Abstract
Dermoscopy is an aiding method in the visualization of the epidermis and dermis. It is usually used to diagnose melanocytic lesions. In recent years, dermoscopy has increasingly been used to diagnose non-melanocytic lesions. Certain vascular structures, their patterns of arrangement and additional criteria may demonstrate lesion-specific characteristics. In this review, vascular structures and their arrangements are discussed separately in the light of conflicting views and an overview of recent literature.
Keywords: Dermoscopy; Skin diseases, Vascular; Skin and connective tissue diseases
INTRODUCTION
Dermoscopes are modified magnifiers,enabling inspection of vessels and pigmented structures in the epidermis and superficial dermis. Unlike traditional dermoscopes which use liquid and gel (contact), modern, hand-held dermoscopes use cross-polarized light that allows monitoring of vascular structures in the skin.1Both systems are commercially available and generally provide 10x magnification.1An advantage of polarized light dermoscope is that no physical contact is required between the skin and glass lens. Nevertheless, compressive contact between the glass lens and tumor surface makes visualization of capillaries on the surface difficult in non-polarized dermoscopes, which limits the opportunityto diagnose pigmented skin tumors.2 Furthermore, it may complicate diagnosis in non-pigmented skin tumors, as the only dermoscopic property to be relied on are vessels in such cases.2
BASICS OF DERMOSCOPIC IMAGING OF VESSELS
Imaging of vascular structures is dependent on optical devices (either contact or non-contact dermoscopes) and dermoscopic imaging techniques.3,4The glass lens should be carefully placed upon the lesion and minimal pressure should be applied when using contact dermoscopes. Low-density liquids such as alcohol and immersion oil can occasionally be used. However, it is better to avoid such applications in contact dermoscopes, since these optical devices may require the application of over-pressure on the lesion to obtain full optical contact. Many dermatology clinics effectively utilize conducive ultrasound gel due to its high density. Ultrasound gel which is applied on the lesion helps the glass lens put less pressure on the lesion. Although non-contact dermoscopes require no physical contact between the skin and glass lens, they may create significant reflections in dry and squamous lesions and inhibit the visualization of vascular lesions. Use of such liquids as water, alcohol and immersion oil, as well as ultrasound gel, helps to reduce reflections on the surface and visualize the vessels.2
THREE-STEP DIAGNOSTIC ALGORITHM FOR NONPIGMENTED SKIN LESIONS
In 2010, Zalaudek et al. suggested a three-step algorithm for dermoscopic evaluation of lesions with vascular involvement.2These three steps are explained below:
1. Morphology of vascular structures: Given that dermoscopy enables horizontal inspection of the skin, vessels that are located in parallel to the skin's surface will appear as a line to the observer, while those located vertically to the skin's surface will present as a dot or node. In this respect, a strong connection between the dominant vascular structure and tumor progression and volume is quite important.4 For example, flat and superficial amelanotic/hypomelanotic melanoma and basal cell carcinoma will display different vascular structures than those of their thick or nodular counterparts.
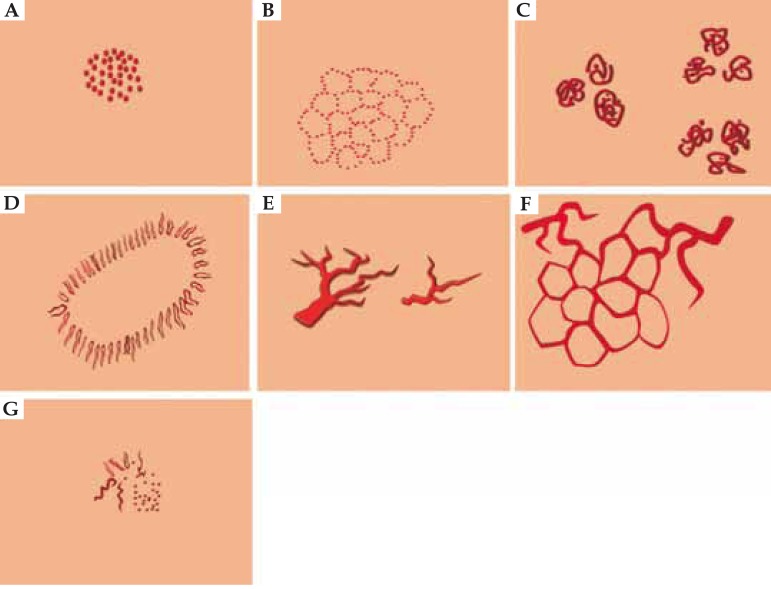
Six main morphological structures are identified among a variety of different vascular structures, namely: comma-like, dotted, linear (linear irregular and linear helical), hairpin-like, glomerular and arborizing, vessels. In addition, there are three specific global structures:crown vessels (located around a whitish core), strawberry vessels and milky red globules.4-7 Furthermore, ring-shaped vessels, spermatozoa-like vessels and red globules have been reported in recent years.
Nevertheless, Kittler et al. have classified vessels into three main morphological groups, namely: dots, clods and linear vessels. They further divide linear vessels into six subcategories: linear - flat (for linear - irregular vessels), linear - loop-like (for hairpin-like vessels), linear - curved (for comma-like vessels), linear - serpiginous (for linear - irregular, arborizing, crown or thin arborizing vessels), linear - helical (for corkscrew vessels) and linear - coiled vessels (for glomerular vessels).8
1. Structural patterns of vessels: Pursuant to a morphological evaluation, the structural patterns of vessels play a critical role in the diagnosis of non-pigmented skin lesions. Zalaudek et al. identified six main groups of structural patterns: regular (homogenous), irregular (non-homogenous), string-like, clustered and glomerular, radial and arborizing.2 As similar types of vessels can exist in various skin lesions, differences in vascular structural patterns may help in differential diagnosis.2,6,8
Nonetheless, Kittler et al. have classified structural patterns of vessels into six main groups as follows:8
a. Non-specificpattern
b. Clustered and glomerular pattern: vascular pattern in which coiled and dotted vessels do not spreadevenly over the lesion but concentrate on certain regions.
c. Serpiginous pattern: vascular pattern where dotted or coiled vessels line up in a linear or arc-like manner.
d. Radial pattern: vascular pattern where linear (flat, looped and curved) vessels at the periphery are orientated towards the core. Vessels do not cross each other.
e. Reticular pattern: pigmented reticular pattern of linear flat vessels. Vessels sometimes cross each other.
f. Arborizing pattern: vascular pattern in which a main vessel arborizes into serpiginous and generally thick sub-branches.
1. Evaluation of additional findings:the following,additional dermoscopic findings provide extra clues for diagnosis:white halo around vessels (typically in keratinized tumors), residual pigmentation (in hypopigmented, melanocytic tumors), hair, central channel patency, superficial squamas and ulceration.4
COMMON MORPHOLOGICAL VESSEL TYPES
1. Arborizing vessels (linear - flat and linear -tortuous)
Arborizing vessels were initially found to be useful for diagnosing BCC in 1990.9 They are large-diameter vessels that arborize into smaller, thinner branches in non-homogenous fashion (Figure 1).10The most striking vascular patterns of this type are seen in BCC. Bloods vessels are located immediately under the epidermis in BCC.11Compared with the pink vessels in the dermal plexus of normal skin, these vessels are much brighter, red in color and perfectly clear. In contrast,vessels located outside the neoplasm arevague and lighter red or pink.12In BCC, main vessels (0.2mm or larger) branch into non-homogenous, terminal capillaries with a diameter of 10µm. These structures also appear in pigmented BCC due to the course of vessels on the tumor's surface. Arborizing vessels generally occur in nodular,cystic or (interestingly)cicatricial, BCC.11 Serpiginous vessels are thicker in such cases. However, shorter (microarborizing) vessels with fewer branches occur in superficial BCC and serpiginous vessels are of smaller diameters, which makes detection difficult.8,12-16
FIGURE 1.
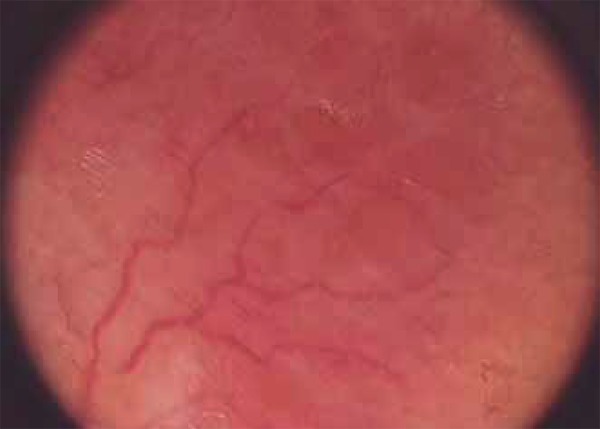
Arborizing vessels and orange areas on the lupus vulgaris lesion
Fibroepithelioma of Pinkus is a rare variant of BCC,involving small-diameter, arborizing vessels. They may be accompanied by pinpoint or chrysalis patterns (whitish and non-homogenous lines).17,18Compared with nodular or cystic BCC, cicatricial BCC hosts thinner, less organized and fewer arborizing vessels. In addition, vessels have vague borders and sit on a white ground in cicatricial BCC whereas they have sharp borders and sit on a pinkish ground in nodular or cystic BCC.12Kreusch et al. have suggested thatthe sensitivity ofthe arborizing vessel pattern is 96.1% while its specificity is 90.6% in BCC.11 Vascular structures in blue nevus, syringoma, nodular melanoma and other tumors with diameters of over 3mm mimic BCC at first glance, but vessels in these tumors display a more homogenous arborization. Moreover, thick, arborizing vessels can appear in juvenile xanthogranuloma, Merkel cell carcinoma, angiohistiocytoma, hidradenoma, intraepidermalporoma and epidermal cysts.19-21
2. Pinpoint or dotted vessels
Dotted vessels generally have a high positive predictive value in melanocytic skin lesions.2 They correspond to the tips of short, vertically arranged capillaries in lesions of smaller diameters and appear in dermoscopy as very small red dots with diameters of 0.01-0.02 mm. They line up side by side in a homogenous and intensive manner. The tips of the small capillary curves may be inspected by greater magnification (30 folds or bigger). They can be mistaken for vascular dots which appear along the tips of hairless skin or normal skin papilla.11
Dotted vessels located in the reticular spaces on the borders of junctional nevi indicate dermal papilla and are not regarded as tumors. In other words, dotted vessels are considered tumoral vessels only when they produce a solid lesion which can be identified upon clinical inspection. Such vessels may appear in many small, verticaldiameter, keratinized tumors such as verruca vularis, actinic keratosis, seborrheic keratosis, Bowen's Disease (BD) and Squamous Cell Carcinoma (SCC).11 In addition, they may appear in dermotofibroma.22-24 Furthermore, they have been reported in juvenile xanthogranuloma cases25, and can occur in Spitz nevus, a melanocytic lesion.11They were detected in 77.8% of Spitz nevi patients in one study.5Hypopigmented Spitz nevus hosts homogenous dotted vessels that sit on a pink ground with reticular depigmentation. Moreover, flat Spitz nevus hosts small dotted vessels that are quite homogenous and intensive, and sit on a pink ground.2 In contrast, nodular and atypical Spitz nevus entails linear - irregular vessels, glomerular vessels or pink globules which generally occur in amelanotic/hypomelanotic melanoma.5
Red Clark nevus, a melanocytic lesion, appears in individuals with skin types 1 or 2.26 Clark nevus displays dotted or comma-like vessels. Unlike Spitz nevus, dotted vessels are less intensive and located on a skin-colored ground.2
Early stage, flat amelanotic/hypomelanotic melanoma (thickness < 1 mm) reveals dotted vessels that are regular,homogenous and which cannot be differentiated from non-pigmented Spitz nevus. On the other hand, amelanotic melanoma of moderate thickness demonstrates dotted vessels and linear - irregular vessels which are non-homogenous and appear in various, rough shapes.2 Theyraise the possibility of invasive melanoma when they sit on a pink ground, with or without reticular depigmentation or chrysalis structures. The positive predictive value of such vessels is reported to be 67.6% in melanoma.2 Thick amelanotic melanoma (thickness >2mm) displays non-homogenous, elongated, linear vessels of different sizes as well as bended and non-homogenous, hairpin-like vessels, corkscrew vessels, polymorphic vessels including arborizing vessels and/or pink globules. Dotted vessels rarely appear in these cases27-30but dotted or corkscrew vessels may appear where melanoma metastasizes into the skin.31
In clear cell acanthoma (CCA), string-like, dotted vessels appear.32,33They are frequently seen in psoriasis vulgaris, pitriasisrosea and lichen planus.34 Dotted vessels also occur in superficial and nodular BCC, fibroepithelioma ofPinkus, pilomatrixoma, chronic dermatitis and mycosis fungoides.13,18,35,36
3. Hairpin-like or linear looped vessels
These are 'U-shaped' blood vessels with open ends side-by-side and closed ends twisting once or more (Figure 2). They have a diameter of approximately 0.01-0.03mm, which remains fixedthroughout the whole lesion.11
FIGURE 2.
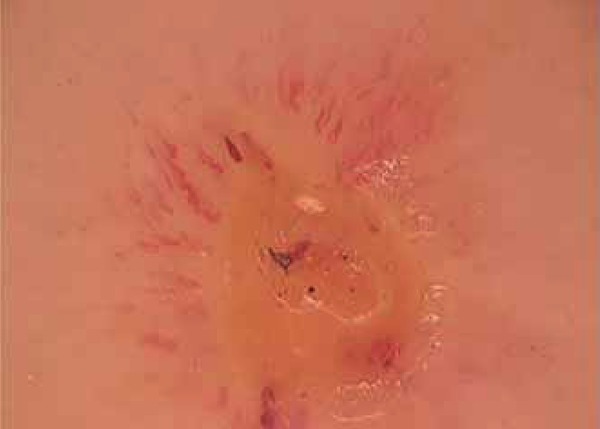
Hairpin-like vessels around excoriating lesion
The blood vessels generally appear in nonmelanocytic tumors like SCC, seborrheic keratosis and keratoacanthoma.4-6They are frequently accompanied by a whitish halo around the lesion.6 However, this halo does not occur in melanocytic lesions.5,27,29,33
Further, these blood vessels are homogenous and monomorphic in seborrheic keratosis.37But their closed end may occasionally be twisted. They appear as dark red, dotted vessels in the flat regions of a lesion.4Hairpin-like vessels occur more frequently in lesions located on the neck and head.2 They can be elongated and twisted, have two branches and appear with different diameters in irritated seborrheic keratosis.1,3
In addition, they may occur in amelanotic melanomas that are thicker than 1mm.2 Hairpin-like vessels have also been detected in eczema-like melanoma.38 These vessels are generally elongated, non-homogenous and coexist with dotted, linear-irregular or glomerular vessels in invasive SCC.11,27,28 They are elongated and sometimes thicker in keratoacanthoma. Furthermore, they occasionally coexist with glomerular or atypical linear vessels.4,39 Zaballos et al. detected hairpin-like vessels in 7 outof 10 pilomatrixoma patients.35
Moreover, hairpin-like vessels appear in polymorphic patterns in superficial BCC, nodular BCC, eccrine poroma and porocarcinoma.13,40-44
4. Comma-like or linear curved vessels
These are slightly bended,somewhat arborizing vessels with a diameter of 1mm or larger (Figure 3). Comma-like vessels are a variant of dotted and hairpin-like vessels. When viewed from above, dotted vessels look smaller than these vessels and appear like a dot as they curve at shorter lengths. Those which curve at longer lengths look like a comma or hairpin. As lesions thicken, thecurves of vessels become longer.11
FIGURE 3.
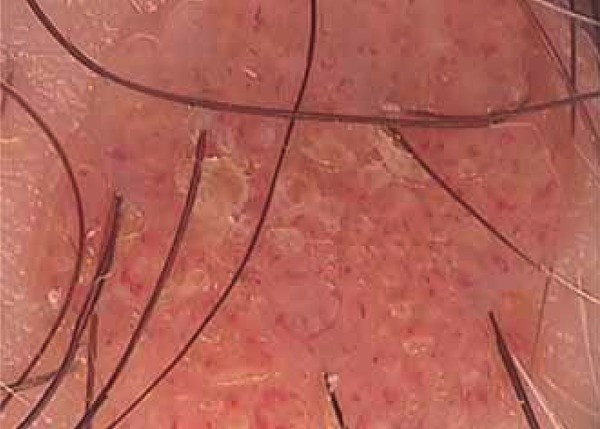
Many comma-like vessels on the dermal nevus
Comma-like vessels are a differential dermoscopic feature in dermal nevus, with a positive predictive value of 94%.45 They generally appear in classical forms in papillomatosis nevus (Unna's nevus) and polymorphic forms in Miescher's nevus.2Furthermore, comma-like vessels can be accompanied by dotted vessels in red and hypopigmented Clark's nevus. Unlike in dermal nevus, comma-like vessels are rather small (0.01-0.03mm) and evenly spread over the lesion in melanoma.11 However, some authors argue that the size of the vessels cannot be relied on in making differential diagnosis between melanoma and dermal nevus.2
These vessels were shown to represent a negative predictive value in amelanotic/hypomelanotic melanoma.27 Some studies have reported that 1-45% of all superficial and nodular types of basal cell carcinomas display comma-like vessels13,46, which have also been described in dermatofibroma.23,24
5. Linear vessels
a. Linear-irregular or linear-tortuous vessels
These are snake-like vessels of different sizes with more than one curve.8 They may coexist with hairpin-like vessels in a white halo, surrounding a central, yellowish-brown, keratin mass in keratoacanthoma.4,38 Two separate studiesof Merkel cell carcinoma patients, one with 10, the other with 2, both reported linear-irregular vessels in all the patients.20,47These vessels can coexist with dotted and hairpin-like vessels in amelanotic melanomas of intermediate sizes (1-2mm).2
In addition, such vessels can appear in mycosis fungoides, pilomatrixoma, metastatic skin melanoma, nodular hyradenoma, dermatofibroma, eczema-like melanoma, actinic keratosis, eccrine poroma and eccrine porocarcinoma.12,23,34,35,36,38,40-44,48,49
b. Linear-helical vessels (Corkscrew vessels)
These vessels curve around a central axis8 and may coexist with hairpin-like vessels in thick amelanotic melanoma (>2 mm).27-30Some studies have suggested that they appear in approximately %31 out of all BCC cases, while others have not noted any such connection.13,46
6. Glomerular vessels (linear coiled or clustered)
These are interpenetrating vessels,appearing as balls of wool which resemble renal glomeruli.8 They coexist with dotted vessels in small, intensive piles in BD.50-52 Zalaudek et al. observed that glomerular vessels appeared in 100% of non-pigmented BD patients and 80% of pigmented BD patients.50
Pan et al. found that glomerular vessels can also occur in superficial BCC.8Moreover,Micantonio et al. concluded that 7.8% of 333 superficial BCC patients and 8.8% of 171 nodular BCC patients displayed glomerular vessels.13
Glomerular vessels may demonstrate a string-like pattern in thicker types of CCA.53-55
Such vessels were also detected in polymorphic forms in invasive SCC and keratoacanthoma.4,39
Kim et al. detected glomerular vessels in 65% of patients with psoriasis and 22% of patients with seborrheic dermatitis.56
In addition, glomerular vessels have been reported in eccrine poroma, Merkel cell carcinoma, eczema-like melanoma, nodular Spitz nevus, stasis dermatitis and actinic keratosis.5,20,38,40-42,57-59
7. Crown vessels
These are homogenously curving,slightly arborizing vessels that surrounding yellowish-whitish,polylobular, sebaceous glands located at the core (Figure 4). Vessels may spread into the core of the lesion. However, they never fully cross the lesion. Occasionally, glandular ostia can appear as small craters60, which typically show up in sebaceous hyperplasia (11), though they have also been reported in sebaceoma and nevus sebaceous of Jadassohn.11,61,62
FIGURE 4.

Crown vessels surround yellowish polilobular sebaceous glands located at the core of sebaseous hyperplasia
They can sometimes be mistaken fortypical BCC vessels.63 However, BCC vessels are brighter, with sharper borders, and frequently enter the core of the lesion.12 Furthermore, crown vessels are surrounded a yellow-white, structureless core in molluscum contagiosum.64
8. Polymorphic vessels
These involve a combination of two or more different, vascular patterns. The most frequent combination comprises linear-irregular vessels and dotted vessels, which is quite specific to amelanotic/hypomelanotic melanoma of thin and medium thickness, particularly when it is localized at the core.65 Combinations of dotted vessels and linear vessels have also been reported in eccrine poroma.41 Furthermore, combinations of linear-irregular vessels and dotted vessels, particularly microarborizing vessels, have been detected in Merkel cell carcinoma.20
Arborizing or linear-helical vessels, in addition to linear-irregular vessels and hairpin-like vessels, are precious findings for thick amelanotic/hypomelanotic melanoma and metastasis of melanoma.27-29 Combinations of linear-irregular vessels and hairpin-like vessels may also occur in SCC and pilomatrixoma.35 Combinations of dotted vessels and comma-like vessels, on the other hand, can occur in red Clark's nevus whereas combinations of dotted vessels and glomerular vessels may show up in CCA.53-55,66Zalaudek et al. detected combinations of thin, arborizing vessels and dotted vessels in 7 (70%) out of 10 patients withfibroepithelioma of Pinkus.17
9. Strawberry pattern
This is a formation of erythema that creates pseudo-networks of red-pink color around hair follicles filled with keratin, which can occur in non-pigmented actinic keratosis. Keratin plugs may appear in the form of targets.59Zalaudek et al. observed this pattern in over 90% of patients with non-pigmented keratosis.59
10. Milky red globules or clods
These are vague, milky red globules or wide regions, corresponding to the parts of a lesion which are raised over the skin.5 They occurespecially in thick amelanotic melanoma (> 2 mm), and their positive predictive value has been found to be 77.8% .5
One study found milky red globules in 7 (4.7%) out of 150 patients with malignant melanoma.It also observed this pattern in two more patients, one with atypical Spitz nevus,the other with BCC.5 Harting et al. detected milky red globules in all 10 patients with Merkel cell carcinoma.20
11. Red globules
These are round or oval,solid, red structures that are larger than dotted vessels.15 Pan et al. detected red globules in 32% of patients with intraepidermal carcinoma, 6% of patients with superficial BCC and 32% of patients with psoriasis.15 Furthermore, these globules have also beenreported in completely regressed melanoma, hemangioma, Kaposi's sarcoma, port-wine stain, eccrine poroma and stasis dermatitis.40-42,58,67-70
12. Twisted Red Loops
These vessels appear ascoils or loops that are numerous, with relatively equal hollows.71 They have been detected in 53-100% of cases with scalp psoriasis and 19-22% of cases with seborrheic dermatitis.56,71In addition, they have been reported in folliculitis decalvans.71
13. Spermatozoa-like vessels
This pattern iscomposed of dotted vessels and short, curved, linear vessels. Lallas et al. detected this vascular pattern in 50% mycosis fungoidescases. It has high specificity and low sensitivity.36 All vascular formations are displayedin figure 5.
FIGURE 5.
Schematic view of common vesselformations. Arborizing (A), hairpin-like (B), linear (C), polymorphic (D), comma-like (E), dotted (F), glomerular (G), corkscrew-like (H), crown-like (J), strawberry pattern (K), milky red globules (L), red globules (M), twisted red loops (N), spermatozoa-like vessels (O)
STRUCTURAL ARRANGEMENTS OF VESSELS
1. Regular (Homogenous)
This is a vascular arrangement whereby similar or different vessels randomly come together without a differential or specific order.8 Dotted vessels in dermal nevus, dotted vessels and (less frequently) linear-irregular vessels in dermatofibroma, hairpin-like vessels and/or dotted vessels in seborrheic keratosis, dotted vessels in Spitz nevus, dotted vessels in psoriasis, dotted vessels and/or linear vessels in eczema, linear vessels in urticariaand glomerular vessels in venous stasis,all display a homogenous arrangement.5,23,24,32,33,72,73
2. String-like
This is a vascular arrangement in which glomerular vessels or dotted vessels demonstrate a linear or arc-like pattern.8 They are highly specific to CCA. Glomerular vessels assume such patterns in thick forms of CCA while dotted vessels demonstrate this arrangement in thinner tumors.53-55 It has a yellowish-whitish ground and is surrounded by a white halo.12
The round or oval and network-like arrangement of dotted vessels can also be termed red globular loops. Although this name is generally used for psoriasis patients, it still represents the same vascular pattern as string-like vessels.15,74 This pattern was reported in anintraepidermal carcinoma case.15
3. Clustered
In this vascular arrangement, glomerular vessels and dotted vessels do not spread homogenously over the lesion but concentrate on certain regions.8 Dotted vessels and glomerular vessels assume this pattern in BD and intraepidermal carcinoma.15,50-52,75
4. Radial
This is a vascular arrangement in which linear-flat, comma-like and hairpin-like vessels at the periphery of a lesion orientate towards the core but do not fully cross the lesion.8
In keratoacanthoma, akeratin mass at the core is enclosed by hairpin-like vessels.8 This radial arrangement may comprise linear-irregular vessels or glomerular vessels.4,39
Sebaceous hyperplasia, sebaceoma, nevus sebaceous of Jadassohn and molluscum contagiosum show crown vessels that enclose a yellowish-whitish,polylobular structure at the core.60-64 Moreover, lichen planus displays white lines at the core and dotted vessels at the periphery whereas psoriasis reveals squama at the core and dotted vessels at the periphery, arranged in a radial pattern.34,76
5. Irregular arborizing
This is a vascular arrangement where by a major, thick vessel generally arborizes into linear, curved vessels.8 It appears in BCC.2,13-16Arborizing vessels can also appear in fibroepithelioma of Pinkus, juvenile xanthogranuloma, Merkel cell carcinoma, angiohistiocytoma, hidradenoma and intraepidermal poroma.17-21
6. Reticular
In this vascular pattern, linear-flat vessels can cross each other, creating a pigmented, network-like pattern.8 It appears in all patients with telangiectasia macularis eruptive perstans and occasionally, in individuals with maculopapular mastocytosis.77 Reticular vessels have also been detected in rosacea78 and they can appear in juvenile xanthogranuloma.25 Similar vessels which are called telangiectasia were also detected in BCC.13
7. Irregular (Non-homogenous)
This vascular arrangement involves lots of similar or different vessels,unevenly spread over a lesion. Linear-irregular vessels in amelanotic melanoma, polymorphic vessels in metastasis of melanoma, arborizing vessels in BCC, linear-irregular vessels and hairpin-like vessels in SCC, linear vessels, hairpin-like vessels and dotted vessels in eccrine porocarcinoma, milky red globules in pyogenic granuloma,dotted vessels in verruca, polymorphic vessels in pityriasisrosea and dotted vessels in lichen aureus,all reveal a non-homogenous arrangement.31,32,43,75,79-83 Prevalent vascular arrangements are displayed in figure 6.
FIGURE 6.
The structural arrangements of vessels. Irregular (homogenous) (A), string-like (B), clustered (C), radial (D), irregular arborising (E), reticular (F), irregular (non-homogenous) (G)
CONCLUSION
The recent characterization of vascular structures in dermoscopy is helpful in diagnosizingmelanocytic and nonmelanocytic lesions. Further studies will provide more information on the diagnosis of such lesions.
Footnotes
Conflict of Interest: None.
Financial Support: None.
How to cite this article: Ayhan E,Ucmak D, Akkurt ZM. Vascular structures in dermoscopy. An Bras Dermatol. 2015; 90(4):545-53.
Work performed at the DinçerlerCaddesiYozgatYoluZile -Tokat, Turkey.
REFERENCES
- 1.Arrazola P, Mullani NA, Abramovits W. DermLite II: an innovative portable instrument for dermoscopy without the need of immersion fluids. Skinmed. 2005;4:78–83. doi: 10.1111/j.1540-9740.2005.04363.x. [DOI] [PubMed] [Google Scholar]
- 2.Zalaudek I, Kreusch J, Giacomel J, Ferrara G, Catricalà C, Argenziano G. How to diagnose nonpigmented skin tumors: A review of vascular structures seen with dermoscopy. Part I. Melanocytic skin tumors. J Am Acad Dermatol. 2010;63:361–374. doi: 10.1016/j.jaad.2009.11.698. [DOI] [PubMed] [Google Scholar]
- 3.Wang SQ, Dusza SW, Scope A, Braun RP, Kopf AW, Marghoob AA. Differences in dermoscopic images from nonpolarized dermoscope and polarized dermoscope influence the diagnostic accuracy and confidence level: a pilot study. Dermatol Surg. 2008;34:1389–1395. doi: 10.1111/j.1524-4725.2008.34293.x. [DOI] [PubMed] [Google Scholar]
- 4.Kreusch J, Koch F. Incident light microscopic characterization of vascular patterns in skin tumors. Hautarzt. 1996;47:264–272. doi: 10.1007/s001050050412. [DOI] [PubMed] [Google Scholar]
- 5.Argenziano G, Zalaudek I, Corona R, Sera F, Cicale L, Petrillo G, et al. Vascular structures in skin tumors. A dermoscopy study. Arch Dermatol. 2004;140:1485–1489. doi: 10.1001/archderm.140.12.1485. [DOI] [PubMed] [Google Scholar]
- 6.Zalaudek I, Argenziano G, Oliviero M, Rabinovitz H. Dermoscopy of nonpigmented skin tumors. In: Thiers BH, Lang PG Jr, editors. Year book of dermatology and dermatologic surgery 2007. Philadelphia: Elsevier Mosby; 2007. pp. 23–38. [Google Scholar]
- 7.Zalaudek I, Argenziano G, Di Stefani A, Ferrara G, Marghoob AA, Hofmann-Wellenhof R, et al. Dermoscopy in general dermatology. Dermatology. 2006;212:7–18. doi: 10.1159/000089015. [DOI] [PubMed] [Google Scholar]
- 8.Kittler H, Riedl E, Rosendahl C, Cameron A. Dermatoscopy of unpigmented lesions of the skin: a new classification of vessel morphology based on pattern analysis. Dermatopathology: Practical e Conceptual; 2008. [2010 Aug 02]. a Available from: http://www.derm101.com. [Google Scholar]
- 9.Bahmer FA, Fritsch P, Kreusch J, Pehamberger H, Rohrer C, Schindera I, et al. Terminology in surface microscopy. J Am Acad Dermatol. 1990;23:1159–1162. [PubMed] [Google Scholar]
- 10.Braun RP, Rabinovitz H, Tzu JE, Marghoob AA. Dermoscopy Research-An Update. Semin Cutan Med Surg. 2009;28:165–171. doi: 10.1016/j.sder.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Kreusch JF. Vascular Patterns in Skin Tumors. Clin Dermatol. 2002;20:248–254. doi: 10.1016/s0738-081x(02)00227-4. [DOI] [PubMed] [Google Scholar]
- 12.Zalaudek I, Kreusch J, Giacomel J, Ferrara G, Catricalà C, Argenziano G. How to diagnose nonpigmented skin tumors: A review of vascular structures seen with dermoscopy. Part II. Nonmelanocytic skin tumors. J Am Acad Dermatol. 2010;63:377–386. doi: 10.1016/j.jaad.2009.11.697. [DOI] [PubMed] [Google Scholar]
- 13.Micantonio T, Gulia A, Altobelli E, Di Cesare A, Fidanza R, Riitano A, et al. Vascular patterns in basal cell carcinoma. J Eur Acad Dermatol Venereol. 2011;25:358–361. doi: 10.1111/j.1468-3083.2010.03734.x. [DOI] [PubMed] [Google Scholar]
- 14.Giacomel J, Zalaudek I. Dermoscopy of Superficial Basal Cell Carcinoma. Dermatol Surg. 2005;31:1710–1713. doi: 10.2310/6350.2005.31314. [DOI] [PubMed] [Google Scholar]
- 15.Pan Y, Chamberlain AJ, Bailey M, Chong AH, Haskett M, Kelly JW. Dermatoscopy aids in the diagnosis of the solitary red scaly patch or plaque-features distinguishing superficial basal cell carcinoma, intraepidermal carcinoma, and psoriasis. J Am Acad Dermatol. 2008;59:268–274. doi: 10.1016/j.jaad.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Felder S, Rabinovitz H, Oliviero M, Kopf A. Dermoscopic differentiation of a superficial basal cell carcinoma and squamous cell carcinoma in situ. Dermatol Surg. 2006;32:423–425. doi: 10.1111/j.1524-4725.2006.32085.x. [DOI] [PubMed] [Google Scholar]
- 17.Zamberk-Majlis P, Velázquez-Tarjuelo D, Avilés-Izquierdo JA, Lázaro-Ochaita P. Dermoscopic characterization of 3 cases of fibroepithelioma of Pinkus. Actas Dermosifiliogr. 2009;100:899–902. [PubMed] [Google Scholar]
- 18.Zalaudek I, Ferrara G, Broganelli P, Moscarella E, Mordente I, Giacomel J, et al. Dermoscopy patterns of fibroepithelioma of pinkus. Arch Dermatol. 2006;142:1318–1322. doi: 10.1001/archderm.142.10.1318. [DOI] [PubMed] [Google Scholar]
- 19.Rubegni P, Mandato F, Fimiani M. Juvenile Xanthogranuloma: dermoscopic pattern. Dermatology. 2009;218:380–380. doi: 10.1159/000172831. [DOI] [PubMed] [Google Scholar]
- 20.Harting MS, Ludgate MW, Fullen DR, Johnson TM, Bichakjian CK. Dermatoscopic vascular patterns in cutaneous Merkel cell carcinoma. J Am Acad Dermatol. 2012;66:923–927. doi: 10.1016/j.jaad.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Sgambato A, Zalaudek I, Ferrara G, Giorgio CM, Moscarella E, Nicolino R, et al. Adnexal tumors: clinical and dermoscopic mimickers of basal cell carcinoma. Arch Dermatol. 2008;144:426–426. doi: 10.1001/archderm.144.3.426. [DOI] [PubMed] [Google Scholar]
- 22.Agero AL, Taliercio S, Dusza SW, Salaro C, Chu P, Marghoob AA. Conventional and Polarized Dermoscopy Features of Dermatofibroma. Arch Dermatol. 2006;142:1431–1437. doi: 10.1001/archderm.142.11.1431. [DOI] [PubMed] [Google Scholar]
- 23.Kilinc Karaarslan I, Gencoglan G, Akalin T, Ozdemir F. Different dermoscopic faces of dermatofibromas. J Am Acad Dermatol. 2007;57:401–406. doi: 10.1016/j.jaad.2006.10.984. [DOI] [PubMed] [Google Scholar]
- 24.Zaballos P, Llambrich A, Ara M, Olazarán Z, Malvehy J, Puig S. Dermoscopic findings of haemosiderotic and aneurysmal dermatofibroma: report of six patients. Br J Dermatol. 2006;154:244–250. doi: 10.1111/j.1365-2133.2005.06844.x. [DOI] [PubMed] [Google Scholar]
- 25.Cavicchini S, Tourlaki A, Tanzi C, Alessi E. Dermoscopy of Solitary Yellow Lesions in Adults. Arch Dermatol. 2008;144:1412–1412. doi: 10.1001/archderm.144.10.1412. [DOI] [PubMed] [Google Scholar]
- 26.Zalaudek I, Leinweber B, Johr R. Nevi with particular pigmentation: black, pink, and white nevus. In: Soyer HP, Argenziano G, Hofmann-Wellenhof R, Johr R, editors. Color atlas of melanocytic lesions of the skin. Berlin-Heidelberg: Springer Verlag; 2007. pp. 142–146. [Google Scholar]
- 27.Menzies SW, Kreusch J, Byth K, Pizzichetta MA, Marghoob A, Braun R, et al. Dermoscopic evaluation of amelanotic and hypomelanotic melanoma. Arch Dermatol. 2008;144:1120–1127. doi: 10.1001/archderm.144.9.1120. [DOI] [PubMed] [Google Scholar]
- 28.Pizzichetta MA, Talamini R, Stanganelli I, Puddu P, Bono R, Argenziano G, et al. Amelanotic/hypomelanotic melanoma: clinical and dermoscopic features. Br J Dermatol. 2004;150:1117–1124. doi: 10.1111/j.1365-2133.2004.05928.x. [DOI] [PubMed] [Google Scholar]
- 29.Argenziano G, Zalaudek I, Ferrara G, Johr R, Langford D, Puig S, et al. Dermoscopy features of melanoma incógnito: indications for biopsy. J Am Acad Dermatol. 2007;56:508–513. doi: 10.1016/j.jaad.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 30.Cavicchini S, Tourlaki A, Bottini S. Dermoscopic vascular patterns in nodular "pure" amelanotic melanoma. Arch Dermatol. 2007;143:556–556. doi: 10.1001/archderm.143.4.556. [DOI] [PubMed] [Google Scholar]
- 31.Bono R, Giampetruzzi AR, Concolino F, Puddu P, Scoppola A, Sera F, et al. Dermoscopic patterns of cutaneous melanoma metastases. Melanoma Res. 2004;14:367–373. doi: 10.1097/00008390-200410000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Vázquez-López F, Kreusch J, Marghoob AA. Dermoscopic semiology: further insights into vascular features by screening a large spectrum of nontumoral skin lesions. Br J Dermatol. 2004;150:226–231. doi: 10.1111/j.1365-2133.2004.05753.x. [DOI] [PubMed] [Google Scholar]
- 33.Argenziano G, Fabbrocini G, Carli P, De Giorgi V, Sammarco E, Delfino M. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions: Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. 1998;134:1563–1570. doi: 10.1001/archderm.134.12.1563. [DOI] [PubMed] [Google Scholar]
- 34.Lallas A, Kyrgidis A, Tzellos TG, Apalla Z, Karakyriou E, Karatolias A, et al. Accuracy of dermoscopic criteria for the diagnosis of psoriasis, dermatitis, lichen planus and pityriasis rosea. Br J Dermatol. 2012;166:1198–1205. doi: 10.1111/j.1365-2133.2012.10868.x. [DOI] [PubMed] [Google Scholar]
- 35.Zaballos P, Llambrich A, Puig S, Malvehy J. Dermoscopic findings of pilomatricomas. Dermatology. 2008;217:225–230. doi: 10.1159/000148248. [DOI] [PubMed] [Google Scholar]
- 36.Lallas A, Apalla Z, Lefaki I, Tzellos T, Karatolias A, Sotiriou E, et al. Dermoscopy of early stage mycosis fungoides. J Eur Acad Dermatol Venereol. 2013;27:617–621. doi: 10.1111/j.1468-3083.2012.04499.x. [DOI] [PubMed] [Google Scholar]
- 37.Braun RP, Rabinovitz HS, Krischer J, Kreusch J, Oliviero M, Naldi L, Kopf AW, Saurat JH. Dermoscopy of pigmented seborrheic keratosis: a morphological study. Arch Dermatol. 2002;138:1556–1560. doi: 10.1001/archderm.138.12.1556. [DOI] [PubMed] [Google Scholar]
- 38.Giacomel J, Zalaudek I, Ferrara G, Argenziano G. Dermoscopy patterns of eczemalike melanoma. Arch Dermatol. 2007;143:1081–1082. doi: 10.1001/archderm.143.8.1081. [DOI] [PubMed] [Google Scholar]
- 39.Argenziano G, Zalaudek I, Corona R, Sera F, Cicale L, Petrillo G, et al. Vascular structures in skin tumors. A dermoscopy study. Arch Dermatol. 2004;140:1485–1489. doi: 10.1001/archderm.140.12.1485. [DOI] [PubMed] [Google Scholar]
- 40.Nicolino R, Zalaudek I, Ferrara G, Annese P, Giorgio CM, Moscarella E, et al. Dermoscopy of eccrine poroma. Dermatology. 2007;215:160–163. doi: 10.1159/000104270. [DOI] [PubMed] [Google Scholar]
- 41.Minagawa A, Koga H. Dermoscopy of pigmented poromas. Dermatology. 2010;221:78–83. doi: 10.1159/000305435. [DOI] [PubMed] [Google Scholar]
- 42.Ferrari A, Buccini P, Silipo V, De Simone P, Mariani G, Marenda S, et al. Eccrine poroma: a clinical-dermoscopic study of seven cases. Acta Derm Venereol. 2009;89:160–164. doi: 10.2340/00015555-0608. [DOI] [PubMed] [Google Scholar]
- 43.Blum A, Metzler G, Bauer J. Polymorphous vascular patterns in dermoscopy as a sign of malignant skin tumors. A case of an amelanotic melanoma and a porocarcinoma. Dermatology. 2005;210:58–59. doi: 10.1159/000081486. [DOI] [PubMed] [Google Scholar]
- 44.Johr R, Saghari S, Nouri K. Eccrine porocarcinoma arising in a seborrheic keratosis evaluated with dermoscopy and treated with Mohs' technique. Int J Dermatol. 2003;42:653–657. doi: 10.1046/j.1365-4362.2003.01779.x. [DOI] [PubMed] [Google Scholar]
- 45.Menzies SW, Westerhoff K, Rabinovitz H, Kopf AW, McCarthy WH, Katz B. Surface microscopy of pigmented basal cell carcinoma. Arch Dermatol. 2000;136:1012–1016. doi: 10.1001/archderm.136.8.1012. [DOI] [PubMed] [Google Scholar]
- 46.Trigoni A, Lazaridou E, Apalla Z, Vakirlis E, Chrysomallis F, Varytimiadis D, et al. Dermoscopic features in the diagnosis of different types of basal cell carcinoma: a prospective analysis. Hippokratia. 2012;16:29–34. [PMC free article] [PubMed] [Google Scholar]
- 47.Ciudad C, Avilés JA, Alfageme F, Lecona M, Suárez R, Lázaro P. Spontaneous regression in merkel cell carcinoma: report of two cases with a description of dermoscopic features and review of the literature. Dermatol Surg. 2010;36:687–693. doi: 10.1111/j.1524-4725.2010.01531.x. [DOI] [PubMed] [Google Scholar]
- 48.Virgili A, Zampino AR, Corazza M. Primary Vulvar Melanoma with Satellite Metastasis: Dermoscopic Findings. Dermatology. 2004;208:145–148. doi: 10.1159/000076490. [DOI] [PubMed] [Google Scholar]
- 49.Yoshida Y, Nakashima K, Yamamoto O. Dermoscopic Features of Clear Cell Hidradenoma. Dermatology. 2008;217:250–251. doi: 10.1159/000148253. [DOI] [PubMed] [Google Scholar]
- 50.Zalaudek I, Argenziano G, Leinweber B, Citarella L, Hofmann-Wellenhof R, Malvehy J, et al. Dermoscopy of Bowen's disease. Br J Dermatol. 2004;150:1112–1116. doi: 10.1111/j.1365-2133.2004.05924.x. [DOI] [PubMed] [Google Scholar]
- 51.Zalaudek I, Di Stefani A, Argenziano G. The specific dermoscopic criteria of Bowen's disease. J Eur Acad Dermatol Venereol. 2006;20:361–362. doi: 10.1111/j.1468-3083.2006.01445.x. [DOI] [PubMed] [Google Scholar]
- 52.Bugatti L, Filosa G, De Angelis R. The specific dermoscopical criteria of Bowen's disease. J Eur Acad Dermatol Venereol. 2007;21:700–701. doi: 10.1111/j.1468-3083.2006.01995.x. [DOI] [PubMed] [Google Scholar]
- 53.Bugatti L, Filosa G, Broganelli P, Tomasini C. Psoriasis-like dermoscopic pattern of clear cell acanthoma. J Eur Acad Dermatol Venereol. 2003;17:452–455. doi: 10.1046/j.1468-3083.2003.00754.x. [DOI] [PubMed] [Google Scholar]
- 54.Akin FY, Ertam I, Ceylan C, Kazandi A, Ozdemir F. Clear cell acanthoma: new observations on dermatoscopy. Indian J Dermatol Venereol Leprol. 2008;74:285–287. doi: 10.4103/0378-6323.41396. [DOI] [PubMed] [Google Scholar]
- 55.Blum A, Metzler G, Bauer J, Rassner G, Garbe C. The dermatoscopic pattern of clear-cell acanthoma resembles psoriasis vulgaris. Dermatology. 2001;203:50–52. doi: 10.1159/000051703. [DOI] [PubMed] [Google Scholar]
- 56.Kim GW, Jung HJ, Ko HC, Kim MB, Lee WJ, Lee SJ, et al. Dermoscopy can be useful in differentiating scalp psoriasis from seborrhoeic dermatitis. Br J Dermatol. 2011;164:652–656. doi: 10.1111/j.1365-2133.2010.10180.x. [DOI] [PubMed] [Google Scholar]
- 57.Vázquez-López F, Kreusch J, Marghoob AA. Dermoscopic semiology: further insights into vascular features by screening a large spectrum of nontumoral skin lesions. Br J Dermatol. 2004;150:226–231. doi: 10.1111/j.1365-2133.2004.05753.x. [DOI] [PubMed] [Google Scholar]
- 58.Zaballos P, Salsench E, Puig S, Malvehy J. Dermoscopy of Venous Stasis Dermatitis. Arch Dermatol. 2006;142:1526–1526. doi: 10.1001/archderm.142.11.1526. [DOI] [PubMed] [Google Scholar]
- 59.Zalaudek I, Giacomel J, Argenziano G, Hofmann-Wellenhof R, Micantonio T, Di Stefani A, et al. Dermoscopy of facial nonpigmented actinic keratosis. Br J Dermatol. 2006;155:951–956. doi: 10.1111/j.1365-2133.2006.07426.x. [DOI] [PubMed] [Google Scholar]
- 60.Zaballos P, Ara M, Puig S, Malvehy J. Dermoscopy of sebaceous hyperplasia. Arch Dermatol. 2005;141:808–808. doi: 10.1001/archderm.141.6.808. [DOI] [PubMed] [Google Scholar]
- 61.Kim NH, Zell DS, Kolm I, Oliviero M, Rabinovitz HS. The dermoscopic differential diagnosis of yellow lobularlike structures. Arch Dermatol. 2008;144:962–962. doi: 10.1001/archderm.144.7.962. [DOI] [PubMed] [Google Scholar]
- 62.Nomura M, Tanaka M, Nunomura M, Izumi M, Oryu F. Dermoscopy of Rippled Pattern Sebaceoma. Dermatol Res Pract. 2010 doi: 10.1155/2010/140486. pii: 140486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bryden AM, Dawe RS, Fleming C. Dermatoscopic features of benign sebaceous proliferation. Clin Exp Dermatol. 2004;29:676–677. doi: 10.1111/j.1365-2230.2004.1612.x. [DOI] [PubMed] [Google Scholar]
- 64.Ianhez M, Cestari Sda C, Enokihara MY, Seize MB. Dermoscopic patterns of molluscum contagiosum: a study of 211 lesions confirmed by histopathology. An Bras Dermatol. 2011;86:74–79. doi: 10.1590/s0365-05962011000100009. [DOI] [PubMed] [Google Scholar]
- 65.Menzies SW, Kreusch J, Byth K, Pizzichetta MA, Marghoob A, Braun R, et al. Dermoscopic evaluation of amelanotic and hypomelanotic melanoma. Arch Dermatol. 2008;144:1120–1127. doi: 10.1001/archderm.144.9.1120. [DOI] [PubMed] [Google Scholar]
- 66.Zalaudek I, Argenziano G, Mordente I, Moscarella E, Corona R, Sera F, et al. Nevus type in dermoscopy is related to skin type in white persons. Arch Dermatol. 2007;143:351–356. doi: 10.1001/archderm.143.3.351. [DOI] [PubMed] [Google Scholar]
- 67.Bories N, Dalle S, Debarbieux S, Balme B, Ronger-Savlé S, Thomas L. Dermoscopy of fully regressive cutaneous melanoma. Br J Dermatol. 2008;158:1224–1229. doi: 10.1111/j.1365-2133.2008.08501.x. [DOI] [PubMed] [Google Scholar]
- 68.Oiso N, Kawada A. The dermoscopic Features in Infantile Hemangioma. Pediatr Dermatol. 2011;28:591–593. doi: 10.1111/j.1525-1470.2011.01385.x. [DOI] [PubMed] [Google Scholar]
- 69.Hu SC, Ke CL, Lee CH, Wu CS, Chen GS, Cheng ST. Dermoscopy of Kaposi's sarcoma: Areas exhibiting the multicoloured 'rainbow pattern'. J Eur Acad Dermatol Venereol. 2009;23:1128–1132. doi: 10.1111/j.1468-3083.2009.03239.x. [DOI] [PubMed] [Google Scholar]
- 70.Vázquez-López F, Coto-Segura P, Fueyo-Casado A, Pérez-Oliva N. Dermoscopy of Port-Wine Stains. Arch Dermatol. 2007;143:962–962. doi: 10.1001/archderm.143.7.962. [DOI] [PubMed] [Google Scholar]
- 71.Ross EK, Vincenzi C, Tosti A. Videodermoscopy in the evaluation of hair and scalp disorders. J Am Acad Dermatol. 2006;55:799–806. doi: 10.1016/j.jaad.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 72.Vázquez-López F, Manjón-Haces JA, Maldonado-Seral C, Raya-Aguado C, Pérez- Oliva N, Marghoob AA. Dermoscopic features of plaque psoriasis and lichen planus: new observations. Dermatology. 2003;207:151–156. doi: 10.1159/000071785. [DOI] [PubMed] [Google Scholar]
- 73.Vázquez-López F, Maldonado-Seral C, Soler-Sánchez T, Perez-Oliva N, Marghoob AA. Surface microscopy for discriminating between common urticaria and urticarial vasculitis. Rheumatology (Oxford) 2003;42:1079–1082. doi: 10.1093/rheumatology/keg301. [DOI] [PubMed] [Google Scholar]
- 74.Vázquez-López F, Zaballos P, Fueyo-Casado A, Sánchez-Martín J. A dermoscopy subpattern of plaque-type psoriasis: red globular rings. Arch Dermatol. 2007;143:1612–1612. doi: 10.1001/archderm.143.12.1612. [DOI] [PubMed] [Google Scholar]
- 75.Zalaudek I, Argenziano G, Di Stefani A, Ferrara G, Marghoob AA, Hofmann- Wellenhof R, et al. Dermoscopy in General Dermatology. Dermatology. 2006;212:7–18. doi: 10.1159/000089015. [DOI] [PubMed] [Google Scholar]
- 76.Zalaudek I, Argenziano G. Dermoscopy subpatterns of inflammatory skin disorders. Arch Dermatol. 2006;142:808–808. doi: 10.1001/archderm.142.6.808. [DOI] [PubMed] [Google Scholar]
- 77.Vano-Galvan S, Alvarez-Twose I, et al. De las Heras E, Morgado JM, Matito A, Sánchez-Muñoz L. Dermoscopic Features of Skin Lesions in Patients With Mastocytosis. Arch Dermatol. 2011;147:932–940. doi: 10.1001/archdermatol.2011.190. [DOI] [PubMed] [Google Scholar]
- 78.Segal R, Mimouni D, Feuerman H, Pagovitz O, David M. Dermoscopy as a diagnostic tool in demodicidosis. Int J Dermatol. 2010;49:1018–1023. doi: 10.1111/j.1365-4632.2010.04495.x. [DOI] [PubMed] [Google Scholar]
- 79.Menzies SW, Ingvar C, Crotty KA, McCarthy WH. Frequency and morphologic characteristics of invasive melanomas lacking specific surface microscopic features. Arch Dermatol. 1996;132:1178–1182. [PubMed] [Google Scholar]
- 80.Schulz H. Epiluminescent microscopy aspects of initial cutaneous melanoma metastases. Hautarzt. 2001;52:21–25. doi: 10.1007/s001050051256. [DOI] [PubMed] [Google Scholar]
- 81.Wolf IH. Dermoscopic diagnosis of vascular lesions. Clin Dermatol. 2002;20:273–275. doi: 10.1016/s0738-081x(02)00222-5. [DOI] [PubMed] [Google Scholar]
- 82.Chuh AA. Collarette scaling in pityriasis rosea demonstrated by digital epiluminescence dermatoscopy. Australas J Dermatol. 2001;42:288–290. doi: 10.1046/j.1440-0960.2001.00538.x. [DOI] [PubMed] [Google Scholar]
- 83.Zaballos P, Puig S, Malvehy J. Dermoscopy of pigmented purpuric dermatoses (lichen aureus): a useful tool for clinical diagnosis. Arch Dermatol. 2004;140:1290–1291. doi: 10.1001/archderm.140.10.1290. [DOI] [PubMed] [Google Scholar]