Abstract
Postoperative cognitive dysfunction (POCD) has been one of the most common problems in elderly patients following surgery. But the specific mechanism of POCD is still not clear. To further understand the reason of these postoperative behavioral deficits, we evaluated the spatial learning memory of both adult (3 months) and aged (18 months) male mice, 3 or 28 days after isoflurane (Iso) exposure for two hours or appendectomy (App). Hippocampal microglia activation and IL-1β, TNF-α, and IFN-γ expression were also evaluated at day 3, day 14 and day 28 after Iso exposure or appendectomy. Results showed that spatial learning memory of aged, but not adult, mice was impaired after Iso exposure or appendectomy, accompanied with more hippocampal microglia activation and IL-1β, TNF-α, and IFN-γ overexpression. These findings suggest that the cognitive deficits of elderly patients who have undergone surgeries are quite possibly caused by hippocampal microglia overactivation and the subsequent inflammation.
Keywords: postoperative cognitive dysfunction, microglia, surgery, isoflurane
Introduction
Postoperative cognitive dysfunction (POCD) is a fairly well-documented clinical phenomenon.1 In addition to affecting patients’ daily activities due to cognitive impairment, POCD has been found to be associated with increased mortality.2 Although the neurobiological basis of POCD remains unknown, major risk factors, such as advanced age, poor education, preexisting cognitive impairment, severity of coexisting illness, duration of anesthesia, respiratory complications, and second operation, have been identified.3–6 Although perioperative morbidity and mortality have been dramatically reduced over the past decades, little progress has been made in alleviating the prevalence of POCD, which imposes a serious burden on the quality of life and healthcare costs.
The pathogenesis of surgery-induced or volatile anesthetic-induced cognitive impairment is not fully understood. However, extensive information gained over the past decade indicates that the excessive release of proinflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6, are involved in cognitive impairment after surgery and anesthesia.7–10 Neuroinflammation has been proposed to contribute to the progression of neurodegenerative diseases and the occurrence of cognitive deficits associated with aging.11,12 Additionally, increased basal levels of the proinflammatory cytokines IL-lβ and IL-6 have been reported in aged animals.13,14 This age-related change in the inflammatory profile of the brain likely results from alterations in the activation status of the brain’s primary immune cells, namely microglia. Research has established that microglia from aged animals are primed to express an inflammatory phenotype.15,16 Microglia, which are myeloid-lineage cells residing in the central nervous system (CNS), are necessary for healthy brain functioning. As a neuron protector, microglia are sensitive to microenvironment and readily become activated in response to immunological stimuli, toxin, or injury.17,18 When sensing adenosine triphosphate (ATP) leak from an injury site, microglia transform to a more motile state and migrate to the site of damage,19 which causes neuroinflammation and subsequent neurodegeneration.20 Activated microglia could significantly induce the production of a large array of inflammatory cytokines, such as IL-lβ, TNF-α,21 and inducible nitric oxide synthase (iNOS),22 leading to the neuronal cell damage of inflammatory surroundings. It has also been suggested that hyperactivation of microglia results in the production of a variety of proinflammatory mediators in the pathogenesis of several neurodegenerative diseases, including Alzheimer’s, Huntington’s, and Parkinson’s disease.23–25 The contribution of microglia to the hippocampus-dependent learning and memory has become a research focus recently. Microglia activation can impair learning and memory via the release of IL-1.26 Inhibiting microglia activation has been found to rescue learning and memory deficits in a murine model of human immunodeficiency virus (HIV) type 1 encephalitis.27 A more recent study found that 50% of elderly patients with mild cognitive impairment had increased microglia activation compared to age-matched controls.12 Bachstetter et al reported that administration of fractalakine, a chemokine that inhibits microglia activation, increased hippocampal neurogenesis in aged rats.28 The elderly are vulnerable to the adverse effects of infections on cognitive function, and the aging process itself is associated with increased neuroinflammatory processes involving microglial activation and production of proinflammatory cytokines.13,29 Existing data indicate that age-related changes in neuroinflammation may contribute to the reductions in neurogenesis. Whether there is a direct link between inflammation-induced reductions in neurogenesis and age-related cognitive decline remains unknown.
The aim of the present study was to determine whether anesthesia with 1.4% isoflurane (Iso) or appendectomy could induce spatial learning memory deficit. We also evaluated the proinflammatory cytokines (TNF-α, IL-lβ, IFN-γ) and Iba-1 (microglia marker) in the hippocampus in an effort to test the hypothesis that surgery and volatile anesthesia activate microglia and then release a large amount of proinflammatory cytokines, thus leading to spatial learning memory deficit.
Materials and Methods
Experimental animals
The animal protocol was approved by the Institutional Animal Care and Use Committee of Fudan University (Shanghai, China). Male C57BL/6 mice were purchased from Shanghai Laboratory Animal Center of the Chinese Academy of Sciences. The median lifespan for C57BL/6 mice is approximately 26 months;30 so to investigate changes that occur from adulthood to what is considered aged, 3-month-old (adult) and 18-month-old (aged) male mice were used. Mice were given ad libitum access to food and housed under a 12-hour light/dark cycle (lights on at 7:00 am). Room temperature was maintained at 22 ± 1°C, and the relative humidity was set at 50 ± 10%. All experiments were performed during the light phase between 7:00 am and 7:00 pm.
Animal groups
Adult mice weighing 25–30 g and aged mice weighing 35–40 g were divided, respectively, into three groups: control, Iso exposure, and surgery. Mice in the Iso-exposed group were exposed to 1.4% Iso for two hours. Mice in the surgery group were subjected to appendectomy. Two sets of experiments were performed. In the first set, mice were subjected to the water maze test at day 3 (D3) and D28 after Iso exposure or appendectomy. In the second set, their hippocampi were harvested at D3, D14, and D28 after Iso exposure or appendectomy for measuring the proinflammatory cytokines (IL-lβ, TNF-α, IFN-γ) and the microglia marker Iba-1.
Anesthesia procedure
Mice that were randomly assigned to the Iso exposure group received 1.4% Iso in 50% oxygen for two hours at a flow rate of approximately 3 L/min in a Plexiglas anesthetizing chamber, which was adjusted to maintain minimum alveolar concentration, oxygen, and carbon dioxide at constant levels. Gases within the anesthetic chamber were monitored continuously, and arterial oxygen saturation was measured noninvasively using a pulse oximeter during anesthesia. The mice were breathing spontaneously, and the temperature of the anesthetizing chamber was controlled at 37 ± 0.5°C using a heating pad. Anesthesia was terminated by discontinuing the gas flow. Then mice were allowed to recover for 20 minutes in a chamber filled with 50% O2, and maintained at 37°C, and were placed in their home cage. The animals were allowed to recover for 48 hours to avoid the confounding influence of any residual anesthetic.
Surgical procedure
Mice that were randomly assigned to the surgical group were anesthetized with pentobarbital sodium under sterile conditions. For all surgical interventions, the animals underwent a standardized procedure, starting with a midline laparotomy, followed by mobilization and exteriorization of the appendix. Division of the appendix was performed between two ligatures that were placed proximal to the border of the cecum and appendix. The cecal stump was irrigated with saline. In the end, the abdominal wall was closed in two layers, using a running suture technique. Mice were breathing spontaneously, and the temperature was controlled at 37 ± 0.5°C using a heating pad. The animals were assessed daily throughout the recovery period of 2 days after surgery.
Morris water maze
We used the Morris water maze (MWM) task to assess hippocampal-dependent spatial memory performance. Mice were tested in the water maze to assess spatial learning. The maze consisted of a circular tub (100 cm diameter) and a clear mesh plastic square platform (8.5 cm). The platform was placed 1 cm below the surface of the water. The water was made opaque with white tempera paint to conceal the platform. Water temperature was maintained at 21 ± 1°C throughout testing. Extra-maze cues were located around the maze. Mice received four trials (up to 60 seconds) per day from different start locations for five consecutive days. Mice that found the platform were allowed to stay on it for 15 seconds. If a mouse did not find the platform within a 60-second period, it was gently guided to the platform and allowed to stay on it for 15 seconds. A video tracking system recorded the swimming motions of the animals, and the data were analyzed using motion-detection software for the MWM (Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, People’s Republic of China). A single probe trial was conducted on D6. The platform was removed, and a mouse was placed in the opposite quadrant. Each mouse was allowed to swim for 60 seconds, and the number of times the animal crossed the original location of the platform was recorded by the tracking system. Specifically, after every trial, each mouse was placed in a holding cage under a heat lamp for 1–2 minutes to dry before being returned to its regular cage.
Western blot analysis
Hippocampal tissues were isolated from mice after they were deeply anesthetized, and the samples were homogenized in RIPA buffer (20 mM Tris, pH 7.4, 150 mM NaCl, 1% NP-40, 2 mM EDTA, 0.1% Na deoxycholate, 0.1% SDS, 50 mM NaF, 1 mM PMSF, 1 mM Na3V04, 10 μg/mL_1 aprotinin, and 10 μg/mL leupeptin) plus protease inhibitors. The homogenates were centrifuged at 12,000 g at 4°C for 15 minutes. The supernatant was saved, and its protein concentration was determined by the BCA (bicinchoninic acid assay) technique (Bio-Rad). Forty micrograms of proteins per lane was subjected to SDS-PAGE (12% gels) and then blotted onto a polyvinylidene difluoride membrane. Membranes were incubated in blocking buffer (TBS/0.1% Tween 20 with 5% nonfat dried milk) for two hours at room temperature. To control for protein loading, a mouse monoclonal anti-GAPDH antibody (1:2000; A2228; Sigma) was used. The primary anti-Iba-1 antibody (1:1000; Abl07159, Abcam) was added to the buffer solution, and the membranes were incubated overnight at 4°C. The membrane was then washed for 10 minutes three times in 1× TBS/0.1% Tween 20. Appropriate secondary antibodies were used. Then membranes were washed with 1× TBS/0.1% Tween 20 several times. Proteins were visualized with an ECL detection kit and analyzed using the Quantity One 1-D Analysis Software (Bio-Rad). The protein band intensities of Iba-1 were normalized by the corresponding band intensities of GAPDH from the same samples. The results from animals under various experimental conditions were then normalized by those of the corresponding control animals.
Immunofluorescence procedures
Mice were sacrificed and perfused transcardially with ice-cold phosphate-buffered solution (PBS) and 4% paraformaldehyde (in 0.1 M phosphate buffer, pH 7.4). The brains were then dissected out; the meninges were carefully removed and then post-fixed in the same fixative overnight, and cryoprotected by first sinking in 10% and then in 30% sucrose (in 0.1 M phosphate buffer) at 4°C. Coronal sections (20 μm) were cut using a microtome. After blocking nonspecific epitopes with PBS containing 10% donkey serum and 0.5% Triton-100 for one hour at room temperature and then in the primary antibody: Iba-1 antibody (abl07159, 1:200; Abcam), four sections (20 μm thickness, 80 mm space) of each mice were incubated for 48 hours at 4°C. Sections were then washed in PBS and incubated for four hours with a 1:2000 dilution of anti-goat IgG secondary antibody (Invitrogen). Nuclear counterstaining was performed with 4′,6-diamidino-2-phenylindole (DAPI, Sigma) for 30 minutes at room temperature before three washes with dH2O for 10 minutes each time, and then protected with a coverslip.
Quantification of IL-lβ, TNF-α, and IFN-γ by Bio-Plex protein array system
Hippocampal tissues of mice were homogenized on ice in 20 mM Tris-HCl buffer (pH = 7.3) containing protease inhibitors (10 μg/mL aproteinin, 5 μg/mL peptastin, 5 μg/mL leupeptin, and 1 mM phenylmethanesulfonylfluoride). The homogenates were centrifuged at 12,000 g for 10 minutes at 4°C. The supernatant was then ultracentrifuged at 150,000 g for two hours 4°C. Bradford protein assay of the supernatant was performed for each sample. IL-lβ, TNF-α, and IFN-γ were measured in the hippocampus as previously described.31 In brief, a customized 3-plex mouse cytokine panel consisting of fluorescent beads for IL-lβ, TNF-α, and IFN-γ (BioRad) was analyzed with a Luminex protein suspension array system (Bioplex 200; Bio-rad) according to manufacturer’s instructions. Due to the large binding surface of the beads, this assay is highly sensitive and has been proven to work well for detecting cytokines from brain tissues.32 All samples were run in triplicate, and data was analyzed with the Bio-Plex Manager software. The results were expressed as pg/mL of brain supernatant.
Quantitative real-time PCR
Total RNA was isolated from the hippocampus of C57BL/6 mice. RNA extraction was performed with the guanidinium isothiocyanate/chloroform-based technique (TRIZOL, Invitrogen) according to the manufacturer’s instructions. The RNA samples from three individual animals per group in the different experiments were used to prepare cDNA for RT-PCR (real-time polymerase chain reaction) using the SuperScript First-strand Synthesis System (Invitrogen). The cDNA was quantified using the SYBR Green PCR master kit. The PCR primers used are described in Table 1. Each reaction was performed in a volume of 20 μL, which contained 100 ng cDNA, 10 μL SYBR Green PCR master mix, and 5 mM of each (forward and reverse) PCR primer. The total mixture (20 μL) was placed in a 0.1 mL tube. Quantitative RT-PCR was performed using an ABI 7000 PCR instrument (Eppendorf) with the two-stage program parameters provided by the manufacturer, as follows: 1 minute at 95°C, and then 40 cycles of 5 seconds at 95°C, and 30 seconds at 60°C. The sequences of the primer sets used for this analysis are given in Table 1. All reactions were performed in triplicate. Gene expression results were calculated using the delta delta cycle threshold (two delta delta CT) method.33 The two delta delta CT method was used to determine the mean fold changes in gene expression between the control and target genes. The results were normalized using GAPDH expression. It has been reported that no age-related change takes place in the central GAPDH mRNA expression.34
Table 1.
Sequences of the primer sets used for the analysis.
| GENE | PRIMER SEQUENCE (5′→3′) (FORWARD) | PRIMER SEQUENCE (5′→3′) (REVERSE) |
|---|---|---|
| IL-1β | CTCACAAGCAGAGCACAAGC | TCCAGCCCATACTTTAGGAAGA |
| TNF-α | CACCACCATCAAGGACTCAA | GAGACAGAGGCAACCTGACC |
| IFN-γ | CTGCTGATGGGAGGAGATGT | TTTGTCATTCGGGTGTAGTCA |
| GAPDH | ACCACAGTCCATGCCATCAC | TCCACCACCCTGTTGCTGTA |
Statistical analysis
All data are presented as mean ± standard error of the mean (SEM). The Statistical Package for the Social Sciences (SPSS) v.19.0 software was used for statistical analyses. Behavioral studies were analyzed using two-way analysis of variance (ANOVA) with repeated measures. Other data were analyzed with one-way ANOVA, followed by a least square difference (LSD) multiple comparison test. A P-value of <0.05 was considered statistically significant.
Results
Iso exposure- or appendectomy-induced cognitive impairment in aged mice
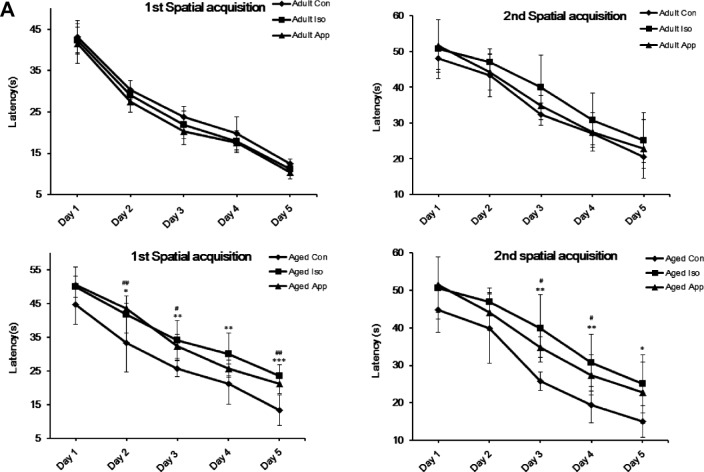
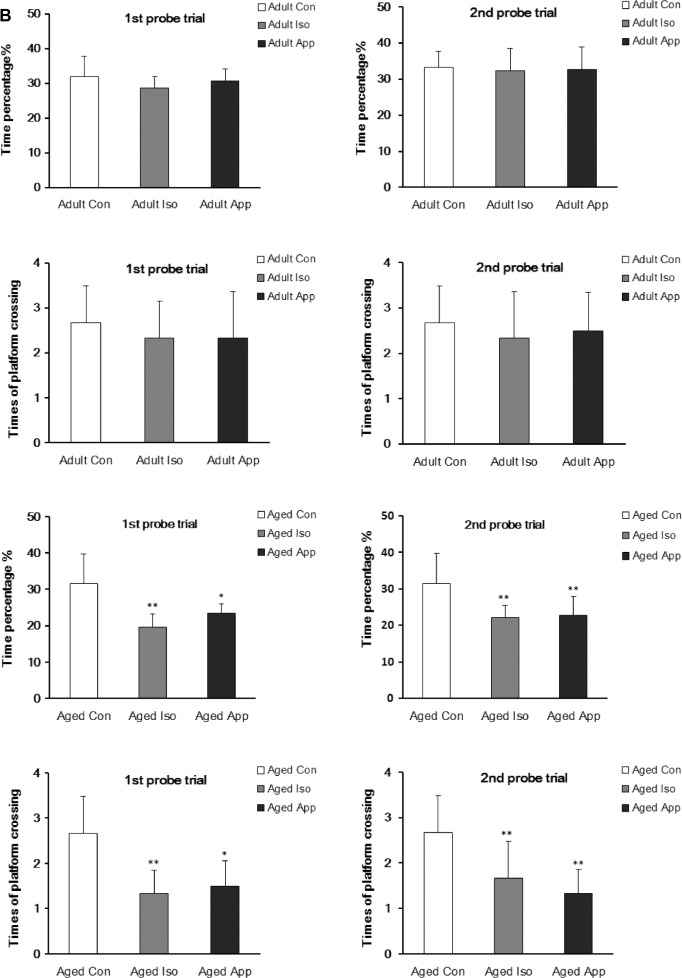
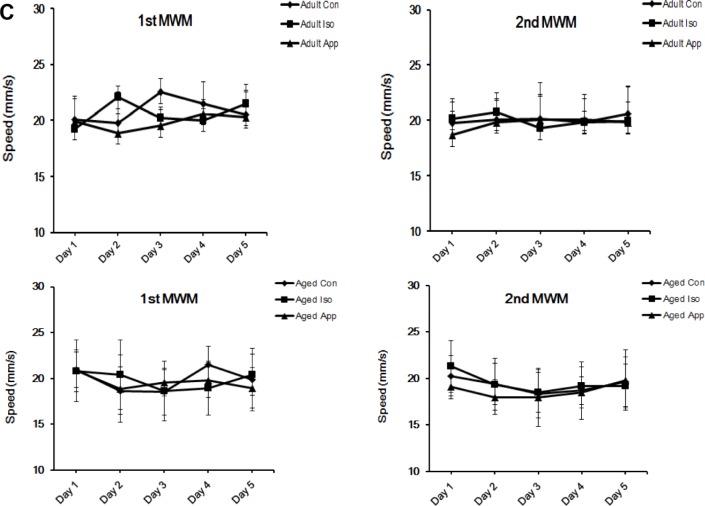
After exposure to 1.4% Iso or appendectomy, mice were tested in the MWM at D3 and D28, which represent recent memory and long-term memory, to evaluate spatial learning and reference memory (Fig. 1). Three groups of adult mice had no significant differences in escape latency, time spent in the target quadrant, and the number of times of crossing the former location of the platform. Aged mice’s spatial memory was impaired after Iso exposure or appendectomy in the two tests. All the three aged groups successfully learned the task, but the Iso-exposure group and appendectomy group had higher escape latency (Fig. 1A). In the Iso-exposure group, the impairment was found on D2, D3, D4, D5, postanesthesia D4, D5, D6, D7 at the first spatial acquisition, and D3, D4, D5, postanesthesia D30, D31, and D32 at the second spatial acquisition. In the appendectomy group, the impairment was found on D2, D3, D5, post appendectomy D4, D5, and D7 at the first spatial acquisition and D3, D4, post appendectomy D30, and D31 at the second spatial acquisition compared to the control mice. During the probe trial, mice subjected to Iso exposure spent less time in the target quadrant searching for the missing platform than did the control mice at postanesthesia D8 and D33. Mice subjected to appendectomy spent less time in the target quadrant searching for the missing platform compared to the control mice at post appendectomy D8 and D33. The decreased number of times the iso-exposure group and appendectomy group crossed the former location of the platform reflected impairment in spatial memory (Fig. 1B). However, mice exposed to both Iso and appendectomy had the same swimming speed compared with control mice (Fig. 1C), indicating that motor deficits did not contribute to differences in escape latencies, number of times of crossing the platform location, or time spent in the target quadrant.
Figure 1.
Effect of isoflurane (Iso) exposure and appendectomy on spatial learning. (A) Mean escape latency (in seconds) to find the hidden platform across training days. There were no significant differences among adult groups. In the aged Iso-exposure group, the significant differences were found on day 2 (D2), D3, D4, and D5 at the first spatial acquisition and D3, D4, and D5 at the second spatial acquisition. In the aged appendectomy group, the significant differences were on D2, D3, and D5 at the first spatial acquisition and D3 and D4 at the second spatial acquisition (*P < 0.05, **P < 0.01, ***P < 0.001; #P < 0.05, ##P < 0.01). (B) Probe trial performance of during testing. No significant differences among adult groups were seen. Both aged Iso and aged appendectomy mice spent less time in the target quadrant searching for the missing platform and less number of times of crossing the former location of the platform than did the controls at postanesthesia D8 and D33. The appendectomy mice spent less time in the target quadrant searching for the missing platform at post appendectomy day 8 and 33 (*P < 0.05, **P < 0.01). (C) There were no differences in the swimming speed during training days (P > 0.05).
Iba-1 expression in the hippocampus
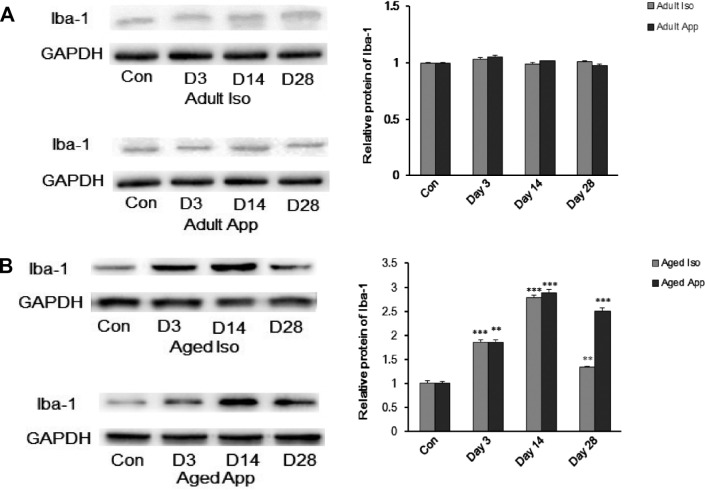
Mice were sacrificed at D3, D14, and D28 after anesthesia or surgery and their Iba-1 levels measured. Western blot analyses and immunofluorescent staining of hippocampal Iba-1 in the iso-exposure group, appendectomy group, and control group were performed. There were no significant differences in Iba-1 expression among adult groups (Fig. 2A). The results showed that, compared with aged control mice, aged iso-exposure mice and aged appendectomy mice exhibited an increase in hippocampal Iba-1. The relative quantity of Iba-1 was significantly different between the iso-exposure group and control group in the hippocampus of mice at D3, D14, and D28. Same was the case with the appendectomy group (Fig. 2B). There was no significant increase in number in Iba-1-positive cells in the hippocampus D3, D14, and D28 among the adult groups (Fig. 3A). Compared with aged control mice, aged Iso-exposure mice and appendectomy mice exhibited an increase in the number of Iba-1-positive cells in the hippocampus at D3, D14, and D28 (Fig. 3B).
Figure 2.
Western blots and graphs illustrating the effect of Iso exposure or appendectomy on relative expression levels of lba-1 (ionized calcium-binding adaptor molecule 1 ; microglia-like cell marker) in adult and aged mice. (A) lba-1 expression had no significant differences among adult groups (P > 0.05). (B) lba-1 levels in aged iso-exposure group and aged appendectomy group significantly increased compared to the aged control group (**P < 0.01, ***P< 0.001).
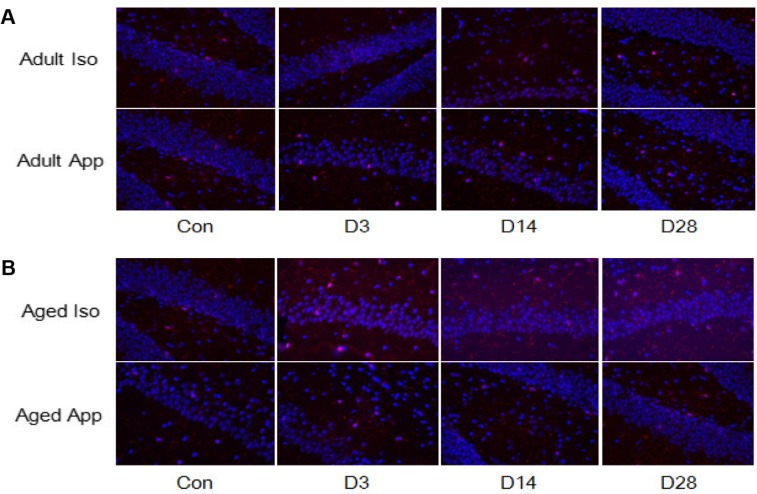
Figure 3.
Immunofluorescent staining of hippocampal lba-1. (A) There were no significant differences in lba-1 expression among adult groups. (B) Aged Iso-exposure group and aged appendectomy group exhibited an increase number in lba-1-positive cells in hippocampus at D3, D14, and D28 compared with aged control group.
Proinflammatory cytokine mRNA expression in the hippocampus
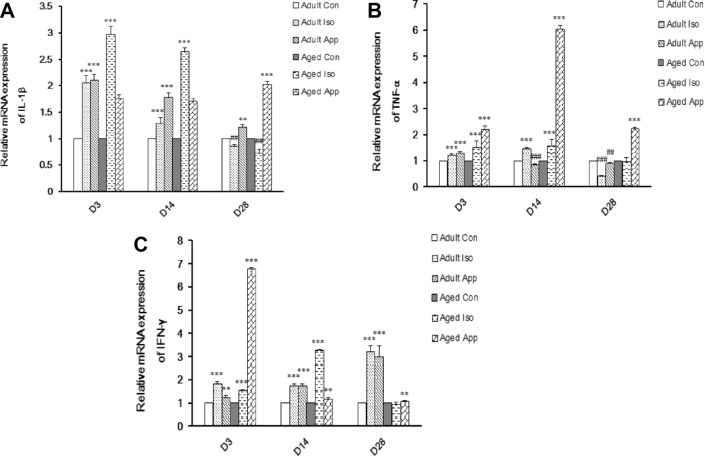
We studied the proinflammatory cytokine mRNA expression levels in the hippocampus at D3, D14, and D28 after Iso exposure or appendectomy (Fig. 4). IL-lβ mRNA expression of adult mice was significantly increased at D3 and D14 after Iso exposure, and then decreased at D28. It was significantly increased at D3, D14, and D28 after appendectomy. IL-lβ mRNA expression of aged mice was significantly increased at D3 and D14 after Iso exposure, and then decreased at D28. It was significantly increased at D3, D14, and D28 after appendectomy (Fig. 4A). TNF-α mRNA expression of adult mice was significantly increased at D3 and D14 after Iso exposure. It had no significance at D28 compared to the control group. It was significantly increased at D3, D14, and D28 after appendectomy. TNF-α mRNA expression of aged mice was significantly increased at D3 and D14 after Iso exposure, then decreased at D28. It was significantly increased at D3 after appendectomy, then decreased at D14 and D28 (Fig. 4B). IFN-γ mRNA expression of adult mice was significantly increased at D3, D14, and D28 after Iso exposure and appendectomy. IFN-γ mRNA expression of aged mice was significantly increased at D3 and D14 after Iso exposure, but had no significance at D28 compared to control group. It was significantly increased at D3, D14, and D28 after appendectomy (Fig. 4C).
Figure 4.
Real-time PCR graphs illustrating the effect of Iso exposure or appendectomy on relative mRNA expression of IL-1β (interleukin-1β), TNF-α (tumor necrosis factor-α), and IFN-γ (interferon-γ). (A) In the adult group, the relative IL-1β mRNA level increased at D3 and D14, and then decreased at D28 after Iso exposure and increased at D3, D14, and D28 after appendectomy. Among aged group, the relative IL-1β mRNA level increased at D3 and D14, and then decreased at D28 after Iso exposure and increased at D3, D14, and D28 after appendectomy (**P< 0.01, ***P< 0.001, ##P< 0.01). (B) In adult group, the relative TNF-α mRNA level increased at D3 and D14, then decreased at D28 after Iso exposure and increased at D3, and then decreased at D14 and D28 after appendectomy. Among aged group, the relative TNF-α mRNA level increased at D3 and D14 after Iso exposure and D3 after appendectomy but decreased at D14 and D28 after appendectomy (***P < 0.001, ##P< 0.01, ###P< 0.001). (C) Among adult group, the relative IFN-γ mRNA level increased at D3, D14, and D28 after Iso exposure and appendectomy. Among aged group, the relative IFN-γ mRNA level increased at D3 and D14 after Iso exposure and D3, D14, and D28 after appendectomy (**P < 0.01, ***P < 0.001).
Proinflammatory cytokine protein level in the hippocampus
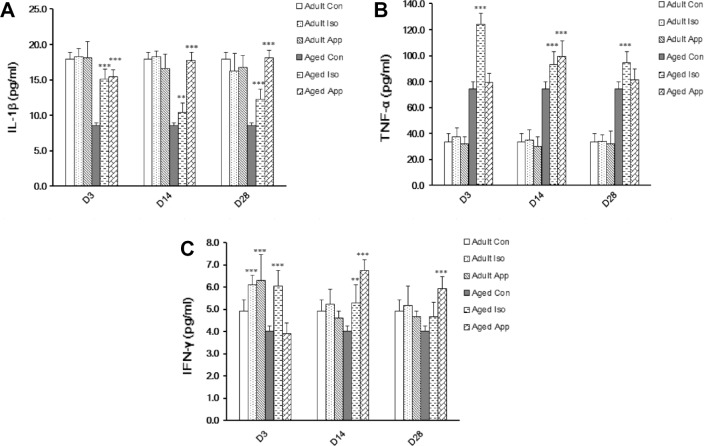
The proinflammatory cytokine protein levels in the hippocampus were studied by Bio-plex at D3, D14, and D28 after Iso exposure or appendectomy (Fig. 5). There were significant differences among the aged groups, but no significant differences of IL-1β or TNF-α could be seen among the adult groups. Among aged groups, IL-1β protein level was significantly increased at D3, D14, and D28 after Iso exposure and appendectomy (Fig. 5A). TNF-α protein level was significantly increased at D3, D14, and D28 after Iso exposure. It was significantly increased at D14 but had no significance at D3 and D28 after appendectomy (Fig. 5B). Among adult groups, IFN-γ protein level increased only at D3 after Iso exposure and appendectomy. Among the aged groups, IFN-γ protein level was significantly increased at D3 and D14 but had no significance at D28 after Iso exposure. It was significantly increased at D14 and D28 but had no significance at D3 after appendectomy (Fig. 5C).
Figure 5.
Bioplex cytokine quantity graphs illustrating the effect of Iso exposure or appendectomy on protein levels of IL-1β, TNF-α, and IFN-γ. (A) IL-1β protein level in aged Iso group and aged appendectomy group increased at D3, D14, and D28 compared with the aged control group (**P< 0.01, ***P < 0.001). There were no significant differences among adult groups (P > 0.05). (B) Among the aged groups, TNF-α protein level increased at D3, D14, and D28 after Iso exposure and D14 after appendectomy (***P < 0.001). No significant differences could be seen among adult groups (P > 0.05). (C) Among adult groups, IFN-γ protein level increased at D3 after Iso exposure and appendectomy. Among aged groups, IFN-γ protein levels increased at D3 and D14 after Iso exposure and D14 and D28 after appendectomy (**P< 0.01, ***P< 0.001).
Discussion
In recent years, there has been increasing concern that general anesthesia/anesthetics may contribute to POCD.35 Many reports have shown spatial memory impairment after Iso exposure.35,36 But the mechanism is not yet clear. In this study, we used adult mice and aged mice to explore the mechanism. Our results showed that aged mice exhibited deficits in hippocampus-dependent learning and memory as manifested by the longer escape latency to reach the platform, the fewer times of original platform crossing, and the less time spent in the target quadrant in the MWM test at D3 and D28 after exposure to 1.4% Iso for two hours or appendectomy, but not adult mice. A recent study showed that exposure of 2-3-month-old mice to 1% Iso, but not 1.5% or 2% Iso, impaired the acquisition of spatial reference tasks but did not affect spatial reference recall/memory when they were tested within 5 days after isoflurane exposure.37 However, another study demonstrated that 2-month-old rats did not have an impairment in fear-conditioning test and spatial reference learning but had improved long-term memory at 4 months after one-MAC isoflurane anesthesia for four hours.38 The reasons for these different findings between our study and the previous studies are not known. Differences in animal species and strains, methods of anesthetic exposure, and time to perform the learning and memory tests may have contributed to these discrepancies.
In our study, after Iso exposure or appendectomy, adult mRNA expression, aged mice mRNA expressions, and protein levels of IL-lβ, TNF-α and IFN-γ increased to different extents, while only adult mice protein level of IFN-γ increased at D3. It has been shown that cytokines are important mediators involved in immune, inflammatory, and immunomodulatory functions.39 Described as double-edged swords, cytokines protect and repair, but also impair, neuronal function during excessive or chronic neuroinflammation.40 Iso may increase the levels of proinflammatory cytokines, which may cause neuroinflammation.10 Iso impaired the long-term spatial reference memory and hippocampus-dependent learning and memory by activating IL-lß, which increased activated caspase 3 in the hippocampus and decreased the neuronal density in the CA1 region. This activation ultimately led to neurodegeneration and cell death in the hippocampus.36,37 The hippocampus, which plays a critical role in both learning and memory and the emotional function, is an important brain region susceptible to anesthetics such as Iso. It highly expresses proinflammatory cytokine receptors, and appears to be more sensitive to excessive or prolonged cytokine exposure.41 Among the many inflammatory triggers and mediators (proinflammatory cytokine), IL-lβ has been suggested as most potent in neurodegeneration, based on both experimental and clinical data.40,42 A recent article of Barrientos et al43 shows that POCD is attenuated by blocking central IL-lβ signaling using an IL-1 receptor antagonist. This suggests that IL-lβ, one of the main cytokines produced by microglia,44 plays a pivotal role in surgery-induced cognitive impairment. In our study, we found the mRNA expression and protein level of IL-lβ in the hippocampus of aged mice increased after Iso exposure or appendectomy. Another proinflammatory cytokine, TNF-α, plays a central role in initiating and regulating the cytokine cascade during an inflammatory response. The levels of TNF-α expression in the healthy brain are low, making it difficult to determine its precise role under physiological conditions. In inflammatory or disease states, TNF-α, along with several other proinflammatory mediators and neurotoxic substances, is predominantly produced by activated microglia. IFN-γ, together with other inflammatory cytokines (IL-lβ, TNF-α), can trigger neurotoxic mechanisms (eg, increase in the expression of other inflammatory factors, influence on the development of oxidative stress).45 Lee et al46 demonstrated that in EAE (experimental autoimmune encephalomyelitis) inhibition of IFN-γ actions mediated through microglial STAT1 is the main cause for the onset of atypical neurological deficits. In our study, IFN-γ protein level in adult mice increased only at D3 after Iso exposure or appendectomy, while no significant differences of IL-lβ or TNF-α were seen. This suggested that individual increase of IFN-γ protein level was not enough to lead to cognitive impairment.
Most studies have found that POCD occurs in the elderly. It has been suggested that aging can facilitate neurobehavioral complications associated with peripheral infections, probably by allowing the overexpression of inflammatory cytokines in brain areas that mediate cognitive processing. Hovens et al argued that elderly patients had an increased behavioral and (neuro) inflammatory response to surgery.47 In addition to increased expression of proinflammatory markers such as IL-1β, IL-6, and TNF-α in the brain, aged mice have more microglia that stain for tomato lectin and higher basal levels of several inflammatory cytokine mRNAs in the hippocampus than young adults.48 This is similar to the results of our study.
Increased microglial activity and proinflammatory cytokine releases may contribute to neuronal dysfunction and death in neurodegenerative diseases. The importance of microglia in protecting the brain is illustrated by the observation that complete blockade of microglial activity exacerbates brain damage in adult and neonatal hypoxic ischemic injury models.49,50 Activated microglia can change from ramified (quiescent) morphology to amoeboid (activated) morphology, secrete a variety of proinflammatory and neurotoxic factors (eg, IL-lβ, IL-6, and TNF-α), and increase the expression of iNOS. Microglia are the primary producers of IL-lβ within the brain.51 Some studies indicate that the release of proinflammatory cytokines from microglia can cause direct cell death of neurons both in vivo and in vitro.52,53 Cunningham et al have argued that microglial priming and the consequent exaggerated CNS inflammatory response may be a key contributor to deleterious cognitive consequences of systemic inflammation in the aged and demented.54,55 Our study suggests that all significant impairments paralleled upregulated cytokine IL-lβ, TNF-α, and IFN-γ expression. Immunohistochemistry assay further showed more hippocampal microglial cell activation in aged mice compared to adults. These findings suggest that Iso exposure or appendectomy results in cognitive function impairment potentiated by aging. The findings of this study indicate that moderate surgical trauma or Iso exposure induces an exaggerated microglia activation response, releasing large amount of proinflammatory cytokines and then leading to an exacerbated cognitive impairment in aged mice when compared to their adult counterparts.
It is natural to think that activation of microglial-IL-lβ-neuronal injury/death pathway may contribute to cognitive impairment after Iso exposure or appendectomy. We might treat POCD by using different therapies related to the corresponding link. Future studies with using specific inhibitors for each component of this pathway are needed to provide evidence for this connection.
Because it is quite difficult to acquire a sufficient volume of arterial blood from mice without anesthesia for analysis, there is currently no record of normal blood gas values for mice. For this reason, many studies do not assess blood gases.56,57 Therefore, we do not know how to define and evaluate the effects of acidosis in mice. However, in the present study, no animals died after two hours of isoflurane exposure, which indicated that even if some minimum physiological changes occurred, they were of little clinical relevance.
Conclusion
In summary, we found that 1.4% Iso exposure or appendectomy impaired hippocampus-dependent learning and memory in aged mice. To explore the mechanism of neurotoxicity induced by Iso or surgery, we found that elevated levels of the proinflammatory cytokines IL-lβ, TNF-α, and IFN-γ in the hippocampus are implicated in learning and memory. Furthermore, we observed an increase in the microglia protein expression and the number of microglia cells in the hippocampus, both of which indicated that microglia was activated. Thus, the pathway of microglia activation and proinflammatory cytokines release contributed to the neurotoxicity and impairment of learning and memory induced by Iso or surgery. This study provides some theoretical basis for the further study of microglia inhibition, which could prevent POCD in elderly patients and decrease the postoperative morbidity and mortality.
Footnotes
ACADEMIC EDITOR: Garry Walsh, Editor in Chief
PEER REVIEW: Five peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1,303 words, excluding any confidential comments to the academic editor.
FUNDING: This project was supported by the Shanghai Municipal Commission of Health and Family Planning Fund (2013-314). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to antiplagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: HF, HLW, ZGX. Performed the experiments: HLW, RHM. Analyzed the data: QWL, HLW. Wrote the first draft of the manuscript: HLW, HF. Contributed to the writing of the manuscript: HF, HLW, QWL, RHM. Made critical revisions and approved final version: HF, ZGX. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Baranov D, Bickler PE, Crosby GJ, et al. Consensus statement: first international workshop on anesthetics and Alzheimer’s disease. Anesth Analg. 2009;108:1627–1630. doi: 10.1213/ane.0b013e318199dc72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110:548–555. doi: 10.1097/ALN.0b013e318195b569. [DOI] [PubMed] [Google Scholar]
- 3.Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators, International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 4.Ancelin ML, Roquefeuil GD, Ledesert B, Bonnel F, Cheminai JC, Ritchie K. Exposure to anesthetic agents, cognitive functioning and depressive symptomatology in the elderly. Br J Psychiatry. 2001;178:360–366. doi: 10.1192/bjp.178.4.360. [DOI] [PubMed] [Google Scholar]
- 5.Rasmussen LS. Postoperative cognitive dysfunction: incidence and prevention. Best Pract Res Clin Anaesthesiol. 2006;20:315–330. doi: 10.1016/j.bpa.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Ramaiah R, Lam AM. Postoperative cognitive dysfunction in the elderly. Anesthesiol Clin. 2009;27:485–496. doi: 10.1016/j.anclin.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Kong F, Chen S, Cheng Y, et al. Minocycline attenuates cognitive impairment induced by isoflurane anesthesia in aged rats. PLoS One. 2013;8:e61385. doi: 10.1371/journal.pone.0061385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin D, Cao L, Wang Z, Li J, Washington JM, Zuo Z. Lidocaine attenuates cognitive impairment after isoflurane anesthesia in old rats. Behav Brain Res. 2012;228(2):319–327. doi: 10.1016/j.bbr.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cibelli M, Fidalgo AR, Terrando N, et al. Role of interleukin-lbeta in postoperative cognitive dysfunction. Ann Neurol. 2010;68:360. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu X, Lu Y, Dong Y, et al. The inhalation anesthetic isoflurane increases levels of proinflammatory TNF-alpha, IL-6, and IL-1beta. Neurobiol Aging. 2012;33(7):1364–1378. doi: 10.1016/j.neurobiolaging.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 12.Okello A, Edison P, Archer HA, et al. Microglial activation and amyloid deposition in mild cognitive impairment: a PET study. Neurology. 2009;72:56–62. doi: 10.1212/01.wnl.0000338622.27876.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol. 1999;93:139–148. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- 14.McLinden KA, Kranjac D, Deodati LE, Kahn M, Chumley MJ, Boehm GW. Age exacerbates sickness behavior following exposure to a viral mimetic. Physiol Behav. 2012;105:1219–1225. doi: 10.1016/j.physbeh.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 15.Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SR. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27:717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55:412–424. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- 17.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19(8):312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 18.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koizumi S, Ohsawa K, Inoue K, Kohsaka S. Purinergic receptors in microglia: functional modal shifts of microglia mediated by P2 and P1 receptors. Glia. 2013;61:47–54. doi: 10.1002/glia.22358. [DOI] [PubMed] [Google Scholar]
- 20.Leung YM. P2X7 receptor as a double-edged sword: neurotrophic and neurotoxic effects. Biomedicine. 2011;1:16–20. [Google Scholar]
- 21.Jensen KD, Wang Y, Wojno ED, et al. Toxoplasma polymorphic effectors determine macrophage polarization and intestinal inflammation. Cell Host Microbe. 2011;9:472–483. doi: 10.1016/j.chom.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurotherapeutics. 2010;7:366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guillot-Sestier MV, Town T. Innate immunity in Alzheimer’s disease: a complex affair. CNS Neurol Disord Drug Targets. 2013;12:593–607. doi: 10.2174/1871527311312050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Politis M, Pavese N, Tai YF, et al. Microglial activation in regions related to cognitive function predicts disease onset in Huntington’s disease: a multimodal imaging study. Hum Brain Mapp. 2011;32:258–270. doi: 10.1002/hbm.21008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qian L, Flood PM, Hong JS. Neuroinflammation is a key player in Parkinson’s disease and a prime target for therapy. J Neural Transm. 2010;117:971–979. doi: 10.1007/s00702-010-0428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka S, Kondo H, Kanda K, et al. Involvement of interleukin-1 in lipopolysaccaride-induced microglial activation and learning and memory deficits. J Neurosci Res. 2011;89(4):506–514. doi: 10.1002/jnr.22582. [DOI] [PubMed] [Google Scholar]
- 27.Keblesh JP, Dou H, Gendelman HE, Xiong H. 4-Aminopyridine improves spatial memory in a murine model of HIV-1 encephalitis. J Neuroimmune Pharmacol. 2009;4:317–327. doi: 10.1007/s11481-009-9161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bachstetter AD, Morganti JM, Jernberg J, et al. Fractalkine and CX 3 CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging. 2011;32:2030–2044. doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richwine AF, Godbout JP, Berg BM, et al. Improved psychomotor performance in aged mice fed diet high in antioxidants is associated with reduced ex vivo brain interleukin-6 production. Brain Behav Immun. 2005;19:512–520. doi: 10.1016/j.bbi.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Morley AA, Trainor KJ. Lack of an effect of vitamin E on lifespan of mice. Biogerontology. 2001;2(2):109–112. doi: 10.1023/a:1011589218219. [DOI] [PubMed] [Google Scholar]
- 31.Luchtman DW, Shao D, Song C. Behavior, neurotransmitters and inflammation in three regimens of the MPTP mouse model of Parkinson’s disease. Physiol Behav. 2009;98:130–138. doi: 10.1016/j.physbeh.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 32.Datta SC, Opp MR. Lipopolysaccharide-induced increases in cytokines in discrete mouse brain regions are detectable using Luminex xMAP((R)) technology. J Neurosci Methods. 2008;75:119–124. doi: 10.1016/j.jneumeth.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using realtime quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Slagboom PE, de Leeuw WJ, Vijg J. Messenger RNA levels and methylation patterns of GAPDH and beta-actin genes in rat liver, spleen and brain in relation to aging. Mech Ageing Dev. 1990;53:243–257. doi: 10.1016/0047-6374(90)90042-e. [DOI] [PubMed] [Google Scholar]
- 35.Bianchi SL, Tran T, Liu C, et al. Brain and behavior changes in 12-month-old Tg2576 and nontransgenic mice exposed to anesthetics. Neurobiol Aging. 2008;29:1002–1010. doi: 10.1016/j.neurobiolaging.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Culley DJ, Baxter MG, Yukhananov R, Crosby G. Long-term impairment of acquisition of a spatial memory task following isoflurane-nitrous oxide anesthesia in rats. Anesthesiology. 2004;100:309–314. doi: 10.1097/00000542-200402000-00020. [DOI] [PubMed] [Google Scholar]
- 37.Valentim AM, Di Giminiani P, Ribeiro PO, Rodrigues P, Olsson IA, Antunes LM. Lower isoflurane concentration affects spatial learning and neurodegeneration in adult mice compared with higher concentrations. Anesthesiology. 2010;113:1099–1108. doi: 10.1097/ALN.0b013e3181f79c7c. [DOI] [PubMed] [Google Scholar]
- 38.Stratmann G, Sall JW, May LD, et al. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834–848. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- 39.Su PY, Liu SJ, Chen YH, Wu SS, Chen YL, et al. Increased IL-8 and IL-lb in the bile of acute cholecystitis patients. Biomedicine. 2013;3:181–185. [Google Scholar]
- 40.Griffin WS, Liu L, Li Y, Mrak R, Barger S. Interleukin-1 mediates Alzheimer and Lewy body pathologies. J Neuroinflammation. 2006;3:5–14. doi: 10.1186/1742-2094-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parnet P, Kelley KW, Bluthe RM, Dantzer R. Expression and regulation of interleukin-1 receptors in the brain: role in cytokines-induced sickness behavior. J Neuroimmunol. 2002;125:5–14. doi: 10.1016/s0165-5728(02)00022-x. [DOI] [PubMed] [Google Scholar]
- 42.Taepavarapruk P, Song C. Reductions of acetylcholine release and nerve growth factor expression are correlated with memory impairment induced by interleukin-1beta administrations: effects of omega-3 fatty acid EPA treatment. J Neurochem. 2010;112:1054–1064. doi: 10.1111/j.1471-4159.2009.06524.x. [DOI] [PubMed] [Google Scholar]
- 43.Barrientos RM, Hein AM, Frank MG, Watkins LR, Maier SF. Intracisterna interleukin-1 receptor antagonist prevents postoperative cognitive decline and neuroinflammatory response in aged rats. J Neurosci. 2012;32:14641–14648. doi: 10.1523/JNEUROSCI.2173-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wynne AM, Henry CJ, Godbout JP. Immune and behavioral consequences of microglial reactivity in the aged brain. Integr Comp Biol. 2009;49:254–266. doi: 10.1093/icb/icp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rubio-Perez JM, Morillas-Ruiz JM. A review: inflammatory process in Alzheimer’s disease, role of cytokines. Scientific World Journal. 2012;2012:756357. doi: 10.1100/2012/756357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee E, Chanamara S, Pleasure D, Soulika AM. IFN-gamma signaling in the central nervous system controls the course of experimental autoimmune encephalomyelitis independently of the localization and composition of inflammatory foci. J Neuroinflammation. 2012;9:7. doi: 10.1186/1742-2094-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hovens IB, Schoemaker RG, van der Zee EA, Heinemanb E, Nyakas C, van Leeuwen BL. Surgery-induced behavioral changes in aged rats. Exp Gerontol. 2013;48:1204–1211. doi: 10.1016/j.exger.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22:301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faustino JV, Wang X, Johnson CE, et al. Microglial cells contribute to endogenous brain defenses after acute neonatal focal stroke. J Neurosci. 2011;31:12992–13001. doi: 10.1523/JNEUROSCI.2102-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Streit WJ, Conde JR, Fendrick SE, Flanary BE, Mariani CL. Role of microglia in the central nervous system’s immune response. Neurol Res. 2005;27:685–691. doi: 10.1179/016164105X49463a. [DOI] [PubMed] [Google Scholar]
- 52.O’Brien R, Wong P. Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci. 2011;34:185–204. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Combs CK, Karlo JC, Kao S-C, Landreth GE. Beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J Neurosci. 2001;21:1179–1188. doi: 10.1523/JNEUROSCI.21-04-01179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cunningham C, Maclullich AM. At the extreme end of the psychoneuroimmunological spectrum: delirium as a maladaptive sickness behavior response. Brain Behav Immun. 2013;28:1–13. doi: 10.1016/j.bbi.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Butterfield NN, Graf P, Ries CR, MacLeod BA. The effect of repeated isoflurane anesthesia on spatial and psychomotor performance in young and aged mice. Anesth Analg. 2004;98:1305–1311. doi: 10.1213/01.ane.0000108484.91089.13. table of contents. [DOI] [PubMed] [Google Scholar]
- 57.Valentim AM, Alves HC, Olsson IA, Antunes L. The effects of depth of isoflurane anesthesia on the performance of mice in a simple spatial learning task. J Am Assoc Lab Anim Sci. 2008;47:16–19. [PMC free article] [PubMed] [Google Scholar]