Abstract
Background
DNA aptamers represent a novel strategy in anti-cancer medicine. These compounds are short sequences of DNA that have protein binding effects via shape specific recognition of a target protein in an interaction which is analogous to antibody-antigen binding. AS1411, a DNA aptamer that targets nucleolin (a protein which is overexpressed in many tumor types), was evaluated in patients with metastatic, predominantly clear-cell, renal cell carcinoma (RCC) who had failed treatment with ≥1 previous tyrosine kinase inhibitor. We present the first manuscript reporting the use of this novel anti-cancer agent in humans.
Methods
In this phase II, single-arm study, AS1411 was administered at 40 mg/kg/day by continuous intravenous infusion on days 1–4 of a 28-day cycle, for two cycles. Primary endpoint was overall response rate; progression-free survival (PFS) and safety were secondary endpoints.
Results
35 patients were enrolled and treated; 33 completed two treatment cycles. Median number of prior therapies was 2 (range 1–7). One patient (2.9%) had a response to treatment. The response was dramatic (84% reduction in the sum of longest diameters of selected target tumor lesions) and durable (the patient remains free of progression 2 years after completing therapy). No responses were seen in the other patients. Median PFS was 4 months. Only 34% of patients had an AS1411-related adverse event, all of which were mild or moderate.
Conclusions
AS1411 appears to have limited activity in unselected patients with metastatic RCC. However, rare, dramatic and durable responses can be observed and toxicity is low. Further studies with nucleolin targeted compounds may benefit from efforts to discover predictive biomarkers of response. Currently, promising pre-clinical studies are ongoing using AS1411 conjugated to traditional cytotoxic agents to selectively deliver these treatments to tumor cells. DNA aptamers represent a novel way to target cancer cells at a molecular level and continue to be developed with a view to improving treatment and imaging in cancer medicine.
Keywords: renal cell carcinoma, novel therapeutics, DNA aptamer, nucleotide aptamer, Bcl2
Within the USA each year, approximately 65,000 new cases of kidney cancer are diagnosed and 14,000 people die from the disease.1 While the treatment of localized renal cell carcinoma (RCC) by complete or partial nephrectomy is potentially curative, current therapies for metastatic RCC are generally palliative. Presently, the expected median survival for patients with metastatic or inoperable locally-advanced RCC is approximately 24 months.2
Agents that target angiogenesis or mammalian target of rapamycin (mTOR) pathways have resulted in longer survival in several studies, although they often are associated with multiple toxicities and ultimately drug resistance is universal.3–6 Therefore, there remains a need to develop new agents in this clinical setting.
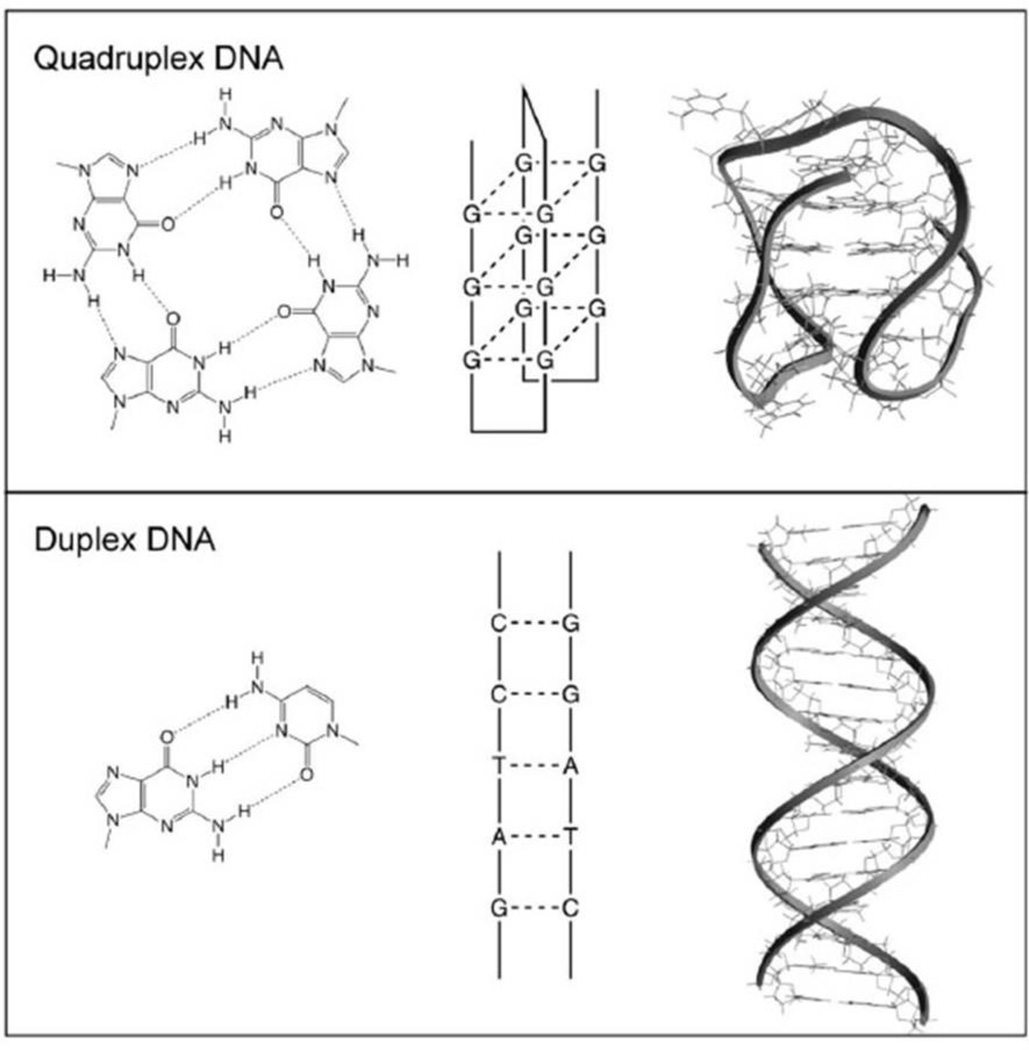
DNA aptamers are short sequences of DNA (oligonucleotides) that have protein binding (aptameric) effects via shape specific recognition of a target protein in an interaction which is analogous to antibody-antigen binding. This property is thought to be due to the propensity of these molecules to form quadruplex structures rather than the usual double helix structure of DNA (Figure 1).7 AS1411 is a first-in-class quadruplex DNA aptamer formed from a single-stranded, G-rich, phosphodiester, 26-mer oligonucleotide. Unlike some monoclonal antibodies however, these molecules are not associated with infusion reactions because they are not immunogenic.8 It targets nucleolin, a multifunctional protein located primarily in the nucleolus, but also found in the cytoplasm and cell membrane.9 Nucleolin is overexpressed in many types of cancer.10 It interacts with many key oncogenes including bcl-2, Rb and p53 and this is may be relevant to a role in cancer development.10 Another of its major functions is to shuttle specific extracellular ligands into the cell by an active transport mechanism, and this appears to be upregulated in stress response.11 After AS1411 binds to tumor cell-surface nucleolin, the AS1411-nucleolin complex is internalized, which leads to significant inhibition of DNA synthesis, subsequent accumulation of cells in S phase, destabilization of bcl2 mRNA, and the induction of apoptosis.12–14 The antiproliferative activity of AS1411 correlates directly with the extent to which the tumor cells are arrested in S phase.
Figure 1.
This figure shows the hydrogen-bonding arrangements(left) for a G-quartet(top) and a G-C base pair(bottom), as well as schematic illustrations(middle) and molecular models(right) for quadruplex and duplex DNA. The quadruplex shown is one possible conformation of the human telomere sequence (PDB accession code 143d). AS1411 has been shown to form a quadruplex and its detailed molecular structure is currently being investigated. (Adapted with permission from Bates PJ et al. Exp Mol Pathol 2009;86: 151–64)
AS1411 has shown antitumour activity in patients with metastatic RCC in a phase I study in patients with solid tumors. At doses ranging from 1 to 40 mg/kg/day given as 4- or 7-day continuous intravenous infusions for up to two cycles of treatment, AS1411 produced one complete response (CR) and one partial response (PR) among twelve patients with metastatic RCC. Seven patients had stable disease (SD) of ≥2 months’ duration.15 No dose-limiting toxicity was observed, and it appeared that AS1411 might represent a less toxic alternative to current treatments. The aim of the present study was to confirm these promising signs of efficacy through a more formal evaluation. Based on previous data showing anti-tumor efficacy in RCC with only 1 or 2 cycles, and the complexity of DNA aptamer manufacture, only two cycles of therapy were administered.
Materials and Methods
Study population
Patients aged 18 years and older, with histologically or cytologically-confirmed metastatic or locally advanced RCC containing predominantly clear-cell histology, who had already received ≥1 approved tyrosine kinase inhibitor, were eligible for enrolment if they satisfied the following criteria: prior nephrectomy; at least one measurable lesion according to the Response Evaluation Criteria in Solid Tumors v1 (RECIST)16; Eastern Cooperative Oncology Group (ECOG) performance status of 0–1; adequate bone marrow, hepatic and renal function; and a negative pregnancy test (with adequate contraception for women of childbearing potential). This trial was conducted prior to the commercial availability of everolimus.
Patients meeting any of the following criteria were excluded from the study: collecting duct, papillary, or chromophobe histology; brain metastases if patients were symptomatic or had received steroids for the brain lesions within 2 weeks of study entry; treatment with a non-approved or investigational product within 4 weeks of starting study therapy; treatment with an approved product within 2 weeks of the starting study therapy; breastfeeding; history of prior or concomitant malignancy (except for nonmelanoma skin cancer, carcinoma in situ of the cervix, or any other cancer from which the patient had been disease free for 3 years); any concurrent medical/psychological condition that would limit ability to provide informed consent or compliance.
Patients were recruited into the study at seven study centers in the USA. The study was approved by local institutional review boards and was conducted in accordance with the Declaration of Helsinki and its amendments. All patients gave written, informed consent before study entry.
Study design
In this open-label, single-arm, study which patients received AS1411 40 mg/kg/day by continuous intravenous infusion on days 1–4 of a 28-day cycle, for two cycles. For toxicity ≥ grade 3 (according to the National Cancer Institute Common Terminology Criteria for Adverse Events Version 3.0 [NCI-CTCAE]), related to AS1411, the current infusion cycle was to be terminated immediately and not recommenced. If toxicity occurred during the first treatment cycle, the relevant adverse events (AEs) were to be followed until resolution or significant improvement before initiation of the second treatment cycle. Dose reduction was permitted in the second cycle for management of drug-related toxicities. Permanent discontinuation of study treatment was required in the event of disease progression, intolerable toxicity, withdrawal of consent, loss to follow-up, investigator decision, or sponsor request.
Assessments
Tumor response was evaluated using RECIST criteria and categorized as CR, PR, SD or progressive disease (PD). Tumor measurements using CT or MRI imaging were obtained at the screening visit and then every 8 weeks from start of treatment until disease progression. In addition to investigator assessments of tumor response, copies of tumor images were submitted for blinded independent assessment (BioClinica Inc., Newtown, PA, USA).
Patients underwent safety assessments with clinical, laboratory and 12 lead electrocardiogram monitoring at weekly intervals throughout treatment. Thereafter patients were assessed bimonthly to end of follow-up.
Pharmacokinetic (PK) studies using blood samples were performed to assess the area under the AS1411 plasma concentration-time curve during cycle 1. The Cmax (maximum observed plasma AS1411 concentration), Css (plasma AS1411 concentration at steady state) and t½, (terminal elimination half-life) were also measured. In addition, peripheral blood samples were collected pre-infusion and then 24, 48, 72, and 96 hours after the commencement of infusion for the measurement of the following pharmacodynamic markers: interferon, IL-2, IL-4, IL-6, IL-10, IL-12, and tumor necrosis factor (TNF).
Statistical analysis
The primary efficacy endpoint was overall response rate (ORR; the proportion of patients reporting a best overall response of CR or PR). The null hypothesis (that the ORR for patients treated with AS1411 would be <5% [p≤0.05]) was tested using a binomial test at an alpha level of 5%. The response rate estimate is presented with a two-sided 95% confidence interval (CI) using the Clopper-Pearson method. A one-sided 95% CI corresponding to the test of the null hypothesis is also presented. Enrollment of 30 patients into the study was required to ensure 27 evaluable patients (assuming that 10% would not be evaluable for response).
The secondary efficacy endpoints were progression free survival (PFS, defined as the time from the date of first infusion of AS1411 to the date of documented progression or death from any cause), time to progression (TTP, defined as the time from the date of first infusion of AS1411 until documented progression), duration of overall response (defined as the time from the first occurrence of CR or PR until the date of first documented disease progression or death) and duration of SD (defined as the time from the date of first infusion of AS1411 to the date of the first documented disease progression or death).
For PK analysis, plasma AS1411 concentration data was plotted using WinNonlin® version 5.2 (Pharsight, Mountain View, CA, USA) or Microsoft® Excel version 2003 SP3. PK evaluations were based on plasma AS1411 concentrations determined using a validated high-performance liquid chromatography procedure with photodiode array detection. PK variables were estimated using the WinNonlin® non-compartmental model 202.
Results
Patient disposition and exposure to AS1411
A total of 37 patients with RCC were enrolled, of whom 35 received AS1411, thereby constituting the full treatment and safety analysis sets. Of these, all 35 patients received the first cycle of AS1411, while 33 (94.3%) received the second cycle of AS1411; two (5.7%) patients failed to receive both cycles because of disease progression. The per protocol analysis set consisted of 34 patients; the patient excluded from this set withdrew consent after completion of the second AS1411 infusion cycle but without having undergone any post-baseline tumor assessment to evaluate response.
Baseline characteristics
Baseline characteristics of patients in the full analysis set are summarized in Table 1. Median age was 61 (range 39–81) and 14 patients (40%) were aged ≥65 years. Previous anticancer treatments are summarized in Table 2. All patients had undergone nephrectomy related to RCC and had received ≥1 tyrosine kinase inhibitor. The median number of prior systemic therapies received was 2 (range 1–7).
Table 1.
Demographic characteristics (safety/full analysis set)
| Characteristic | |
|---|---|
| N | 35 |
| Mean age ± StD, years | 61.1 ± 10.65 |
| Median age (range), years | 61 (39–81) |
| Age <65 years, n (%) | 21 (60.0) |
| Age ≥65 years, n (%) | 14 (40.0) |
| Men, n (%) | 27 (77.1) |
| Women, n (%) | 8 (22.9) |
| Race, n (%) | |
| White/Caucasian | 32 (91.4) |
| Black | 2 (5.7) |
| Asian | 1 (2.9) |
| Mean weight ± StD, kg | 91.8 ± 18.03 |
| Mean height ± StD, cm | 174.6 ± 9.54 |
| Cancer stage at diagnosis, n (%) | |
| I | 1 (2.9) |
| II | 4 (11.4) |
| III | 8 (22.9) |
| IV | 22 (62.9) |
| Mean time from diagnosis to first infusion ± StD, months | 53.6 ± 40.17 |
| Median time from diagnosis to first infusion (range), months | 43.7 (7–169) |
Abbreviations: StD, standard deviation.
Table 2.
Previous anticancer therapies (safety/full analysis set)
| Patients with previous anticancer therapy, n (%) | 35 (100.0) |
| Mean no. of anticancer therapies ± StD | 2.9 ± 1.72 |
| Median no. of anticancer therapies (range) | 2.0 (1–7) |
| Tyrosine kinase inhibitors, n (%) | 35 (100.0) |
| Sunitinib | 27 (77.1) |
| Sorafenib | 22 (62.9) |
| Gefitinib | 2 (5.7) |
| Erlotinib | 1 (2.9) |
| AZD2171 | 3 (8.6) |
| Other antineoplastic agents, n (%) | |
| Temsirolimus (mTOR inhibitor) | 10 (28.6) |
| Interferon, n (%) | 7 (20.0) |
| Interleukin-2, n (%) | 7 (20.0) |
| Anti-VEGF monoclonal antibodies, n (%) | |
| Bevacizumab | 6 (17.1) |
| Pyrimidine analogues, n (%) | |
| Capecitabine | 3 (8.6) |
| Gemcitabine | 1 (2.9) |
| Other alkylating agents, n (%) | |
| Temozolomide | 3 (8.6) |
| Other immunosuppressive agents, n (%) | |
| Thalidomide | 3 (8.6) |
| Selective immunosuppressive agents | |
| Everolimus | 3 (8.6) |
| Investigational drugs, n (%) | 7 (20.0) |
| All other therapies, n (%) | 8 (22.9) |
Abbreviations: mTOR, mammalian target of rapamycin; StD, standard deviation; VEGF, vascular endothelial growth factor.
Safety
No deaths occurred during the study treatment period and no death was assessed as being related to AS1411 therapy.
All patients had ≥1 treatment-emergent AE. Most patients (80%) had only mild or moderate AEs (grade 1 or 2). Seven patients (20%) had severe AEs (grade 3). Constipation, fatigue, and peripheral edema were the most common AEs (Table 3). None of the grade 3–4 AEs based on laboratory abnormalities were judged as being related to AS1411 (Table 4).
Table 3.
Adverse events occurring in ≥3 patients (safety analysis set)
| Adverse event | Number of patients (%) | |||
|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Total† | |
| Any* | 12 (34.3) | 16 (45.7) | 7 (20.0) | 35 (100.0) |
| Constipation | 10 (28.6) | 2 (5.7) | 0 | 12 (34.3) |
| Fatigue | 8 (22.9) | 2 (5.7) | 0 | 10 (28.6) |
| Peripheral edema | 6 (17.1) | 1 (2.9) | 0 | 7 (20.0) |
| Nausea | 6 (17.1) | 0 | 0 | 6 (17.1) |
| Pyrexia | 4 (11.4) | 0 | 0 | 4 (11.4) |
| Arthralgia | 2 (5.7) | 1 (2.9) | 1 (2.9) | 4 (11.4) |
| Musculoskeletal pain | 2 (5.7) | 2 (5.7) | 0 | 4 (11.4) |
| Anemia | 0 | 3 (8.6) | 1 (2.9) | 4 (11.4) |
| Headache | 4 (11.4) | 0 | 0 | 4 (11.4) |
| Insomnia | 4 (11.4) | 0 | 0 | 4 (11.4) |
| Upper abdominal pain | 2 (5.7) | 0 | 1 (2.9) | 3 (8.6) |
| Vomiting | 2 (5.7) | 1 (2.9) | 0 | 3 (8.6) |
| Coughing | 2 (5.7) | 1 (2.9) | 0 | 3 (8.6) |
| Rash | 1 (2.9) | 2 (5.7) | 0 | 3 (8.6) |
Highest grade recorded.
No ≥ grade 4 adverse events were recorded.
Table 4.
Laboratory abnormalities reported in >10% of patients (safety analysis set)
| Variable | Number of patients (%) | |||
|---|---|---|---|---|
| Grades 1–2 | Grade 3 | Grade 4 | Total | |
| Hematology | ||||
| Hemoglobin | 33 (94.3) | 1 (2.9) | 0 | 34 (97.1) |
| Lymphocytes | 16 (45.7) | 6 (17.1) | 1 (2.9) | 23 (65.7) |
| Leukocytes | 7 (20.0) | 0 | 0 | 7 (20.0) |
| Platelets | 5 (14.3) | 0 | 0 | 5 (14.3) |
| Clinical chemistry | ||||
| Albumin | 27 (77.1) | 0 | 0 | 27 (77.1) |
| Hyperglycemia† | 25 (75.8) | 0 | 1 (3.0) | 26 (78.8) |
| Creatinine | 23 (65.7) | 0 | 0 | 23 (65.7) |
| Aspartate aminotransferase | 22 (62.9) | 0 | 0 | 22 (62.9) |
| Hyponatremia | 15 (42.9) | 0 | 1 (2.9) | 16 (45.7) |
| Gamma-glutamyltransferase | 12 (34.3) | 2 (5.7) | 0 | 14 (40.0) |
| Hyperkalemia | 12 (34.3) | 2 (5.7) | 0 | 14 (40.0) |
| Hypocalcemia | 14 (40.0) | 0 | 0 | 14 (40.0) |
| Bicarbonate | 12 (34.3) | 0 | 0 | 12 (34.3) |
| Hypomagnesemia | 12 (34.3) | 0 | 0 | 12 (34.3) |
| Alkaline phosphatase | 10 (28.6) | 1 (2.9) | 0 | 11 (31.4) |
| Hypercalcemia | 7 (20.0) | 1 (2.9) | 0 | 8 (22.9) |
| Alanine aminotransferase | 7 (20.0) | 0 | 0 | 7 (20.0) |
| Hypermagnesemia | 5 (14.3) | 1 (2.9) | 0 | 6 (17.1) |
No grade 5 laboratory abnormalities were recorded.
n = 33.
Twelve (34%) patients had AEs that were judged by the investigator as being related to AS1411; all were grade 1 or 2, and only headache (n = 3), increased aspartate aminotransferase (AST) (n = 2), and fatigue (n = 2) were reported with greater than single patient incidence
Five (14%) patients reported serious AEs, none of which was judged by the investigator as being related to AS1411.
Efficacy
One patient had a PR, resulting in an ORR of 2.9 %(Table 5). The two-sided 95% CIs for response rate and their associated p- values (full analysis set, 0.1–14.9%; per protocol analysis set, 0.1–15.3%) did not permit rejection of the null hypothesis that the ORR for patients treated with AS1411 is <5%.
Table 5.
Efficacy results
| Variable | Investigator assessment |
Independent assessment |
|---|---|---|
| Best overall response rate | ||
| Full analysis set (n = 35) | ||
| CR, n (%) | 0 | 0 |
| PR, n (%) | 1 (2.9) | 1 (2.9) |
| SD, n (%) | 12 (34.3) | 21 (60.0) |
| PD, n (%) | 21 (60.0) | 10 (28.6) |
| Missing* | 1 (2.9) | 3 (8.6) |
| ORR (CR + PR) | ||
| Responders, n | 1 | 1 |
| Estimate of response rate, % | 2.9 | 2.9 |
| Two-sided 95% CI, % | 0.1–14.9 | 0.1–14.9 |
| Per protocol analysis set (n = 34) | ||
| CR, n (%) | 0 | 0 |
| PR, n (%) | 1 (2.9) | 1 (2.9) |
| SD, n (%) | 12 (35.3) | 21 (61.8) |
| PD, n (%) | 21 (61.8) | 10 (29.4) |
| Missing* | 0 | 2 (5.9) |
| ORR (CR + PR) | ||
| Responders, n | 1 | 1 |
| Estimate of response rate, % | 2.9 | 2.9 |
| Two-sided 95% CI, % | 0.1–15.3 | 0.1–15.3 |
| Progression-free survival | ||
| Full analysis set (n = 35) | ||
| Patients with event†, n (%) | 29 (82.9) | 14 (40.0) |
| Censored patients, n (%) | 6 (17.1) | 21 (60.0) |
| Median (95%CI) PFS, days | 59.0 (55.0–114.0) | 120.0 (59.0–261.0) |
| PFS rate (Kaplan-Meier estimate), % 6 months | 9.0 (0.0–20.1) | 31.9 (2.5–61.3) |
| Time to progression | ||
| Full analysis set (n = 35) | ||
| Patients with event‡, n (%) | 29 (82.9) | 14 (40.0) |
| Censored patients, n (%) | 6 (17.1) | 21 (60.0) |
| Median (95%CI) TTP, days | 59.0 (55.0–114.0) | 120.0 (59.0–261.0) |
| TTP rate (Kaplan-Meier estimate), % 6 months | 9.0 (0.0–20.1) | 31.9 (2.5–61.3) |
| Duration of stable disease | ||
| Full analysis set (n = 35) | ||
| Patients with event§, n (%) | 9 (25.7) | 4 (11.4) |
| Censored patients, n (%) | 3 (8.6) | 17 (48.6) |
| Median (95%CI) SD, days | 165.0 (114.0–173.0) | 173.0 (173.0–261.0) |
| SD rate (Kaplan-Meier estimate), % 6 months | 14.1 (00–39.0) | 39.0 (0.0–94.7) |
No after baseline tumor assessment.
Event is the earlier of documented disease progression or death from any cause.
Event is documented disease progression.
Event is documented disease progression or death.
Abbreviations: CI, confidence interval; CR, complete response; ORR, overall response rate; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease; TPP, time to progression.
Secondary efficacy endpoints for patients in the full analysis set are also summarized in Table 5. Median PFS was 2 months (95% CI 1.8–3.8) as determined by investigator assessment and 4 months (95% CI 2.0–8.7) by independent review. Median duration of SD was 5.5 months (95% CI 3.8–5.8) based on investigator assessment and 5.8 months (95% CI 5.8–8.7) months based on independent assessment. As a treatment response was reported for only one patient, no formal analysis of duration of overall response was possible; however this patient currently remains free of progression a full 2 years after completion of study therapy.
Pharmacokinetics
Summary PK variables derived from plasma AS1411 concentrations during and after continuous infusion are shown in Table 6. There was large variability in plasma AS1411 concentrations between patients. After the cessation of infusion, the plasma AS1411 concentration declined rapidly in a monophasic manner. The disposition of AS1411 followed linear kinetics. The estimated mean systemic CL of 111 ± 40.9 mL/h/kg was approximately the same as the glomerular filtration rate of 107 mL/h/kg, suggesting that renal filtration may be the major clearance route. Vss ranged from 38.0 to 2520 mL/kg and was highly variable between patients (CV%, 94.2); this may indicate different distribution patterns in different patients.
Table 6.
Plasma pharmacokinetic variables for AS1411 during and after continuous intravenous infusion in cycle 1 (pharmacokinetic analysis set)
| Variable | Mean ± StD | CV, % |
|---|---|---|
| tmax, h | 44.1 ± 21.4 | 48.4 |
| Cmax, µg/mL | 25.4 ± 11.5 | 45.2 |
| t½, h | 1.71 ± 0.45 | 26.3 |
| AUC(0–last), µg/h/mL | 1600 ± 537 | 33.6 |
| AUC(0–inf), µg/h/mL | 1630 ± 557 | 34.2 |
| CL, mL/h/kg | 111 ± 40.9 | 36.9 |
| Vss, mL/kg | 689 ± 649 | 94.2 |
Abbreviations: AUC(0–inf), the area under the plasma AS1411 concentration-time curve from time 0 to infinity; AUC(0–last), the area under the plasma AS1411 concentration-time curve from time 0 to the last quantifiable time point; CV, coefficient of variability; CL, total body clearance; Cmax, maximum observed plasma AS1411 concentration; StD, standard deviation; t½, terminal elimination half-life; tmax, time to reach Cmax; Vss, volume of distribution at steady state.
The mean Css of 20.6 ± 7.00 µg/mL was similar to the IC50 concentration identified for renal cancer cell lines in vitro. Exposure to AS1411 in the patient with a PR, as assessed by Cmax, Css, and AUC, was similar to that in other patients, indicating that the response observed in this patient was not a result of greater exposure to study drug.
Pharmacodynamics
In general, circulating levels of cytokines over the 96 hours from the commencement of AS1411 infusion in cycle 1 were similar to preinfusion levels. Slight increases in IL-2 and TNF levels during AS1411 infusion in one patient coincided with the rash and pruritus that resulted in temporary interruption of infusion.
Discussion
We provide the first published manuscript describing the use of this novel anti-cancer agent in humans. In an open-label, single-arm, multicenter, phase II study, we investigated the efficacy and safety of the DNA aptamer AS1411 in the treatment of patients with advanced RCC who had failed tyrosine kinase inhibitor therapy.
Although only one patient achieved a PR (ORR 2.9%), there was a dramatic response to treatment with an 84% decrease in the sum diameters of target tumor lesions. That patient remains in PR at 24 months from the end of study therapy with no evidence of disease progression and no requirement for additional therapy. Interestingly, this patient was primary refractory to prior TKI therapies. The durability of this response may lead one to suspect an immune driven mode of action. However, there were no signs of constitutive immune activation either clinically (no rash, pruritis or other autoimmune phenomena) or on laboratory testing (leukocyte and lymphocyte counts were within normal range with no marked elevations after starting therapy). In the phase 1 study of AS1411 a similar dramatic response was seen in a patient with metastatic RCC who had a near CR to treatment of 18 months duration.15
Median PFS was 4 months by independent assessment and 2 months by investigator assessment. The marked differences between the independent and investigator reviews are different from what is usually expected, where investigator bias is usually associated with improved PFS rather than shorter PFS as seen in this study. Although this agent had limited activity in RCC it was well tolerated with minimal toxicity.
Despite the disappointing overall efficacy results in this trial DNA aptamers remain promising molecules with potential utility in cancer medicine. In recent months, a Her2 targeted DNA aptamer conjugated to doxorubicin was shown to selectively deliver this cytotoxic agent to Her2 overexpressing cancer cells in vitro.17 Another study used AS1411 tagged drug-loaded nanoparticles to deliver targeted cytotoxic therapy to nucleolin overexpressing cancer cells in vitro with promising results.18 Furthermore, the potential of AS1411 in novel cancer imaging techniques was shown in a study where it was conjugated to a magnetic fluorescence micro-RNA molecule which allowed cancer cell uptake and imaging of intracellularly expressed micro-RNA.19
The oncology community is increasingly aware of the fact that drugs which target specific molecular abnormalities within a tumor need to be restricted to patients with relevant signaling pathway aberrations to show their full potential.20, 21 Such a strategy leads to improved risk-benefit and cost-benefit ratios for novel drugs. Molecular and genetic discoveries are demonstrating the heterogeneity of cancer and compartmentalizing it into “a thousand rare diseases” meaning such rational selection of appropriate patients will become increasingly relevant in future trials of novel agents.22 Perhaps, restricting enrollment in this trial to patients who had tumors overexpressing nucleolin would have produced higher response rates. Unfortunately, archival tissue was not routinely collected on this trial.
In conclusion, this study provides evidence that although AS1411 has a low level of activity in unselected patients with metastatic RCC, dramatic and durable responses can be observed and toxicity is low. The nucleolin pathway may still represent a relevant target in selected patients with metastatic RCC and AS1411-conjugated compounds are being investigated. DNA aptamers represent a novel way to target cancer cells at a molecular level and continue to be developed with a view to improving treatment and imaging in cancer medicine.
Acknowledgments
Grant Support
This study was sponsored by Antisoma Research, Ltd.
Footnotes
Disclosure of Potential Conflicts of Interest
Frederik Erlandsson – employee of Antisoma
ClinicalTrials.gov Identifier: NCT00740441
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Hutson TE, Tomczak P, et al. Overall Survival and Updated Results for Sunitinib Compared With Interferon Alfa in Patients With Metastatic Renal Cell Carcinoma. Journal of Clinical Oncology. 2009;27(22):3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 4.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370(9605):2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 5.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 7.Bates PJ, Laber DA, Miller DM, Thomas SD, Trent JO. Discovery and development of the G-rich oligonucleotide AS1411 as a novel treatment for cancer. Exp Mol Pathol. 2009;86(3):151–164. doi: 10.1016/j.yexmp.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esposito CL, Catuogno S, de Franciscis V, Cerchia L. New insight into clinical development of nucleic acid aptamers. Discov Med. 2011;11(61):487–496. [PubMed] [Google Scholar]
- 9.Abdelmohsen K, Gorospe M. RNA-binding protein nucleolin in disease. RNA Biol. 2012;9(6) doi: 10.4161/rna.19718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Z, Joshi N, Agarwal A, et al. Knocking down nucleolin expression in gliomas inhibits tumor growth and induces cell cycle arrest. J Neurooncol. 2012;108(1):59–67. doi: 10.1007/s11060-012-0827-2. [DOI] [PubMed] [Google Scholar]
- 11.Hovanessian AG, Soundaramourty C, El Khoury D, Nondier I, Svab J, Krust B. Surface expressed nucleolin is constantly induced in tumor cells to mediate calcium-dependent ligand internalization. PLoS One. 2010;5(12):e15787. doi: 10.1371/journal.pone.0015787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W, Sridharan V, Soundararajan S, et al. Activity and Mechanism of Action of AS1411 in Acute Myeloid Leukemia Cells. Blood. 2007;110:1604. [Google Scholar]
- 13.Xu X, Hamhouyia F, Thomas SD, et al. Inhibition of DNA replication and induction of S phase cell cycle arrest by G-rich oligonucleotides. J Biol Chem. 2001;276(46):43221–43230. doi: 10.1074/jbc.M104446200. [DOI] [PubMed] [Google Scholar]
- 14.Soundararajan S, Chen W, Spicer EK, Courtenay-Luck N, Fernandes DJ. The nucleolin targeting aptamer AS1411 destabilizes Bcl-2 messenger RNA in human breast cancer cells. Cancer Res. 2008;68(7):2358–2365. doi: 10.1158/0008-5472.CAN-07-5723. [DOI] [PubMed] [Google Scholar]
- 15.Miller DM, Laber DA, Bates PJ, et al. Extended phase I study of AS1411 in renal and non-small cell lung cancers. Ann Oncol. 2006;17(suppl 9):ix144–ix157. (450P). [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z, Duan JH, Song YM, et al. Novel HER2 Aptamer Selectively Delivers Cytotoxic Drug to HER2-positive Breast Cancer Cells in Vitro. J Transl Med. 2012;10(1):148. doi: 10.1186/1479-5876-10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aravind A, Jeyamohan P, Nair R, et al. AS1411 aptamer tagged PLGA-lecithin-PEG nanoparticles for tumor cell targeting and drug delivery. Biotechnol Bioeng. 2012 doi: 10.1002/bit.24558. [DOI] [PubMed] [Google Scholar]
- 19.Kim JK, Choi KJ, Lee M, Jo MH, Kim S. Molecular imaging of a cancer-targeting theragnostics probe using a nucleolin aptamer- and microRNA-221 molecular beacon-conjugated nanoparticle. Biomaterials. 2012;33(1):207–217. doi: 10.1016/j.biomaterials.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 20.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerr D. Is Oncology Ready for 1000 Rare Diseases? 2012 Available from URL: http://www.medscape.com/viewarticle/766796. [Google Scholar]