Abstract
Some weaknesses of conventional glass ionomer cement (GIC) as dental materials, for instance the lack of bioactive potential and poor mechanical properties, remain unsolved.
Objective
The purpose of this study was to investigate the effects of the partial replacement of CaO with MgO or ZnO on the mechanical and biological properties of the experimental glass ionomer cements.
Material and Methods
Calcium fluoro-alumino-silicate glass was prepared for an experimental glass ionomer cement by melt quenching technique. The glass composition was modified by partial replacement (10 mol%) of CaO with MgO or ZnO. Net setting time, compressive and flexural properties, and in vitro rat dental pulp stem cells (rDPSCs) viability were examined for the prepared GICs and compared to a commercial GIC.
Results
The experimental GICs set more slowly than the commercial product, but their extended setting times are still within the maximum limit (8 min) specified in ISO 9917-1. Compressive strength of the experimental GIC was not increased by the partial substitution of CaO with either MgO or ZnO, but was comparable to the commercial control. For flexural properties, although there was no significance between the base and the modified glass, all prepared GICs marked a statistically higher flexural strength (p<0.05) and comparable modulus to control. The modified cements showed increased cell viability for rDPSCs.
Conclusions
The experimental GICs modified with MgO or ZnO can be considered bioactive dental materials.
Keywords: Glass ionomer cement, Strength, Cell viability
INTRODUCTION
The development of glass-ionomer cement (GIC) materials was originated from the combination of two different dental cements, silicate (for aluminosilicate glass powder) and zinc polycarboxylate (for polyacrylic acid liquid) 2 , 25 . The conventional GICs are set by acid-base reaction of ion-leachable glass with aqueous solution of polyacrylic acid. These materials have been widely used in clinical dentistry as tooth-colored luting and restorative materials with some modifications in their composition 24 , 26 . Further, they are considered potential biomaterials for orthopedic application for their ability to adhere to bone and metals, stability in an aqueous environment, and lack of exothermic reaction 23 . However, some weaknesses of conventional GICs as dental materials, such as lack of bioactive potential and poor mechanical properties, remain unsolved 7 , 16 , 17 .
Considerable studies have been performed to improve the mechanical or biological properties of conventional GIC by incorporating bioactive ceramic particles, glass powders, and other chemicals to the GIC powder 20 . However, much of these efforts were found to be unsatisfied in enhancing mechanical properties without compromising other properties of the cements. A distinct strengthening can only be obtained by compounding short glass fibers of similar composition with the GICs 11 , 15 , 18 .
Since the properties of GIC are dependent on the specific chemical and physical formulation of glasses 22 , the development of new glasses and preparation methods are important to improve GIC properties. The addition of MgO or ZnO oxides to the calcium-aluminosilicate glasses has proven effective in improving elastic modulus and chemical durability 27 . Zinc has also been shown to have a stimulatory action on bone formation both in vitro and in vivo 29 , and antibacterial activity, similarly to silver 8 . Moreover, the addition of MgO to glasses increased cell proliferation particularly for those glasses containing 7 mole% MgO or more 10 . Thus, it would be wise to incorporate magnesium and zinc to glass cements as potential elements to enhance the mechanical and biological properties of GIC. In previous studies, experimental glass ionomer cements based on ZnO and/or MgO-base glasses showed great potential for biomedical application. However, their mechanical properties were generally much lower than the conventional GIC based on aluminosilicate glasses 4 , 6 , 14 , demonstrating that aluminum plays a key role in the GIC hardening and strengthening 19 .
The purpose of this study was to investigate the effects of glass modification with MgO or ZnO on the mechanical and biological properties of the experimental glass ionomer cements. A calcium fluoro-alumino-silicate glass batch, similar to conventional GIC composition, was prepared by melt quenching technique. The glass composition was modified by the partial replacement of CaO with MgO or ZnO to enhance the mechanical and biological properties of the prepared GIC. The hypothesis tested is that MgO/CaO or ZnO/CaO modifications in the glass composition do not enhance mechanical and biological properties of the prepared GIC. Results of the prepared cements were compared with those of a conventional GIC available in the market.
MATERIAL AND METHODS
Glass preparation
Glasses batches for GIC powder were produced using a melt-quench route. In the base glass system CaO-Al2O3-SiO2-P2O5-CaF2 (denoted glass G1), 10 mol% of calcium oxide was partially replaced by the equal amounts of magnesium (G2) or zinc oxides (G3). The details of the chemical composition are given in Table 1. Reagent grade SiO2, Al2O3, NH4H2PO4, CaCO3, CaF2, MgO and ZnO powders (Sigma-Aldrich, St. Louis, MO, USA) were used as starting materials. The mixtures of chemicals were melted at 1400°C-1450°C for 1.5 h using alumina crucibles in an electric furnace. After melting, the glasses were rapidly quenched into distilled water to prevent crystallization, and dried in an electrical oven. The glass frit was grounded using a vibratory mill (Gyro Mill, Glen Creston, London, UK) for 30 min and sieved using a 38 μm mesh test sieve (CISA, Barcelona, Spain). The thermal behavior of the finely powdered glass samples was monitored by differential scanning calorimeter (DSC 404, Netzsch, Selb, Germany) at a heating rate of 10°C·min−1 in air. The glass transition temperature (Tg) and crystallization temperature (Tc) were studied to determine the effect of the substitution of calcium with magnesium and zinc in the glass. X-ray powder diffraction (Ultima IV, Rigaku, Tokyo, Japan) was used to identify the amorphous phase present in the glasses using CuKα irradiation.
Table 1. Glass composition and differential scanning calorimeter (DSC) data of the experimental glasses for glass ionomer cement.
| Glass samples | Oxide constituents (mole %) | DSC data | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CaO | MgO | ZnO | SiO2 | Al2O3 | P2O5 | CaF2 | Tg | TC | |
| G1 | 37.27 | --- | --- | 36.12 | 18.05 | 6.42 | 2.14 | 766 | 944 |
| G2 | 27.27 | 10 | --- | 36.12 | 18.05 | 6.42 | 2.14 | 746 | 959 |
| G3 | 27.27 | --- | 10 | 36.12 | 18.05 | 6.42 | 2.14 | 734 | 901 |
Tg-Glass transitional temperature, Tc-Crystallization temperature
Cement formation
The prepared glass powders were mixed with an aqueous solution of polyacrylic acid (PAA) of commercial product (HY-BOND Glasionomer CX Liquid, Shofu, Kyoto, Japan) to form GICs. The cement liquid was a 40~50% solution of PAA (average molar weight 62,500 g/mol) and 1~10 % tartaric acid in water 14 . The powder liquid ratio was fixed at 2:1 and mixed for 30 s. The commercial powder and liquid (HY-BOND Glasionomer kit, Shofu) was also mixed to prepare control sample. The net setting time of the cements was measured by the method according to ISO 9917-1:2007 (Dental water-based cement) 12 , recorded as the time elapsed between the start of mixing and the time when the flat-end needle (1 mm in diameter) of indentor (400 g) does not form a complete circular indentation on the cement within a humid cabinet maintained at 37°C. The mean setting time of each group was recorded after three replicate tests.
Mechanical properties
Cylindrical specimens (4 mmΦ x 6 mm) for compressive strength testing, and bend bar specimens (2 mm x 2 mm x 25 mm) for flexural testing were made using stainless steel split molds according to ISO 9917-1. The samples were then kept in relative humidity of approximately 90% at 37°C for 1 h, removed from the molds and immersed into 50 mL of distilled water, then maintained at 37°C in an incubator for further 23 h, 7 days and 14 days prior to mechanical testing 14 . Ten specimens were made for each immersion time. After the appropriate storage time, cylindrical specimens were loaded to measure compressive strength on a universal testing machine (8871 Model, Instron, Norwood, MA, USA) at a crosshead speed of 1.0 mm·min-1.
Bar specimens were subjected to a three-point flexural test with a supporting span of 20 mm. The specimens were loaded at a crosshead speed of 1.0 mm·min-1 on a universal testing machine (3344 Model, Instron). The flexural strength (σ) was calculated from the maximum load (F) using the following equation: σ=3Fl/2bh 2 where l is the span length, b is the width, and h is the height of specimen. The flexural modulus (E) was determined from the following equation: E=l 3 m/4bh 3 where m is the slope (N/mm) of the linear portion of the load–displacement graph 14 .
Cell viability test
The cell viability of the prepared GICs was evaluated using an indirect method with rat dental pulp stem cells (rDPSC) and an extracted solution of the cements. The GIC samples (15 mmΦ x 6 mm) were separately soaked in 2.5 ml culture medium (α-MEM; Gibco, USA) at 37°C for 24 h to extract ionic products from the GICs, which were then prepared in a series of dilutions (1:5, 1:10, 1:50 and 1:100).
Rat dental pulp cells (rDPSC) were harvested from incisors of 6-week-old male Sprague Dawley rats. The primary rDPSCs were cultured by the procedure described in a previous study 21 . The protocol of the in vitro primary cell cultures was approved by the Institutional Animal Care and Use Committee of Dankook University, Republic of Korea. Cells at passage 3 or 4 were used for cell proliferation test using CCK-8 test (Cell Counting Kit-8, Dojindo, Tokyo, Japan) according to the manufacturer’s instruction. Briefly, cells (1,000) were seeded onto each well of 96-well plate and incubated for 24 h to allow cell adhesion, and then added with the extract dilutions of the cements. After 3 days of culture, cell viability was measured by the CCK assay. For each group, five replicated were made per dilution, and cells cultured without the cement extract were used as control. The results were normalized to the values obtained from the control dish. The morphologies of rDPSC grown on the culture plates were taken by confocal laser scanning microscope (M700, Carl Zeiss, Jena, Germany). Details of the procedures of cell test and CLSM were described elsewhere 13 , 14 .
Statistical analysis
Statistical differences between groups at each immersion time and dilution (CCK-assay) were analyzed with a one-way ANOVA and Tukey HSD post hoc test (IBM SPSS v.21, USA). A significance was set at p<0.05.
RESULTS
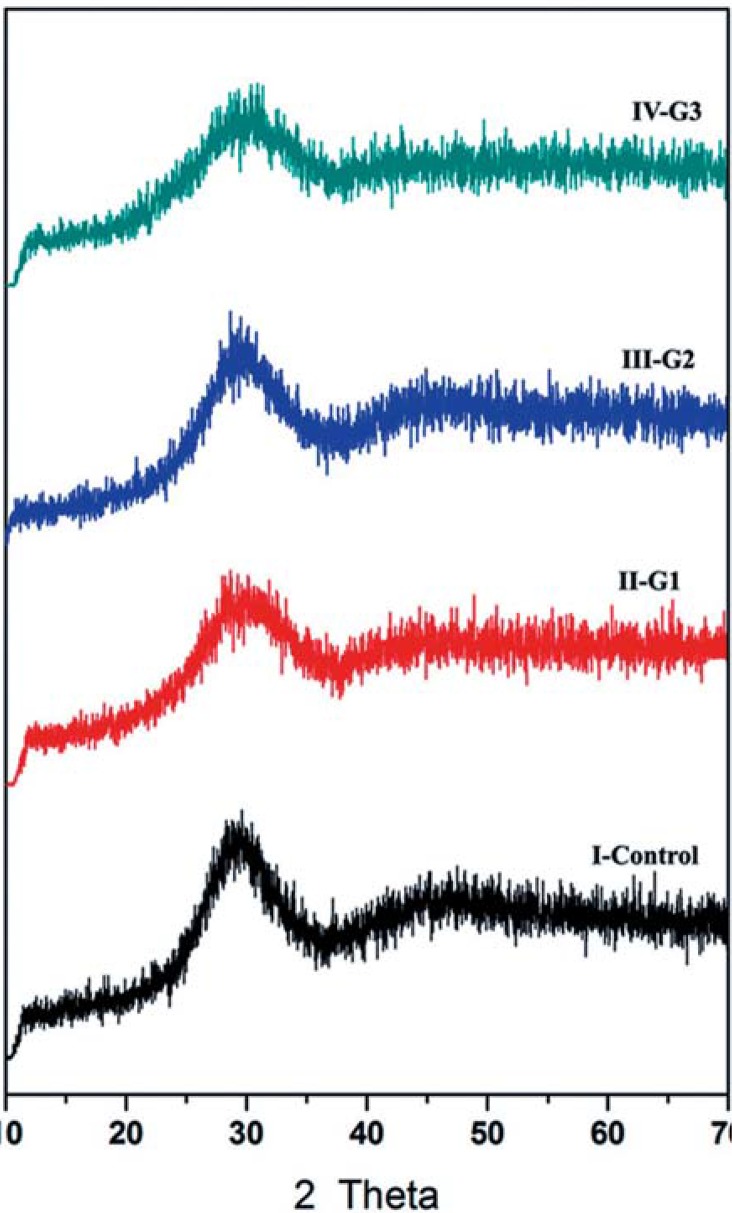
A typical amorphous feature of the as-quenched glasses with broad X-ray diffraction halos appeared between 20° and 30° (2θ), as shown in Figure 1, indicating that a fully glassy structure was obtained from the glassy melts and absence of any crystalline phase. DSC analyses of the studied glasses are presented in Table 1. The endothermic reactions indicating glass transition temperature (Tg) were recorded at the temperature range of 734°C -766°C on the DSC of the glasses. Exothermic effects at 901°C-959°C indicating crystallization reaction in the glasses were also recorded.
Figure 1. Powder x-ray diffraction patterns control (commercial GIC) and the prepared glasses (G1, G2, G3).
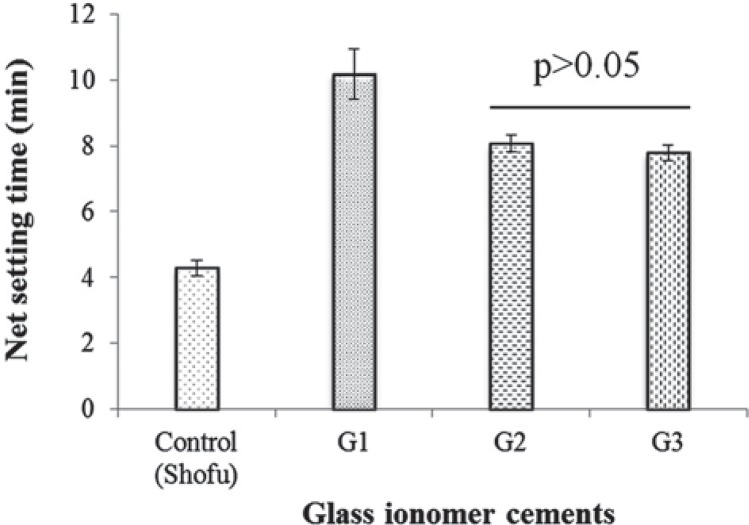
Figure 2 shows variation in the setting time for cements formed at the powder liquid ratio of 2:1. The setting time of the base glass GIC (10.2 min) was much longer than that of the control GIC (4.3 min). However, the setting time decreased with partial substitution of CaO for MgO (8.1 min) or ZnO (7.9 min).
Figure 2. Net setting time of glass ionomer cements. Horizontal bar denotes no significant difference (Tukey test).
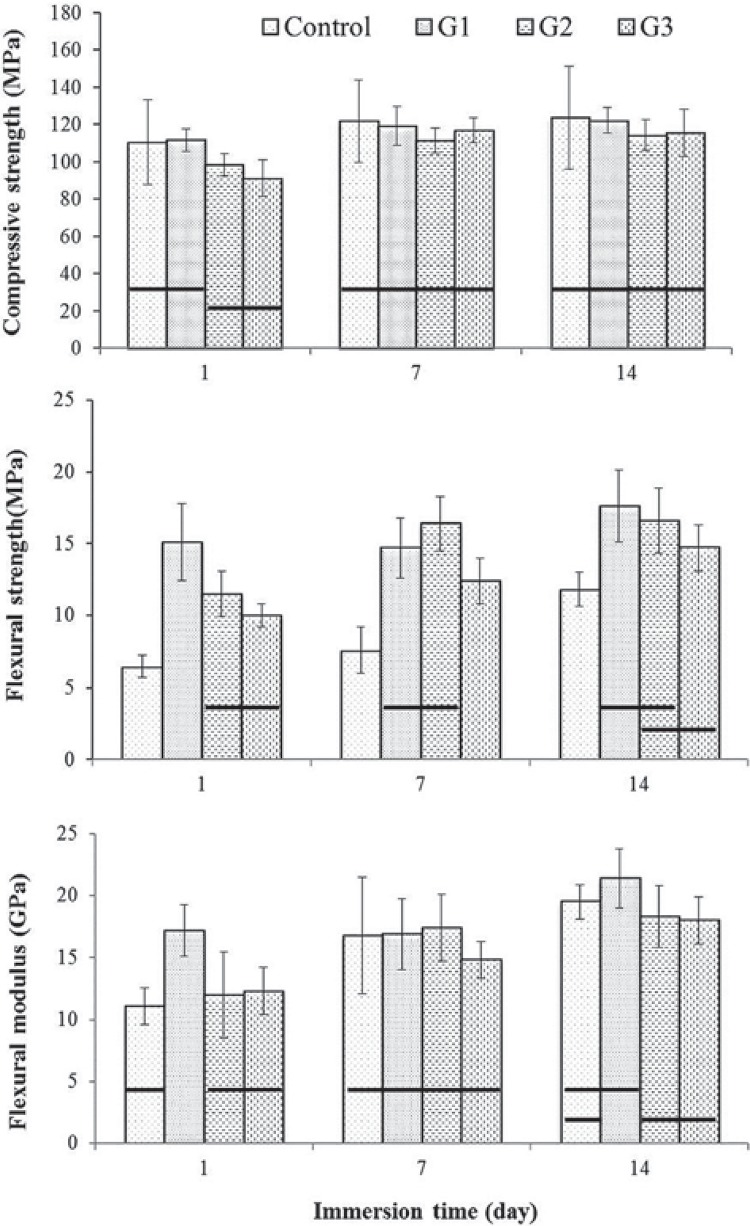
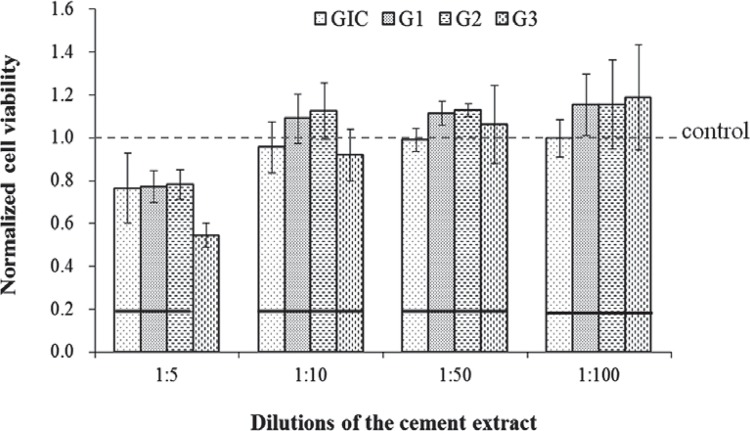
The mechanical properties of the prepared GICs were displayed in Figure 3. The mean compressive strength (CS) values of the GIC specimens ranged from 91.3 to 122.2 MPa (Figure 3A). The mean flexural strength and modulus values of specimen groups are displayed as a function of storage time in Figure 3B and Figure 3C. The initial flexural strength of the control cement after 1 day was 6.5 MPa, while the prepared GIC materials ranged between 11.5 and 15.1 MPa. Similarly to CS, the initial (1 d) flexural properties of the modified GICs (G2, G3) were significantly lower than those of the base GIC (G1, p<0.05), however, the differences were decreased after 7 days, and particularly G2 showed no significant differences with G1 (p>0.05). Scanning electron micrographs of the fractured surfaces of the cements after immersion in water for 7 days are shown in Figure 4. No significant difference or pattern was observed between the commercial GIC and the prepared glass ionomer cement. Figure 5 presents results of the cell viability test to rDPSC. No statistical differences were observed between groups except for high dilution of cement extract (1:5) (p>0.05). Figure 6 shows the morphologies of rDPSCs cultivated in the culture well.
Figure 3. Mechanical properties of the glass ionomer cements as a function of immersion time. Horizontal bars indicate no significant difference between groups at each immersion time (Tukey test, p<0.05).
Figure 4. Scanning electron microscopy micrographs of the fractured surface of the cements matured for 7 days in water.
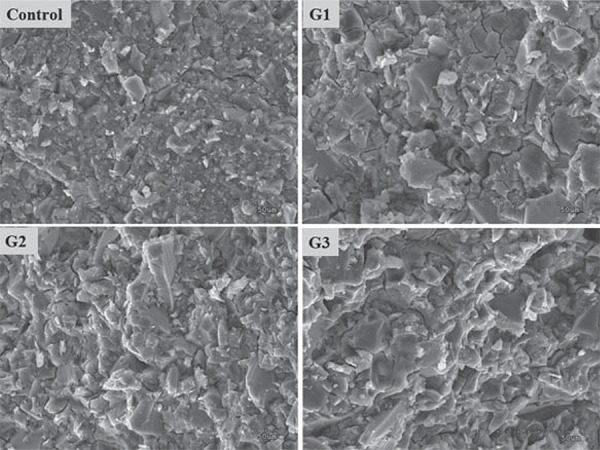
Figure 5. Results of the normalized cell viability to the control (no extract) by CCK-8 test. Horizontal bars indicate no significant difference between groups at each dilution (Tukey test, p<0.05).
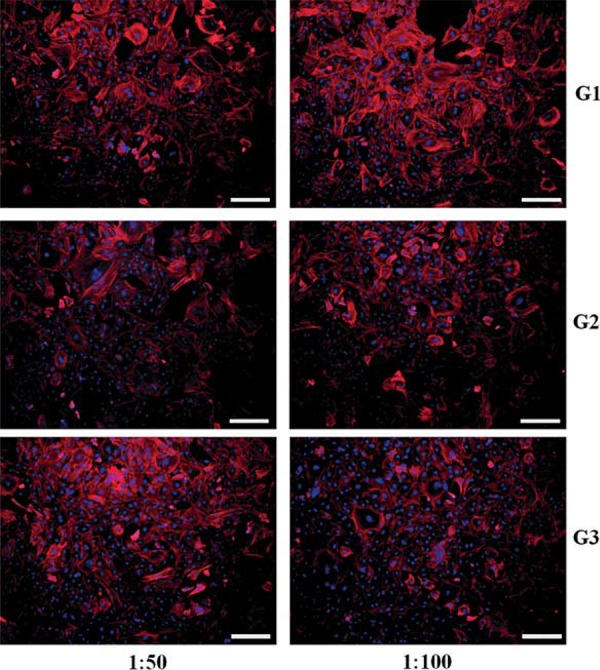
Figure 6. Morphologies of rat dental pulp stem cells cultured in dilutions of cement extract (G1-G3) by CLSM (stains of nuclei in blue, and of alpha-actin filaments in red). Scale bars denote 200 µm.
DISCUSSION
The X-ray diffraction analysis was performed on each sample to check that all the glasses produced were amorphous and not crystalline in nature. The Tg values of G2 glass were decreased by MgO/CaO partial substitution, possibly as a result of the magnesium entering the glass network. The formation of a weaker bond between the oxygen and the magnesium regarding a Si-O bond (3.35 eV vs. 8.10 eV) could result in a reduction in the Tg as less energy is required to break the bonds 1 . On the other hand, the decrease in the Tg by ZnO/CaO replacement may be attributed to the fact that zinc acts as a network modifier, and not as a network former in calcium zinc silicate glasses 5 .
Although the GIC setting reaction has not been fully elucidated, a number of factors are known to influence the rate of the setting reaction of glass-ionomer cement 3 . These include molar mass of the poly acid, concentration of the polymeric solution, powder-to-liquid ratio and the presence of chelating agents such as tartaric acid 28 . The maturation to form a stable and insoluble glass ionomer cement has been associated with the development of trivalent cross-linking bonds (e.g., by aluminium) within the glass-ionomer matrix, which follow the much faster divalent cross-linking (e.g., by calcium and magnesium) 9 .
The mean net setting time of the prepared GICs were higher than that of the control (4.3 min) (Figure 2). The mean setting time of GIC of base glass batch (G1) is decreased with MgO/CaO (G2) and ZnO/CaO (G3) partial replacement. This may be attributed to the different dissolution rate of the ions from the glasses. Although the experimental GICs set slowly than the commercial product, their extended net setting times are within the maximum net setting time (8 min) specified in ISO 9917-1.
Compressive strength (CS) of the prepared GICs was comparable to commercial product (control). Although G3 cement showed a lower CS than the control and base GIC at early stage (1 day), there were no statistical differences between groups after maturation for 7 days (p>0.05), indicating that the cement reaction still continues after one day, and a more disrupted network allows the reaction to crosslink more in the cement-matrix. Overall results indicate that mechanical enhancement of the experimental cement by the CaO partial replacement with MgO or ZnO was not demonstrated in terms of compressive property. Nevertheless, the CS value of the experimental GICs (90~120 MPa) prepared in this study was significantly higher than those of free-aluminum GICs (20~40 MPa) prepared by zinc/strontium-containing glasses 4 , 6 , 14 .
Flexural properties of all groups were increased or sustained with the aging in water up to 14 days. During maturation periods, flexural strength of the prepared GICs was significantly higher than the one of the control cement (p<0.05), whereas no differences were observed in flexural modulus. Similarly to CS, the initial (1 d) flexural properties of the modified GICs (G2, G3) were significantly lower than those of the base GIC (G1), however, the differences were decreased after 7 days, and G2 showed no significant differences with G1, which indicates slow maturation rate.
Benefits in mechanical properties of the prepared GIC with CaO/MgO or CaO/ZnO partial substitutions were not clearly evident in this study. However, the compressive strength values of the base and modified cements, after 7 days, were sufficiently higher than the relevant limits specified in the ISO 9917-1 standard for GIC; 50 MPa (for luting and base) and 100 MPa (for restoration). Furthermore, all prepared GICs marked a significantly higher flexural strength than the commercial product. Thus, the GICs prepared in this study may be satisfactory for dental applications.
Investigation of the change in strength after aging the cement gives an indication of the stability of this material. Figure 4 shows the typical microstructures of the fractured GIC specimens. The control sample showed a microstructure more fine-grained than the experimental GICs, probably due to the difference in particle size. The prepared GICs in this study showed no degradation in the microstructure of specimens, which was appeared in aluminum-free GICs with decrease in mechanical properties after aging 14 .
A similar trend was observed between commercial and experimental GICs in the rDPSC viability increasing with a low dose of cement extract. At high concentration of extract (1:5), all GIC samples showed low cell viability in comparison with no cement extract control, particularly in G3 sample. However, although it was not statistically significant, an increased cell viability of the prepared cements (G1-G3) was evident at 1:10, 1:50 and 1:100 in comparison with control cement, suggesting positive roles of eluted ions in cell proliferative potential. It was confirmed that at low doses, the rDPSCs were actively grown in the cement extracts (Figure 6). This initial cell test provides some promising results, but they should be demonstrated in further studies. The overall results failed to reject the null hypothesis of this study; however, a further modification of conventional GIC composition was encouraged.
CONCLUSION
In this study, a glass composition for conventional GIC was modified by partial replacement of 10 mol% CaO with MgO or ZnO to enhance the mechanical and biological properties of the cements. The prepared GICs retained compressive strengths 91-122 MPa, flexure strengths 10-18 MPa, flexural moduli 12-21 GPa, and net setting times 8-10 min. Compressive and flexural properties of the prepared GICs with CaO/MgO or CaO/ZnO partial replacements were not improved in comparison with the unmodified GIC. However, all prepared GICs marked mechanical properties statistically higher than a commercial GIC, or comparable ones. Moreover, the cements showed an increased viability of rat dental pulp stem cells. Based on this study, the experimental GIC with partial replacement of MgO or ZnO can be considered bioactive dental materials.
AKNOWLEDGEMENTS
This research was supported by grants from National Research Foundation of Korea (Priority Research Centers Program: 2009-0093829, Basic Science Research Program: 2010-0010841).
REFERENCES
- 1.Abo-Mosallam HA, Salama SN, Salman SM. Formulation and characterization of glass-ceramics based on Na2Ca2Si3O9-Ca5(PO4)3F-Mg2SiO4-system in relation to their biological activity. J Mater Sci Mater Med. 2009;20:2385–2394. doi: 10.1007/s10856-009-3811-4. [DOI] [PubMed] [Google Scholar]
- 2.Bayne SC, Thompson JY, Taylor DA. Dental materials. In: Roberson TM, Heymann HO, Swift EJJ, editors. Art & Science of operative dentistry. 4th ed. St. Louis: Mosby; 2002. pp. 133–234. [Google Scholar]
- 3.Billington RW, Williams JA, Pearson GJ. Ion processes in glass ionomer cements. J Dent. 2006;34:544–555. doi: 10.1016/j.jdent.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Boyd D, Towler MR. The processing, mechanical properties and bioactivity of zinc based glass ionomer cements. J Mater Sci Mater Med. 2005;16:843–850. doi: 10.1007/s10856-005-3578-1. [DOI] [PubMed] [Google Scholar]
- 5.Boyd D, Towler MR, Law RV, Hill RG. An investigation into the structure and reactivity of calcium-zinc-silicate ionomer glasses using MAS-NMR spectroscopy. J Mater Sci Mater Med. 2006;17:397–402. doi: 10.1007/s10856-006-8465-x. [DOI] [PubMed] [Google Scholar]
- 6.Brauer DS, Gentleman E, Farrar DF, Stevens MM, Hill RG. Benefits and drawbacks of zinc in glass ionomer bone cements. Biomed Mater. 2011;6: doi: 10.1088/1748-6041/6/4/045007. [DOI] [PubMed] [Google Scholar]
- 7.Choi JY, Lee HH, Kim HW. Bioactive sol-gel glass added ionomer cement for the regeneration of tooth structure. J Mater Sci Mater Med. 2008;19:3287–3294. doi: 10.1007/s10856-008-3464-8. [DOI] [PubMed] [Google Scholar]
- 8.Coughlan A, Scanlon K, Mahon BP, Towler MR. Zinc and silver glass polyalkenoate cements: an evaluation of their antibacterial nature. Bio-Med Mater Eng. 2010;20:99–106. doi: 10.3233/BME-2010-0620. [DOI] [PubMed] [Google Scholar]
- 9.Czarnecka B, Limanowska-Shaw H, Nicholson JW. Buffering and ion-release by a glass-ionomer cement under near-neutral and acidic conditions. Biomaterials. 2002;23:2783–2788. doi: 10.1016/s0142-9612(02)00014-5. [DOI] [PubMed] [Google Scholar]
- 10.Franks K, Salih V, Knowles JC, Olsen I. The effect of MgO on the solubility behavior and cell proliferation in a quaternary soluble phosphate based glass system. J Mater Sci Mater Med. 2002;13:549–556. doi: 10.1023/a:1015122709576. [DOI] [PubMed] [Google Scholar]
- 11.Hammouda IM. Reinforcement of conventional glass-ionomer restorative material with short glass fibers. J Mech Behav Biomed Mater. 2009;2:73–81. doi: 10.1016/j.jmbbm.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 12.International Organization for Standardization . ISO 9917-1:2007. Dentistry - Water-based cements - Part 1: Powder/liquid acid-base cements. Geneva: ISO; 2007. [Google Scholar]
- 13.Jin GZ, Kim M, Shin US, Kim HW. Neurite outgrowth of dorsal root ganglia neurons is enhanced on aligned nanofibrous biopolymer scaffold with carbon nanotube coating. Neurosci Lett. 2011;501:10–14. doi: 10.1016/j.neulet.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 14.Kim DA, Abo-Mosallam HA, Lee HY, Kim GR, Kim HW, Lee HH. Development of a novel aluminum-free glass ionomer cement based on magnesium/strontium-silicate glasses. Mater Sci Eng C Mater Biol Appl. 2014;42:665–671. doi: 10.1016/j.msec.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi M, Kon M, Miyai K, Asaoka K. Strengthening of glass-ionomer cement by compounding short fibres with CaO-P2O5-SiO2-Al2O3 glass. Biomaterials. 2000;21:2051–2058. doi: 10.1016/s0142-9612(00)00096-x. [DOI] [PubMed] [Google Scholar]
- 16.Kovarik RE, Haubenreich JE, Gore D. Glass ionomer cements: a review of composition, chemistry, and biocompatibility as a dental and medical implant material. J Long Term Eff Med Implants. 2005;15:655–671. doi: 10.1615/jlongtermeffmedimplants.v15.i6.80. [DOI] [PubMed] [Google Scholar]
- 17.Lohbauer U. Dental glass ionomer cements as permanent filling materials? - Properties, limitations and future trends. Materials. 2010;3:79–96. [Google Scholar]
- 18.Lohbauer U, Walker J, Nikolaenko S, Werner J, Clare A, Petschelt A, et al. Reactive fibre reinforced glass ionomer cements. Biomaterials. 2003;24:2901–2907. doi: 10.1016/s0142-9612(03)00130-3. [DOI] [PubMed] [Google Scholar]
- 19.Matsuya S, Maeda T, Ohta M. IR and NMR analyses of hardening and maturation of glass-ionomer cement. J Dent Res. 1996;75:1920–1927. doi: 10.1177/00220345960750120201. [DOI] [PubMed] [Google Scholar]
- 20.Moshaverinia A, Roohpour N, Chee WW, Schricker SR. A review of powder modifications in conventional glass-ionomer dental cements. J Mater Chem. 2011;21:1319–1328. [Google Scholar]
- 21.Nam SY, Won JE, Kim CH, Kim HW. Odontogenic differentiation of human dental pulp stem cells stimulated by the calcium phosphate porous granules. J Tissue Eng. 2011: doi: 10.4061/2011/812547. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicholson JW. Chemistry of glass-ionomer cements: a review. Biomaterials. 1998;19:485–494. doi: 10.1016/s0142-9612(97)00128-2. [DOI] [PubMed] [Google Scholar]
- 23.Nicholson JW, Braybrook JH, Wasson EA. The biocompatibility of glass-poly(alkenoate) (Glass-Ionomer) cements: a review. J Biomater Sci Polym Ed. 1991;2:277–285. doi: 10.1163/156856291x00179. [DOI] [PubMed] [Google Scholar]
- 24.Sidhu SK. Glass-ionomer cement restorative materials: a sticky subject? Aust Dent J. 2011;56:23–30. doi: 10.1111/j.1834-7819.2010.01293.x. [DOI] [PubMed] [Google Scholar]
- 25.Smith DC. Development of glass-ionomer cement systems. Biomaterials. 1998;19:467–478. doi: 10.1016/s0142-9612(97)00126-9. [DOI] [PubMed] [Google Scholar]
- 26.Tyas MJ. Clinical evaluation of glass-ionomer cement restorations. J Appl Oral Sci. 2006;14:10–13. doi: 10.1590/s1678-77572006000700003. [DOI] [PubMed] [Google Scholar]
- 27.Varshneya AK. Fundamentals of inorganic glasses. San Diego: Academic Press; 1994. [Google Scholar]
- 28.Wilson AD, McLean JW. Glass-ionomer cement. Chicago: Quintessence Publishing Co; 1998. [Google Scholar]
- 29.Yamaguchi M, Yamaguchi R. Action of zinc on bone metabolism in rats. Increases in alkaline phosphatase activity and DNA content. Biochem Pharmacol. 1986;35:773–777. doi: 10.1016/0006-2952(86)90245-5. [DOI] [PubMed] [Google Scholar]