Abstract
Objective
RetroMTA® is a new hydraulic bioceramic indicated for pulp capping, perforations or root resorption repair, apexification and apical surgery. The aim of this study was to compare the radiopacity, pH variation and cytotoxicity of this material to ProRoot® MTA.
Material and Methods
Mixed cements were exposed to a digital x-ray along with an aluminum stepwedge for the radiopacity assay. pH values were verified after incubation period of 3, 24, 48, 72 and 168 hours. The cytotoxicity of each cement was tested on human periodontal ligament fibroblasts using a multiparametric assay. Data analysis was performed using ANOVA and Tukey’s post hoc in GraphPad Prism.
Results
ProRoot® MTA had higher radiopacity than RetroMTA® (p<0.001). No significant differences were observed for the pH of the materials throughout experimental periods (p>0.05) although pH levels of both materials reduced over time. Both ProRoot® MTA and RetroMTA® allowed for significantly higher cell viability when compared with the positive control (p<0.001). No statistical difference was observed between ProRoot® MTA and RetroMTA® cytotoxicity level in all test parameters, except for the ProRoot® MTA 48-hour extract media in the NR assay (p<0.05).
Conclusion
The current study provides new data about the physicochemical and biological properties of Retro® MTA concerning radiopacity, pH and cytotoxic effects on human periodontal ligaments cells. Based on our findings, RetroMTA® meets the radiopacity requirements standardized by ANSI/ADA number 572, and similar pH values and biocompatibility to ProRoot® MTA. Further studies should be performed to evaluate additional properties of this new material.
Keywords: Dental materials, Endodontics, Physical and chemical properties, Cytotoxicity
INTRODUCTION
Mineral trioxide aggregate (MTA) is considered a gold standard material for several endodontic applications such as pulp capping, perforations or root resorption repair, apexification and apical surgery 9 . The main elemental components of MTA are Portland cement, bismuth oxide and gypsita 23 , 24 . MTA is commercially available as ProRoot® MTA (Dentsply Tulsa Dental, Tulsa, OK, USA) and MTA-Angelus® (Angelus, Londrina, PR, Brazil) in white and gray colors. However, studies have reported on MTA as having difficult handling characteristics 18 , delayed setting time (around 150 min) 14 , elevated cost 15 , 18 and tooth discoloration 19 .
The possibility of tooth discoloration associated with the use of MTA has been attributed to the presence of bismuth oxide (radiopacifying agent) in its composition, and raises a major concern in clinical practice as it may negatively impact the patients’ anterior esthetics. Marciano, et al. 19 (2014) analyzed the color change in the tooth structures induced by bismuth oxide and white MTA-Angelus®, as well as the interaction of the radiopacifying agent (bismuth oxide) with collagen, the main constituent of the dentin. Spectrophotometer was used for the color assessment of the tooth structure, and visual observation was used for the color assessment of the chemical interaction between bismuth oxide and collagen. The authors concluded that the white MTA-Angelus® caused discoloration and dentin stain. Further, they showed that collagen reacts with bismuth oxide, resulting in a grayish color, and therefore suggested the use of an alternative radiopacifier in MTA formulations 19 .
RetroMTA® (BioMTA, Seoul, Korea) has recently been introduced in the market as a new hydraulic bioceramic material proposed for use in similar endodontic applications as MTA (pulp capping, perforations or root resorption repair, apexification and apical surgery). However, unlike MTA, this material does not contain Portland cement, and hydraulic calcium zirconia is included as a radiopacifying agent. According to the manufacturer, RetroMTA® is ideal for aesthetic repair, since it has no discoloration and has a fast setting (initial setting time of 150 seconds), which would be beneficial considering the moist environment of the oral cavity.
There are only two studies in literature reporting on some of the physicochemical and biological properties of RetroMTA®. Kang, et al. 15 (2015) compared the discoloration of ProRoot® MTA with MTA-Angelus®, ENDOCEM Zr® (Maruchi, Wonju, Korea) and RetroMTA®. Test samples of the four materials were analyzed regarding changes in color after being irradiated with light for 15 and 30 minutes. In vitro tooth discoloration was also evaluated after filling the pulp chambers with the materials and measured for a 16-week period. From their results, these authors concluded that RetroMTA® and ENDOCEM Zr® showed less discoloration than ProRoot® MTA and MTA-Angelus® in both experiments 15 . Ghorbanzadeh, et al. 11 (2015) evaluated the marginal adaptation of ProRoot® MTA, OrthoMTA® (BioMTA, Seoul, Korea) and RetroMTA® when used as retrofilling materials. Single-rooted teeth were retrofilled with either ProRoot® MTA, OrthoMTA® or RetroMTA® and stored in phosphate buffer saline for one week or for two months until evaluation of the marginal adaptation of each test material to dentin under scanning electron microscope. Results showed that all of the tested materials presented similar marginal adaptation for both time periods 11 .
The composition of RetroMTA® seems to be promising in several aspects, such as fast setting time and no discoloration, hence it could be a possible substitute to MTA. Therefore, the aim of this study was to evaluate the radiopacity, pH variation and cytotoxicity of RetroMTA® in comparison to ProRoot® MTA.
MATERIAL AND METHODS
Material
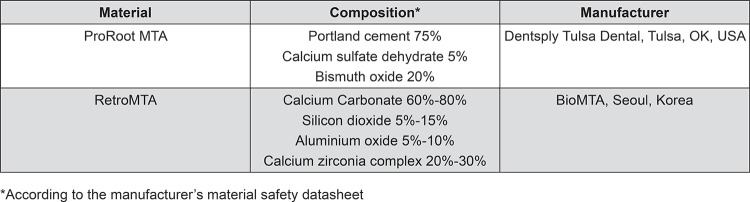
The chemical composition of ProRoot® MTA and RetroMTA® are presented in Figure 1. Both materials were mixed according to the manufacturer’s instructions. Briefly, the manufacturers’ instructions for RetroMTA® manipulation cite mixing 0.3 g of powder with 3 drops of the liquid for 20 seconds with the use of a plastic spatula.
Figure 1. Chemical composition of the materials used in the study.
Radiopacity assays
Mixed samples (n=3 per group) were placed into stainless steel rings (10 mm in diameter and 1 mm in height) and incubated at 37±1°C and 95% relative humidity for 24 hours. The samples were placed onto an occlusal phosphor plate along with an aluminum stepwedge with 1 mm of increments (1 to 9 mm). Radiographic images were taken with FocusTM X-ray (Instrumentarium Dental, Tuusula, Finland) at 70 kVp and 7 mA, and a 30 cm focus-film distance. Images were analyzed with AxioVision Rel. 4.6 Software (Zeiss, Jena, Germany). The gray pixel values of three points from each sample image and the aluminum step from the stepwedge were measured, and the averages calculated. The average pixel values were converted into millimeters of Aluminum (mm Al), as previously described 6 . Then, a graph of the radiographic density versus the thickness of the aluminium stepwedge was plotted to all obtained radiographs, and a calibration curve was generated using logarithmic regression. The obtained equation was used to calculate the radiopacity of the materials in mm of Al 6 .
pH analysis assays
Mixed samples (n=5 per group) were placed into plastic tubes (1.0 mm of internal diameter and 1 cm of length) using an endodontic file. Samples were immersed in a glass vial with 10 ml of distilled water and incubated for 3, 24, 48, 72 hours and 7 days. After each experimental period, the pH was evaluated with a pH meter (Accumet basic AB 15, Fisher Scientific, Pittsburgh, PA, USA) and samples were placed in a new vial with fresh water.
Multi-parametric cytotoxicity assays
Preparation of cement elutes (extract media)
Cement elutes were prepared as previously described 29 . In short, cements were mixed inside a laminar flow hood and inserted into 1000 µl pipette tips (VWR, Radnor, PA, USA) that were cut at 2.2 cm from their final segment. The tip with the cement was attached to the lid of a microcentrifuge tube using an o-ring (5 mm in diameter and 2 mm in height). Upon closing of the lid, the tip containing the cement was immersed in the tube containing 0.5 ml of Dulbecco’s Modified Eagle Medium (DMEM) (ATCC, Manassas, VA, USA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS) (Gibco, Grand Island, NY), 100 U/mL penicillin (PEN) and 100 mg/mL streptomycin (STREP) (Sigma-Aldrich, Saint Louis, MO, USA). Each cement sample was incubated at 37°C, 95% humidity and 5% CO2 for 24 and 48 hours. After each incubation period, the samples were removed from the media and the extract media were briefly vortexed, transferred to a new microcentrifuge tube and stored at -20oC until further use.
Cell culture
Human periodontal ligament fibroblasts (HPDL) were cultured in DMEM medium supplemented with 10% FBS, 100 U/mL PEN and 100 mg/mL STREP and incubated at 37°C, 95% humidity and 5% CO2.
Cell viability assays
HPDL fibroblasts were seeded at a density of 2x10 4 cells/well in a 96-well plate and incubated for 24 hours. Next, 200 µl of extract media of each test material was added to each well and incubated for an additional 24 hours. Fresh culture media was used as a negative control, and 0.1% sodium dodecyl sulfate (SDS) (Bio-Rad, Hercules, CA, USA) as a positive control. All experiments were performed in triplicate and in three independent reactions.
The cytotoxicity of the test materials was evaluated using a multi-parametric assay kit (In Cytotox XTT-NR-CVDE, Xenometrix, Allschwill, Switzerland) as follows: first, the XTT [2,3-bis(2-methoxy-4-nitro-5-sulfopheny)-2H-tetrazolium-5-carboxyanilide inner salt] assay measures the ability of viable cells to convert XTT, a tetrazolium salt, into formazan by the succinate dehydrogenase, which belongs to the mitochondrial respiratory chain; second, the Neutral Red assay (NR) measures the ability of viable cells to incorporate and bind NR within lysosomes; and finally, the Crystal Violet Dye Elution (CVDE) assay stains viable DNA and provides quantitative information about the cell density 8 . The results were analyzed using an ELISA plate reader (Dynex Tecnologies, Chantilly, VA, USA). Cell viability was calculated in function of the negative control [cell viability % = mean optical density (OD) of test sample × 100 / mean OD of negative control]. According to the ISO standard number 10993-5 13 , a reduction in cell viability by more than 30% is considered to have a cytotoxic effect.
Statistical analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test as implemented in GraphPad Prism 6.0 (GraphPad Software Inc., La Jolla, CA, USA). The significance level was established at 5% (p≤0.05).
RESULTS
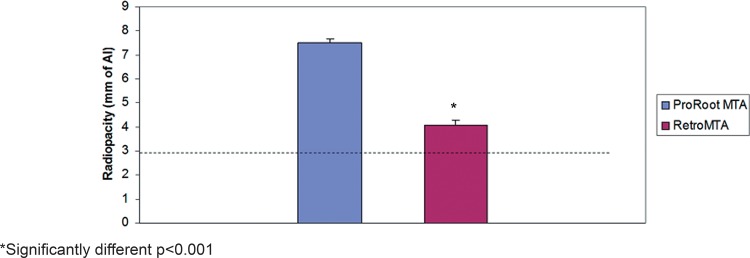
Figure 2 shows the mean values and standard deviations of the radiopacity levels for ProRoot® MTA and RetroMTA® in mm of Al. While both materials achieved the minimum required radiopacity value of 3 mm of Al, as recommended by ANSI/ADA 2 , ProRoot® MTA showed significantly higher radiopacity values (7.52±0.15 mm of Al) when compared to RetroMTA® (4.07±0.20 mm of Al) (p<0.001).
Figure 2. Radiopacity of ProRoot® MTA and RetroMTA® in mm of Al. Dashed line represents the radiopacity of 3 mm of Al as recommended by ANSI/ADA No. 572.
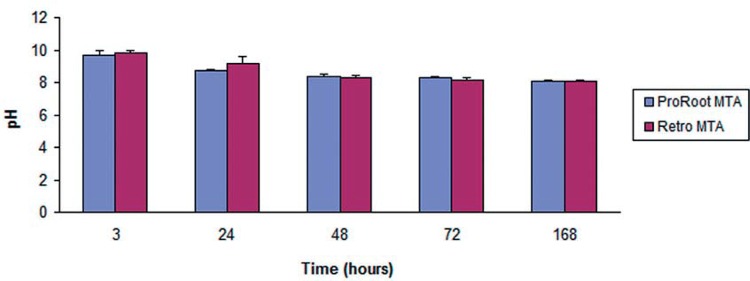
There were no significant differences in the pH levels of ProRoot® MTA and RetroMTA® throughout the experimental periods (p>0.05). The pH of ProRoot® MTA varied from 9.93 to 8, while the values for RetroMTA® varied from 9.93 to 7.9. It is worth noting that the pH of both materials tended to decrease over time (Figure 3).
Figure 3. Results of pH analysis over time.
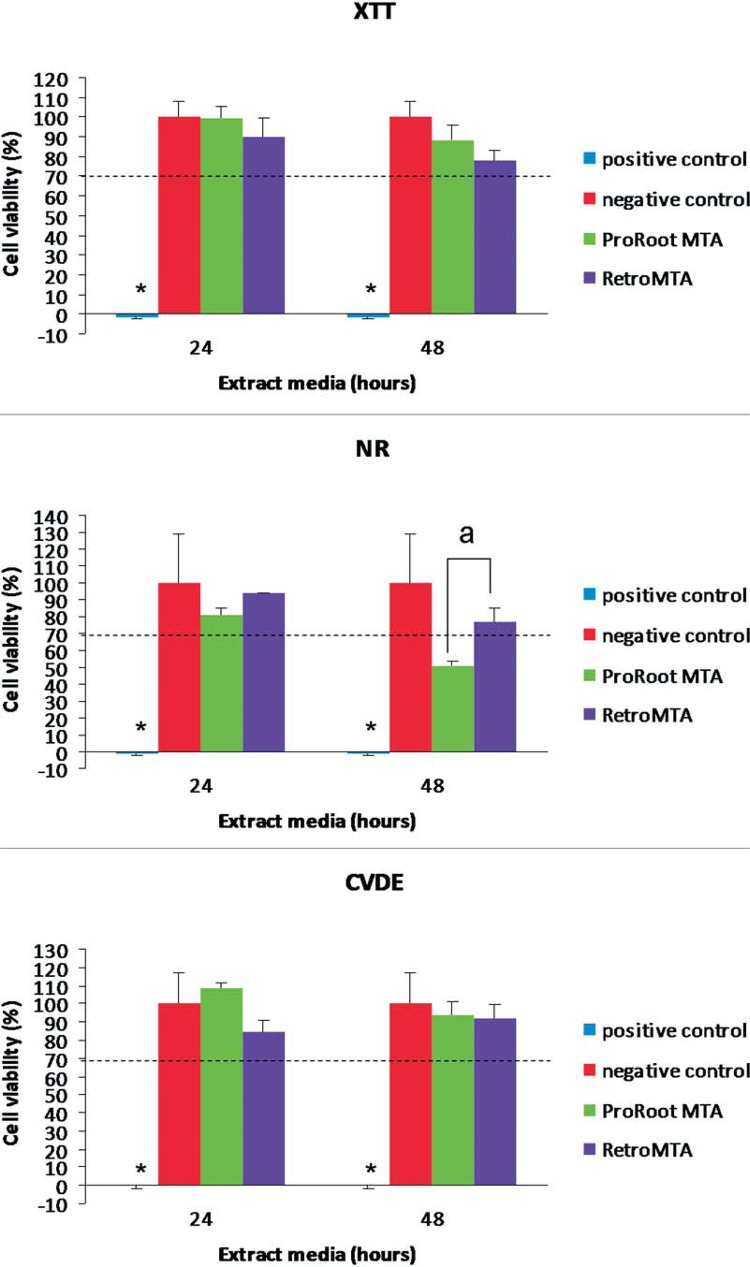
Both ProRoot® MTA and RetroMTA® allowed for significantly higher cell viability when compared with the positive control (p<0.001). When comparing the XTT, NR and CVDE values for ProRoot® MTA and RetroMTA® among the experimental periods, no significant differences were found, except for the 48-hour extract media in the NR assay, in which ProRoot® MTA showed significantly lower cell viability (p≤0.05) (Figure 4).
Figure 4. Cell viability (%) after exposure to each material extract media. *Significantly different from all groups (p<0.0001). a, significantly different between test materials (p<0.05). Dashed line represents the 70% cut-off level established by ISO 10993-513.
DISCUSSION
In this study, we evaluated the radiopacity, pH variation and cytotoxicity of RetroMTA® and ProRoot® MTA, and provided novel information on some physicochemical and biological properties of this new material.
ANSI/ADA specification number 57 2 recommends that an endodontic material must have a radiopacity value higher than 3 mm of Al. Both ProRoot® MTA and RetroMTA® met the established criteria, however ProRoot® MTA showed significantly higher radiopacity when compared to RetroMTA®. Our results for the radiopacity of ProRoot® MTA are in agreement with previous reports using similar methodology 4 , 5 , 14 . Although this is the first report on the radiopacity of RetroMTA® in the scientific literature, our values observed for radiopacity are similar to the values presented by the manufacturer (5 mm of Al).
Moreover, according to the manufacturers, the pH of ProRoot® MTA is ~12, and the pH of RetroMTA® is initially 12.5, decreasing to pH 8 after four weeks. To evaluate the pH of the materials at different time periods, distilled water was changed after each reading, thus the reading would reflect the increase over that period 27 . In the present study, the pH of the tested materials was alkaline, varying from 9.93 to 8 for ProRoot® MTA and from 9.93 to 7.9 for RetroMTA®. The lower pH levels observed here when compared to the manufacturers values may be explained by the different methodological approaches used. In this study, to simulate the surface area of the material exposed during clinical use, the cements were placed in plastic tubes for the analysis, thus decreasing the surface area of the material in contact with the liquid 20 , 27 , which may have contributed to the lower pH values observed. Our findings corroborate previous studies with similar sample sizes in which lower pH values for the ProRoot® MTA were observed when compared to the manufacturer’s product specifications 10 , 22 , 26 . It is worth noting that both materials showed a decrease in the pH over time. The cements were immersed in distilled water while fresh, allowing the material surface to be exposed before setting of the material. Further, this allows the release of hydroxyl ions, thus increasing the pH. Over time, the pH decreases, probably because the setting is complete 27 .
We also evaluated the cytotoxicity of ProRoot® MTA and RetroMTA® using a multiparametric cytotoxicity assay which allows the simultaneous evaluation of the toxic effects of tested materials in the same sample through three different parameters: mitochondrial metabolism and respiratory toxicity (XTT), lysossomal integrity and membrane permeability (NR), and cell proliferation and presence of DNA (CVDE). Our results showed that both ProRoot® MTA and RetroMTA® promoted significantly higher cell viability when compared to the positive control. No statistically significant differences were observed when comparing XTT, NR and CVDE values for ProRoot® MTA and RetroMTA®, except for the 48-hour period in the NR assay. At this experimental period in the neutral red assay, the cell viability decreased in the ProRoot® MTA group, although no major decrease in the XTT and CVDE values were observed, corroborating the findings of De Deus, et al. 8 (2009). The observed lower viability in the neutral red assay may indicate some adverse effect of ProRoot® MTA on membrane integrity that could contribute to possible cell toxicity in vitro. Nonetheless, the effect alone may not be enough to provoke damage to normal cell function, as mitochondrial activity values were within normal limits and no effects of DNA damage were suspected in the XTT and CVDE assays. Moreover, the excellent in vivo biocompatibility of ProRoot® has been reported on several studies 17 , 21 , 25 , 30 . The main advantage of this multiparametric assay in comparison to other commonly used cytotoxicity assays [i.e. MTT, (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)] is that it provides information about the specific biological mechanisms through which the materials may be cytotoxic 8 , 9 . Although the MTT assay measures the mitochondrial metabolic activity rate as an estimate of cell viability, an observed excess of metabolic activity may also represent a response to increased cellular stress due to toxicity, thus caution is recommended with the use of MTT as its estimation of cell viability may be misleading 28 , 29 . Moreover, the MTT requires the cells to be killed, making it impossible to follow up on the cells in culture 1 , and yet requires an extended incubation time with lower sensitivity than the XTT 3 . Our experimental model designed to obtain extract media for the cell viability assay and the use of human periodontal ligament cells resembles a clinical scenario, simulating the amount and surface area of the material that usually comes in contact with surrounding tissues in most clinical applications 8 , 9 , 12 , 29 . Further, the use of the extract media also simulates the scenario in which toxic elements released from the materials could be leaching into the periodontal ligament 16 . Recently, Chung, et al. 7 (2015) also investigated the cytotoxicity of calcium-silicate cements including ProRoot® MTA and RetroMTA® on human pulp-derived cells using a XTT assay. Human pulp-derived cells were grown in direct contact with the material, Dycal, or no cement for seven days. Initial cell attachment, viability, calcium release, and the levels of vascular endothelial growth factor (VEGF), angiogenin, and basic fibroblast growth factor (FGF-2) were evaluated. The cell viability was tested with freshly mixed and set materials after three and seven days. These authors reported that the overall biocompatibility of RetroMTA® was similar to those of the control and ProRoot® MTA, corroborating our findings.
CONCLUSION
The current study provides new and important data about the physicochemical and biological properties of RetroMTA® concerning radiopacity, pH and cytotoxic effects on human periodontal ligaments cells. Based on our findings, RetroMTA® meets the radiopacity requirements stipulated by ANSI/ADA 2 , and presents similar pH values and biocompatibility to ProRoot® MTA. Further studies should be performed to evaluate additional properties of this new material.
ACKNOWLEDGEMENTS
This work was performed with financial support from CAPES - Coordination of Higher Education and Graduate Training (BEX: 1099/12-4) to Leticia Souza. This study was supported by UTSD - University of Texas Health Science Center at Houston School of Dentistry startup funds to Ariadne Letra. HPDL cells were kindly provided by Dr. Isabel Gay, Department of Periodontics, University of Texas Health Science Center at Houston School of Dentistry. Thanks to Min Zhao for technical assistance.
REFERENCES
- 1.Al-Nasiry S, Geusens N, Hanssens M, Luyten C, Pijnenborg R. The use of alamar blue assay for quantitative analysis of viability, migration and invasion of choriocarcinoma cells. Hum Reprod. 2007;22:1304–1309. doi: 10.1093/humrep/dem011. [DOI] [PubMed] [Google Scholar]
- 2.American National Standards Institute. American Dental Association . ANSI/ADA Specification N° 57: Endodontic sealing material. Chicago: ANSI/ADA; 2000. [Google Scholar]
- 3.Atay A, Bozok Cetintas V, Cal E, Kosova B, Kesercioglu A, Guneri P. Cytotoxicity of hard and soft denture lining materials. Dent Mat J. 2012;31:1082–1086. doi: 10.4012/dmj.2012-209. [DOI] [PubMed] [Google Scholar]
- 4.Camilleri J. Evaluation of the physical properties of an endodontic Portland cement incorporating alternative radiopacifiers used as root-end filling material. Int Endod J. 2010;43:231–240. doi: 10.1111/j.1365-2591.2009.01670.x. [DOI] [PubMed] [Google Scholar]
- 5.Camilleri J, Gandolfi MG. Evaluation of the radiopacity of calcium silicate cements containing different radiopacifiers. Int Endod J. 2010;43:21–30. doi: 10.1111/j.1365-2591.2009.01621.x. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho JR, Junior, Correr L, Sobrinho, Correr AB, Sinhoreti MAC, Consani S, Sousa MD., Neto Radiopacity of root filling materials using digital radiography. Int Endod J. 2007;40:514–520. doi: 10.1111/j.1365-2591.2007.01246.x. [DOI] [PubMed] [Google Scholar]
- 7.Chung CJ, Kim E, Song M, Park JW, Shin SJ. Effects of two fast-setting calcium-silicate cements on cell viability and angiogenic factor release in human pulp-derived cells. Odontology. 2015 doi: 10.1007/s10266-015-0194-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.De-Deus G, Canabarro A, Alves G, Linhares A, Senne MI, Granjeiro JM. Optimal cytocompatibility of a bioceramic nanoparticulate cement in primary human mesenchymal cells. J Endod. 2009;35:1387–1390. doi: 10.1016/j.joen.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 9.De-Deus G, Canabarro A, Alves GG, Marins JR, Linhares AB, Granjeiro JM. Cytocompatibility of the ready-to-use bioceramic putty repair cement iRoot BP Plus with primary human osteoblasts. Int Endod J. 2012;45:508–513. doi: 10.1111/j.1365-2591.2011.02003.x. [DOI] [PubMed] [Google Scholar]
- 10.Duarte MA, Demarchi AC, Yamashita JC, Kuga MC, Fraga SC. pH and calcium ion release of 2 root-end filling materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:809–815. doi: 10.1067/moe.2003.12. [DOI] [PubMed] [Google Scholar]
- 11.Ghorbanzadeh A, Shokouhinejad N, Fathi B, Raoof M, Khoshkhounejad M. An in vitro comparison of marginal adaptation of MTA and MTA-like materials in the presence of PBS at one-week and two-month intervals. J Dent. 2014;11:560–568. Tehran. [PMC free article] [PubMed] [Google Scholar]
- 12.Gomes Cornélio AL, Salles LP, Paz MC, Cirelli JA, Guerreiro-Tanomaru JM, Tanomaru M., Filho Cytotoxicity of Portland cement with different radiopacifying agents: a cell death study. J Endod. 2011;37:203–210. doi: 10.1016/j.joen.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 13.International Organization for Standardization . ISO 10993-5:2009. Biological evaluation of medical devices – Part 5: Tests for in vitro cytotoxicity. Geneva: ISO; 2009. [Google Scholar]
- 14.Islam I, Chng HK, Yap AUJ. Comparison of the physical and mechanical properties of MTA and Portland cement. J Endod. 2006;32:193–197. doi: 10.1016/j.joen.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 15.Kang SH, Shin YS, Lee HS, Kim SO, Shin Y, Jung IY, et al. Color changes of teeth after treatment with various mineral trioxide aggregate-based materials: an ex vivo study. J Endod. 2015;41:737–741. doi: 10.1016/j.joen.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Keiser K, Johnson CC, Tipton DA. Cytotoxicity of mineral trioxide aggregate using human periodontal ligament fibroblasts. J Endod. 2000;26:288–291. doi: 10.1097/00004770-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Koçak S, Erten H, Baris E, Türk S, Alaçam T. Evaluation of the biocompatibility of experimentally manufactured portland cement: an animal study. J Clin Exp Dent. 2014;6:e17–e21. doi: 10.4317/jced.51210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komabayashi T, Spångberg LS. Comparative analysis of the particle size and shape of commercially available mineral trioxide aggregates and Portland cement: a study with a flow particle image analyzer. J Endod. 2008;34:94–98. doi: 10.1016/j.joen.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Marciano MA, Costa RM, Camilleri J, Mondelli RFL, Guimarães BM, Duarte MA. Assessment of color stability of white mineral trioxide aggregate Angelus and bismuth oxide in contact with tooth structure. J Endod. 2014;40:1235–1240. doi: 10.1016/j.joen.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 20.Massi S, Tanomaru M, Filho, Silva GF, Duarte MA, Grizzo LT, Buzalaf MA, et al. pH, calcium ion release, and setting time of an experimental mineral trioxide aggregate-based root canal sealer. J Endod. 2011;37:844–846. doi: 10.1016/j.joen.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 21.Menezes R, Bramante CM, Letra A, Carvalho VG, Garcia RB. Histologic evaluation of pulpotomies in dog using two types of mineral trioxide aggregate and regular and white Portland cement as wound dressings. Oral Surg Oral Med Oral Med Oral Pathol Oral Radiol Endod. 2004;98:376–379. doi: 10.1016/S107921040400215X. [DOI] [PubMed] [Google Scholar]
- 22.Saghiri MA, Asatourian A, Orangi J, Lotfi M, Soukup JW, Garcia-Godoy F, et al. Effect of particle size on calcium release and elevation of pH of endodontic cements. Dent Traumatol. 2015;31:196–201. doi: 10.1111/edt.12160. [DOI] [PubMed] [Google Scholar]
- 23.Song JS, Mante FK, Romanow WJ, Kim S. Chemical analysis of powder and set forms of Portland cement, gray ProRoot MTA, white ProRootMTA, and gray MTA-Angelus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:809–815. doi: 10.1016/j.tripleo.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 24.Storm B, Eichmiller FC, Tordik PA, Goodell GG. Setting expansion of gray and white mineral trioxide aggregate and Portland cement. J Endod. 2008;34:80–82. doi: 10.1016/j.joen.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Tawil PZ, Trope M, Curran AE, Caplan DJ, Kirakozova A, Duggan DJ, et al. Periapical microsurgery: an in vivo evaluation of endodontic root-end filling materials. J Endod. 2009;35:357–362. doi: 10.1016/j.joen.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Vasconcelos BC, Bernardes RA, Cruz SM, Duarte MA, Padilha PM, Bernardineli N, et al. Evaluation of pH and calcium íon release of new root-end filling materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:135–139. doi: 10.1016/j.tripleo.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 27.Vivan RR, Zapata RO, Zeferino MA, Bramante CM, Bernardineli N, Garcia RB, et al. Evaluation of the physical and chemical properties of two commercial and three experimental root-end filling materials. Oral Surg Oral Med Oral Med Oral Pathol Oral Radiol Endod. 2010;110:250–256. doi: 10.1016/j.tripleo.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 28.Wang P, Henning SM, Heber D. Limitations of MTT and MTS-based assays for measurement of antiproliferative activity of green tea polyphenols. PLoS ONE. 2010;5: doi: 10.1371/journal.pone.0010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yadlapati M, Souza LC, Dorn S, Garlet GP, Letra A, Silva RM. Deleterious effect of triple antibiotic paste on human periodontal ligament fibroblasts. Int Endod J. 2014;47:769–775. doi: 10.1111/iej.12216. [DOI] [PubMed] [Google Scholar]
- 30.Zarrabi MH, Javidi M, Jafarian AH, Joushan B. Histologic assessment of human pulp response to capping with mineral trioxide aggregate and a novel endodontic cement. J Endod. 2010;36:1778–1781. doi: 10.1016/j.joen.2010.08.024. [DOI] [PubMed] [Google Scholar]