cortices.
Despite increasing evidence about the uniformity of atypical brain structure and function in AB, it has yet to be objectively determined which brain regions are commonly affected. Functional and structural neuroimaging studies are crucial for the understanding of the phenotype and aetiology of AB. However, most results and interpretations are based on individual neuroimaging studies and present various limitations (e.g. small sample sizes, low reliability, dependency on task chosen [51,52,53]). Furthermore, very few imaging studies have yet investigated brain structure and function in the same population. Activation likelihood estimation (ALE) meta-analyses allow the identification of consistent findings of brain activation and structure across multiple data sets. Hereby, ALE quantitatively investigates communalities between reported foci based on modelling them as probability distributions centered around the corresponding coordinates. The resulting probability maps mirror the likelihood of morphological change and/or activation on a voxel-wise level across an entire set of studies [51]. ALE has been successfully applied in meta-analyses of various neuropsychiatric disorders to date [54,55,56,57,58] and provides a promising tool for a more unified investigation of pathophysiologic changes in disease.
Therefore, the present paper intends to close this gap in research and aims to aggregate all structural and functional neuroimaging studies conducted in adolescent AB to date. In a first step, we planned to conduct a systematic literature review of neuroimaging findings in adolescents with AB. Secondly two separate meta-analyses looking at gray matter volume reductions as well as hypoactivations during emotion processing tasks in AB were carried out. Finally, we decided to run a conjunction analysis to identify potential overlaps in deviant brain structure and function in adolescents with AB.
Method
Participants
We decided to focus our analysis on adolescents with aggressive behaviour (AB) in general as opposed to a specific clinical diagnosis. By including both community samples and clinical samples in the present meta-analyses we adhere to the heterogeneity in juvenile aggression. This heterogeneity is further reflected by different behavioural symptoms of aggression and antisocial tendencies, such as oppositional behaviour, impulsive hot-tempered quarrels or premeditated violent acts, the presence of callous unemotional/psychopathic traits or co-morbid conditions in CD and ODD patients. All studies were conducted during childhood and/or adolescence and share the communality of aggression and antisocial tendencies within the populations studied. Thus, AB as defined here may be considered an umbrella term for children and adolescents with a range of subclinical and clinically relevant symptoms of pathological aggression.
Study Selection
For the structural and functional neuroimaging meta-analyses we used PubMed and Google Scholar to systematically search for neuroimaging literature in AB. Literature searches were conducted and reviewed by several research team members (NMR, WMM, LVF, ET) and adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA; S1 Table) guidelines and the revised Quality Of Reporting Of Meta-analyses (QUOROM) statement [59]. Our main search (see Fig 1) conducted through PubMed included the following key words: “conduct disorder”, “conduct problems”, “disruptive behaviour disorder”, “oppositional defiant disorder” and “aggression”, each in combination with methodologically relevant terms including “VBM”, “fMRI” and/or “neuroimaging”. Moreover, a number of review articles published on conduct disorder, antisocial behaviour and aggression in adolescents were considered (e.g. [11,60,61,62,63,64,65]). Finally, additional publications were explored by searching the reference list of the articles obtained to assure integration of all data available. Studies were included in our meta-analyses if the following criteria were given: (I) included at least one clinical group with described aggressive behaviour, (II) in combination with a healthy control sample, (III) conducted during adolescence, (IV) reported whole brain gray matter volume alterations or whole brain functional neuroimaging data, (V) results are described using a standard reference space (Talairach or MNI) and (VI) the same threshold was used throughout the whole brain analysis. All structural studies included employed a standard VBM analysis protocol. In both meta-analysis of structural and functional brain alterations in adolescents with AB versus controls, no studies providing results based on a priori region-of-interest analysis only were included (since they violate the assumption, under the null hypothesis, that the likelihood of locating activated foci is equal at every voxel). Similarly, no animal studies or case reports were included in any meta-analysis and only studies from peer-reviewed journals that are written in English were considered. Data is current up to July 2015.
Fig 1. Systematic literature research.
Literature research according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the revised Quality Of Reporting Of Meta-analyses (QUOROM) statement (59) resulting in 17 neuroimaging studies included in the current meta-analyses.
Of the 1021 studies identified through our systematic review (see Fig 1), we screened 930 (after removal of duplicates) and consequently assessed the full texts of 173 articles. 156 studies had to be excluded from the functional or structural meta-analysis in adolescents with AB, because they did not meet the criteria listed above (for detailed exclusion reasons, see Fig 1). Looking more closely at our review on structural research studies in AB revealed that only five studies reported on gray matter volume increases in AB (four reported de- and increases, one study only reported increases). Therefore we did not conduct a separate meta-analysis for gray matter volume increases in AB. Consequently, eight studies were included in our meta-analysis about gray matter volume reductions, together reporting data from 408 research participants (224 AB, 184 typically developing controls = TD), and 50 foci of gray matter volume decreases in youths with AB (Table 1 [21,22,23,66,67,68,69,70]).
Table 1. Characteristics of the studies in adolescents with AB included in the current structural meta-analysis.
| # | First author | Year | Method | Diagnosis [N] | Sex [m/f] | Average age and [range] in years |
|---|---|---|---|---|---|---|
| 1 | Huebner | 2008 | VBM | CD, early-onset [23] | [23/0] | CD, early-onset: 14.5 |
| TD [23] | [23/0] | TD: 14.2 | ||||
| [12–17] | ||||||
| 2 | De Brito | 2009 | VBM | CP/CU+ [23] | CP/CU+: 11.5 | |
| TD [25] | TD: 11.8 | |||||
| [10–13] | ||||||
| 3 | Dalwani | 2011 | VBM | CP+SUD [25] | [25/0] | CP+SUD: 16.6 |
| TD [19] | [19/0] | TD: 16.6 | ||||
| [14–18] | ||||||
| 4 | Fahim | 2011 | VBM | DBD [22; 11CD/11ODD] | [22/0] | DBD: 8.4 |
| TD [25] | [25/0] | TD: 8.4 | ||||
| 5 | Fairchild | 2011 | VBM | CD, early-onset [36] | [36/0] | CD, early-onset: 17.7 |
| CD, late-onset [27] | [27/0] | CD, late-onset: 17.9 | ||||
| TD [27] | [27/0] | TD: 18.5 | ||||
| [16–21] | ||||||
| 6 | Stevens | 2012 | VBM | CD [24] | [16/8] | CD: 16.0 |
| TD [24] | [16/8] | TD: 16.0 | ||||
| [12–18] | ||||||
| 7 | Fairchild | 2013 | VBM | CD [22] | [0/22] | CD: 17.6 |
| TD [20] | [0/20] | TD: 17.2 | ||||
| [14–20] | ||||||
| 8 | Dalwani | 2015 | VBM | CP [22] | [0/22] | CP: 16.7 |
| TD [21] | [0/21] | TD: 16.1 | ||||
| [14–18] |
CD = Conduct disorder. DBD = Disruptive behaviour disorders. CU+ = with high callous-unemotional traits. SUD = Substance use disorder. TD = Typically developing participants. VBM = Voxel-based morphometry.
Our systematic literature review of functional neuroimaging studies in youths with AB identified experiments targeting emotion processing [34,35,36,37,38,39,40,41,42,43], empathy [41,44,45], theory of mind [46], passive avoidance [47], decision making [48,49] or executive functioning [40,42,50]. We decided to restrict our functional meta-analysis to tasks only including emotionally loaded and visually presented stimuli (e.g. tasks of emotion processing and empathy). In case of sample overlap, the study with the highest subject number meeting all other criteria listed above was selected. In case of comparisons between AB and TD in more than one contrast, only foci from the contrast putting the highest demand on emotion processing, were included. The majority of studies indicated hypoactivations in AB. Only six studies that fulfilled all other criteria listed above reported hyperactivations in AB compared to TD. Therefore, we did not conduct a separate meta-analysis on functional overactivations in AB. Consequently nine studies suggesting hypoactivations in adolescents with AB compared to TD were selected (Table 2; [34,39,40,41,43,44,71,72,73]). Together the selected studies report data from 375 research participants (215 AB, 160 TD) and describe 58 foci of hypoactivation in AB compared to TD.
Table 2. Characteristics of the studies in adolescents with AB included in current functional meta-analysis.
| # | First author | Year | Stimuli | Diagnosis [N] | Sex [m/f] | Average age and [range] in years |
|---|---|---|---|---|---|---|
| 1 | Sterzer | 2005 | Neutral or negative | CD [13] | [13/0] | CD: 12.9 |
| pictures (IAPS) | TD [14] | [14/0] | TD: 12.7 | |||
| [9–15] | ||||||
| 2 | Passamonti | 2010 | Pictures of angry, sad | CD, early-onset [27] | [27/0] | CD, early-onset: 17.7 |
| and neutral faces | CD, late-onset [25] | [25/0] | CD, late-onset: 17.1 | |||
| TD [23] | [23/0] | TD: 17.8 | ||||
| [16–21] | ||||||
| 3 | Marsh | 2011 | Emotional words | CD/ODD+PT [14] | [8/6] | CD/ODD+PT: 14.4 |
| (categorization task) | TD [14] | [11/3] | TD: 13.5 | |||
| 4 | White | 2012 | Pictures of fearful and | CD/ODD+PT [15] | [12/3] | CD/ODD+PT: 15.7 |
| neutral faces | TD [17] | [9/8] | TD: 14.5 | |||
| [10–17] | ||||||
| 5 | Lockwood | 2013 | Pictures of others in | CD [37] | [37/0] | CD: 14.05 |
| pain or no pain | TD [18] | [18/0] | TD: 13.68 | |||
| [10–16] | ||||||
| 6 | Marsh | 2013 | Pictures of others in | CD/ODD+PT [14] | [8/6] | CD/ODD+PT: 15.4 |
| pain or no pain. | TD [21] | [15/6] | TD: 14.3 | |||
| [10–17] | ||||||
| 7 | Fairchild | 2014 | Pictures of emotional | CD [20] | [0/20] | CD: 17.0 |
| or neutral faces | TD [20] | [0/20] | TD: 17.6 | |||
| 8 | O'Nions | 2014 | Cartoons (affective | CP/CU+ [16] | [16/0] | CP/CU+: 14.2 |
| picture series) | TD [16] | [16/0] | TD: 13.5 | |||
| [10–16] | ||||||
| 9 | Sebastian | 2014 | Pictures of fearful and | CP/CU+ [17] | [17/0] | CP/CU+: 14.0 |
| calm facial expressions | CP/CU- [17] | [17/0] | CP/CU-: 14.5 | |||
| TD [17] | [17/0] | TD: 13.5 | ||||
| [10–16] |
CD = Conduct disorder. CP = Conduct problems. ODD = Oppositional defiant disorder. PT = with psychopathic traits. CU+ = with high callous-unemotional traits. CU- = with low callous-unemotional traits. TD = Typically developing participants.
ALE Meta-Analysis Procedure
We conducted two separate meta-analyses on gray matter volume alterations and functional hypoactivations in adolescents with AB. Data analysis was carried out using the revised version of the ALE approach for coordinate-based meta-analysis of neuroimaging data (GingerALE software, version 2.3; available from http://brainmap.org/ale/ [51,74,75,76]). In short, this new approach implements a random-effects model, a quantitative uncertainty model to determine the FWHM and an exclusive gray matter mask (for further details, see also [51,53,74,75,76]). Most importantly, instead of testing for an above-chance clustering between foci, the revised ALE algorithm assesses above-chance clustering between experiments. The spatial relationship between foci in a given experiment is now assumed to be fixed and ALE results are assessed against a nulldistribution of random spatial association between experiments. Prior to running any analyses, coordinates reported in Talairach space were transformed to MNI space using the tal2icbm algorithm [77,78]. The here employed revised ALE approach identifies areas of convergence of activation across various experiments, minimizing the within-groups effects (approach by Turkeltaub and colleagues [75]). Each focus is represented as a centre for 3D Gaussian probability distributions, where the standard deviation depends on group size (capturing spatial uncertainty) rather than single time points. First, the probabilities of all activation foci in a given experiment are combined for each voxel, which is represented in modelled activation maps (fMRI) or modelled anatomical maps (VBM). Secondly, the ALE method combines all modelled maps (fMRI and VBM separately) on a voxel-by-voxel basis to form an ALE image containing all unthresholded voxel ALE values. In the last step, this ALE image is tested against the null hypothesis under the assumption that all activated voxels are homogeneously distributed in the brain, independent of the experiments. This null-hypothesis model (a distribution map made by multiple permutations of random voxel activation) was created using a random-effects statistical method and tested against the original ALE image according to the selected significance threshold. Therefore, the nulldistribution is constructed reflecting a random spatial association between different studies. Comparing the “true” ALE score to this distribution allows a focused inference on convergence between studies while preserving the relationship between individual foci within each study. Critically, this change from fixed- (foci-based) to random-effects (testing between study effects) inference in ALE analysis allows generalisation of the results to the entire population of studies from which the analysed ones were drawn. This more conservative approach with an increased specificity [51,76] does also accommodate the idea of convergence across heterogeneous studies. We used a statistical threshold of p<0.05 False Discovery Rate (FDR) corrected for multiple comparisons and a minimum cluster size of 500mm3. ALE maps are overlaid onto a standard brain in MNI space (Colin27 available at http://www.brainmap.org/ale/) using the Multi-image Analysis GUI (Mango available at http://ric.uthscsa.edu/mango/mango.html) and clusters were anatomically labelled by cross-referencing the Talairach Daemon [79,80] and aal [81]. In order to further investigate possible overlaps between the structural (VBM) and functional (fMRI) meta-analysis in adolescent AB, a formal conjunction analysis was performed by multiplying binarized versions of the individually thresholded ALE maps.
Results
Our meta-analysis of structural neuroimaging studies in adolescents with AB revealed 19 clusters of significant convergence between the studies (see Table 3; Fig 2). The largest clusters were found in the right inferior frontal lobe (inferior frontal/precentral gyrus), right precuneus and left-hemispheric insula. Further smaller clusters were found bilaterally in the frontal (e.g. dorsolateral and medial frontal gyrus), parietal (e.g. precuneus) and temporal lobe (e.g. middle/superior temporal gyrus) as well as the cerebellum (e.g. culmen). Our meta-analysis of functional hypoactivation in adolescents with AB revealed 8 clusters of significant convergence between the studies with the largest clusters in the right middle/superior frontal gyrus, left thalamus and basal ganglia, as well as left-hemispheric insula (see Table 3, Fig 2). Beyond others, further clusters included the right anterior cingulate, left middle temporal gyrus and right amygdala.
Table 3. Results of the structural and functional ALE-meta analyses and conjunction analysis of structure and functional alterations in adolescents with AB.
| Region | BA | H | Volume | Local Maxima | |||
| Structural Meta-Analysis (TD>AB) | |||||||
| 1 | inferior frontal/precentral gyrus, | 13, | R | 1952 | 54 | 16 | 10 |
| insula | 44, 45 | 62 | 20 | 6 | |||
| 56 | 26 | 16 | |||||
| 2 | subcallosal gyrus, putamen, | 34 | R | 1672 | 26 | 4 | -16 |
| lateral globus pallidus, amygdala | 22 | 4 | -8 | ||||
| 14 | 10 | -12 | |||||
| 3 | inferior frontal gyrus | 45, 47 | R | 1304 | 52 | 26 | -10 |
| 4 | insula | 13 | L | 1144 | -38 | 8 | 8 |
| -38 | 4 | -2 | |||||
| 5 | middle/superior frontal gyrus | 9,8 | R | 1112 | 34 | 48 | 30 |
| 40 | 38 | 30 | |||||
| 6 | middle/inferior frontal gyrus | 10,46 | L | 1040 | -36 | 48 | -2 |
| -46 | 48 | 2 | |||||
| 7 | putamen, claustrum | R | 688 | 34 | 2 | -2 | |
| 8 | thalamus | R | 560 | 20 | -30 | 8 | |
| 9 | subcallosal/middle frontal gyrus, cingulate | 25 | R | 528 | 10 | 14 | -22 |
| 10 | cingulate/middle frontal gyrus | 32 | L | 528 | -10 | 24 | 42 |
| 11 | claustrum | L | 520 | -24 | 20 | 8 | |
| 12 | claustrum, insula | R | 520 | 32 | 14 | 10 | |
| 13 | subcallosal/parahippocampal gyrus, amygdala | 34 | L | 512 | -30 | 4 | -18 |
| 14 | culmen, declive | R | 512 | 4 | -58 | -16 | |
| 15 | caudate | R | 512 | 10 | 14 | 2 | |
| 16 | thalamus | L | 512 | -8 | -16 | 15 | |
| 17 | inferior frontal gyrus | 47 | R | 504 | 46 | 26 | -30 |
| 18 | middle temporal gyrus | 37 | R | 504 | 54 | -68 | 12 |
| 19 | superior frontal gyrus | 9 | R | 504 | 18 | 56 | 20 |
| All x, y, z-coordinates represent local maxima in MNI space | AB=Aggressive Behaviour | ||||||
| Volume=Volume (mm3) | TD=Typically developing controls | ||||||
| H=Hemisphere | BA= Brodmann areas | ||||||
| R=Right; L=Left | |||||||
| Region | BA | H | Volume | Local Maxima | |||
| Functional Meta-Analysis (TD>AB) | |||||||
| 1 | middle/superior frontal gyrus, | 8, 9, | R/L | 3728 | 14 | 44 | 30 |
| anterior cingulate gyrus | 10, 32 | 8 | 36 | 28 | |||
| 22 | 48 | 22 | |||||
| 32 | 50 | 14 | |||||
| 0 | 36 | 24 | |||||
| 2 | thalamus, lentiform nucleus, | L | 1944 | -6 | -12 | -4 | |
| putamen, medial globus pallidus | -26 | -8 | -12 | ||||
| amygdala | -16 | -8 | -4 | ||||
| 3 | claustrum, insula | 13 | L | 1896 | -28 | 20 | 0 |
| -38 | 20 | 12 | |||||
| 4 | middle frontal gyrus, | 11, 24 | R | 1328 | 12 | 30 | -20 |
| anterior cingulate | 4 | 30 | -14 | ||||
| 5 | inferior/middle temporal gyrus | 21 | L | 1288 | -48 | -8 | -26 |
| 6 | amygdala, parahippocampal | 28 | R | 1224 | 30 | -4 | -28 |
| gyrus | 20 | -2 | -30 | ||||
| 7 | claustrum, putamen, insula | 13 | R | 776 | 28 | 20 | 0 |
| 30 | 24 | -2 | |||||
| 8 | superior, middle frontal gyrus | 9 | R | 552 | 14 | 60 | 16 |
| Conjunction: Structural (TD>AB) ∩ Functional (TD>AB) | |||||||
| 1 | superior frontal gyrus (dmPFC) | 9 | R | 128 | 16 | 58 | 18 |
| 2 | claustrum, insula | L | 8 | -26 | 20 | 4 | |
| 3 | claustrum, insula | L | 8 | -28 | 18 | 6 | |
All x, y, z-coordinates represent local maxima in MNI space. AB = Aggressive Behaviour. Volume = Volume (mm3). TD = Typically developing controls. H = Hemisphere. BA = Brodmann areas. R = Right; L = Left.
Fig 2. Neuronal alterations in adolescents with aggressive behaviour (TD>AB): Results from an ALE meta-analysis.
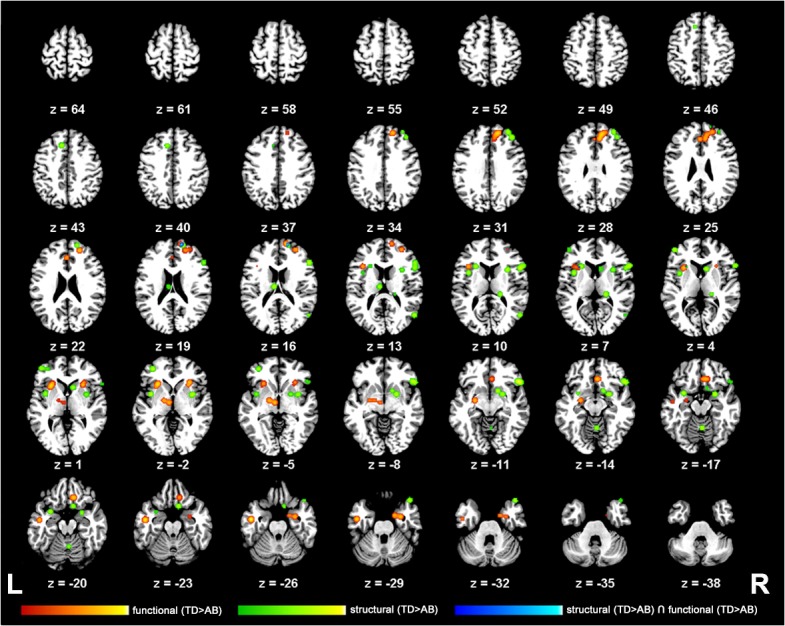
2-D axial slices displaying the thresholded and binarized ALE maps of significant overlap (P<0.05, FDR-corrected) in studies of structural (green) and functional (red) alterations in adolescent AB (TD>AB) as well as a conjunction analysis (blue) overlaid on the Colin T1-template in MNI space. Z-slices depicting the results range from z = 21 to 120 and are displayed in neurological view using the Multi-image Analysis GUI (Mango available at http://ric.uthscsa.edu/mango/mango.html).
A formal conjunction analysis using the thresholded ALE maps from the structural and functional meta-analysis discovered three areas of regional overlap (Table 3, Fig 3 ). The biggest area of functional and structural overlap (128mm3) in adolescents with AB was identified within the right dmPFC. Additionally, the analysis exposed two smaller, close-lying clusters of convergence with a peak in the left claustrum, extending into the insular cortex.
Fig 3. Structural and functional neuroimaging findings in youths with AB co-localize in right dorsomedial prefrontal cortex (dmPFC) and left insular cortex.
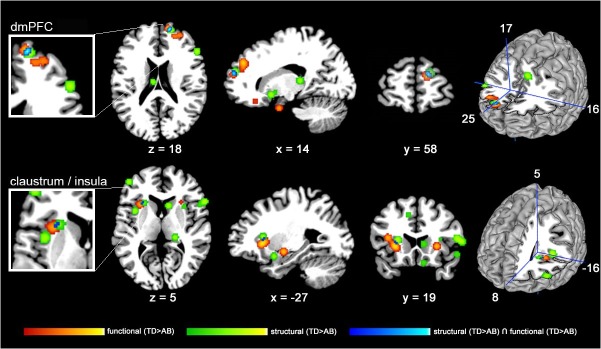
2-D slices displaying the thresholded and binarized ALE maps of significant overlap (P<0.05, FDR-corrected) in studies of structural (green) and functional (red) alterations in adolescents with AB (TD>AB) as well as a conjunction analysis (blue) overlaid on the Colin T1-template in MNI space. The upper-row including left cut-out as well as right surface-model highlight the right dmPFC where structural and functional alterations co-localize. The lower-row including left cut-out as well as right surface-model illustrate left insular cortex/claustrum where structural and functional alterations overlap.
Discussion
To our knowledge, the current work provides the first quantitative summary of functional hypoactivations and gray matter volume reductions in adolescents with AB by summarizing findings of eight structural and nine functional neuroimaging studies in a total of 783 participants (408 [224 AB/184 TD] and 375 [215 AB/160 TD] for structural and functional analysis respectively). Our findings indicate 19 structural and eight functional foci of significant alterations in AB, mainly located within the emotion processing and regulation network of the human brain (including orbitofrontal, dorsolateral/medial prefrontal cortex and limbic brain regions; for reviews on emotion processing and regulation see also [82,83,84]). Conjunction analysis reveal that functional and structural alterations in AB overlap in three areas, with the largest cluster centered in the right dmPFC and two smaller clusters that encompass the left insula.
In the following sections we will review structural and functional neuroanatomical evidence derived from healthy participants as well as those with aggressive behaviour (e.g. conduct problems, CD, ODD) for the key areas implicated here (orbitofrontal and dorsomedial prefrontal cortex, insula, cingulate cortex, amygdala).
Orbitofrontal and Dorsomedial Prefrontal Cortex
Our findings identify prefrontal brain regions including orbitofrontal and dorsomedial prefrontal cortex as main locations of aberrant brain function and structure in youths with AB. Furthermore, an overlap in the foci representing structural and functional changes that co-localize in AB is centered in the right dmPFC. While the orbitofrontal as well as the dorsomedial prefrontal cortex can be differentiated based on quantitative as well as qualitative markers [85], both have equally been suggested in emotion processing and working memory/inhibitory control [86]. The medial prefrontal cortex in particular has been implicated in emotional self-regulation [87], general self-referential activities [88] and emotion-related decision making [89]. Meta-analytic evidence suggests a more generic role of the dmPFC in emotion processing (e.g. appraisal, evaluation, experience, response), non-specific to a particular emotion [90]. In addition, lesion, neurophysiological and neuroimaging evidence have linked the orbitofrontal and dorsomedial prefrontal cortex to stimulus-reinforcement association learning [91]. The ability to rapidly decode and readjust values of different input signals is likely to be crucial to emotional behaviour and may ultimately influence emotional learning. It has been suggested that the observed deficits in decision making may directly result from aberrant emotion processing as for example observed after frontal brain damage [91]. Research has for instance demonstrated that aberrant self-monitoring abilities may be responsible to preclude the generation of social emotions typically associated with the resolution of social mistakes [92]. Finally, a whole line of evidence (e.g. [18,93,94]) has linked the prefrontal cortex to aggression. In its extreme, antisocial personality disorder and psychopathy are exemplary for individuals displaying increased aggressive behaviour and studies of both have linked structural [95,96] and functional [97,98] changes to the prefrontal cortex.
Insula
Both our functional and structural AB meta-analysis have found significant clusters of hypoactivations or altered brain structure within the insula. In addition to that, two smaller clusters reached significance in the left insular cortex during our conjunction analysis, mapping structural and functional alterations in youths with AB. The insula or insular cortex is part of the cerebral cortex forming the base of the lateral sulcus (or sylvian fissure [99]). From a neurodevelopmental perspective it is the first region of the cortex to develop and differentiate around 6 weeks of fetal life [100]. The insula is bi-directionally connected to various brain regions, including the orbitofrontal cortex, anterior cingulate, supplementary motor areas, parietal and temporal cortices, but also to subcortical structures such as the amygdala, basal ganglia and thalamus [99,101]. Connectivity to and from the insula is divided, in that the anterior part of the insula has greater connectivity with the frontal lobe, while posterior parts are more strongly connected to the parietal lobe. Neuroimaging evidence has suggested that the insula may play a key role in the awareness of bodily sensations and affective feelings [84,102]. Meta-analytic data supports this idea, and suggests that the insula is a key player in the evaluation, experience or expression of internally generated emotions [90]. Particularly the left insula, along with frontal and temporal brain regions, is associated with anger [84]. Furthermore, an emotion-specific role of the insula for disgust [103] has been discussed. However, the majority of neuroimaging findings and meta-analytic reviews to date support a generic role of the insula in emotional behaviour (e.g. [84,104]).
Atypical neuronal functioning of the insula (e.g. during tasks of emotion processing and empathy) are linked to AB (e.g. [41,97]). However, so far, both hyper- [45,71] and hypoactivations [39,41,105] are observed during tasks of empathy, face or pain processing. In psychopathy particularly fear conditioning has been linked to aberrant insula activation [106]. Functional atypicalities within the insula are further observed in borderline personality disorder [107], schizophrenia [108], depression [109] or anorexia nervosa [110]. Gray matter volume alterations within the insula are associated with various psychiatric conditions beyond antisocial populations (e.g. [24,96]), including bipolar disorder [111], schizophrenia [56], drug dependence [112], major depression [113] or anorexia nervosa [114].Therefore, the neuronal and structural alterations within the insula may reflect a characteristic of psychiatric conditions per se [99].
Cingulate Cortex
The cingulate cortex showed functional as well as structural foci of significance in each of our two meta-analyses individually. Cytoarchitectonically, the cingulate gyrus may be divided into four functionally independent but interconnected subregions, including the anterior cingulate cortex (emotion), the midcingulate cortex (response selection), the posterior cingulate cortex (personal orientation), and the retrosplenial cortex (memory formation and access) [115]. Overall the cingulate cortex has been implicated in the regulation of cognitive as well as emotional processes [90,115] (e.g. processing of acute pain [116] or affective stimulus material [115]), most likely through an interaction with the prefrontal cortex, anterior insula, premotor area, the striatum and cerebellum [115,117]. We here particularly identified regions within the bilateral anterior cingulate as foci of interest through both our functional and structural meta-analysis. While dorsal aspects of the anterior cingulate have been linked to tasks of executive functioning [118,119], the anterior part of the cingulate is part of the emotion processing network [119,120]. It is further suggested that the cingulate gyrus may serve as a transition and/or interaction zone between affective and cognitive processing [90].
Studies in AB and antisocial personality disorder have found both gray and white matter increases as well as decreases within the cingulate (e.g. [23,68,121,122]); the developmental pathway within this region thus still needs further assessment. Hypoactivation in AB within the cingulate has been reported during tasks of emotion processing [34,35], empathy [41,67], response inhibition [85] and sustained attention [105]. Similarly, individuals with antisocial personality disorder or psychopathic tendencies show reduced activation within the cingulate during tasks of emotion processing and conflict resolution, as for example observed in moral decision making [123,124], deception [125], frustration [126] and emotion processing [127].
Amygdala
Both our functional and structural meta-analyses have identified the right and left-hemispheric amygdala as significant foci of interest, even though this area has not reached significance in our conjunction analysis. The amygdala is crucial for the perception and encoding of emotionally loaded stimulus material and has been suggested as the brain locus of fear (e.g. detection, generation, maintenance of fear and coordination of response in the danger of such) [84,128]. To summarize the existing fMRI evidence, neuronal activation within the amygdala has been observed in healthy individuals in tasks that include arousing stimulus material (e.g. emotionally loaded images [129,130], facial expressions [131,132,133] or words [134,135]), during tasks of empathy [136,137], moral reasoning [138] or when processing potential threats [139]). A range of tasks investigating amygdala responses to different evocative stimulus material led to the suggestion that increased activation within the amygdala may particularly mirror affective processing under acute danger or threat, rather than fear per se [90]. Furthermore, neuronal activation is thought to mirror dispositional affective style [90,140], whereby increased amygdala activity correlates with affective reactivity to negative stimuli. Interestingly, amygdala activation in response to emotionally loaded stimuli may be attenuated by task demand [120,141,142] or comorbid anxiety and depression symptoms [34]. For example, concurrent goal-directed processing can disrupt amygdala activation that is evoked by emotional images [142]. This is in line with meta-analytic evidence indicating that studies employing a cognitive task during affect processing are less likely to demonstrate amygdala activation [90].
Because of its role in aversive conditioning, instrumental learning and fear processing, the amygdala is often chosen as a region of interest in investigations targeting AB, antisocial personality disorder or psychopathy [18]. Amygdala dysfunction is suggested to be one of the core features in the symptomatology of antisocial disorders (e.g. [18,34,43,143]). Structurally, the amygdala is altered in AB similarly as in antisocial personality disorders and psychopathy (e.g. [24,144,145]). Finally, it is to note that the amygdala is strongly interconnected with the orbitofrontal brain regions and alterations in the connectivity between these two centers have been reported in AB and psychopathy (e.g. connectivity between key regions of the emotion processing and regulation network (e.g. [146,147], for a further discussion see following section).
Structure-Function Relationship and Connectivity Findings
While neuroplasticity is known to potentially range from synaptic plasticity to more complex changes (e.g. shrinkage in cell size, neural or glial cell genesis, spine density or even changes in blood flow or interstitial fluid [148]), the neurophysiological basis of experience-induced neuroplasticity is still a matter of extensive research [14]. Some studies indicate that functional and structural measures of plasticity may be related. For example it could be hypothesized that experience-related gray matter volume changes correspond to task-specific processing, or, more precisely, synaptic remodelling within specific processing areas [149]. Another possibility may be that impaired connectivity between key regions leads to the functional alterations observed. For example researchers have argued that the social and emotional deficits seen in AB may be mediated by impaired connectivity between the emotion processing and regulation network [146,147]. These system-specific deficits may be observed by diffusion tensor imaging and tractography measurements. For example, the uncinate fasciculus is a white-matter tract connecting the amygdala and neighbouring anterior temporal lobe with the orbitofrontal cortex and it thus may be involved in facilitating empathy, emotion regulation and socio-cognitive processes [150]. Such models would for example explain why local changes in brain structure cannot always be inferred from purely functional models. For example in individuals with reactive aggression aberrant amygdala activity but intact amygdala structure is observed [151]. In such cases it is possible that impaired fibre connections (e.g. reduced functional anisotropy in the uncinate fasciculus) to and from this area cause the neuronal differences observed [151]. In line with evidence in AB [151] significant differences in the fractional anisotropy (FA) measures of the uncinate fasciculus have been demonstrated in adolescents with conduct disorder [29,39] as well as in adult psychopathy [144,152]. Similarly, studies of intrinsic connectivity (resting state) explore functional networks that are non-stimulus driven and may inform about the basic functional brain architecture while implicating anatomical connectivity of the regions involved [153]. In individuals with antisocial personality disorder this intrinsic connectivity between highly interconnected brain centres is disrupted [154].
Independent of the precise neurophysiological nature of structure-function associations, our results have indicated co-localized structural and functional deficits in right dmPFC and left insular cortex. Based on today’s structure-function knowledge we thus hypothesize that decreased synaptic density may have led to a co-localized decrease within the BOLD response measured through fMRI. However, it has to be noted that here we only investigate co-localized structure-function findings that are based on gray matter volume reductions and functional hypoactivations in AB. This limitation (no volume increases or hyperactivity investigated) is due to the nature of the existing neuroimaging evidence, with only five studies reporting gray matter volume increases and six studies providing evidence for functional hyperactivations in individuals with AB. Further studies comparing adolescents with AB compared to controls are needed in order to examine functional hypoactivations and gray matter volume increases more extensively. Furthermore, only longitudinal research studies will be able to show the precise developmental trajectory of these alterations in detail.
Limitations
Meta-analytic approaches such as the current one have a number of limitations in need for discussion. The presented analyses are first of all limited by the detail and quality of the original research studies. This includes problems of variations within the significance threshold of data reported, insufficient information on possible coordinate transformations and variation in group sizes. Additionally, even though psychosocial factors have been significantly linked to brain structure in AB, none of the studies to date systematically studied the influence of these within their designs. Furthermore, only a small number of studies to date have examined brain structure and function in youths with AB on a whole brain level. We decided that a more stringent inclusion criteria is beneficial over the absolute number of studies entering the analyses, especially in regards to the attempt to truly capture the neuronal and structural phenotype of adolescents with AB. The number of studies entering each analysis therefore is on the lower limit. Contrast analyses are ideally contain a minimum of 15 studies in each dataset to obtain sufficient statistical power (http://brainmap.org/ale/ [51,74,75,76]). Therefore, the current analysis runs the risk of being under-powered.
Most of the studies included here consisted of only, or majority of, male participants (see Tables 1 and 2). Some of the included study designs considered sex-matched clinical and control groups, while others applied a gender covariate within their design (e.g. [26,69]). Two VBM [21,70] and one fMRI [71] study included only female participants. These studies were nevertheless included in the current meta-analyses because the structural alterations observed in girls with CD broadly overlapped with those previously reported in male samples only [21]. But while the current population included mirrors the occurrence of AB in the general population (e.g. higher number of males with AB [19]), research has shown that it may be crucial to differentiate clinical cases based on gender in future research studies (e.g. [155]). Specifically, to determine possible gender related differences of structural and functional characteristics in individuals with AB, a comparison between meta-analyses of studies examining females and those examining males separately would have been of interest, but was not possible due to the small number of studies that are available for each group individually.
Another potential caveat is the fact that clinical and subclinical forms of aggressive behaviour are often associated with comorbid diagnoses, most prominently attention-deficit hyperactivity disorder (ADHD; reported in up to 69% of CD patients [156]) and anxiety [19]. To date there is no neuroimaging evidence investigating pure diagnosis of clinical manifestations of aggressive behaviour (e.g. CD or ODD) [157]. Researchers argue whether aggressive behaviour in combination with ADHD even posits a distinct subtype or not [158] and common neurobiological pathways are considered [157]. Overall it can be concluded that neuroimaging research studies on aggressive behaviour in children and adolescents to date are characterized by diverse approaches in regards to the sample selection and definition, all of which have their justification and pitfalls [159]. Ultimately, only a comparisons of both, pure and comorbid groups will be able to inform about the specificity and predictive value of either definition. Here we included adolescents with clinical and subclinical forms of aggressive behaviour, most of which have comorbid ADHD symptoms (e.g. [21,22,23,26,34,39,40,41,43,44,66,67,71,72,73]. Many of the included studies report no differences in results when controlling for ADHD (through exclusion or a covariate within the study design; [34,39,43,44,48,71,72]).
Similar problems are IQ differences, drug use or socioeconomic status, all of which are a characteristic of populations with aggressive behaviour. Studies included in the current meta-analysis have all matched their participants according to IQ measures [22,39,40,41,43,44,66,68,69,71,72,73] or used IQ as a covariate within their study design [21,23,25,26,34,67]. Drug use and socio-economic status were controlled for in some, but not all, studies and further research is needed using a more careful sample characterisation in order to inform about the impact of these variables on brain structure and function.
It is also to consider that the diagnosis of conduct disorder (clinical manifestation of AB) may encompass at least two clinically relevant subgroups. While the first group exhibits callous-unemotional traits (e.g. reduced guilt, callousness, uncaring behaviour and reduced empathy) and heightened risk of persistent antisocial behaviour, the second group is characterized by heightened threat sensitivity and reactive aggression [1,160]. Callous-unemotional traits are highly heritable [161], expressed as early as at two years of age [162] and are predictive of the most severe and persistent variant of conduct disorder [163,164]. Studies also indicate that this severity may significantly impact the neuronal alterations observed [22,39,71,165]. To summarize, while we were unable to constrain the current meta-analysis based on potential subtypification and gender variables, these factors may pose an exciting view on data analysis strategies and interpretations for future studies. For all the reasons noted, the current results have to be interpreted with caution. However, multimodal neuroimaging methods combining two or more functional (fMRI and/or EEG) and structural (MRI and/or DTI) approaches are suggested to provide a more sensitive measure in comparison to unimodal imaging for disease classification [166]. Furthermore, we think that the confounding variables discussed here have influenced the functional and structural meta-analyses similarly.
Overall, we could demonstrate that structural and functional alterations in adolescents with AB co-localize within key regions of the emotion processing and regulation network (e.g. prefrontal and insular cortex). Thus, our current analysis, using an activation likelihood estimation approach, provides an important step towards a more focused method of neuroimaging in AB. Future studies need to determine whether the here identified convergent clusters of neuronal and structural alterations may be applicable for clinical purposes (for example an improved pathophysiological description of individuals with AB) or whether a further specification (e.g. based on subtypes and gender) may be needed. However, the coordinates presented here can serve as non-independent regions of interest for future studies in AB, conduct disorder or in individuals with AB or antisocial/psychopathic tendencies.
Summary and Conclusion
Aggressive behaviour constitutes a major issue of public health and increased knowledge about the behavioural and neuronal underpinnings of AB are crucial for the development of novel and implementation of existing treatment strategies. However, single site studies often suffer problems of small sample size and thus power issues. Quantitative meta-analysis techniques using activation likelihood estimations as implemented here offer a unique opportunity to investigate consistency of results between several studies investigating the same research question and population. We have implicated several brain regions of the emotion processing and regulation network to show hypoactivations and gray matter volume reductions in adolescents with AB (including prefrontal brain regions, amygdala, insular and cingulate cortex) and demonstrated that functional and structural alterations in AB co-localize within right dmPFC and left insular cortex.
Overall, we are in line with meta-analytic work as well as structural, functional and connectivity findings that make a strong point for the involvement of a network of brain areas responsible for emotion processing and regulations. This network is impacted in individuals with AB and antisocial personality disorder/psychopathy. However, much still needs to be investigated. For example, study findings differ in regards to hypo- or hyperactivations and gray matter volume reductions or increases in different regions of the emotion processing and regulation network. Due to power constraints, the current meta-analysis only investigated hypoactivations and gray matter volume reductions in youths with AB and no hyperactivations or increases in brain structure. Future studies implementing longitudinal designs may be able to shed more light on the developmental pathway as well as onto typical and atypical trajectories within the regions reported. Such longitudinal designs will further allow the investigation of the bidirectional influence of biological and psychosocial influences in AB.
Supporting Information
(DOC)
Acknowledgments
We thank Kübra Özoglu and Lea Klüwer for their help during the manuscript preparation.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Blair RJ, Leibenluft E, Pine DS (2014) Conduct disorder and callous-unemotional traits in youth. N Engl J Med 371: 2207–2216. 10.1056/NEJMra1315612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Association AP (2013) Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC.
- 3. Scott S, Knapp M, Henderson J, Maughan B (2001) Financial cost of social exclusion: follow up study of antisocial children into adulthood. BMJ 323: 191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ravens-Sieberer U, Wille N, Erhart M, Bettge S, Wittchen HU, et al. (2008) Prevalence of mental health problems among children and adolescents in Germany: results of the BELLA study within the National Health Interview and Examination Survey. Eur Child Adolesc Psychiatry 17 Suppl 1: 22–33. 10.1007/s00787-008-1003-2 [DOI] [PubMed] [Google Scholar]
- 5. Pedersen W, Mastekaasa A (2011) Conduct disorder symptoms and subsequent pregnancy, child-birth and abortion: a population-based longitudinal study of adolescents. J Adolesc 34: 1025–1033. 10.1016/j.adolescence.2010.11.005 [DOI] [PubMed] [Google Scholar]
- 6. Bardone AM, Moffitt TE, Caspi A, Dickson N, Stanton WR, et al. (1998) Adult physical health outcomes of adolescent girls with conduct disorder, depression, and anxiety. J Am Acad Child Adolesc Psychiatry 37: 594–601. [DOI] [PubMed] [Google Scholar]
- 7. Lahey BB, Loeber R, Burke JD, Applegate B (2005) Predicting future antisocial personality disorder in males from a clinical assessment in childhood. J Consult Clin Psychol 73: 389–399. [DOI] [PubMed] [Google Scholar]
- 8. Gao Y, Glenn AL, Schug RA, Yang Y, Raine A (2009) The neurobiology of psychopathy: a neurodevelopmental perspective. Can J Psychiatry 54: 813–823. [DOI] [PubMed] [Google Scholar]
- 9. Glenn AL, Raine A (2008) The neurobiology of psychopathy. Psychiatr Clin North Am 31: 463–475, vii. 10.1016/j.psc.2008.03.004 [DOI] [PubMed] [Google Scholar]
- 10. Frick PJ, Viding E (2009) Antisocial behavior from a developmental psychopathology perspective. Dev Psychopathol 21: 1111–1131. 10.1017/S0954579409990071 [DOI] [PubMed] [Google Scholar]
- 11. Vloet TD, Konrad K, Huebner T, Herpertz S, Herpertz-Dahlmann B (2008) Structural and functional MRI- findings in children and adolescents with antisocial behavior. Behav Sci Law 26: 99–111. 10.1002/bsl.794 [DOI] [PubMed] [Google Scholar]
- 12. McEwen BS (2003) Early life influences on life-long patterns of behavior and health. Ment Retard Dev Disabil Res Rev 9: 149–154. [DOI] [PubMed] [Google Scholar]
- 13. Raine A, Yang Y (2006) Neural foundations to moral reasoning and antisocial behavior. Soc Cogn Affect Neurosci 1: 203–213. 10.1093/scan/nsl033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmidt-Wilcke T, Rosengarth K, Luerding R, Bogdahn U, Greenlee MW (2010) Distinct patterns of functional and structural neuroplasticity associated with learning Morse code. Neuroimage 51: 1234–1241. 10.1016/j.neuroimage.2010.03.042 [DOI] [PubMed] [Google Scholar]
- 15. Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, et al. (2000) Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A 97: 4398–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, et al. (2004) Neuroplasticity: changes in grey matter induced by training. Nature 427: 311–312. [DOI] [PubMed] [Google Scholar]
- 17. Driemeyer J, Boyke J, Gaser C, Buchel C, May A (2008) Changes in gray matter induced by learning—revisited. PLoS One 3: e2669 10.1371/journal.pone.0002669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blair RJ (2003) Neurobiological basis of psychopathy. Br J Psychiatry 182: 5–7. [DOI] [PubMed] [Google Scholar]
- 19. Loeber R, Burke JD, Lahey BB, Winters A, Zera M (2000) Oppositional defiant and conduct disorder: a review of the past 10 years, part I. J Am Acad Child Adolesc Psychiatry 39: 1468–1484. [DOI] [PubMed] [Google Scholar]
- 20. Crowe SL, Blair RJR (2008) The development of antisocial behavior: What can we learn from functional neuroimaging studies? Development and Psychopathology 20: 1145–1159. 10.1017/S0954579408000540 [DOI] [PubMed] [Google Scholar]
- 21. Fairchild G, Hagan CC, Walsh ND, Passamonti L, Calder AJ, et al. (2013) Brain structure abnormalities in adolescent girls with conduct disorder. J Child Psychol Psychiatry 54: 86–95. 10.1111/j.1469-7610.2012.02617.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fairchild G, Passamonti L, Hurford G, Hagan CC, von dem Hagen EA, et al. (2011) Brain structure abnormalities in early-onset and adolescent-onset conduct disorder. Am J Psychiatry 168: 624–633. 10.1176/appi.ajp.2010.10081184 [DOI] [PubMed] [Google Scholar]
- 23. De Brito SA, Mechelli A, Wilke M, Laurens KR, Jones AP, et al. (2009) Size matters: increased grey matter in boys with conduct problems and callous-unemotional traits. Brain 132: 843–852. 10.1093/brain/awp011 [DOI] [PubMed] [Google Scholar]
- 24. Sterzer P, Stadler C, Poustka F, Kleinschmidt A (2007) A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. Neuroimage 37: 335–342. [DOI] [PubMed] [Google Scholar]
- 25. Hyatt CJ, Haney-Caron E, Stevens MC (2012) Cortical thickness and folding deficits in conduct-disordered adolescents. Biol Psychiatry 72: 207–214. 10.1016/j.biopsych.2011.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wallace GL, White SF, Robustelli B, Sinclair S, Hwang S, et al. (2014) Cortical and subcortical abnormalities in youths with conduct disorder and elevated callous-unemotional traits. J Am Acad Child Adolesc Psychiatry 53: 456–465 e451. 10.1016/j.jaac.2013.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Passamonti L, Fairchild G, Fornito A, Goodyer IM, Nimmo-Smith I, et al. (2012) Abnormal anatomical connectivity between the amygdala and orbitofrontal cortex in conduct disorder. PLoS One 7: e48789 10.1371/journal.pone.0048789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Finger EC, Marsh A, Blair KS, Majestic C, Evangelou I, et al. (2012) Impaired functional but preserved structural connectivity in limbic white matter tracts in youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. Psychiatry Res 202: 239–244. 10.1016/j.pscychresns.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sarkar S, Craig MC, Catani M, Dell'acqua F, Fahy T, et al. (2013) Frontotemporal white-matter microstructural abnormalities in adolescents with conduct disorder: a diffusion tensor imaging study. Psychol Med 43: 401–411. 10.1017/S003329171200116X [DOI] [PubMed] [Google Scholar]
- 30. Haney-Caron E, Caprihan A, Stevens MC (2014) DTI-measured white matter abnormalities in adolescents with Conduct Disorder. J Psychiatr Res 48: 111–120. 10.1016/j.jpsychires.2013.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang J, Zhu X, Wang X, Gao J, Shi H, et al. (2014) Increased structural connectivity in corpus callosum in adolescent males with conduct disorder. J Am Acad Child Adolesc Psychiatry 53: 466–475 e461. 10.1016/j.jaac.2013.12.015 [DOI] [PubMed] [Google Scholar]
- 32. Zhang J, Gao J, Shi H, Huang B, Wang X, et al. (2014) Sex differences of uncinate fasciculus structural connectivity in individuals with conduct disorder. Biomed Res Int 2014: 673165 10.1155/2014/673165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li TQ, Mathews VP, Wang Y, Dunn D, Kronenberger W (2005) Adolescents with disruptive behavior disorder investigated using an optimized MR diffusion tensor imaging protocol. Ann N Y Acad Sci 1064: 184–192. [DOI] [PubMed] [Google Scholar]
- 34. Sterzer P, Stadler C, Krebs A, Kleinschmidt A, Poustka F (2005) Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biol Psychiatry 57: 7–15. [DOI] [PubMed] [Google Scholar]
- 35. Stadler C, Sterzer P, Schmeck K, Krebs A, Kleinschmidt A, et al. (2007) Reduced anterior cingulate activation in aggressive children and adolescents during affective stimulation: association with temperament traits. J Psychiatr Res 41: 410–417. [DOI] [PubMed] [Google Scholar]
- 36. Herpertz SC, Huebner T, Marx I, Vloet TD, Fink GR, et al. (2008) Emotional processing in male adolescents with childhood-onset conduct disorder. J Child Psychol Psychiatry 49: 781–791. 10.1111/j.1469-7610.2008.01905.x [DOI] [PubMed] [Google Scholar]
- 37. Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, et al. (2008) Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry 165: 712–720. 10.1176/appi.ajp.2007.07071145 [DOI] [PubMed] [Google Scholar]
- 38. Jones AP, Laurens KR, Herba CM, Barker GJ, Viding E (2009) Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. Am J Psychiatry 166: 95–102. 10.1176/appi.ajp.2008.07071050 [DOI] [PubMed] [Google Scholar]
- 39. Passamonti L, Fairchild G, Goodyer IM, Hurford G, Hagan CC, et al. (2010) Neural abnormalities in early-onset and adolescence-onset conduct disorder. Arch Gen Psychiatry 67: 729–738. 10.1001/archgenpsychiatry.2010.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. White SF, Williams WC, Brislin SJ, Sinclair S, Blair KS, et al. (2012) Reduced activity within the dorsal endogenous orienting of attention network to fearful expressions in youth with disruptive behavior disorders and psychopathic traits. Dev Psychopathol 24: 1105–1116. 10.1017/S0954579412000569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lockwood PL, Sebastian CL, McCrory EJ, Hyde ZH, Gu X, et al. (2013) Association of callous traits with reduced neural response to others' pain in children with conduct problems. Curr Biol 23: 901–905. 10.1016/j.cub.2013.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mathews VP, Kronenberger WG, Wang Y, Lurito JT, Lowe MJ, et al. (2005) Media violence exposure and frontal lobe activation measured by functional magnetic resonance imaging in aggressive and nonaggressive adolescents. J Comput Assist Tomogr 29: 287–292. [DOI] [PubMed] [Google Scholar]
- 43. Sebastian CL, McCrory EJ, Dadds MR, Cecil CA, Lockwood PL, et al. (2014) Neural responses to fearful eyes in children with conduct problems and varying levels of callous-unemotional traits. Psychol Med 44: 99–109. 10.1017/S0033291713000482 [DOI] [PubMed] [Google Scholar]
- 44. Marsh AA, Finger EC, Fowler KA, Adalio CJ, Jurkowitz IT, et al. (2013) Empathic responsiveness in amygdala and anterior cingulate cortex in youths with psychopathic traits. J Child Psychol Psychiatry 54: 900–910. 10.1111/jcpp.12063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Decety J, Michalska KJ, Akitsuki Y, Lahey BB (2009) Atypical empathic responses in adolescents with aggressive conduct disorder: a functional MRI investigation. Biol Psychol 80: 203–211. 10.1016/j.biopsycho.2008.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sebastian CL, McCrory EJ, Cecil CA, Lockwood PL, De Brito SA, et al. (2012) Neural responses to affective and cognitive theory of mind in children with conduct problems and varying levels of callous-unemotional traits. Arch Gen Psychiatry 69: 814–822. 10.1001/archgenpsychiatry.2011.2070 [DOI] [PubMed] [Google Scholar]
- 47. Finger EC, Marsh AA, Blair KS, Reid ME, Sims C, et al. (2011) Disrupted reinforcement signaling in the orbitofrontal cortex and caudate in youths with conduct disorder or oppositional defiant disorder and a high level of psychopathic traits. Am J Psychiatry 168: 152–162. 10.1176/appi.ajp.2010.10010129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. White SF, Pope K, Sinclair S, Fowler KA, Brislin SJ, et al. (2013) Disrupted expected value and prediction error signaling in youths with disruptive behavior disorders during a passive avoidance task. Am J Psychiatry 170: 315–323. 10.1176/appi.ajp.2012.12060840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dalwani MS, Tregellas JR, Andrews-Hanna JR, Mikulich-Gilbertson SK, Raymond KM, et al. (2014) Default mode network activity in male adolescents with conduct and substance use disorder. Drug Alcohol Depend 134: 242–250. 10.1016/j.drugalcdep.2013.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rubia K, Halari R, Smith AB, Mohammed M, Scott S, et al. (2008) Dissociated functional brain abnormalities of inhibition in boys with pure conduct disorder and in boys with pure attention deficit hyperactivity disorder. Am J Psychiatry 165: 889–897. 10.1176/appi.ajp.2008.07071084 [DOI] [PubMed] [Google Scholar]
- 51. Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, et al. (2009) Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 30: 2907–2926. 10.1002/hbm.20718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Raemaekers M, du Plessis S, Ramsey NF, Weusten JM, Vink M (2012) Test-retest variability underlying fMRI measurements. Neuroimage 60: 717–727. 10.1016/j.neuroimage.2011.11.061 [DOI] [PubMed] [Google Scholar]
- 53. Stark CE, Squire LR (2001) When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci U S A 98: 12760–12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kollndorfer K, Krajnik J, Woitek R, Freiherr J, Prayer D, et al. (2013) Altered likelihood of brain activation in attention and working memory networks in patients with multiple sclerosis: an ALE meta-analysis. Neurosci Biobehav Rev 37: 2699–2708. 10.1016/j.neubiorev.2013.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fusar-Poli P, Borgwardt S, Crescini A, Deste G, Kempton MJ, et al. (2011) Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci Biobehav Rev 35: 1175–1185. 10.1016/j.neubiorev.2010.12.005 [DOI] [PubMed] [Google Scholar]
- 56. Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, et al. (2008) Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry 64: 774–781. 10.1016/j.biopsych.2008.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Linkersdorfer J, Lonnemann J, Lindberg S, Hasselhorn M, Fiebach CJ (2012) Grey matter alterations co-localize with functional abnormalities in developmental dyslexia: an ALE meta-analysis. PLoS One 7: e43122 10.1371/journal.pone.0043122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schwindt GC, Black SE (2009) Functional imaging studies of episodic memory in Alzheimer's disease: a quantitative meta-analysis. Neuroimage 45: 181–190. 10.1016/j.neuroimage.2008.11.024 [DOI] [PubMed] [Google Scholar]
- 59. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339: b2535 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cappadocia MC, Desrocher M, Pepler D, Schroeder JH (2009) Contextualizing the neurobiology of conduct disorder in an emotion dysregulation framework. Clin Psychol Rev 29: 506–518. 10.1016/j.cpr.2009.06.001 [DOI] [PubMed] [Google Scholar]
- 61. Viding E, McCrory EJ (2012) Genetic and neurocognitive contributions to the development of psychopathy. Dev Psychopathol 24: 969–983. 10.1017/S095457941200048X [DOI] [PubMed] [Google Scholar]
- 62. Dolan MC (2010) What imaging tells us about violence in anti-social men. Crim Behav Ment Health 20: 199–214. 10.1002/cbm.771 [DOI] [PubMed] [Google Scholar]
- 63. Nguyen TN, Faulkner D, Rampono J, Blair E (2010) In utero exposure to antidepressant medication and neonatal growth outcomes: closer examination of the evidence is needed. Aust N Z J Psychiatry 44: 766–767. [DOI] [PubMed] [Google Scholar]
- 64. Anderson NE, Kiehl KA (2014) Psychopathy: developmental perspectives and their implications for treatment. Restor Neurol Neurosci 32: 103–117. 10.3233/RNN-139001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fairchild G, van Goozen SH, Calder AJ, Goodyer IM (2013) Research review: evaluating and reformulating the developmental taxonomic theory of antisocial behaviour. J Child Psychol Psychiatry 54: 924–940. 10.1111/jcpp.12102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Huebner T, Vloet TD, Marx I, Konrad K, Fink GR, et al. (2008) Morphometric brain abnormalities in boys with conduct disorder. J Am Acad Child Adolesc Psychiatry 47: 540–547. 10.1097/CHI.0b013e3181676545 [DOI] [PubMed] [Google Scholar]
- 67. Dalwani M, Sakai JT, Mikulich-Gilbertson SK, Tanabe J, Raymond K, et al. (2011) Reduced cortical gray matter volume in male adolescents with substance and conduct problems. Drug Alcohol Depend 118: 295–305. 10.1016/j.drugalcdep.2011.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fahim C, He Y, Yoon U, Chen J, Evans A, et al. (2011) Neuroanatomy of childhood disruptive behavior disorders. Aggress Behav 37: 326–337. 10.1002/ab.20396 [DOI] [PubMed] [Google Scholar]
- 69. Stevens MC, Haney-Caron E (2012) Comparison of brain volume abnormalities between ADHD and conduct disorder in adolescence. J Psychiatry Neurosci 37: 389–398. 10.1503/jpn.110148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dalwani MS, McMahon MA, Mikulich-Gilbertson SK, Young SE, Regner MF, et al. (2015) Female adolescents with severe substance and conduct problems have substantially less brain gray matter volume. PLoS One 10: e0126368 10.1371/journal.pone.0126368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fairchild G, Hagan CC, Passamonti L, Walsh ND, Goodyer IM, et al. (2014) Atypical neural responses during face processing in female adolescents with conduct disorder. J Am Acad Child Adolesc Psychiatry 53: 677–687 e675. 10.1016/j.jaac.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. O'Nions E, Sebastian CL, McCrory E, Chantiluke K, Happe F, et al. (2014) Neural bases of Theory of Mind in children with autism spectrum disorders and children with conduct problems and callous-unemotional traits. Dev Sci 17: 786–796. 10.1111/desc.12167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Marsh AA, Finger EC, Fowler KA, Jurkowitz ITN, Schechter JC, et al. (2011) Reduced amygdala-orbitofrontal connectivity during moral judgments in youths with disruptive behavior disorders and psychopathic traits. Psychiatry Research-Neuroimaging 194: 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, et al. (2005) ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp 25: 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, et al. (2012) Minimizing within-experiment and within-group effects in Activation Likelihood Estimation meta-analyses. Hum Brain Mapp 33: 1–13. 10.1002/hbm.21186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT (2012) Activation likelihood estimation meta-analysis revisited. Neuroimage 59: 2349–2361. 10.1016/j.neuroimage.2011.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, et al. (2007) Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp 28: 1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Laird AR, Robinson JL, McMillan KM, Tordesillas-Gutierrez D, Moran ST, et al. (2010) Comparison of the disparity between Talairach and MNI coordinates in functional neuroimaging data: validation of the Lancaster transform. Neuroimage 51: 677–683. 10.1016/j.neuroimage.2010.02.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, et al. (2000) Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10: 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, et al. (1997) Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Hum Brain Mapp 5: 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, et al. (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- 82. Rubia K (2011) "Cool" inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus "hot" ventromedial orbitofrontal-limbic dysfunction in conduct disorder: a review. Biol Psychiatry 69: e69–87. 10.1016/j.biopsych.2010.09.023 [DOI] [PubMed] [Google Scholar]
- 83. Ochsner KN, Silvers JA, Buhle JT (2012) Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci 1251: E1–24. 10.1111/j.1749-6632.2012.06751.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF (2012) The brain basis of emotion: a meta-analytic review. Behav Brain Sci 35: 121–143. 10.1017/S0140525X11000446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zald DH (2007) Orbital versus dorsolateral prefrontal cortex: anatomical insights into content versus process differentiation models of the prefrontal cortex. Ann N Y Acad Sci 1121: 395–406. [DOI] [PubMed] [Google Scholar]
- 86. Golkar A, Lonsdorf TB, Olsson A, Lindstrom KM, Berrebi J, et al. (2012) Distinct contributions of the dorsolateral prefrontal and orbitofrontal cortex during emotion regulation. PLoS One 7: e48107 10.1371/journal.pone.0048107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Davidson RJ, Jackson DC, Kalin NH (2000) Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychol Bull 126: 890–909. [DOI] [PubMed] [Google Scholar]
- 88. D'Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, et al. (2007) Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. J Cogn Neurosci 19: 935–944. [DOI] [PubMed] [Google Scholar]
- 89. Euston DR, Gruber AJ, McNaughton BL (2012) The role of medial prefrontal cortex in memory and decision making. Neuron 76: 1057–1070. 10.1016/j.neuron.2012.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Phan KL, Wager T, Taylor SF, Liberzon I (2002) Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 16: 331–348. [DOI] [PubMed] [Google Scholar]
- 91. Bechara A, Damasio H, Damasio AR (2000) Emotion, decision making and the orbitofrontal cortex. Cereb Cortex 10: 295–307. [DOI] [PubMed] [Google Scholar]
- 92. Beer JS, John OP, Scabini D, Knight RT (2006) Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion-cognition interactions. J Cogn Neurosci 18: 871–879. [DOI] [PubMed] [Google Scholar]
- 93. Beyer F, Munte TF, Gottlich M, Kramer UM (2014) Orbitofrontal Cortex Reactivity to Angry Facial Expression in a Social Interaction Correlates with Aggressive Behavior. Cereb Cortex. [DOI] [PubMed] [Google Scholar]
- 94. Potegal M (2012) Temporal and frontal lobe initiation and regulation of the top-down escalation of anger and aggression. Behav Brain Res 231: 386–395. 10.1016/j.bbr.2011.10.049 [DOI] [PubMed] [Google Scholar]
- 95. Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P (2000) Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Arch Gen Psychiatry 57: 119–127; discussion 128–119. [DOI] [PubMed] [Google Scholar]
- 96. Ermer E, Cope LM, Nyalakanti PK, Calhoun VD, Kiehl KA (2012) Aberrant paralimbic gray matter in criminal psychopathy. J Abnorm Psychol 121: 649–658. 10.1037/a0026371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Decety J, Skelly LR, Kiehl KA (2013) Brain response to empathy-eliciting scenarios involving pain in incarcerated individuals with psychopathy. JAMA Psychiatry 70: 638–645. 10.1001/jamapsychiatry.2013.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Liu H, Liao J, Jiang W, Wang W (2014) Changes in low-frequency fluctuations in patients with antisocial personality disorder revealed by resting-state functional MRI. PLoS One 9: e89790 10.1371/journal.pone.0089790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gasquoine PG (2014) Contributions of the insula to cognition and emotion. Neuropsychol Rev 24: 77–87. 10.1007/s11065-014-9246-9 [DOI] [PubMed] [Google Scholar]
- 100. Afif A, Bouvier R, Buenerd A, Trouillas J, Mertens P (2007) Development of the human fetal insular cortex: study of the gyration from 13 to 28 gestational weeks. Brain Struct Funct 212: 335–346. [DOI] [PubMed] [Google Scholar]
- 101. Dupont S, Bouilleret V, Hasboun D, Semah F, Baulac M (2003) Functional anatomy of the insula: new insights from imaging. Surg Radiol Anat 25: 113–119. [DOI] [PubMed] [Google Scholar]
- 102. Craig AD (2009) How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci 10: 59–70. 10.1038/nrn2555 [DOI] [PubMed] [Google Scholar]
- 103. Phillips ML, Young AW, Senior C, Brammer M, Andrew C, et al. (1997) A specific neural substrate for perceiving facial expressions of disgust. Nature 389: 495–498. [DOI] [PubMed] [Google Scholar]
- 104. Phan KL, Wager TD, Taylor SF, Liberzon I (2004) Functional neuroimaging studies of human emotions. CNS Spectr 9: 258–266. [DOI] [PubMed] [Google Scholar]
- 105. Rubia K, Smith AB, Halari R, Matsukura F, Mohammad M, et al. (2009) Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. Am J Psychiatry 166: 83–94. 10.1176/appi.ajp.2008.08020212 [DOI] [PubMed] [Google Scholar]
- 106. Birbaumer N, Veit R, Lotze M, Erb M, Hermann C, et al. (2005) Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Arch Gen Psychiatry 62: 799–805. [DOI] [PubMed] [Google Scholar]
- 107. Koenigsberg HW, Siever LJ, Lee H, Pizzarello S, New AS, et al. (2009) Neural correlates of emotion processing in borderline personality disorder. Psychiatry Res 172: 192–199. 10.1016/j.pscychresns.2008.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Manoliu A, Riedl V, Doll A, Bauml JG, Muhlau M, et al. (2013) Insular Dysfunction Reflects Altered Between-Network Connectivity and Severity of Negative Symptoms in Schizophrenia during Psychotic Remission. Front Hum Neurosci 7: 216 10.3389/fnhum.2013.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Manoliu A, Meng C, Brandl F, Doll A, Tahmasian M, et al. (2014) Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Frontiers in Human Neuroscience 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bar KJ, Berger S, Schwier C, Wutzler U, Beissner F (2013) Insular dysfunction and descending pain inhibition in anorexia nervosa. Acta Psychiatrica Scandinavica 127: 269–278. 10.1111/j.1600-0447.2012.01896.x [DOI] [PubMed] [Google Scholar]
- 111. Selvaraj S, Arnone D, Job D, Stanfield A, Farrow TF, et al. (2012) Grey matter differences in bipolar disorder: a meta-analysis of voxel-based morphometry studies. Bipolar Disord 14: 135–145. 10.1111/j.1399-5618.2012.01000.x [DOI] [PubMed] [Google Scholar]
- 112. Garavan H (2010) Insula and drug cravings. Brain Struct Funct 214: 593–601. 10.1007/s00429-010-0259-8 [DOI] [PubMed] [Google Scholar]
- 113. Bora E, Fornito A, Pantelis C, Yucel M (2012) Gray matter abnormalities in Major Depressive Disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord 138: 9–18. 10.1016/j.jad.2011.03.049 [DOI] [PubMed] [Google Scholar]
- 114. Nunn K, Frampton I, Fuglset TS, Torzsok-Sonnevend M, Lask B (2011) Anorexia nervosa and the insula. Med Hypotheses 76: 353–357. 10.1016/j.mehy.2010.10.038 [DOI] [PubMed] [Google Scholar]
- 115. Vogt BA (2005) Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 6: 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, et al. (2011) The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci 12: 154–167. 10.1038/nrn2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Derbyshire SW (2000) Exploring the pain "neuromatrix". Curr Rev Pain 4: 467–477. [DOI] [PubMed] [Google Scholar]
- 118. Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S (2004) The role of the medial frontal cortex in cognitive control. Science 306: 443–447. [DOI] [PubMed] [Google Scholar]
- 119. Botvinick MM (2007) Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci 7: 356–366. [DOI] [PubMed] [Google Scholar]
- 120. Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J (2006) Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 51: 871–882. [DOI] [PubMed] [Google Scholar]
- 121. Yeh PH, Zhu HT, Nicoletti MA, Miles GA, Baloch H, et al. (2009) Different Regional Patterns of Gray Matter Volume and Cortical Thickness between Adolescent Bipolar and Major Depressive Disorders. Biological Psychiatry 65: 241s–241s. [Google Scholar]
- 122. Wu DX, Zhao Y, Liao J, Yin HF, Wang W (2011) White matter abnormalities in young males with antisocial personality disorder Evidence from voxel-based morphometry-diffeomorphic anatomical registration using exponentiated lie algebra analysis. Neural Regeneration Research 6: 1965–1970. [Google Scholar]
- 123. Glenn AL, Raine A, Schug RA (2009) The neural correlates of moral decision-making in psychopathy. Mol Psychiatry 14: 5–6. 10.1038/mp.2008.104 [DOI] [PubMed] [Google Scholar]
- 124. Prehn K, Schlagenhauf F, Schulze L, Berger C, Vohs K, et al. (2013) Neural correlates of risk taking in violent criminal offenders characterized by emotional hypo- and hyper-reactivity. Soc Neurosci 8: 136–147. 10.1080/17470919.2012.686923 [DOI] [PubMed] [Google Scholar]
- 125. Jiang W, Liu H, Liao J, Ma X, Rong P, et al. (2013) A Functional Mri Study of Deception among Offenders with Antisocial Personality Disorders (Vol 244, Pg 90, 2013). Neuroscience 252: 125–125. [DOI] [PubMed] [Google Scholar]
- 126. Pawliczek CM, Derntl B, Kellermann T, Gur RC, Schneider F, et al. (2013) Anger under Control: Neural Correlates of Frustration as a Function of Trait Aggression. Plos One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, et al. (2001) Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biol Psychiatry 50: 677–684. [DOI] [PubMed] [Google Scholar]
- 128. LeDoux JE (2000) Emotion circuits in the brain. Annu Rev Neurosci 23: 155–184. [DOI] [PubMed] [Google Scholar]
- 129. Irwin W, Davidson RJ, Lowe MJ, Mock BJ, Sorenson JA, et al. (1996) Human amygdala activation detected with echo-planar functional magnetic resonance imaging. Neuroreport 7: 1765–1769. [DOI] [PubMed] [Google Scholar]
- 130. Garavan H, Pendergrass JC, Ross TJ, Stein EA, Risinger RC (2001) Amygdala response to both positively and negatively valenced stimuli. Neuroreport 12: 2779–2783. [DOI] [PubMed] [Google Scholar]
- 131. Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, et al. (1998) Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 18: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Vuilleumier P, Armony JL, Driver J, Dolan RJ (2001) Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron 30: 829–841. [DOI] [PubMed] [Google Scholar]
- 133. Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, et al. (1998) A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain 121 (Pt 1): 47–57. [DOI] [PubMed] [Google Scholar]
- 134. Kensinger EA, Schacter DL (2006) Processing emotional pictures and words: effects of valence and arousal. Cogn Affect Behav Neurosci 6: 110–126. [DOI] [PubMed] [Google Scholar]
- 135. Hamann SB, Ely TD, Hoffman JM, Kilts CD (2002) Ecstasy and agony: activation of the human amygdala in positive and negative emotion. Psychol Sci 13: 135–141. [DOI] [PubMed] [Google Scholar]
- 136. Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL (2003) Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci U S A 100: 5497–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, et al. (1999) Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci 11: 1891–1898. [DOI] [PubMed] [Google Scholar]
- 138. Luo Q, Nakic M, Wheatley T, Richell R, Martin A, et al. (2006) The neural basis of implicit moral attitude—an IAT study using event-related fMRI. Neuroimage 30: 1449–1457. [DOI] [PubMed] [Google Scholar]
- 139. Phelps EA, O'Connor KJ, Gatenby JC, Gore JC, Grillon C, et al. (2001) Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci 4: 437–441. [DOI] [PubMed] [Google Scholar]
- 140. Davidson RJ, Irwin W (1999) The functional neuroanatomy of emotion and affective style. Trends Cogn Sci 3: 11–21. [DOI] [PubMed] [Google Scholar]
- 141. Mitchell DG, Nakic M, Fridberg D, Kamel N, Pine DS, et al. (2007) The impact of processing load on emotion. Neuroimage 34: 1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Blair KS, Smith BW, Mitchell DGV, Morton J, Vythilingam M, et al. (2007) Modulation of emotion by cognition and cognition by emotion. Neuroimage 35: 430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Dolan MC, Fullam RS (2009) Psychopathy and functional magnetic resonance imaging blood oxygenation level-dependent responses to emotional faces in violent patients with schizophrenia. Biol Psychiatry 66: 570–577. 10.1016/j.biopsych.2009.03.019 [DOI] [PubMed] [Google Scholar]
- 144. Craig MC, Catani M, Deeley Q, Latham R, Daly E, et al. (2009) Altered connections on the road to psychopathy. Mol Psychiatry 14: 946–953, 907. 10.1038/mp.2009.40 [DOI] [PubMed] [Google Scholar]
- 145. Boccardi M, Frisoni GB, Hare RD, Cavedo E, Najt P, et al. (2011) Cortex and amygdala morphology in psychopathy. Psychiatry Res 193: 85–92. 10.1016/j.pscychresns.2010.12.013 [DOI] [PubMed] [Google Scholar]
- 146. Blair RJ (2007) The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends Cogn Sci 11: 387–392. [DOI] [PubMed] [Google Scholar]
- 147. van Honk J, Schutter DJ (2006) Unmasking feigned sanity: a neurobiological model of emotion processing in primary psychopathy. Cogn Neuropsychiatry 11: 285–306. [DOI] [PubMed] [Google Scholar]
- 148. May A, Hajak G, Ganssbauer S, Steffens T, Langguth B, et al. (2007) Structural brain alterations following 5 days of intervention: dynamic aspects of neuroplasticity. Cereb Cortex 17: 205–210. [DOI] [PubMed] [Google Scholar]
- 149. Ilg R, Wohlschlager AM, Gaser C, Liebau Y, Dauner R, et al. (2008) Gray matter increase induced by practice correlates with task-specific activation: a combined functional and morphometric magnetic resonance imaging study. J Neurosci 28: 4210–4215. 10.1523/JNEUROSCI.5722-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR (2013) Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain 136: 1692–1707. 10.1093/brain/awt094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Bobes MA, Ostrosky F, Diaz K, Romero C, Borja K, et al. (2013) Linkage of functional and structural anomalies in the left amygdala of reactive-aggressive men. Soc Cogn Affect Neurosci 8: 928–936. 10.1093/scan/nss101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Motzkin JC, Newman JP, Kiehl KA, Koenigs M (2011) Reduced Prefrontal Connectivity in Psychopathy. Journal of Neuroscience 31: 17348–17357. 10.1523/JNEUROSCI.4215-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT (2011) The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 106: 2322–2345. 10.1152/jn.00339.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Tang Y, Jiang W, Liao J, Wang W, Luo A (2014) Identifying Individuals with Antisocial Personality Disorder Using Resting-State fMRI (vol 8, e60652, 2013). Plos One 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Berkout OV, Young JN, Gross AM (2011) Mean girls and bad boys: Recent research on gender differences in conduct disorder. Aggression and Violent Behavior 16: 503–511. [Google Scholar]
- 156. Klein RG, Abikoff H, Klass E, Ganeles D, Seese LM, et al. (1997) Clinical efficacy of methylphenidate in conduct disorder with and without attention deficit hyperactivity disorder. Arch Gen Psychiatry 54: 1073–1080. [DOI] [PubMed] [Google Scholar]
- 157. Banaschewski T, Hollis C, Oosterlaan J, Roeyers H, Rubia K, et al. (2005) Towards an understanding of unique and shared pathways in the psychopathophysiology of ADHD. Dev Sci 8: 132–140. [DOI] [PubMed] [Google Scholar]
- 158. Banaschewski T, Brandeis D, Heinrich H, Albrecht B, Brunner E, et al. (2003) Association of ADHD and conduct disorder—brain electrical evidence for the existence of a distinct subtype. J Child Psychol Psychiatry 44: 356–376. [DOI] [PubMed] [Google Scholar]
- 159. Sterzer P, Stadler C (2009) Neuroimaging of aggressive and violent behaviour in children and adolescents. Front Behav Neurosci 3: 35 10.3389/neuro.08.035.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Euler F, Jenkel N, Stadler C, Schmeck K, Fegert JM, et al. (2014) Variants of Girls and Boys with Conduct Disorder: Anxiety Symptoms and Callous-Unemotional Traits. J Abnorm Child Psychol. [DOI] [PubMed] [Google Scholar]
- 161. Viding E, Seara-Cardoso A, McCrory EJ (2014) Antisocial and callous behaviour in children. Curr Top Behav Neurosci 17: 395–419. 10.1007/7854_2013_266 [DOI] [PubMed] [Google Scholar]
- 162. Waller R, Gardner F, Hyde LW, Shaw DS, Dishion TJ, et al. (2012) Do harsh and positive parenting predict parent reports of deceitful-callous behavior in early childhood? J Child Psychol Psychiatry 53: 946–953. 10.1111/j.1469-7610.2012.02550.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Rowe R, Maughan B, Moran P, Ford T, Briskman J, et al. (2010) The role of callous and unemotional traits in the diagnosis of conduct disorder. J Child Psychol Psychiatry 51: 688–695. 10.1111/j.1469-7610.2009.02199.x [DOI] [PubMed] [Google Scholar]
- 164. Dandreaux DM, Frick PJ (2009) Developmental pathways to conduct problems: a further test of the childhood and adolescent-onset distinction. J Abnorm Child Psychol 37: 375–385. 10.1007/s10802-008-9261-5 [DOI] [PubMed] [Google Scholar]
- 165. Ducharme S, Hudziak JJ, Botteron KN, Ganjavi H, Lepage C, et al. (2011) Right anterior cingulate cortical thickness and bilateral striatal volume correlate with child behavior checklist aggressive behavior scores in healthy children. Biol Psychiatry 70: 283–290. 10.1016/j.biopsych.2011.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Sui J, Huster R, Yu Q, Segall JM, Calhoun VD (2014) Function-structure associations of the brain: evidence from multimodal connectivity and covariance studies. Neuroimage 102 Pt 1: 11–23. 10.1016/j.neuroimage.2013.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.