Abstract
Standard first-line therapy for older patients with high-risk MDS consists of hypomethylating agents such as azacitidine (AZA). However, the only approach with curative potential remains allogeneic hematopoietic cell transplantation (HCT). So far no direct comparison of both strategies has been carried out. The outcomes of two well balanced cohorts of high-risk MDS patients defined by age (60–70 years), performance status (ECOG ≤2) and donor availability (yes/no) were compared including 103 patients undergoing HCT and 75 patients without this option who received AZA. The estimated 2-year overall survival (OS) after the start of treatment was 39% (95% CI, 30% to 50%) for the HCT patients and 23% (95% CI, 14 to 40%) for the AZA patients. In a multivariate Cox regression analysis of all (n=178) patients ECOG score (0 vs. 1 vs. 2, HR: 2.9/3.9, p<0.001), cytogenetics (good vs. intermediate vs. poor, HR: 1.2/1.7, p=0.026) and type of treatment (HCT vs. AZA, HR: 0.3, p=0.007) were associated with OS.
This retrospective cohort analysis suggests a survival advantage with allogeneic HCT compared to treatment with AZA in medically fit high-risk MDS patients 60 to 70 years of age, Prospective controlled studies are warranted.
Keywords: MDS, secondary AML, allogeneic transplantation, comorbidity, azacitidine
Introduction
Myelodysplastic syndromes (MDS) occur primarily in older individuals, in whom treatment with hypomethylating agents (HMA) such as azacitidine (AZA) has resulted in a survival advantage1 compared to patients treated with supportive care (SC)2. The only therapy with proven curative potential, however, remains allogeneic hematopoietic stem cell transplantation (HCT)3. Published data regarding the use of allogeneic HCT in older patients with high-risk MDS have been limited until recently. Many of these patients were not considered as candidates for allogeneic HCT, because of concerns about increased transplant-related toxicity and excess of non-relapse mortality (NRM), a challenging problem primarily in older individuals. As the extramedullary toxicity of transplant conditioning contributes significantly to early NRM, the development of reduced-intensity conditioning (RIC) regimens and the use of alternative donor sources has allowed the successful application of HCT in older patients with MDS and AML4.
Today, there is considerable evidence that allogeneic HCT can be successful in older patients with MDS5–8 and it is conceivable that it provides a survival advantage compared to SC only or other non-transplant approaches9. No controlled prospective data are available and no direct randomized comparison between HCT and the efficacy of HMA has been presented so far10. Given the heterogeneity of MDS, the potential complications associated with HCT, and the availability of therapeutic non-transplant alternatives, for example with HMA, the decision of when and in whom to perform allogeneic HCT remains difficult for both the patient and the treating physician. In fact, a recent retrospective analysis demonstrated that, in contrast to younger MDS patients, a survival benefit might be obtained in higher-risk MDS patients only with a certain delay after HCT compared to HMA11. Although retrospective studies are subject to selection bias, they can provide important information to facilitate clinical decision-making12. Therefore, we analysed the treatment results of a well defined group of patients 60 to 70 years of age with de novo high-risk MDS undergoing allogeneic HCT. The intent was to compare the outcome after allogeneic HCT with that observed in a similar cohort of patients who had received first-line treatment with AZA in the absence of a donor.
Design and Methods
Study design
First, the participating transplant centers in Germany and the US (GMDS-SG, GCTSG, FHCRC) provided data on patients 60 to 70 years of age with the initial diagnosis of high-risk MDS, defined as either refractory anemia with excess of blasts (RAEB), RAEB in transformation (RAEB-t) or chronic myelomonocytic leukemia (CMML) according to the French-American-British (FAB) classification or INT-2/HIGH risk MDS according to the IPSS2 who received allogeneic HCT. The analysis was restricted to patients with RAEB, RAEB-t or CMML and at least 5% marrow blasts at diagnosis as these disease categories generally would be considered indications for HCT in younger individuals. Only patients with at least intermediate intensity conditioning (excluding patients conditioned with fludarabine plus 2 Gy TBI) and patients with ECOG scores <3 were included in order to avoid a selection of “medically unfit” patients with comorbidities that would presumably exclude them from consideration for intensive treatment approaches including allogeneic HCT. Then, the HCT cohort was compared to a French cohort of patients who had received treatment with AZA and did not undergo allogeneic HCT, because of the lack of a suitably HLA matched donor or because the patient was not considered for HCT by the treating physician due to guidelines not to offer transplantation to high-risk MDS patients above the age of 60 years. As for the HCT cohort the analysis was restricted to patients 60–70 years of age with ≥ 5% blasts (RAEB, RAEB-t or CMML) in the bone marrow at diagnosis. Data were provided by the registry of the GFM (groupe francophone de myelodysplasie), which currently contains information on 735 patients who received at least 1 cycle of AZA and were treated at 42 French centers, including 282 patients with IPSS INT-2 or HIGH risk MDS and patients with AML with ≤ 30% marrow myeloblasts (RAEB-t), as described previously13.
Definitions
Patients were also classified according to the WHO classification. Cytogenetic subgroups and red-blood cell transfusion dependency (TD) were defined according to the IPSS2 and WPSS scoring systems8. Conditioning regimens were defined according to a study of the European Group for Blood and Marrow Transplantation (EBMT) as “conventional” intensity (CIC) with myeloablative intent14. All other regimens where considered to be of reduced intensity (RIC).
Response criteria and parameters for progression were assessed according to the IWG15. Event-free (event defined either as death, relapse or progression) and overall survival times were calculated from start of treatment (AZA vs. HCT, respectively). Relapse and progression were both considered for the calculation of relapse incidence. The definition of NRM as competing event for relapse incidence was applied to both HCT and AZA therapy according to Cheson et al15. However, patients with either CR, PR, HI or SD in response to AZA were considered for NRM of the AZA cohort.
Statistical Methods
Estimates for overall and relapse-free survival were calculated by the method of Kaplan and Meier. For univariate comparisons the log-rank test was used. Incidences of relapse/progression and NRM were calculated using competing event statistics, and the Gray-test was employed for univariate comparisons16. Approximate 95%-confidence intervals are provided for point estimates of overall survival (OS), event-free survival (EFS), relapse incidence (RI) and NRM. Variables for the multivariate analysis were selected a priori based on literature data to assure that the estimated treatment effects were adjusted for the most important confounders. Survival data were censored at three years after start of treatment. Complete case analyses were performed. The proportional hazard (PH) assumption was checked for each multivariate model by analysis of the scaled Schoenfeld residuals17. Cox regression models were fitted for OS and EFS. Age, time from diagnosis to treatment, ECOG, WHO classification, cytogenetic results and type of treatment were entered into the multivariate model. Scaled Schoenfeld residuals were analysed in order to check the proportional hazard assumption for the Cox regression models for OS and EFS. The global tests for a change of the hazard rates over time indicated non-proportionality for both models (p=0.02 for the Cox model for OS and p=0.002 for the Cox model for EFS). Further analysis indicated that this was the result of a variation of the treatment effect (AZA versus HCT) over time (data not shown). Scaled Schoenfeld residuals can be interpreted as time-dependent beta-coefficients. Higher values of these residuals with increasing observation times indicated an increasing hazard-rate for patients in the AZA group compared to the HCT group. The systematic deviation of the smoothing splines from a horizontal line is indicative of non-proportional hazards in the two treatment groups. Proportional hazards are, however, a basic assumption for Cox regression analysis. Since proportionality of the hazard could not be assumed for this simple model, a dichotomous time-dependent covariate was introduced into the Cox model, which allowed for the calculation of different treatment effects in the first year after the start of treatment (AZA or HCT) and the subsequent time period.
Computations were done with SPSS statistics, version 18.0.0. (SPSS Inc., Chicago, IL, USA) and R, version 2.12.1 including the software packages for survival (2.36–5)18,19. This retrospective study was performed according to the declaration of Helsinki. All patients signed informed consent for participation in research studies, and the use of data of the MDS registry had been approved by the local IRB of the University of Dresden, the Fred Hutchinson Cancer Research Center, Seattle, WA as well as the GFM group.
Results
Characteristics of the study cohorts
Transplantation Cohort
Overall, 103 patients with de novo high-risk MDS, transplanted from 1995 through 2008 were identified at participating centers. As shown in Table 1, all patients had RAEB, RAEB-t or CMML, and 67 (65%) met the IPSS INT-2/HIGH risk criteria. Forty-two patients (41%) had received induction chemotherapy (IC, CR rate: 39%), six patients (6%) various treatments including erythropoiesis stimulating agents (ESA) or HMA alone (3 patients achieved hematological improvement while the remainder obtained stable disease), and 51 patients (49%) received SC only.
Table 1. Disease and patient characteristics at diagnosis and prior to therapy.
Patients were grouped according to the current classification and scoring systems used in MDS, including French-American-British (FAB), World Health Organization (WHO), International Prognostic Scoring System (IPSS) and cytogenetic risk groups defined by the IPSS; RAEB=RA with excess of blasts, RAEB-t= RAEB in transformation, AML=acute myeloid leukemia, CMML=chronic myelomonocytic leukemia, NA=not available, HCT=hematopoietic cell transplantation, AZA=azacitidine, RBC TD= red blood cell transfusion dependency
| AZA (%) | HCT (%) | AZA (%) | HCT (%) | |||
|---|---|---|---|---|---|---|
| P | at diagnosis N=75 |
at diagnosis N=103 |
P | prior to AZA n=75 |
prior to HCT n=103 |
|
| Gender | ||||||
| male/female | 0.95 | 55/20 | 76/27 | 0.95 | 55/20 | 76/27 |
| Age | ||||||
| median (range) | 0.01 | 65 (56–70) | 63 (54–69) | 0.02 | 66 (60–70) | 64 (60–70) |
| FAB | 0.33 | <0.01 | ||||
| RAEB | 60 (80) | 75 (73) | 45 (60) | 41 (40) | ||
| RAEB-T | 11 (15) | 16 (16) | 21 (28) | 10 (10) | ||
| AML | 0 | 0 | 7 (9) | 43 (42) | ||
| CMML | 4 (5) | 12 (12) | 2 (3) | 9 (9) | ||
| WHO | 0.18 | <0.01 | ||||
| RAEB-1/CMML-1 | 31 (41) | 41 (40) | 16 (21) | 15 (15) | ||
| RAEB-2/CMML-2 | 30 (40) | 49 (48) | 31 (41) | 28 (27) | ||
| AML | 11 (15) | 13 (13) | 28 (37) | 51 (50) | ||
| Unknown | 3 (4) | 0 | 0 | 9 (9) | ||
| IPSS | 0.78 | <0.01 | ||||
| INT-1 | 14 (19) | 20 (19) | 4 (5) | 9 (9) | ||
| INT-2 | 30 (40) | 36 (35) | 29 (39) | 23 (22) | ||
| HIGH | 23 (31) | 31 (30) | 37 (49) | 19 (18) | ||
| AML | 0 | 0 | 0 | 43 (42) | ||
| Unknown | 8 (11) | 16 (16) | 5 (7) | 9 (9) | ||
| Cytogenetics | 0.78 | 0.14 | ||||
| Good | 37 (49) | 53 (52) | 28 (37) | 51 (50) | ||
| Intermediate | 10 (13) | 16 (16) | 12 (16) | 16 (16) | ||
| Poor | 23 (31) | 25 (24) | 32 (43) | 28 (27) | ||
| Unknown | 5 (7) | 9 (9) | 3 (4) | 8 (8) | ||
| RBC TD | — | <0.01 | ||||
| Yes | NA | NA | 42 (56) | 73 (71) | ||
| No | NA | NA | 16 (21) | 28 (27) | ||
| Unknown | NA | NA | 17 (23) | 2 (2) | ||
| ECOG | — | 0.06 | ||||
| 0 | NA | 28 (27) | 24 (32) | 23 (22) | ||
| 1 | NA | 43 (42) | 38 (51) | 70 (68) | ||
| 2 | NA | 2 (2) | 13 (17) | 10 (10) | ||
| Median time from diagnosis (months) | — | NA | NA | 0.10 | 6.0 (0–141) | 7.6 (1.3–112) |
At the time of diagnosis and pre-HCT the marrow blast counts were 5%–30% (median 11%) and 0%–80% (median 10%), respectively. The pre-transplant hematopoietic cell transplantation specific comorbidity index (HCT-CI) could be determined in 94 patients, of whom 37 (39%), 16 (17%), 16 (17%), and 25 (27%) had scores of 0, 1, 2 and ≥3, respectively20.
Allogeneic HCT was performed at 1.3–112 (median 7.6) months from MDS diagnosis. Sixty-one patients (59%) underwent RIC while 45 patients (41%) received CIC, followed by peripheral blood stem cells (PBSC, n=94) or bone marrow (n=4) (information missing on 5 patients) from unrelated (n=63; 61%) or related (n=40; 39%) donors without further manipulation. The donors were HLA-identical for 78 patients (76%) and single allele mismatched in 17 patients.
AZA cohort
There were 75 patients with a date of diagnosis between 2004 and 2009. The time from diagnosis to first-line treatment with AZA was 0–141 (median 6) months. The marrow blast counts at diagnosis and prior to AZA were 5% to 30% (median 12%) and 6% to 59% (median 17%), respectively. A search for a suitable (at least 9 out of 10 allele match) donor was initiated at the start of AZA for the majority (n=60, 80%) of patients but remained unsuccessful. In the other patients (20%) HCT was not considered due to guidelines not to offer allogeneic HCT to patients above the age of 60 years.
Patients received a median of 6 (range 1–52) cycles of AZA therapy. There were only 6 patients with less than 3 cycles of treatment. The reason for the limited number of cycles administered was early progression to higher stage MDS or AML in all of them. The ECOG score was not available at the time of diagnosis for the AZA group but was 0–2 (median 1) at the time of in initiating AZA. Thirty-four patients (45%) responded to AZA treatment with CR, PR or HI, 18 (24%) had stable disease and 14 (19%) had primary disease progression. The extent of response was unknown in 5 patients (7%), and 4 patients (6%) were not evaluable due to early death. Finally, during the later course of the disease 16 patients (21%) underwent induction chemotherapy (IC), because of no response or progressive disease while receiving AZA.
Comparison of AZA and HCT cohorts
Characteristics at diagnosis
As shown in Table 1, at diagnosis the two patient cohorts (HCT or AZA) were comparable with regards to disease subtype (FAB or WHO), IPSS and cytogenetic characteristics. There was no difference in the gender distribution, while AZA treated patients were slightly older than HCT patients (median 65 vs. 63 years; p=0.01).
Characteristics at onset of treatment
The cohorts were similar with regards to cytogenetic risk, but there were more patients with advanced disease (by FAB, WHO or IPSS) in the HCT cohort. In fact, the majority of HCT patients had progressed to a higher disease stage prior to HCT, with 43 (42%) vs. 7 (9%), and 51 (50%) vs. 28 patients (37%) in the AZA group having evolved to AML (by FAB or WHO criteria, respectively). Also, more patients in the HCT group were TD (n=73, 71%) than in the AZA group (n=42, 56%, p<0.01). There was no statistically significant difference in the performance status between the two cohorts (Table 1) as well as the overall rate of induction chemotherapy (in the AZA cohort given only after AZA failure).
Outcome
All patients undergoing HCT achieved primary engraftment. With a follow-up of 7–154 (median 39) months for surviving patients the estimated 2-year OS and EFS were 39% (95% CI, 30% to 50%) and 37% (95% CI, 28% to 48%), and relapse and NRM were 30% (95% CI, 21% to 39%) and 33% (95% CI, 23% to 42%), for the HCT cohort, respectively. The 5-year OS and EFS were 35% (95% CI, 26% to 47%) and 36% (95% CI, 27% to 47%), respectively (Figures 1 and 2). At last follow-up, 40 patients, 62 – 77 (median 68) years of age, were alive in remission after HCT.
Figure 1.
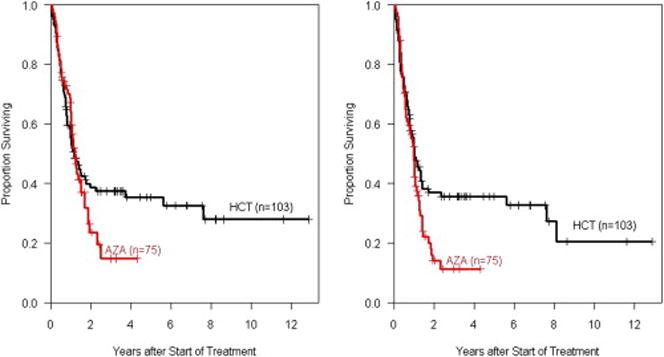
Overall (OS) and event-free survival (EFS) among MDS patients followed from start of therapy according to treatment approach
Figure 2.
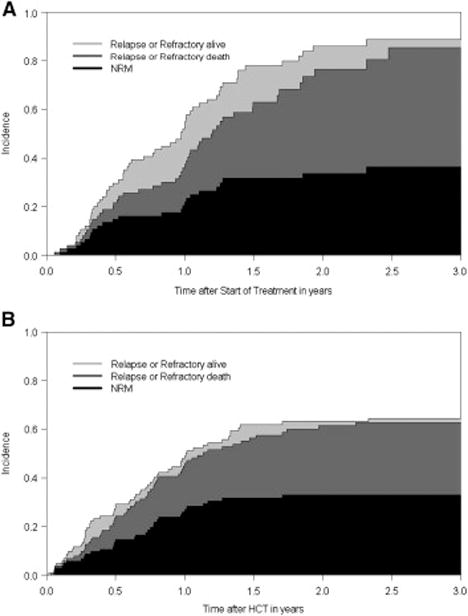
Stacked cumulative incidence curves from a competing risk model with relapse and death as competing risks, with the study population substratified according to type of treatment AZA: upper figure, HCT: lower figure
In the AZA group, with a follow-up of 1–52 (median 13) months from start of AZA therapy the 2-year OS, EFS, relapse/progression incidence and NRM were 23% (95% CI, 14% to 40%), 14% (95% CI, 7% to 27%), 52% (95% CI, 40% to 65%) and 34% (95% CI, 22% to 45%), respectively (Figure 1 and 2). At last follow-up, 16 patients with a follow up of 3 to 51 months (median 16 months), with 62 – 70 (median 67) years of age, were alive in that group.
Multivariate Cox regression analysis of the total cohort
The final models are shown in Table 2. ECOG performance score and cytogenetics significantly contributed to the prediction of OS and EFS. In the first year after start of the intervention no effect on OS was detectable (hazard ratio [HR] of HCT versus AZA, 1.3, p=0.3) while after one year HCT was associated with a strong protective effect (HR of HCT versus AZA, 0.3, p=0.007). The same pattern was observed for EFS.
Table 2.
Multivariate analysis of factors impacting on 2-year overall survival (OS) and event-free survival (EFS) in the total cohort of patients undergoing either allogeneic HCT (n=103) or treatment with AZA (n=75)
| OS | EFS | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (continuous) | 1.0 | 0.93 – 1.09 | 0.9 | 0.99 | 0.92 – 1.07 | 0.8 |
| Time from diagnosis | 0.94 | 0.84 – 1.06 | 0.3 | 0.93 | 0.83 – 1.04 | 0.2 |
| Disease stage | ||||||
| RAEB-1/CMML-1 | 1 | 1 | ||||
| RAEB-2/CMML-2 | 1.4 | 0.7 – 2.6 | 0.3 | 1.4 | 0.8 – 2.5 | 0.3 |
| RAEB-t/AML | 1.5 | 0.8 – 2.8 | 0.2 | 1.5 | 0.8 – 2.6 | 0.2 |
| ECOG | ||||||
| 0 | 1 | 1 | ||||
| 1 | 2.9 | 1.6 – 5.1 | <0.001 | 2.3 | 1.4 – 3.9 | 0.001 |
| 2 | 3.9 | 1.9 – 8.0 | <0.001 | 3.0 | 1.6 – 5.6 | 0.001 |
| Cytogenetics | ||||||
| good | 1 | 1 | ||||
| intermediate | 1.2 | 0.7 – 2.2 | 0.45 | 1.3 | 0.7 – 2.2 | 0.4 |
| poor risk | 1.7 | 1.1 – 2.8 | 0.026 | 1.7 | 1.1 – 2.6 | 0.02 |
| HCT versus AZA in the 1st year after start of treatment | 1.3 | 0.8 – 2.3 | 0.3 | 0.9 | 0.5 – 1.4 | 0.6 |
| HCT versus AZA from 1 year on | 0.3 | 0.1 – 0.7 | 0.007 | 0.4 | 0.2 – 0.97 | 0.04 |
Discussion
The present results show that HCT was superior to conventional treatment with AZA in achieving improved long-term survival. However, the survival curves of the two cohorts did not separate until about two years after start of therapy, reflecting an advantage for transplanted patients only with a delay after HCT which is consistent with the recent analysis by Koreth and colleagues11. The delayed separation of the curves was at least in part the result of NRM after allogeneic HCT related to GVHD and associated infectious complications. On the other hand, NRM at 2 years was basically identical 34% (95% CI, 22% to 45%) in the AZA group compared to 33% (95% CI, 23% to 42%) in the HCT group. Therefore, the present data also suggest that, contrary to a frequent perception, NRM following HCT may not significantly exceed mortality after conventional treatment with HMA. Additionally, our study supports the concept that chronological age alone should not serve as a barrier to allogeneic HCT in patients with advanced MDS who have a suitable donor. However, in agreement with previous reports on patients undergoing HCT or AZA, respectively, we confirmed the predictive value of the performance status of patients on the probability of success of a given treatment7,13,20. Until recently, supportive care was considered the standard of treatment for most older patients with high-risk MDS, while allogeneic HCT was restricted to a minority of patients selected, presumably, on the basis of biologic age and fitness. New developments of disease-modifying agents and innovations in allogeneic HCT have changed this paradigm4,21–27, although only limited data have been published on patients undergoing allogeneic HCT above 60 years of age. Our data also demonstrate that allogeneic HCT, in contrast to treatment with AZA, can lead to long-term disease control and possibly cure in a substantial proportion of patients with advanced MDS in the 7th decade of life. In agreement with observations in younger patients and recent retrospective analyses in older patients with MDS6,7 who underwent allogeneic HCT, high risk cytogenetics were associated with adverse outcome secondary to an increased incidence of relapse3,4,8,28,29. Thus, relapse remains a problem, primarily in patients with high risk cytogenetics and a high blast count at the time of HCT.
A shortcoming of the present study is that we can presumably not exclude a selection bias of the two cohorts analyzed. Additionally, patients were primarily matched only for the disease stage at diagnosis but not at the time point when the respective therapy started. However, in light of the fact that patients in the HCT group had more advanced disease prior to HCT, their survival benefit compared to the AZA cohort further strengthens the results. The worse median survival of our AZA cohort compared to the AZA001 study1 is presumably a result of a registry-based analysis as well as major differences in patient characteristics including more patients with moderate performance status (ECOG 0; 32% vs. AZA001 44%) and poor risk cytogenetics (43% vs. AZA001 28%) in our study. Patients groups were also matched for ECOG performance status, but not for comorbidities according to the HCT-CI, simply because such comorbidity scores were not available for patients in the AZA group. Therefore, we cannot exclude the possibility that the presence of certain comorbidities of patients in the AZA cohort might have dissuaded patients and their physicians from pursuing a more aggressive management, including HCT. Nevertheless, two thirds of HCT patients had HCT-CI scores of one or higher, which argues against the possibility that only totally “medically fit” patients were transplanted.
Taken together the current analysis suggests a benefit of allogeneic HCT compared to AZA in older patients with high-risk MDS or secondary AML. While these findings must be interpreted with caution, they lead to the provocative hypothesis that HCT offers a survival advantage to those patients, although the present data do not present proof of superiority of HCT over AZA. In particular, our analysis cannot be considered a true “donor versus no-donor” comparison. The inclusion of patients who had a donors identified, but who did not undergo allogeneic HCT e.g. because of toxicities related to prior therapy, might have further strengthened our findings. Unfortunately, that information was not available to us. Nevertheless, interpretation of the results is based on the assumption that the risk profiles of the two patient cohorts were captured correctly, and differences in outcome cannot be explained by confounders which were not included in the model. We believe, therefore, that this kind of retrospective analysis can still provide relevant clinical information in the absence of prospective trials12. Since the common availability of HMA has now changed strategies in preparation for transplantation30, future randomized studies should evaluate allogeneic HCT preceded by HMA in comparison to HMA alone in older patients with high-risk MDS.
Acknowledgments
We thank all nurses and physicians who were involved in primary patient care. We thank Wendy Wilson, Irmgard Matt and Mohamed Sorror for help with data collection. Supported in part by grant HL036444. UP developed the study design, included patients, collected patient’s data, analyzed the results and wrote the manuscript; JS performed the statistical analyses and edited the manuscript. All other authors collected patient data, analyzed data and reviewed/edited the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimersthat apply to the journal pertain.
Financial disclosure: UP, PF, GK, MB and LA had received honoraria from Celgene.
Reference List
- 1.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncology. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 3.Deeg HJ. Optimization of Transplant Regimens for Patients with Myelodysplastic Syndrome (MDS) Hematology Am Soc Hematol Educ Program. 2005:167–173. doi: 10.1182/asheducation-2005.1.167. [DOI] [PubMed] [Google Scholar]
- 4.Sockel K, Hofbauer LC, Ehninger G, Platzbecker U. Optimizing outcome of MDS patients post transplantation. Expert Reviews in Hematology. 2011;11:12–18. doi: 10.1586/ehm.11.58. [DOI] [PubMed] [Google Scholar]
- 5.Bertz H, Potthoff K, Finke J. Allogeneic stem-cell transplantation from related and unrelated donors in older patients with myeloid leukemia. J Clin Oncol. 2003;21:1480–1484. doi: 10.1200/JCO.2003.09.110. [DOI] [PubMed] [Google Scholar]
- 6.Lim Z, Brand R, Martino R, et al. Allogeneic Hematopoietic Stem-Cell Transplantation for Patients 50 Years or Older With Myelodysplastic Syndromes or Secondary Acute Myeloid Leukemia. Journal of Clinical Oncology. 2010;28:405–411. doi: 10.1200/JCO.2009.21.8073. [DOI] [PubMed] [Google Scholar]
- 7.McClune BL, Weisdorf DJ, Pedersen TL, et al. Effect of Age on Outcome of Reduced-Intensity Hematopoietic Cell Transplantation for Older Patients With Acute Myeloid Leukemia in First Complete Remission or With Myelodysplastic Syndrome. Journal of Clinical Oncology. 2010;28:1878–1887. doi: 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alessandrino EP, Della Porta MG, Bacigalupo A, et al. WHO classification and WPSS predict posttransplantation outcome in patients with myelodysplastic syndrome: a study from the Gruppo Italiano Trapianto di Midollo Osseo (GITMO) Blood. 2008;112:895–902. doi: 10.1182/blood-2008-03-143735. [DOI] [PubMed] [Google Scholar]
- 9.Estey E, de Lima M, Tibes R, et al. Prospective feasibility analysis of reduced-intensity conditioning (RIC) regimens for hematopoietic stem cell transplantation (HSCT) in elderly patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS) Blood. 2007;109:1395–1400. doi: 10.1182/blood-2006-05-021907. [DOI] [PubMed] [Google Scholar]
- 10.Stone RM. How I treat patients with myelodysplastic syndromes. Blood. 2009;113:6296–6303. doi: 10.1182/blood-2008-09-038935. [DOI] [PubMed] [Google Scholar]
- 11.Koreth J, Pidala J, Deeg HJ, et al. A decision analysis of reduced-intensity conditioning allogeneic hematopoietic stem cell transplantation for older patients with de-novo myelodysplastic syndrome (MDS): early transplantation offers survival benefit in higher-risk MDS. Blood. 2011 [Google Scholar]
- 12.Cutler CS, Lee SJ, Greenberg P, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104:579–585. doi: 10.1182/blood-2004-01-0338. [DOI] [PubMed] [Google Scholar]
- 13.Itzykson R, Thepot S, Quesnel B, et al. Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood. 2011;117:403–411. doi: 10.1182/blood-2010-06-289280. [DOI] [PubMed] [Google Scholar]
- 14.Ringden O, Labopin M, Ehninger G, et al. Reduced Intensity Conditioning Compared With Myeloablative Conditioning Using Unrelated Donor Transplants in Patients With Acute Myeloid Leukemia. Journal of Clinical Oncology. 2009;27:4570–4577. doi: 10.1200/JCO.2008.20.9692. [DOI] [PubMed] [Google Scholar]
- 15.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 16.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of A Competing Risk. Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 17.Grambsch PM, Therneau TM. Proportional Hazards Tests and Diagnostics Based on Weighted Residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 18.Therneau Terry M. Package ‘Survival’. R package version 2.36-2. 2011 [program]. http://r-forger-project.org.
- 19.Gray Robert J. Subdistribution Analysis of Competing Risks. R package version 2.2-1. 2011 [program]. http://biowww.dfci.harvard.edu/~gray.
- 20.Zipperer E, Pelz D, Nachtkamp K, et al. The hematopoietic stem cell transplantation comorbidity index is of prognostic relevance for patients with myelodysplastic syndrome. Haematologica-the Hematology Journal. 2009;94:729–732. doi: 10.3324/haematol.2008.002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoudjhane M, Labopin M, Gorin NC, et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT) Leukemia. 2005;19:2304–2312. doi: 10.1038/sj.leu.2403967. [DOI] [PubMed] [Google Scholar]
- 22.Scott BL, Sandmaier BM, Storer B, et al. Myeloablative vs nonmyeloablative allogeneic transplantation for patients with myelodysplastic syndrome or acute myelogenous leukemia with multilineage dysplasia: a retrospective analysis. Leukemia. 2006;20:128–135. doi: 10.1038/sj.leu.2404010. [DOI] [PubMed] [Google Scholar]
- 23.Tauro S, Craddock C, Peggs K, et al. Allogeneic stem-cell transplantation using a reduced-intensity conditioning regimen has the capacity to produce durable remissions and long-term disease-free survival in patients with high-risk acute myeloid leukemia and myelodysplasia. Journal of Clinical Oncology. 2005;23:9387–9393. doi: 10.1200/JCO.2005.02.0057. [DOI] [PubMed] [Google Scholar]
- 24.Giralt S, Thall PF, Khouri I, et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood. 2001;97:631–637. doi: 10.1182/blood.v97.3.631. [DOI] [PubMed] [Google Scholar]
- 25.Ho AYL, Pagliuca A, Kenyon M, et al. Reduced-intensity allogeneic hematopoietic stem cell transplantation for myelodysplastic syndrome and acute myeloid leukemia with multilineage dysplasia using fludarabine, busulphan, and alemtuzumab (FBC) conditioning. Blood. 2004;104:1616–1623. doi: 10.1182/blood-2003-12-4207. [DOI] [PubMed] [Google Scholar]
- 26.Kroger N, Bornhauser M, Ehninger G, et al. Allogeneic stem cell transplantation after a fludarabine/busulfan-based reduced-intensity conditioning in patients with myelodysplastic syndrome or secondary acute myeloid leukemia. Ann Hematol. 2003;82:336–342. doi: 10.1007/s00277-003-0654-9. [DOI] [PubMed] [Google Scholar]
- 27.Martino R, Iacobelli S, Brand R, et al. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using BLA-identical sibling donors in myelodysplastic syndromes. Blood. 2006;108:836–846. doi: 10.1182/blood-2005-11-4503. [DOI] [PubMed] [Google Scholar]
- 28.Chang CK, Storer BE, Scott BL, et al. Hematopoietic cell transplantation in patients with myelodysplastic syndrome or acute myeloid leukemia arising from myelodysplastic syndrome: similar outcomes in patients with de novo disease and disease following prior therapy or antecedent hematologic disorders. Blood. 2007;110:1379–1387. doi: 10.1182/blood-2007-02-076307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armand P, Kim HT, DeAngelo DJ, et al. Impact of cytogenetics on outcome of de novo and therapy-related AML and MDS after allogeneic transplantation. Biology of Blood and Marrow Transplantation. 2007;13:655–664. doi: 10.1016/j.bbmt.2007.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lubbert M, Bertz H, Ruter B, et al. Non-intensive treatment with low-dose 5-aza-2′-deoxycytidine (DAC) prior to allogeneic blood SCT of older MDS/AML patients. Bone Marrow Transplantation. 2009;44:585–588. doi: 10.1038/bmt.2009.64. [DOI] [PubMed] [Google Scholar]


